Method for preparing pharmaceutical cocrystals through suspension crystallization
A technology for preparing drugs and crystallization, which is applied in the field of preparation of drug co-crystals by suspension crystallization, can solve problems such as insufficient understanding of process mechanism and product impurity, and achieve the effects of reducing solvent recycling, reducing energy consumption, and saving costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
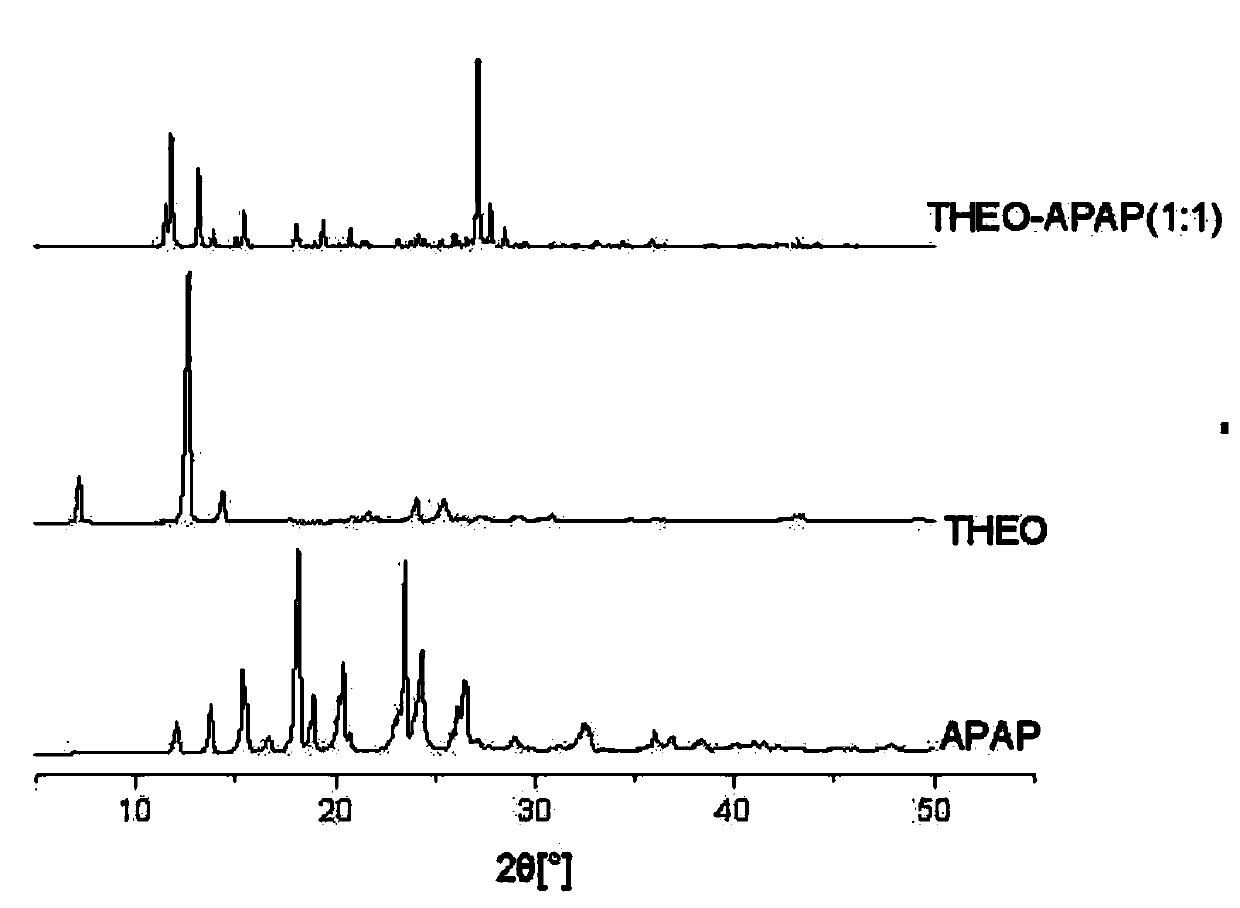
[0035] Preparation of theophylline-acetaminophen (THEO-APAP) co-crystal in six different solvents: methanol, ethanol, acetonitrile, acetone, butanone, and nitromethane:
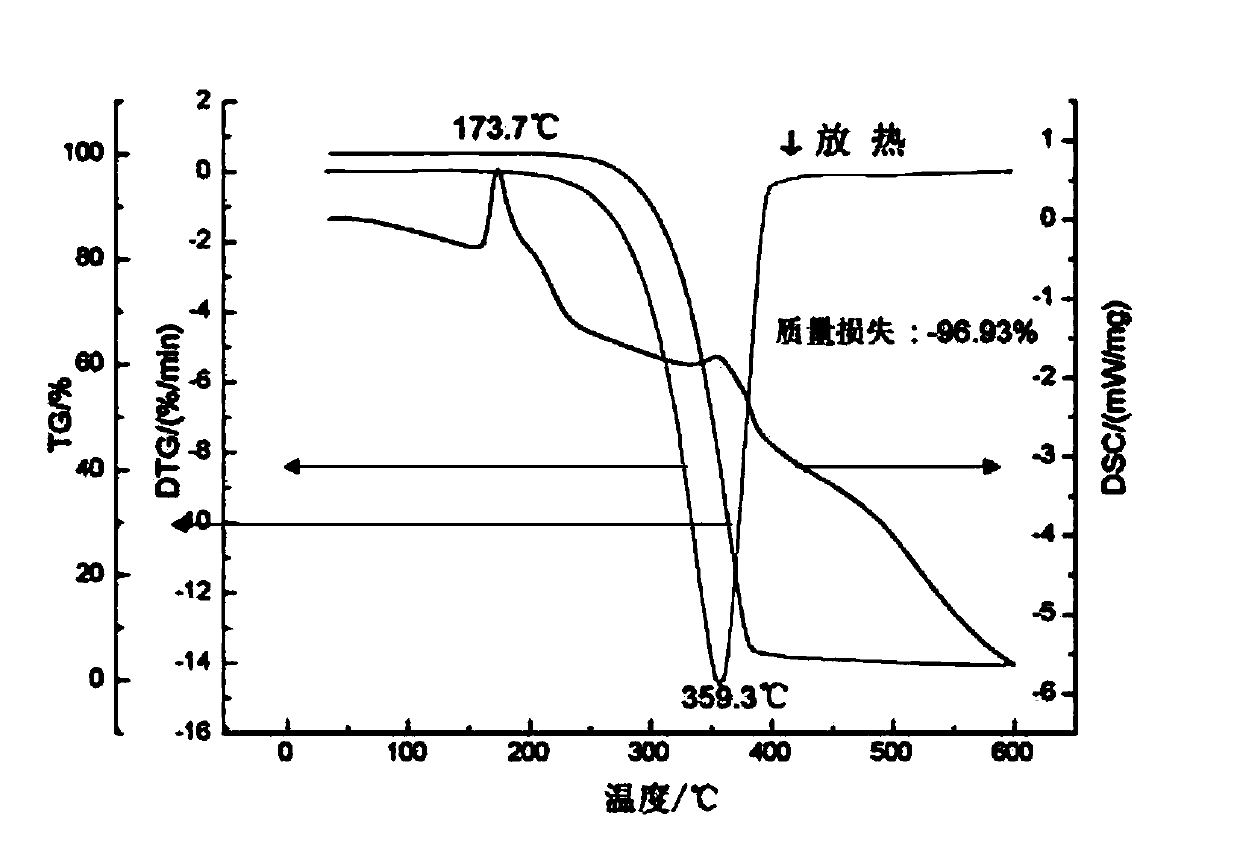
[0036] Among these six solvents, theophylline (THEO) is used as API, and acetaminophen (APAP) is used as CCF. There is no solvate in THEO and APAP in the six solvents. 1:1 co-crystal theophylline-acetaminophen. First prepare 1000 mL of a saturated solution of APAP paracetamol. In the six solvents of methanol, ethanol, acetonitrile, acetone, butanone, and nitromethane, the solubility of APAP acetaminophen is 216.3, 139.9, 22.2, 73.6, 48.0, 3.9 mg / mL, and the solubility of THEO theophylline is respectively 6.1, 3.4, 1.6, 2.3, 1.6, 1.5 mg / mL. Add 18.0g of theophylline THEO (0.1mol) and 15.1g of acetaminophen APAP (0.1mol) into the saturated solution of APAP, so that each of THEO and APAP put into the CCF saturated solution is 0.1mol, and the solid loading rate is 33.1g / L. After the suspension was thoroughly...
Embodiment 2
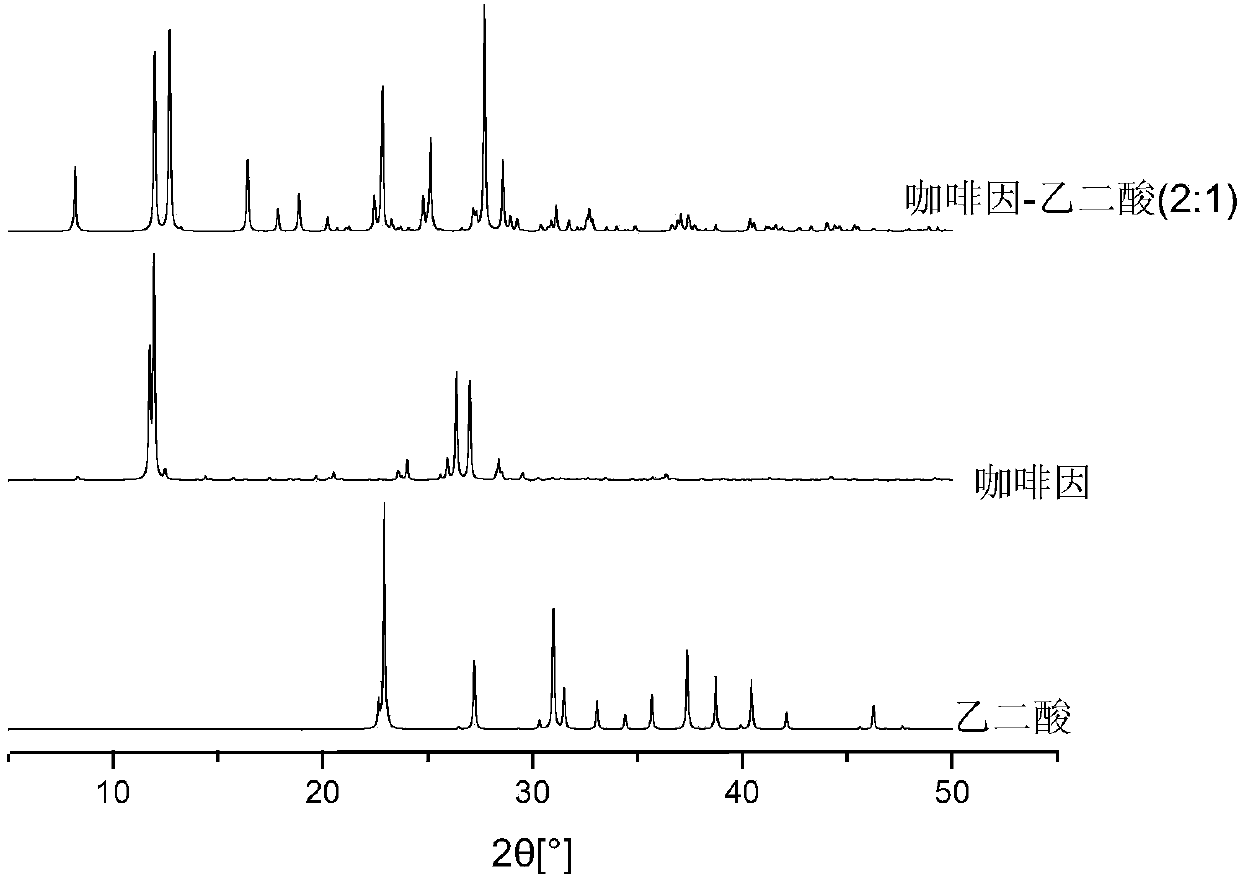
[0038] Caffeine and oxalic acid, maleic acid, and malonic acid form eutectics with a molar ratio of 2:1 in acetonitrile
[0039] In acetonitrile, caffeine (CAF) was used as API, oxalic acid (OXA), maleic acid (MEI), and malonic acid (MON) were used as CCF respectively, and neither API nor the three different CCFs were present in acetonitrile solvate.
[0040] Prepare 1000 mL of a saturated solution of oxalic acid OXA. Add 77.7g caffeine (0.4mol) and 18.0g oxalic acid (0.2mol) into the saturated solution, so that the molar ratio of CAF and OXA put into the CCF saturated solution is 2:1, and the solid loading rate is 95.7g / L. After fully mixing and stirring at 25° C. for 6 hours, the solid was taken for XRD test, which proved that the solid was a pure eutectic phase. The resulting solid is vacuum filtered, washed with CCF saturated solution, and the solid obtained after the drying process is 2CAF-OXA ( image 3 ). The XRD characteristic peaks of all substances are consistent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com