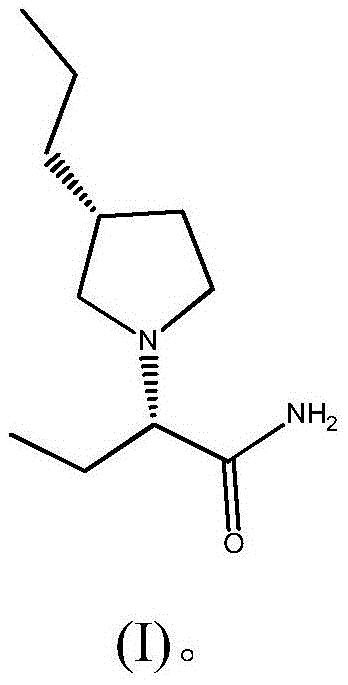
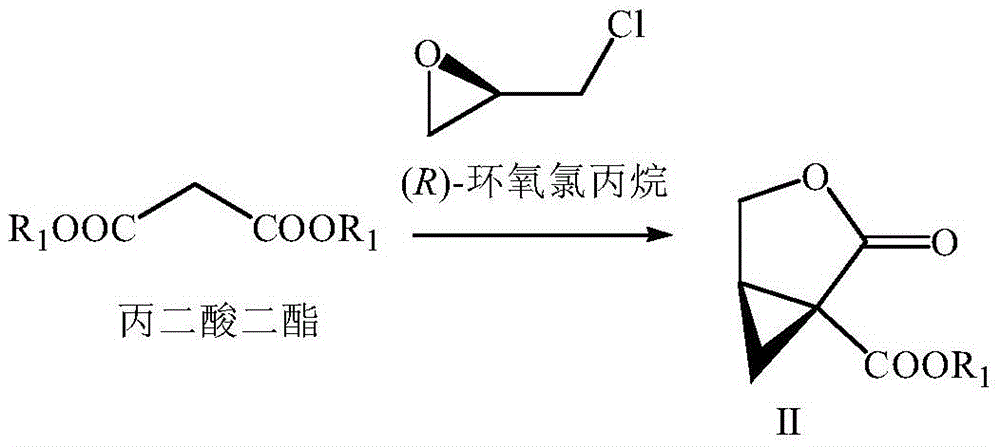
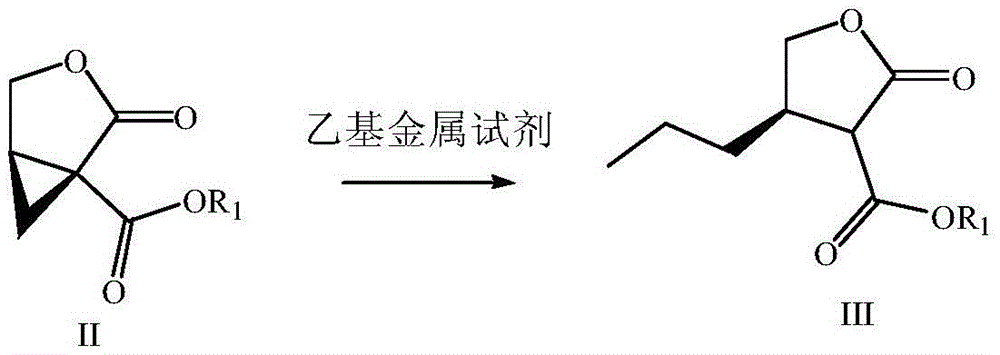
Preparation method of brivaracetam
A reaction and selection technology, applied in the field of drug synthesis, can solve the problems of high production cost, cumbersome separation and purification steps, and unsuitability for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] In this embodiment, Buvaracetam is prepared by the following method, which specifically includes the following steps:
[0074] (1) Lactone (II) (wherein R 1 For the preparation of phenyl)
[0075] Add toluene (300mL) and sodium amide (8.58g, 0.22mol) in a 1000mL three-necked flask with mechanical stirring, and cool down to 10°C, slowly drop diphenyl malonate (25.62g, 0.1mol), control The temperature does not exceed 15°C. After the drop is completed, stir at 10-15°C for 60 minutes. (R)-Epichlorohydrin (10.18g, 0.11mol) is slowly added dropwise to control the temperature not exceeding 30°C. Stir for 5 hours, slowly add water (100mL) to control the temperature not to exceed 30°C, separate layers, water (100mL) and saturated brine (100mL) in the toluene layer, concentrate part of the toluene, then cool down to precipitate a solid, filter and dry to obtain 19.96g of Yellow solid lactone (II) (R 1 For phenyl), the yield is 91%, ee (enantiomeric excess percentage)>99%.
[...
Embodiment 2
[0089] In this embodiment, Buvaracetam is prepared by the following method, which specifically includes the following steps:
[0090] (1) Lactone (II) (wherein R 1 For the preparation of phenyl)
[0091] Add toluene (300mL) and sodium amide (8.58g, 0.22mol) in a 1000mL three-necked flask with mechanical stirring, and cool down to 10°C, slowly drop diphenyl malonate (25.62g, 0.1mol), control The temperature does not exceed 15°C. After the drop is completed, stir at 10-15°C for 60 minutes. (R)-Epichlorohydrin (10.18g, 0.11mol) is slowly added dropwise to control the temperature not to exceed 30°C. Stir at ℃ for 5 hours, slowly add water (100mL) to control the temperature not to exceed 30℃, separate layers, water (100mL) and saturated brine (100mL) in the toluene layer, concentrate part of the toluene, then cool down to precipitate a solid, filter and dry to obtain 19.96g Pale yellow solid lactone (II) (R 1 For phenyl), the yield is 91%, ee>99%.
[0092] Step (1) product NMR ...
Embodiment 3
[0106] In this embodiment, Buvaracetam is prepared by the following method, which specifically includes the following steps:
[0107] (1) Lactone (II) (wherein R 1 For the preparation of phenyl)
[0108] Add toluene (300mL) and sodium amide (8.58g, 0.22mol) in a 1000mL three-necked flask with mechanical stirring, and cool down to 10°C, slowly drop diphenyl malonate (25.62g, 0.1mol), control The temperature does not exceed 15°C. After the addition, stir at 10-15°C for 60 minutes. (R)-Epichlorohydrin (9.25g, 0.1mol) is slowly added dropwise to control the temperature not exceeding 30°C. Stir for 8 hours, slowly add water (100mL) to control the temperature not to exceed 30°C, separate layers, water (100mL) and saturated brine (100mL) in the toluene layer, concentrate part of the toluene, then cool down to precipitate a solid, filter and dry to obtain 19.74g Yellow solid lactone (II) (R 1 For phenyl), the yield is 90%, ee>99%.
[0109] Step (1) product NMR characterization res...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com