Synthesis method of brivaracetam intermediate and brivaracetam
A synthetic method and intermediate technology, applied in the field of medicine, can solve the problems of not being suitable for industrial production, difficult amide condensation, high production cost, etc., and achieve the advantages of synthetic cost advantages, easy control of reaction conditions, and large processing capacity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
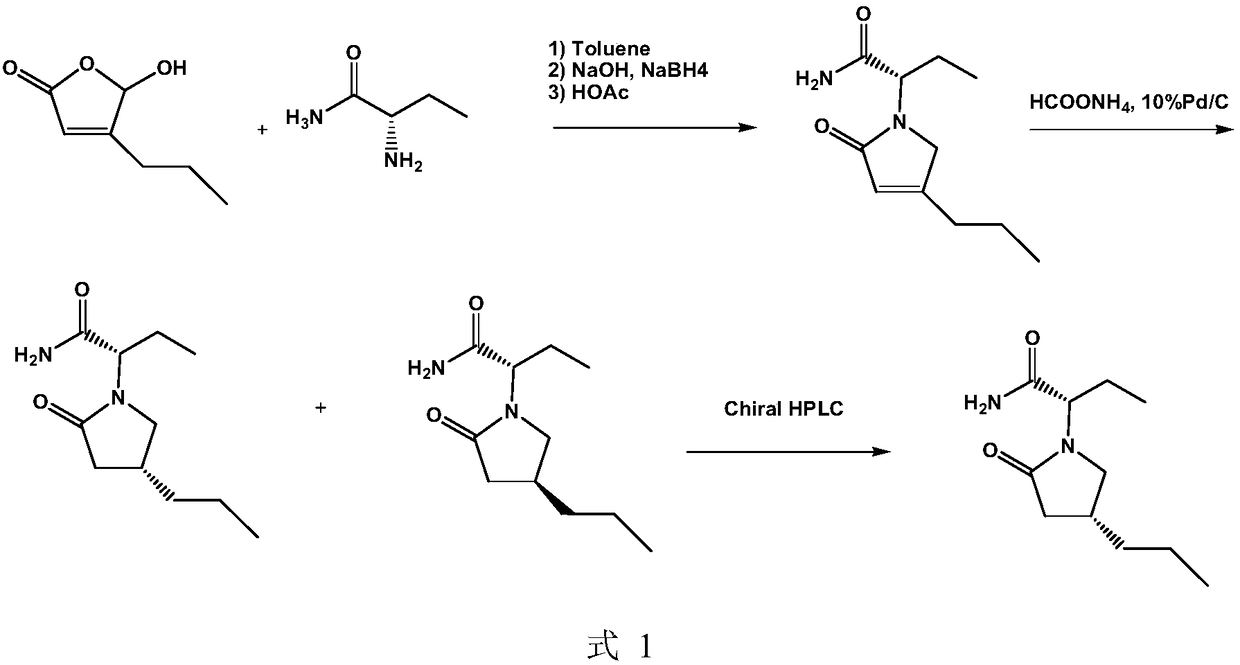
[0086] Preparation of Compound 2: Add Compound 1 (50g, 352mmol) into toluene (400ml), slowly add an aqueous solution of sodium borohydride (13.30g, 352mmol) dropwise under nitrogen atmosphere at 0°C, and react at room temperature for 4h. The reaction was quenched by slow dropwise addition of acetic acid. The aqueous phase was extracted twice with toluene. The organic phases were combined, washed with 10% sodium bicarbonate solution, washed with brine, and concentrated in vacuo to obtain an orange oil, namely compound 2.
[0087] The yield is 80%, HPLC>99%, 1 H NMR (300MHz, CDCl 3 ): δ5.83(s,1H), 4.73(s,2H), 2.38(t,2H), 1.55–1.70(m,2H), 1.00(t,3H).
[0088] The preparation of compound 3: put compound 2 (10.00g, 126.2mmol) and 80ml methanol into the hydrogenation kettle, drop into Raney nickel (0.20g) under nitrogen atmosphere, nitrogen replacement 3 times, hydrogen replacement 3 times, 0.45MPa hydrogen pressure, Reaction at 45°C for 5h. It was filtered through celite and c...
Embodiment 2
[0098] Preparation of compound 2: Add compound 1 (50g, 352mmol) into toluene (400ml), slowly add potassium borohydride (16.2g, 300mmol) aqueous solution dropwise under nitrogen atmosphere at 0°C, and react at room temperature for 4h. The reaction was quenched by slow dropwise addition of acetic acid. The aqueous phase was extracted twice with toluene. The organic phases were combined, washed with 10% sodium bicarbonate solution, washed with brine, and concentrated in vacuo to obtain an orange oil, namely compound 2.
[0099] Yield is 78%, HPLC>99%, 1 H NMR (300MHz, CDCl 3 ): δ5.83(s,1H),4.73(s,2H),2.38(t,J 1 / 4 7.6Hz, 2H), 1.55–1.70(m, 2H), 1.00(t, J 1 / 4 7.0Hz, 3H).
[0100]The preparation of compound 3: put compound 2 (10.00g, 126mmol) and 80ml methanol into the hydrogenation kettle, put 10% palladium carbon (0.20g) under nitrogen atmosphere, nitrogen replacement 3 times, hydrogen replacement 3 times, 1.2MPa hydrogen pressure, Reaction at room temperature for 4h....
Embodiment 3
[0110] Preparation of compound 2: Add compound 1 (75g, 528mmol) into toluene (600ml), under nitrogen atmosphere at 0°C, slowly add an aqueous solution of lithium borohydride (9.5g, 450mmol) dropwise, and react at room temperature for 5h. The reaction was quenched by slow dropwise addition of acetic acid. The aqueous phase was extracted twice with toluene. The organic phases were combined, washed with 10% sodium bicarbonate solution, washed with brine, and concentrated in vacuo to obtain an orange oil, namely compound 2.
[0111] Yield is 71%, HPLC>99%, 1 H NMR (300MHz, CDCl 3 ): δ5.83(s,1H),4.73(s,2H),2.38(t,J 1 / 4 7.6Hz, 2H), 1.55–1.70(m, 2H), 1.00(t, J 1 / 4 7.0Hz, 3H).
[0112] The preparation of compound 3: put compound 2 (20.00g, 252mmol) and 160ml methanol into the hydrogenation kettle, put 5% palladium carbon (0.45g) under nitrogen atmosphere, nitrogen replacement 3 times, hydrogen replacement 3 times, 2.0MPa hydrogen pressure, Reaction at room temperature fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com