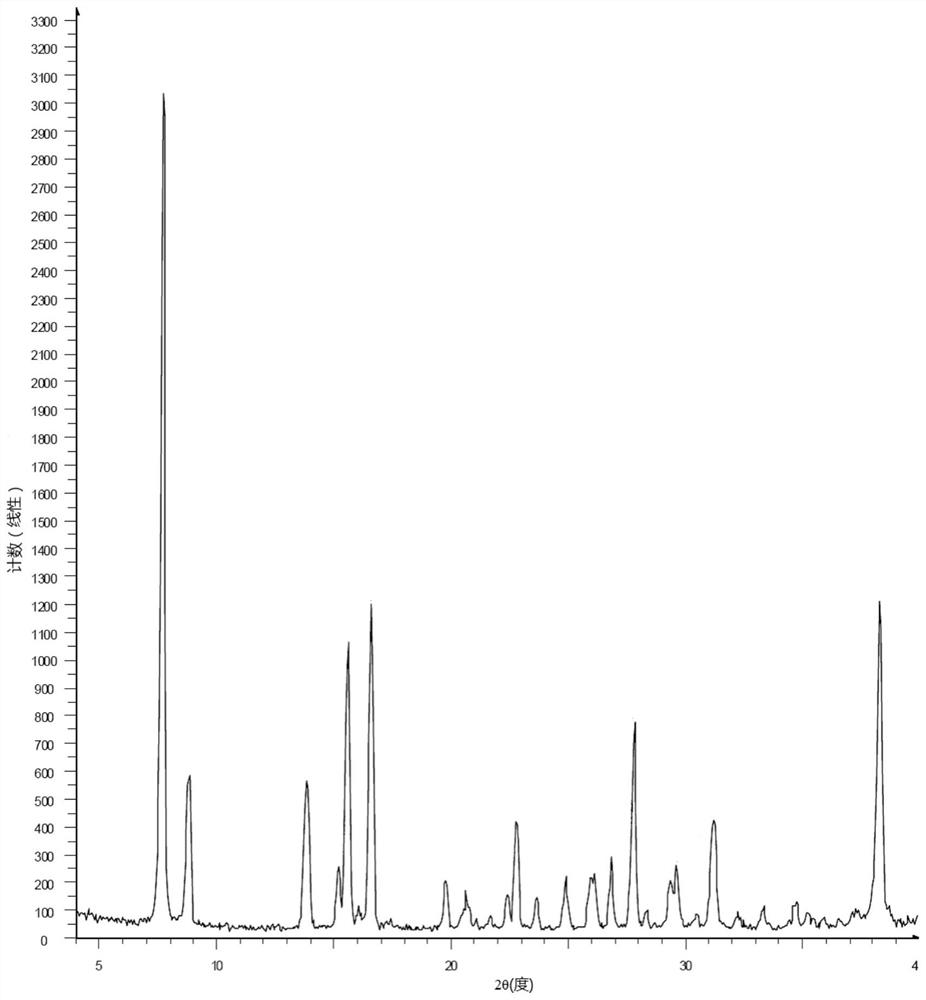
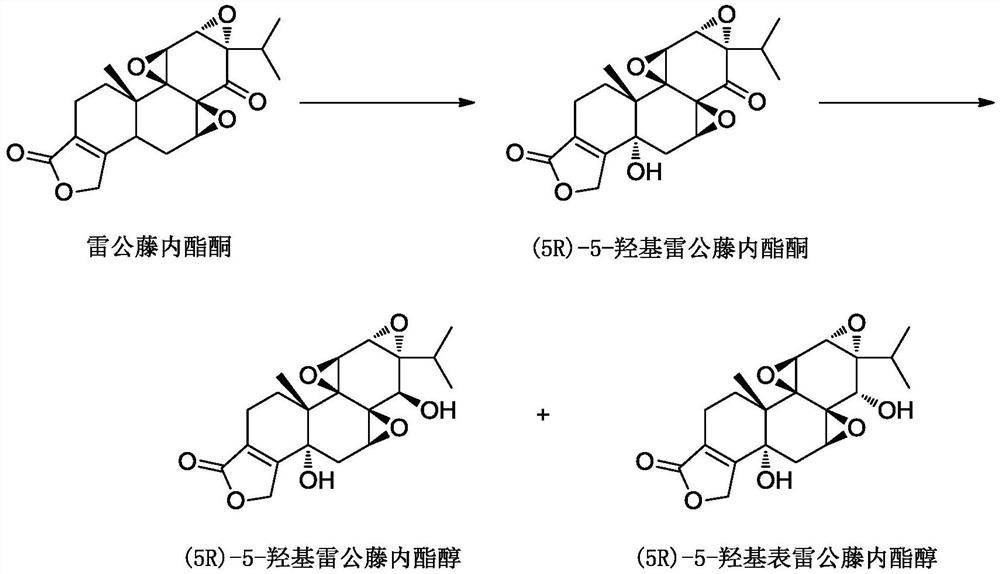
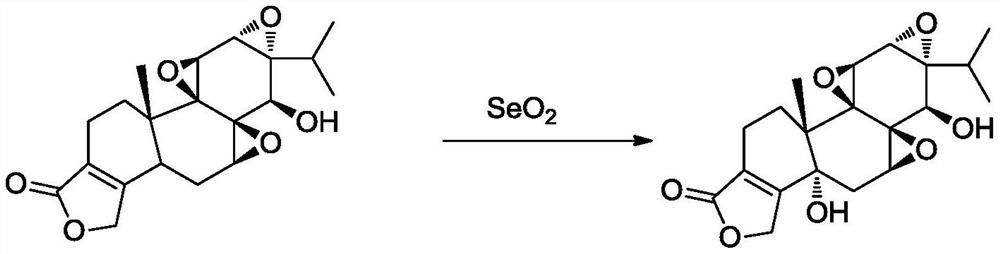
The preparation method of (5r)-5-hydroxy triptolide
A technology of lactone and hydroxysuccinimide, applied in the direction of organic chemistry, organic chemistry, etc., can solve the problems of using highly toxic chemicals, low yield, needing chiral separation, etc., to avoid chiral separation , good yield, easy industrial production effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The preparation of embodiment 1 O-acetyl triptolide
[0059]
[0060] At room temperature, 300 mL of pyridine was added into a 1000 mL three-necked flask, and 30 g of triptolide (T2) and 0.3 g of p-dimethylaminopyridine (DMAP) were added at one time under stirring. After the solution was clear, 75g of acetic anhydride was added dropwise. After 15 minutes of dripping, keep the reaction at 25±5°C. After 80 minutes, TLC (petroleum ether: ethyl acetate = 1:1, kedde color development) detected that the raw material T2 disappeared. The reaction solution was transferred to a rotary evaporator and evaporated to dryness below 40°C. Add 300mL of ethyl acetate to dissolve, wash with 150mL x 3 water in the separatory funnel, dry the ethyl acetate layer with 12g of anhydrous magnesium sulfate for 30 minutes, filter, then rinse with 60mL of ethyl acetate, combine the ethyl acetate, and rotary evaporate to dryness Obtained 35.5 g of off-white foamy solid, purity: 88.5%.
[0061...
Embodiment 2
[0062] Example 2 Preparation of O-acetyl-(5R)-5-hydroxy triptolide
[0063]
[0064] At room temperature, add 600 mL of acetonitrile and 60 mL of water into a 1000 mL eggplant-shaped bottle, add 35.5 g of O-Ac-T2 obtained in Example 1, and stir to dissolve. Add 9.6g of N-hydroxysuccinimide (HOSu) at one time, and then add CrO 3 41.7g, heated to 35±2°C and reacted for 20 hours. TLC (petroleum ether: ethyl acetate = 1:1, kedde color development) detected the disappearance of O-Ac-T2, and the reaction was terminated.
[0065] Transfer the reaction solution into a 5L separatory funnel, add 300mL of water, extract once with 1500mL of ethyl acetate, once with 300mL, and wash the combined ethyl acetate with 300mL of 5% aqueous sodium bisulfite solution once, once with 150mL in the separatory funnel, and then Wash with water 300 mL x 3. The ethyl acetate layer was dried with 60 g of anhydrous magnesium sulfate for 30 minutes, filtered, rinsed with 180 mL of ethyl acetate, and t...
Embodiment 3
[0067] The preparation of embodiment 3 (5R)-5-hydroxy triptolide
[0068]
[0069] At room temperature, 510 mL of methanol was added to a 2-liter flask, 30.7 g of O-Ac-T8 obtained in Example 2 was added, stirred and dissolved, and the temperature was lowered to -1°C. Add 360 mL of 50% hydrazine hydrate dropwise, and control the temperature at -1 to 1°C. After 30 minutes of dripping, keep the warm reaction for 30 minutes, TLC (DCM: ethyl acetate = 3:1, kedde color detection) O-Ac-T8 disappeared. Cool down to -20°C, add 4N sulfuric acid solution dropwise, keep the internal temperature not exceeding -10°C, the end point is pH 5-6, spin the solution below 40°C to remove methanol, extract with ethyl acetate 500mL x 3, and combine the extracts Then washed with 300 mL of water, and the ethyl acetate layer was rotary evaporated to constant weight. (5R)-5-Hydroxytriptolide (T8) 14.9 g was obtained by rapid separation on a silica gel column, with a purity of 99.6%.
[0070] The ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com