Method for preparing (S)-2-aminobutanamide hydrochloride
A technology of aminobutyramide and hydrochloride, which is applied in the field of preparation of levetiracetam intermediates, can solve the problems of potential safety hazards, high temperature and pressure, and high cost of desalination, and can avoid chiral separation, process The effect of controllable stability enhancement and avoiding a large number of outgassing reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A preparation method of (S)-2-aminobutyramide hydrochloride, comprising the following steps: (1) using n-propionaldehyde as raw material to react with sodium bisulfite, potassium cyanide and R-phenylglycinol to obtain normal Intermediate 1;
[0033] The ingredient ratio is:
[0034] Product name Dosage (g) Specification Remarks
[0035] Potassium cyanide 385 30%
[0036] R-Phenylglycine 236 Industrial
[0037] Sodium bisulfite 200 Industrial
[0038] Propionaldehyde 100 Industrial
[0039] Ethyl acetate 600 Industrial Recyclable1
[0040] Petroleum ether 600 Industrial
[0041] The specific operation steps are: add 385g of potassium cyanide with a mass fraction of 30% and 100g of n-propanal in a 2L three-necked flask, add 200g of sodium bisulfite after stirring, stir to dissolve, cool in a water bath, and dropwise add 236g of R-phenylglycinol aqueous solution was dropped within about 1 hour, then stirred at 70-80°C for 6 hours, and the propionaldehyde basically d...
Embodiment 2
[0059] A preparation method of (S)-2-aminobutanamide hydrochloride, comprising the following steps: (1) reacting n-propanal, sodium bisulfite, potassium cyanide and R-phenylglycinol as raw materials to prepare Intermediate 1; the mass ratio of n-propionaldehyde, sodium bisulfite, potassium cyanide and R-phenylglycinol is 1:1:1.1:1.1, and the reaction temperature is 40°C;
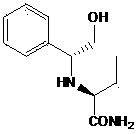
[0060] The structural formula of intermediate 1 is:
[0061]
[0062] The reaction equation is:
[0063] ;
[0064] (2) Hydrolysis: add sodium hydroxide solution to intermediate 1, and hydrolyze to obtain intermediate 2; the mass fraction of sodium hydroxide solution is 30%, and the amount of sodium hydroxide added is 1 g of sodium hydroxide per gram of n-propionaldehyde solution, the reaction temperature is 100°C;
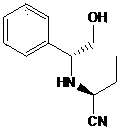
[0065] The structural formula of intermediate 2 is:
[0066]
[0067] The reaction equation is:
[0068] ;
[0069] (3) Hydrogenation reaction: add a catalyst, and hydrogenate the ...
Embodiment 3
[0073] A preparation method of (S)-2-aminobutanamide hydrochloride, comprising the following steps: (1) reacting n-propanal, sodium bisulfite, potassium cyanide and R-phenylglycinol as raw materials to prepare Intermediate 1; the mass ratio of n-propionaldehyde, sodium bisulfite, potassium cyanide and R-phenylglycinol is 1:1.5:1:1.5, and the reaction temperature is 50°C;
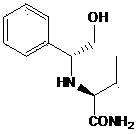
[0074] The structural formula of intermediate 1 is:
[0075]
[0076] The reaction equation is:
[0077] ;
[0078] (2) Hydrolysis: add sodium hydroxide solution to intermediate 1, and hydrolyze to obtain intermediate 2; the mass fraction of sodium hydroxide solution is 30%, and the amount of sodium hydroxide added is 1.5g of hydroxide per gram of n-propionaldehyde Sodium solution, the reaction temperature is 120°C;
[0079] The structural formula of intermediate 2 is:
[0080]
[0081] The reaction equation is:
[0082] ;
[0083] (3) Hydrogenation reaction: add a catalyst, and hydrogenate t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com