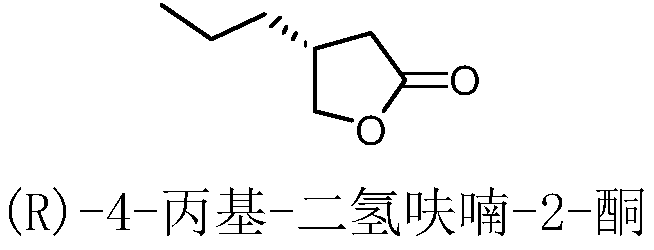
Method for preparing brivaracetam intermediate
An intermediate and reaction system technology, applied in the field of biological enzyme catalysis, can solve the problem of high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] The synthesis of embodiment 1 compound 3
[0113]
[0114] Dissolve 30g (1.09eq) of morpholine in 90mL of n-heptane, cool down to 5°C, add dropwise 50% glyoxylic acid aqueous solution (46g, 1.0eq) to control the temperature not to exceed 40°C, and control the temperature for 25-30 ℃ reaction 2h. Slowly add 29g of n-valeraldehyde dropwise, and control the temperature not to be higher than 40°C, and react at 40°C for 16h after the drop is completed. Cool down to 20°C, add 50 mL of concentrated hydrochloric acid, extract with n-heptane, adjust the pH to 1.0 with aqueous sodium hydroxide solution, extract three times with tertiary methyl ether, combine the organic phases and concentrate under reduced pressure to obtain compound 3 (43 g, yield 96%). (refer to patent WO2005028435A1)
[0115] 1 H NMR (300MHz, CDCl 3 )δ6.00(s,1H),5.84(t,J=1.8Hz,1H),4.65(bs,1H,exchange with D 2 O),2.25–2.52(m,2H),1.52–1.77(m,2H),1.00(t,J=7.6Hz,3H).
[0116] 13 C NMR (75MHz, CDCl 3 )δ1...
Embodiment 2
[0117] The synthesis of embodiment 2 compound 2
[0118]
[0119] Compound 3 (14.2 g) was dissolved in 70 mL of ethanol, 0.7 g of palladium carbon (10% Pd) was added to replace the nitrogen, and then hydrogen gas (1 atmosphere) was introduced to react at room temperature for 6 h. The reaction was complete by GC detection. The palladium carbon was removed by filtration, and the ethanol was removed by concentration under reduced pressure to obtain compound 2 (14 g, yield 100%).
Embodiment 3
[0120] The preparation of embodiment 3 alcohol dehydrogenase
[0121] 3.1 Acquisition of enzyme gene
[0122] Alcohol dehydrogenase (ADH) in the following table was retrieved from NCBI, according to the sequence of the enzyme gene, the whole synthetase gene.
[0123] Table 1 Enzymes
[0124]
[0125]
[0126] 3.2 Expression of enzyme gene
[0127] Link the enzyme gene to pET28a, the restriction site NdeI&HindIII, and the carrier with the enzyme to transform the host Escherichia coli BL21 competent cells; the strain is inoculated with LB culture at 37°C, 200rpm shaker, when the OD600 reaches about 0.8, Take the bacterial solution and add sterile glycerol with a final concentration of 25%, after numbering, store it in a -80°C low-temperature refrigerator for future use.
[0128] 3.3 Cultivation of enzyme strains
[0129] The composition of LB liquid medium: peptone 10g / L, yeast powder 5g / L, NaCl 10g / L, dissolved in deionized water and then constant volume, sterilized a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com