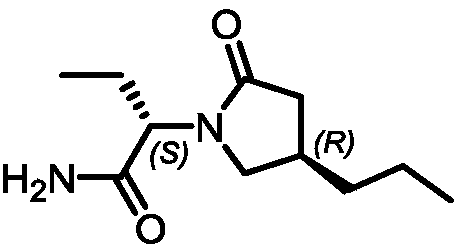
Preparation method of (R)-3-propyl-gamma-butyrolactone
A technology of butyrolactone and propyl group is applied in the field of preparation of antiepileptic drug brivaracetam intermediate-3-propyl-γ-butyrolactone, and can solve the problems of chiral column separation, low yield and chemical selection. problems such as poor performance, to achieve the effects of simple operation, high yield, good application value and economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (a) Preparation of (S)-3-p-toluenesulfonyl-γ-butyrolactone
[0045] In a 250mL single-necked flask, the raw material (S)-3-hydroxy-γ-butyrolactone (10.0g, 98mmol) was dissolved in 20.0mL of dichloromethane, the temperature was controlled at -30°C, and triethylamine (20.0g, 198mmol), and stirred evenly. p-Toluenesulfonyl chloride (30.0 g, 157 mmol) was dissolved in 40.0 mL of dichloromethane, slowly added dropwise to the above reaction system, stirred overnight at room temperature (12 hours of reaction), and TLC detected that the reaction of the raw materials was complete. The pH was adjusted to 5 with 3 mol / L hydrochloric acid, the liquid was separated, the organic phase was washed with water and saturated brine, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and purified by column chromatography to obtain 20.3 g of a white solid with a yield of 81%.
[0046] 1 H NMR (DMSO, 400MHz) δ: 7.72-7.81(d,2H), 7.44-7.53(d,2H), 5.32-5.48(m,1H), 4.33-4...
Embodiment 2
[0054] (a) Preparation of (S)-3-p-toluenesulfonyl-γ-butyrolactone
[0055] In a 250mL single-necked flask, take the raw material (S)-3-hydroxy-γ-butyrolactone (13.0g, 127mmol), dissolve it in 26.0mL toluene, put it in an ice bath, and add N-methylmorpholine (25.8g, 255mmol) , stir well. Dissolve p-toluenesulfonyl chloride (48.5g, 254mmol) in 70.0mL toluene, and slowly add it dropwise to the above reaction system, the reaction produces a large amount of solid triethylamine hydrochloride, and the enlarged reaction requires mechanical stirring, and the reaction is stirred for 12 hours. TLC detects that the reaction of raw materials is complete.
[0056] Suction filtration, wash the filter cake with 13mL toluene, adjust the pH of the filtrate to 5 with 3mol / L hydrochloric acid, neutralize the excess triethylamine, continue suction filtration, separate the filtrate, and extract the water layer with toluene (26.0mL) for 1 ~2 times, combine the organic phases. Washed with water, w...
Embodiment 3
[0061] (a) Preparation of (S)-3-p-toluenesulfonyl-γ-butyrolactone
[0062] In a 250mL single-necked flask, take the raw material (S)-3-hydroxy-γ-butyrolactone (13.0g, 127mmol), dissolve it in 26.0mL of tetrahydrofuran, put it in an ice bath, add pyridine (20.2g, 255mmol), and stir well. Dissolve p-toluenesulfonyl chloride (24.3g, 127mmol) in 70.0mL tetrahydrofuran, and slowly add it dropwise to the above reaction system, the reaction produces a large amount of solid triethylamine hydrochloride, and the enlarged reaction requires mechanical stirring, and the reaction is stirred for 15 hours. TLC detects that the reaction of raw materials is complete.
[0063] Suction filtration, wash the filter cake with 13mL tetrahydrofuran, adjust the pH of the filtrate to 5 with 3mol / L hydrochloric acid, neutralize the excess triethylamine, continue suction filtration, separate the filtrate, and extract the water layer with tetrahydrofuran (26.0mL) for 1 ~2 times, combine the organic phases...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com