Brivaracetam controlled-release preparation and preparation method thereof
A technology of membrane control and excipient, applied in the field of anti-epileptic drug briracetam controlled-release tablet and its preparation, can solve the problems of solvent residual environment and pollution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
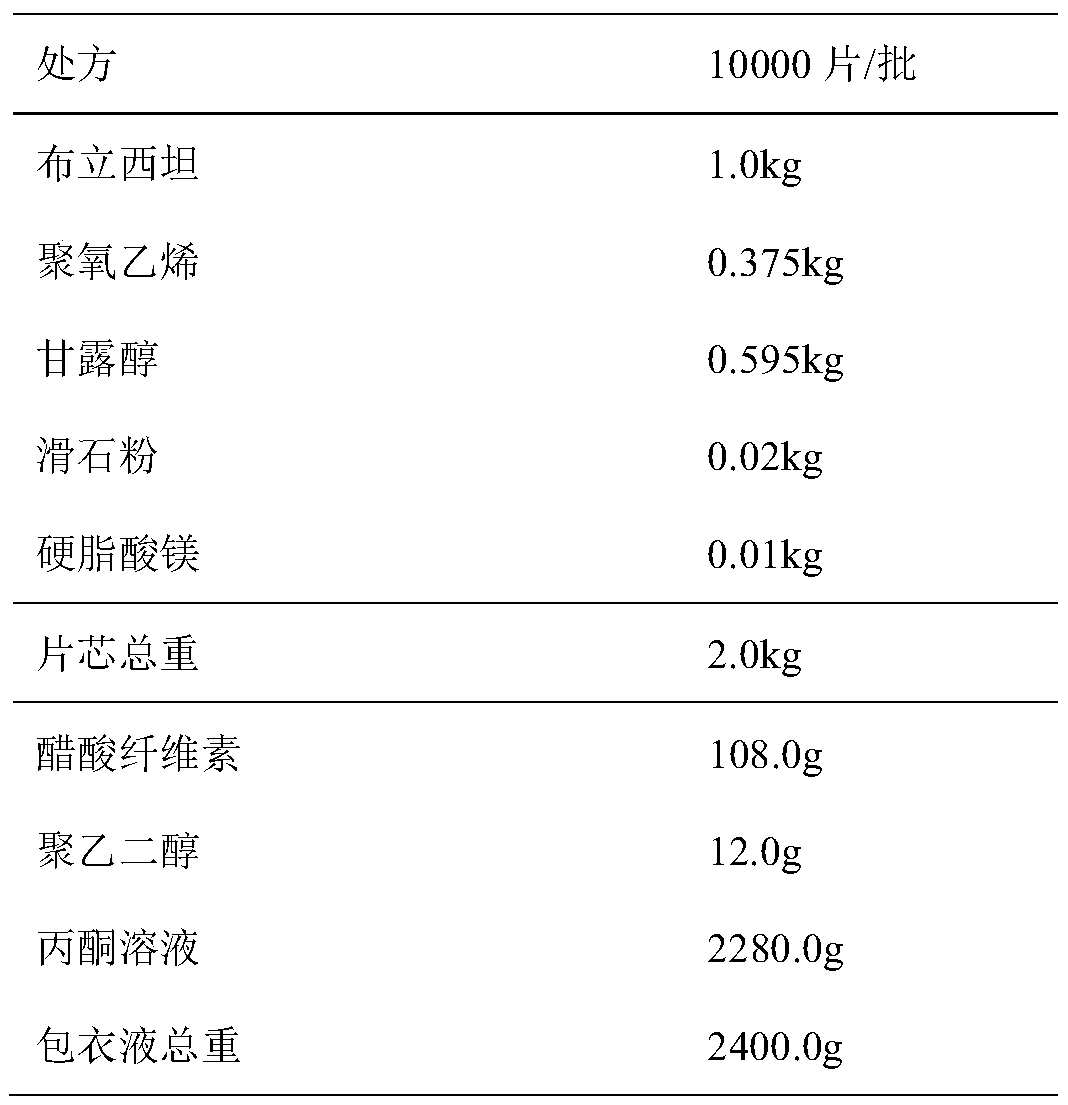
[0027] Cellulose acetate as film-forming material
[0028]
[0029] Process:
[0030] (1) After pre-mixing polyoxyethylene as a hydrophilic matrix with mannitol for 5 minutes, add the bulk drug of crystal form A and mix for 5 minutes, then add talc powder and magnesium stearate in sequence, mix for 2 minutes and 1 minute respectively, and press into tablets. That is the tablet core.
[0031] (2) Add polyethylene glycol and cellulose acetate in sequence to the acetone solution, stir and dissolve to obtain a semi-permeable membrane coating solution.
[0032] (3) Place the tablet core in a high-efficiency coating machine for semi-permeable membrane coating, spray pressure 0.1mPa, host speed 10rpm, air inlet temperature 35-40°C, material temperature 28-32°C, liquid spray rate 4-40°C 6g / min, take out the coated tablet when the coating weight gain reaches 5%, place it in a 40°C oven for static aging for 24 hours, and punch a hole in the center of the front of the coated special-s...
Embodiment 2
[0034] Acrylic resin polymer as film-forming material
[0035]
[0036] Process:
[0037] (1) After premixing hypromellose as a hydrophilic matrix with sorbitol and microcrystalline cellulose for 5 minutes, add the raw material drug of crystal form A and mix for 5 minutes, and then add colloidal silicon dioxide and magnesium stearate in sequence , mixed for 2 minutes and 1 minute respectively, and pressed into tablets to obtain tablet cores.
[0038] (2) Pour talcum powder and triethyl citrate into water, homogenize with a high-shear homogenizer, pour into Eudragit RL30D / RS30D water dispersion before use, and filter through a 40-mesh sieve. Stir continuously during the coating process to obtain a semi-permeable membrane coating solution.
[0039] (3) Place the tablet core in a high-efficiency coating machine for semi-permeable membrane coating, spray pressure 0.1mPa, host speed 10rpm, air inlet temperature 35-40°C, material temperature 28-32°C, liquid spray rate 4-40°C 6...
Embodiment 3
[0040] Embodiment 3 Drug release hole position research
[0041] Using the prescription process in Example 2, holes were respectively punched on the side of the short axis and the side of the long axis of the coated special-shaped tablet (12.6*5.4 mm), with a hole diameter of 0.6 mm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com