Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
90 results about "Chlorosulfonyl isocyanate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chlorosulfonyl isocyanate is the chemical compound ClSO₂NCO, known as CSI. This compound is a versatile reagent in organic synthesis.
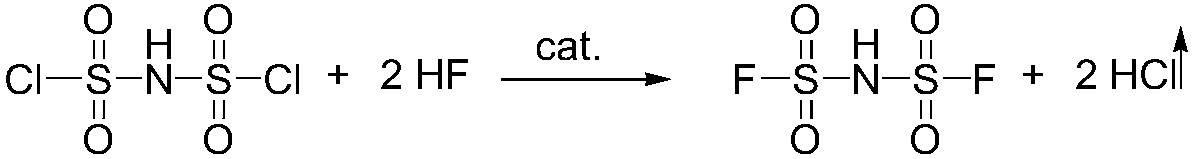
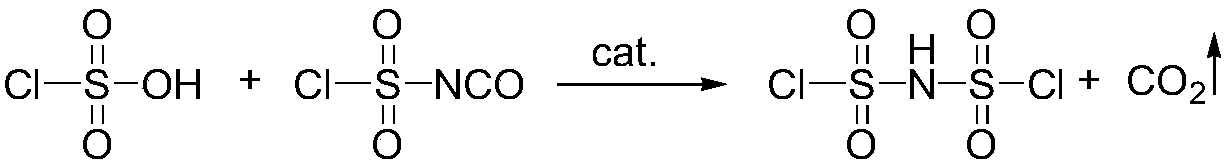
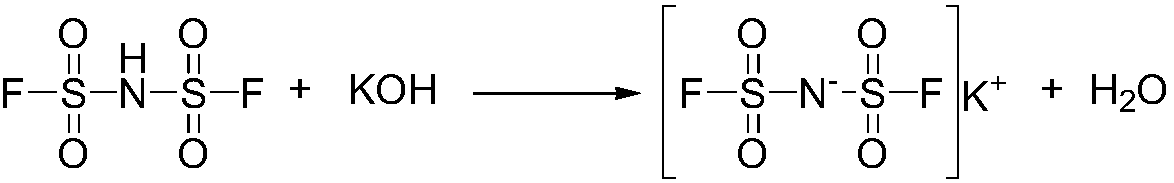
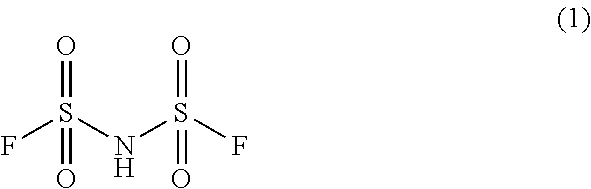
Preparation method of imidodisulfuryl fluoride lithium salt
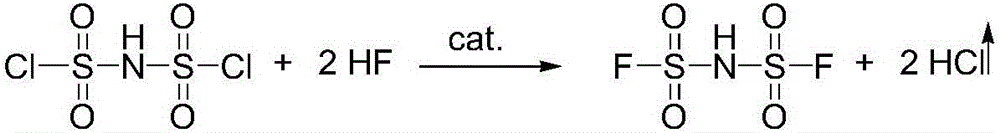
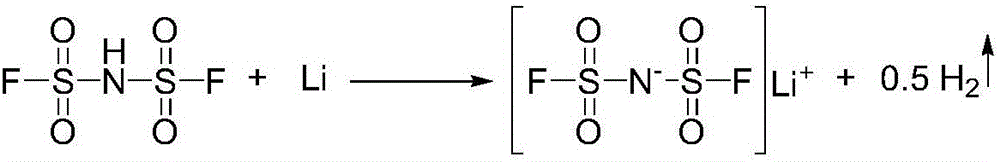
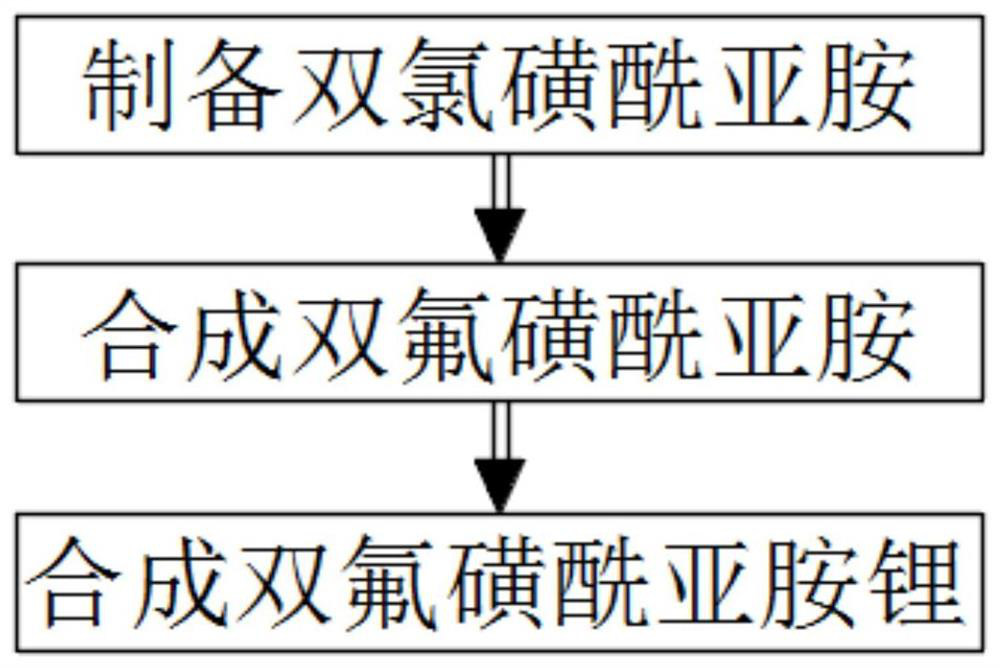
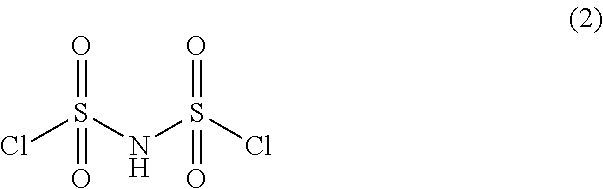
The invention relates to the field of chemical synthesizing, in particular to a preparation method of imidodisulfuryl fluoride lithium salt. The preparation method includes the steps of firstly, allowing chlorosulfonic acid and chlorosulfonyl isocyanate to react in the presence of catalyst to obtain dichlorosulfimide; secondly, allowing dichlorosulfimide and hydrogen fluoride to react in the presence of catalyst to obtain imidodisulfuryl fluoride; thirdly, allowing imidodisulfuryl fluoride to react with compound containing lithium to obtain the imidodisulfuryl fluoride lithium salt. The preparation method has the advantages that the chlorosulfonic acid and chlorosulfonyl isocyanate are used as the raw materials in the first-step reaction, the generation of waste gases such as SO2 and HCl is avoided, and environment protection requirements are satisfied.
Owner:SHANGHAI CHEMSPEC CORP +1
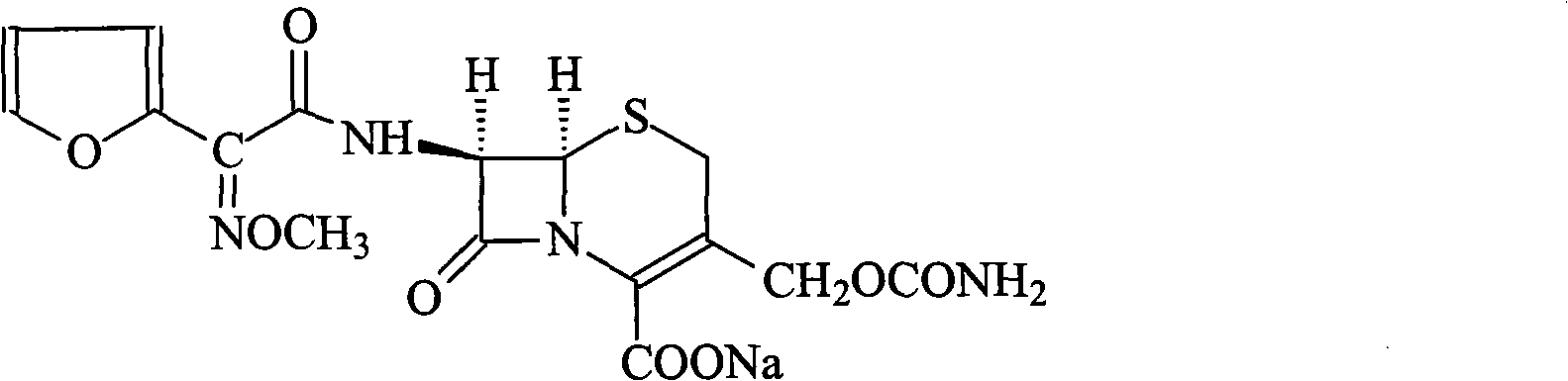
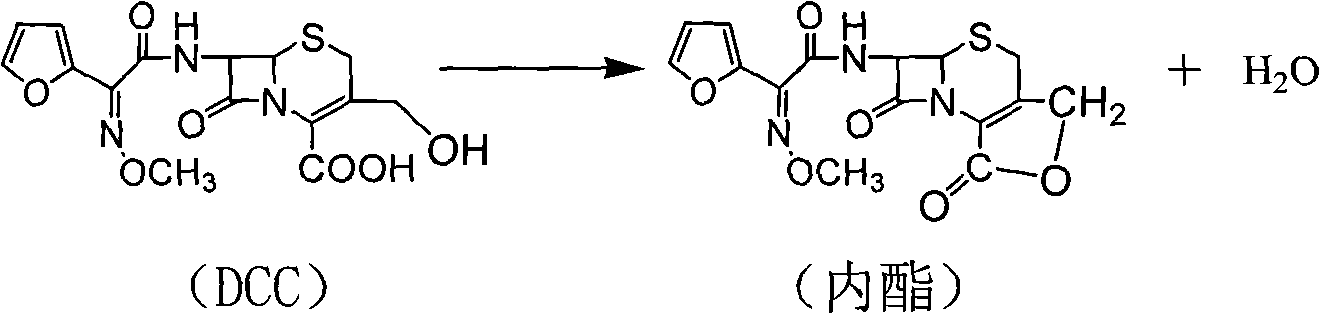
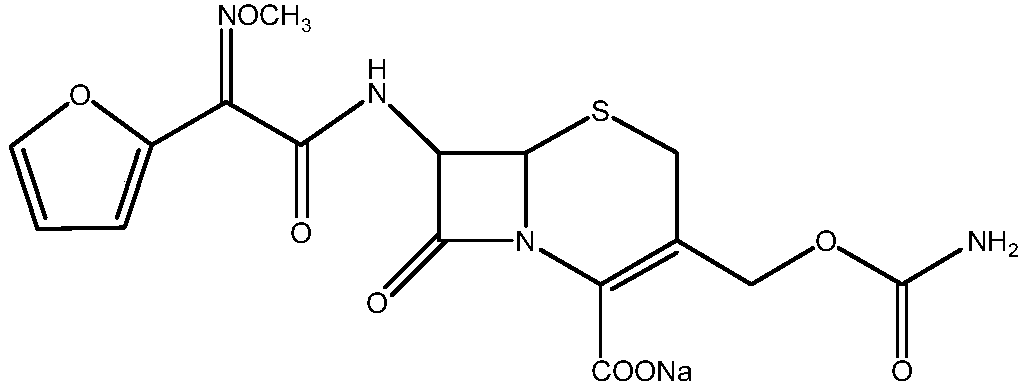
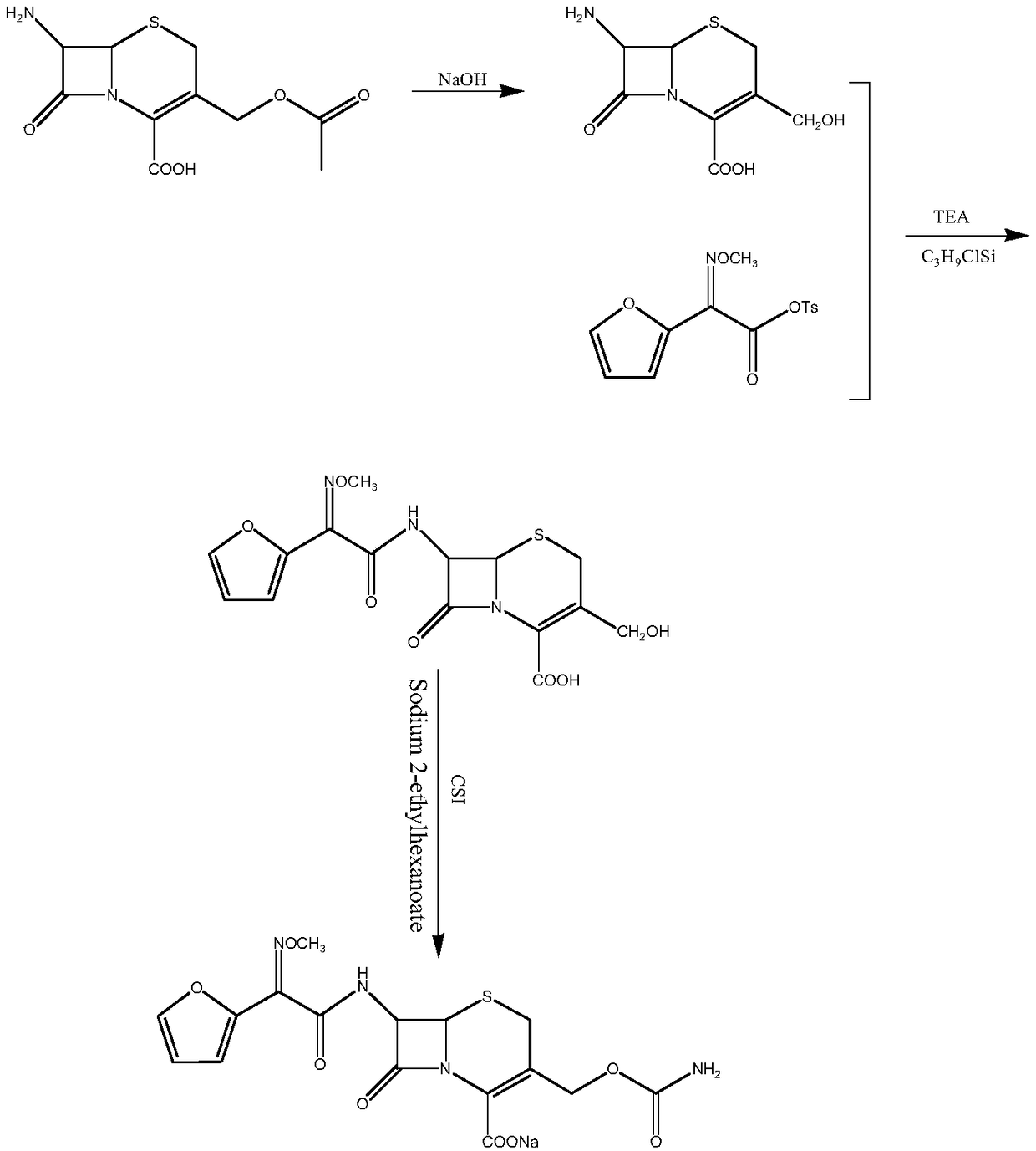
Preparation method of high-purity cefuroxime acid
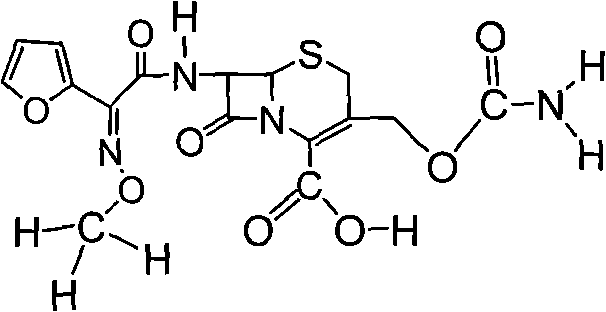
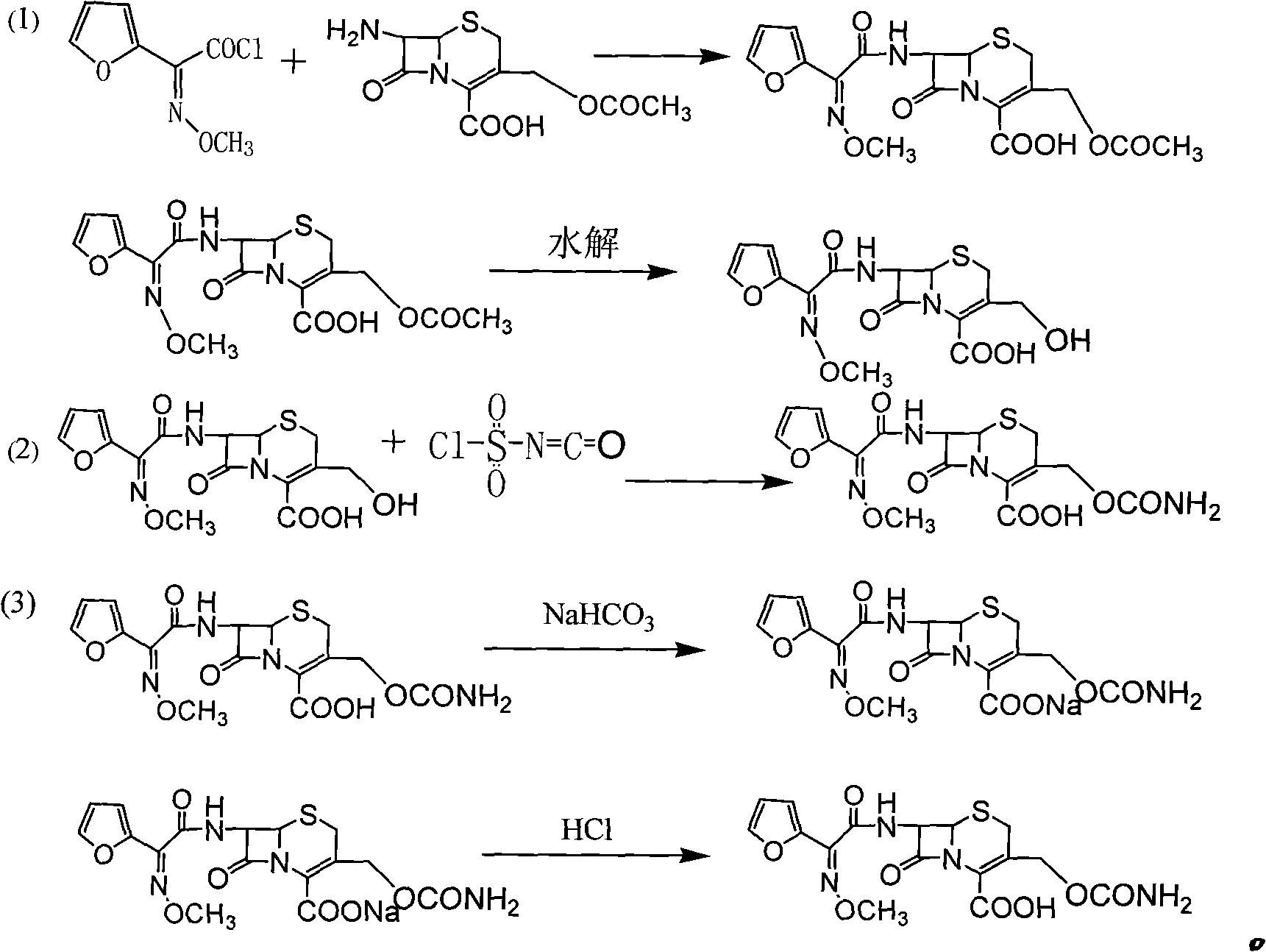
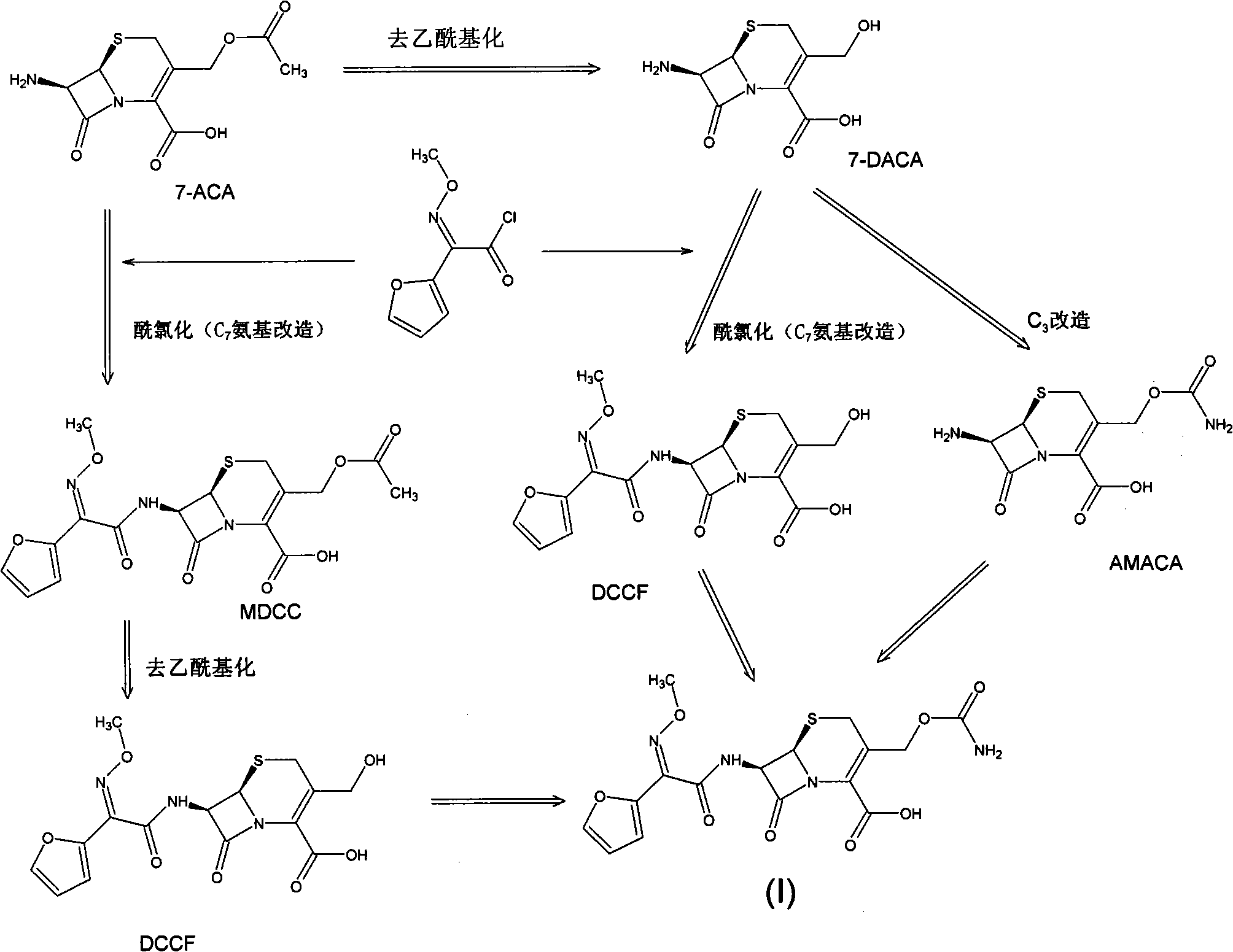
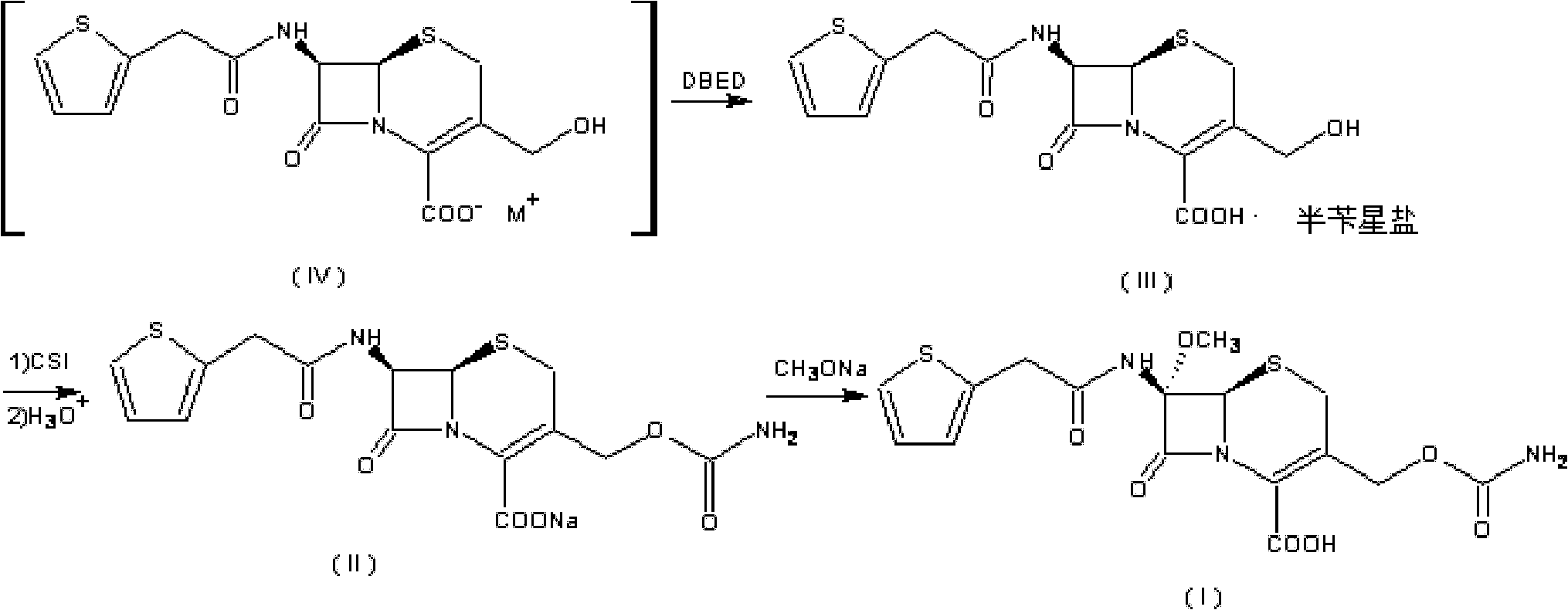
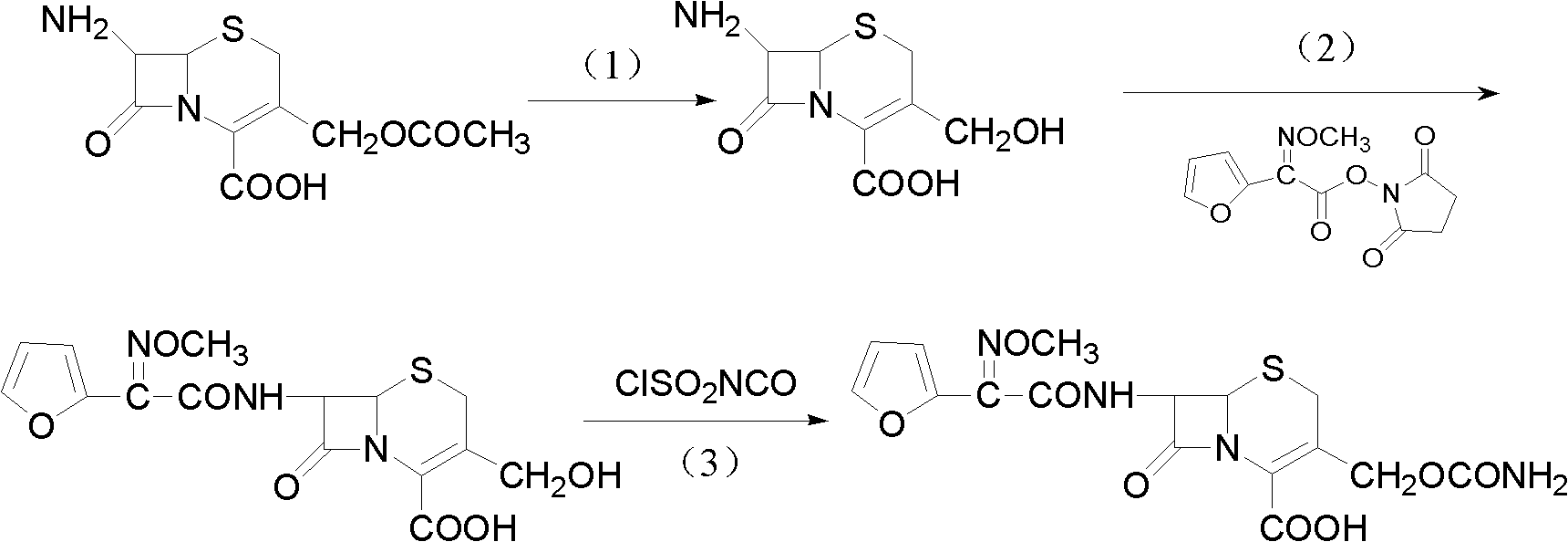
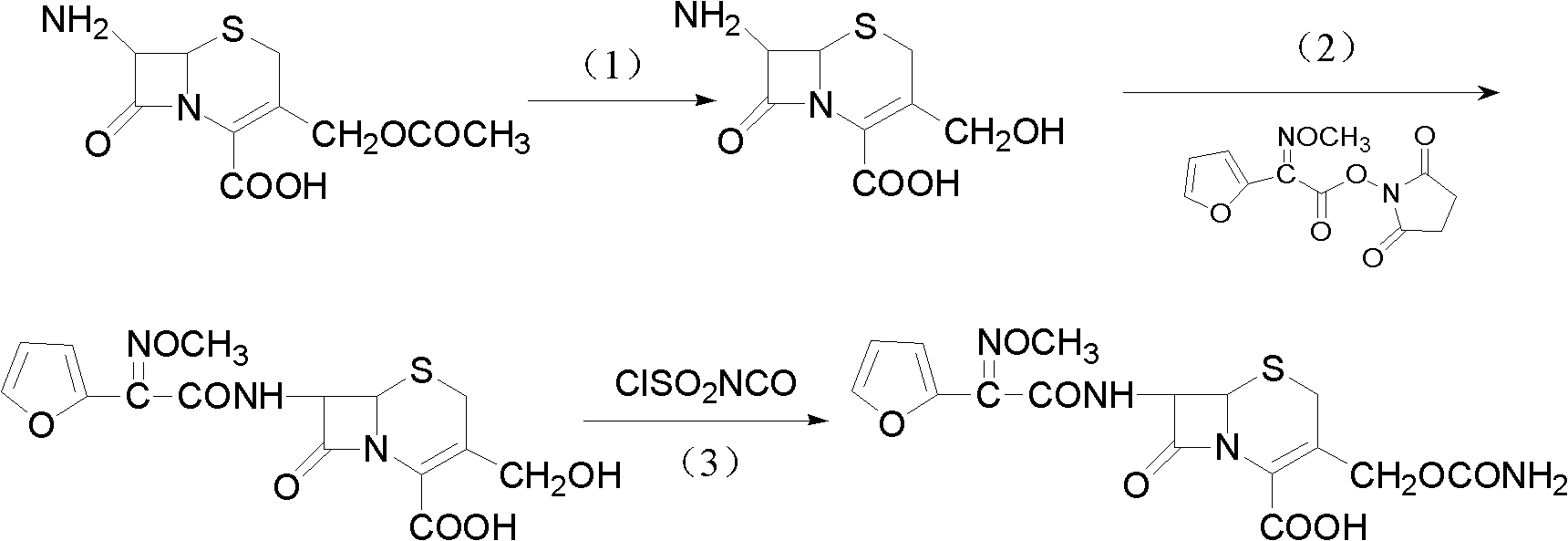
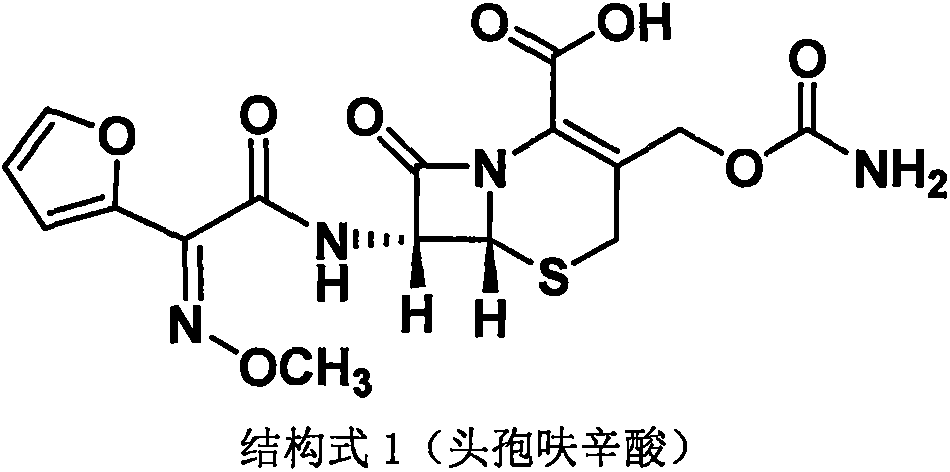
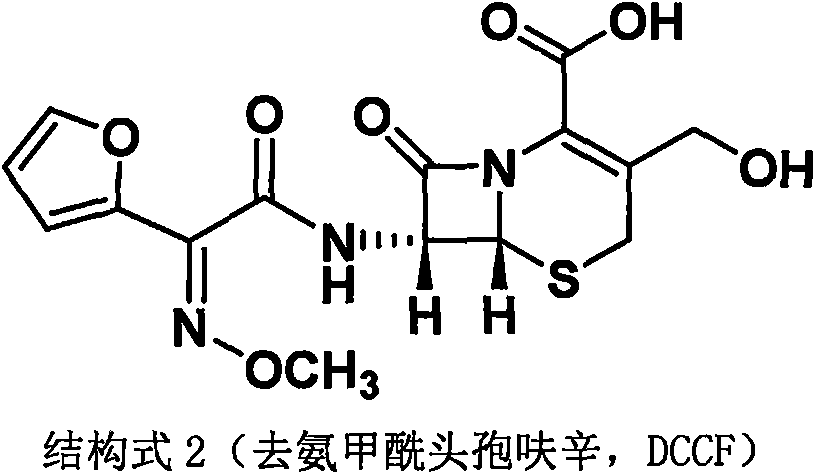
The invention discloses a preparation method of high-purity cefuroxime acid which is an intermediate for synthesizing second-generation cephalosporins cefuroxime sodium and cefuroxime axetil. The preparation method comprises the following steps: based on 7-aminocephalosporanic acid (7-ACA) as a raw material, carrying out an N-acylation reaction on the 7-ACA and furoyl acetylcholine at the 7-position; at a low temperature, hydrolyzing 3-acetyl with a sodium hydroxide solution, crystallizing, filtering and drying so as to obtain the intermediate 3-deformamido cefuroxime acid (DCC); quantitatively adding the DCC in a tetrahydrofuran solvent, dropwise adding chlorosulfonyl isocyanate for a nucleophilic addition reaction so as to generate chlorosulfonyl cefuroxime acid, and adding purified water for hydrolysis so as to prepare a cefuroxime acid reaction liquid; adding sodium bicarbonate for salifying; removing by-reactant lactone and other unsaponifiable impurities in the reaction liquid with a ternary compound extracting agent of dichloromethane, ethyl acetate and tetrahydrofuran, layering, and adding hydrochloric acid in a water phase for acidification; adding the ternary compound extracting agent to extract and separate out the cefuroxime acid; and removing water-soluble impurities, crystallizing and filtering a distilled organic phase, and then drying so as to obtain the high-purity cefuroxime acid with the purity of more than or equal to 99%.
Owner:四平市精细化学品有限公司
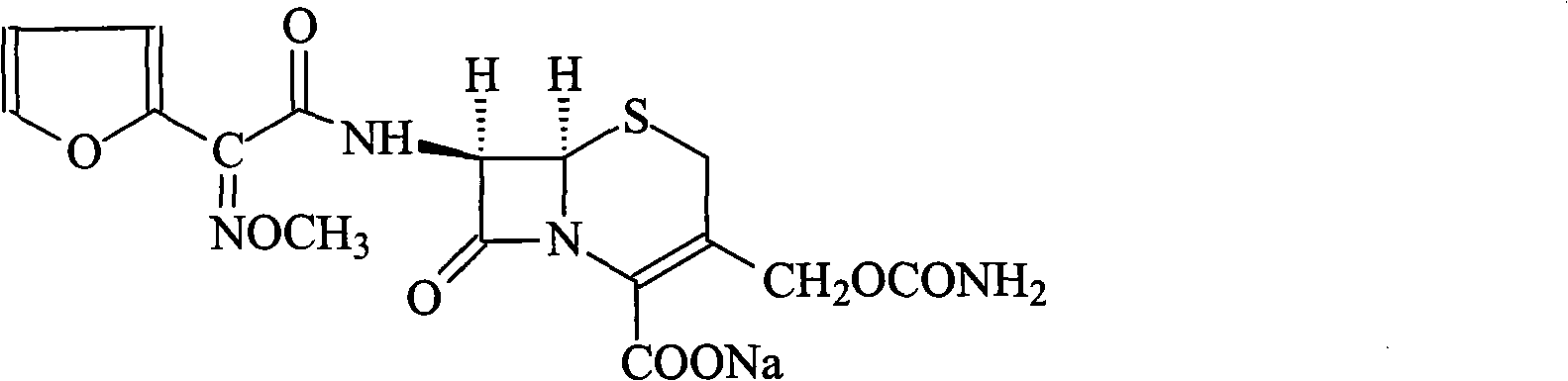
Method for synthesizing cefuroxime sodium
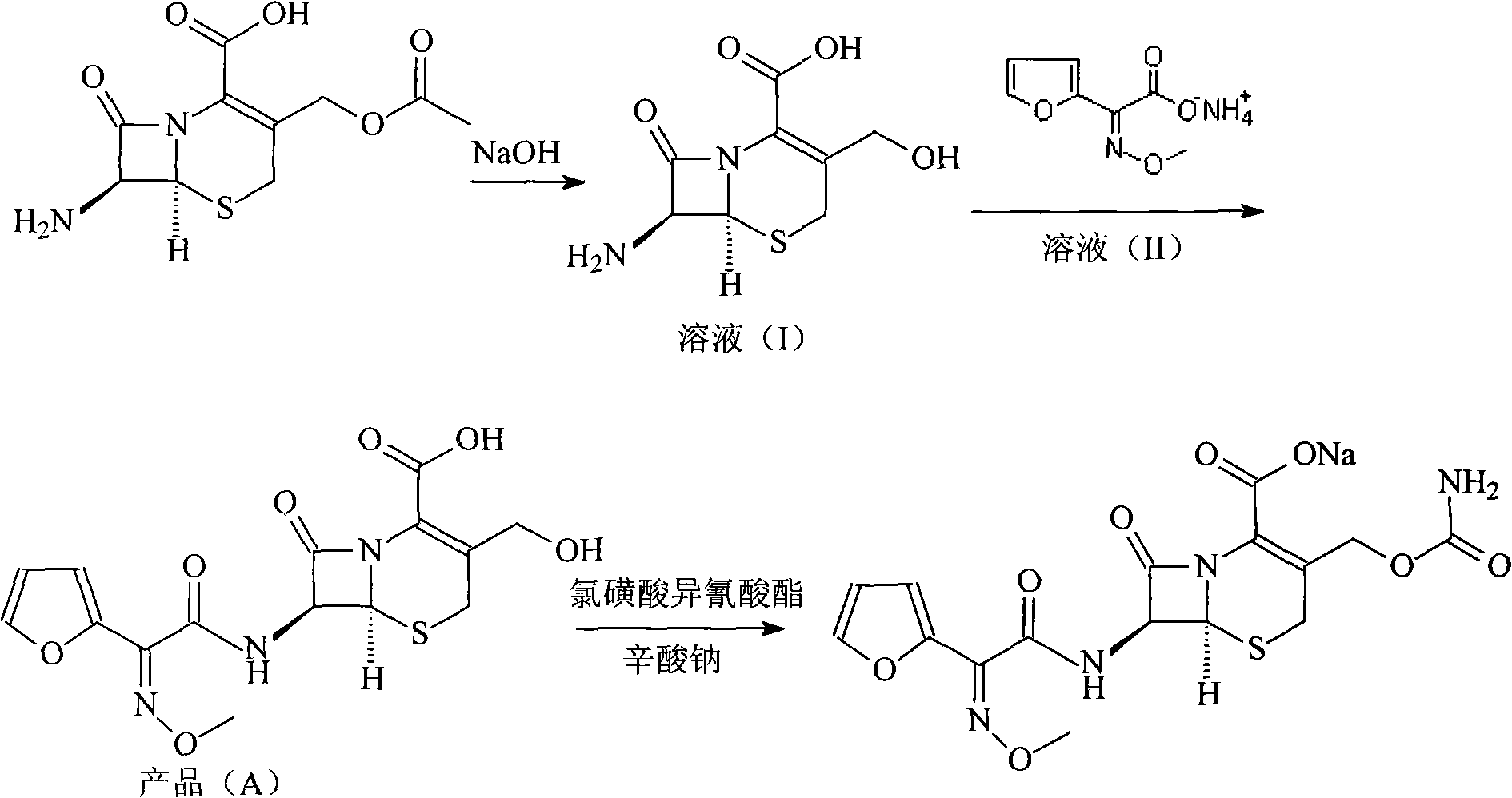
The invention relates to a method for synthesizing cefuroxime sodium, which comprises the following steps: 1, performing the N- acylation reaction of 3-deacetyl-7aminocephalosporanic acid and methoxyaminofuranyl ammonium salt which serve as raw materials and adjusting the pH value with hydrochloric acid to less than 7 in a mixed solvent phase to precipitate crystals to obtain 3-deoxyformyl cefuroxime acid; 2, performing the addition reaction of the 3-deoxyformyl cefuroxime acid and chlorosulfonyl isocyanate serving as a strong ammonia formylating agent in an organic solvent to obtain chlorosulfonyl cefuroxime acid, dehydrating the chlorosulfonyl cefuroxime acid to obtain the cefuroxime acid, decarburizing and concentrating the cefuroxime acid, crystallizing the cefuroxime acid in a solvent phase, and drying the crystals under vacuum to obtain a solid product of cefuroxime acid; and 3, dissolving the cefuroxime acid in alkaline solution, decarburizing the resulting product, crystallizing the resulting product in a mixed solvent phase, filtering crystals, and drying the crystals under vacuum to obtain the cefuroxime sodium.
Owner:哈药集团股份有限公司 +1
Cefuroxime sodium and preparation method thereof
The invention provides cefuroxime sodium and a preparation method thereof. The preparation method comprises the following steps that: (1) 7-aminocephalosporanic acid reacts with 2-(furan-2-base)-2-(methoxyimino) acetyl chloride to produce 3-deacety-7-aminocephalosporanic acid; (2) crystallization is conducted after the 3-deacety-7-aminocephalosporanic acid reacts with chlorosulfonyl isocyanate to produce cefuroxime acid; and (3) the cefuroxime acid is salified to obtain the cefuroxime sodium, wherein solvent for crystallization is selected from one or more of petroleum ether, normal hexane, cyclohexane, solvent oil and tetrahydrofuran. Since the preparation method adopts solvents such as petroleum ether for crystallization in the process of the preparation of the cefuroxime acid, the invention has the advantages that the yield of the product is effectively improved, the product purity is further improved, the impurity content is reduced, the product quality is compliant with Chinese Pharmacopoeia of version 2005, the operation of the method is simple, the raw materials can be easily obtained, the cost is relatively low and the industrial production can be realized easily.
Owner:LIVZON PHARM GRP INC
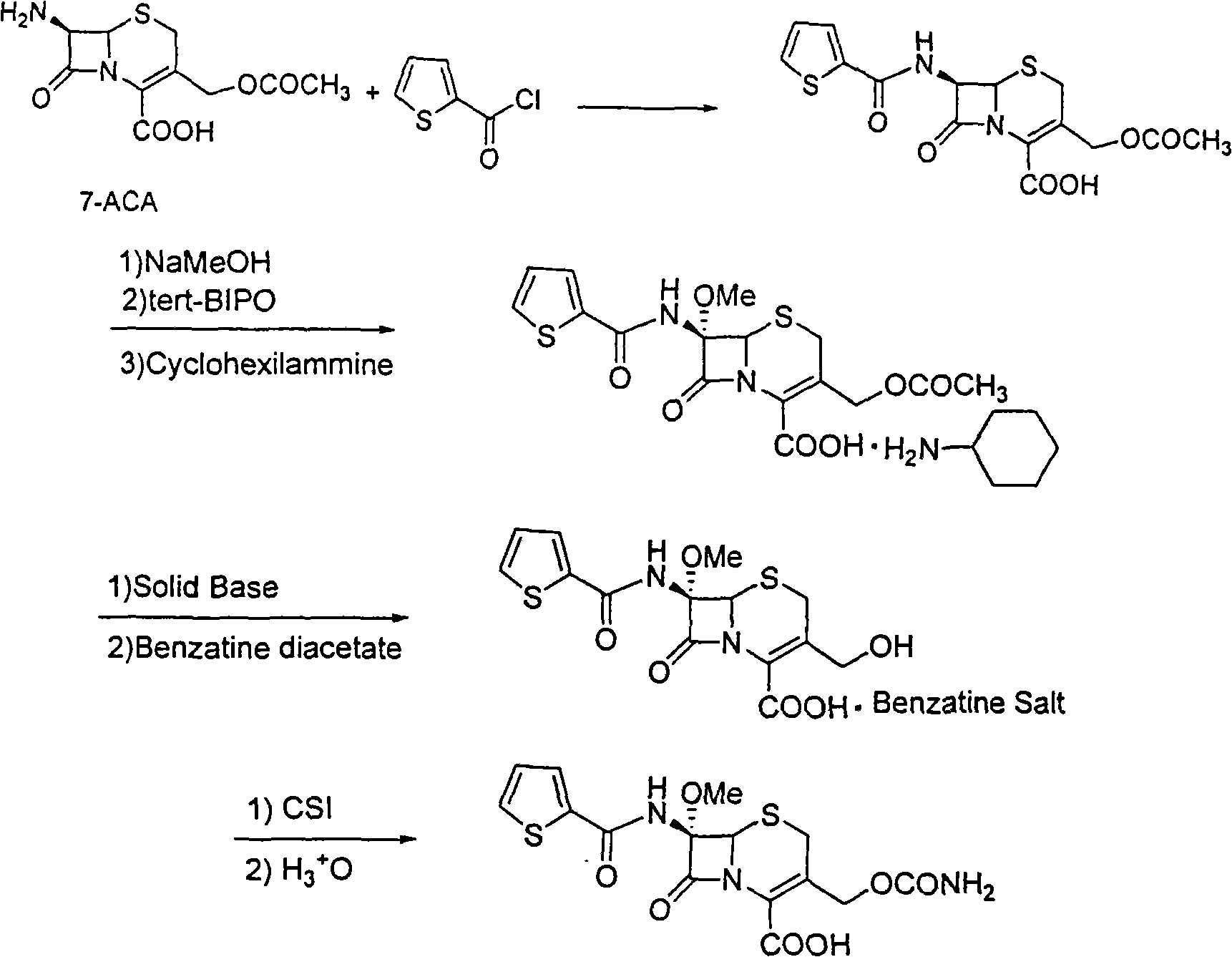
Synthetic method of antibiotic cefoxitin
ActiveCN101555252AEmission reductionEliminate emissionsAntibacterial agentsOrganic chemistryCefoxitinAlkaloid
The invention relates to a synthetic method of antibiotic cefoxitin. 7-ACA is taken as a starting material and firstly reacts with thiopheneacetyl chloride, cephalothin acid is obtained by separation; the cephalothin acid is not purified, and the methoxylation is directly carried out on the cephalothin acid to obtain an intermediate A which introduces methoxy on the position of 7, the intermediate A obtains 7-alpha-methoxy cephalothin cyclohexylamine salt under the action of cyclohexylamine; the 7-alpha-methoxy cephalothin cyclohexylamine salt reacts with benzathine diacetate under the catalysation of solid alkaloid to obtain 7-alpha-methoxy-3-deacetoxy cephalothin benzathine salt; and the carbamylation is carried out under the action of chlorosulfonyl isocyanate to obtain the cefoxitin. The synthetic method has the advantages that compared with the prior art, the synthetic method simplifies the operation process, has high product yield, reduces the production cycle, eliminates the discharge of waste water containing organic solvent and reduces the production cost. The product quality is stable, and the synthetic method is applicable to the large-scale industrial production.
Owner:国药集团致君(苏州)制药有限公司
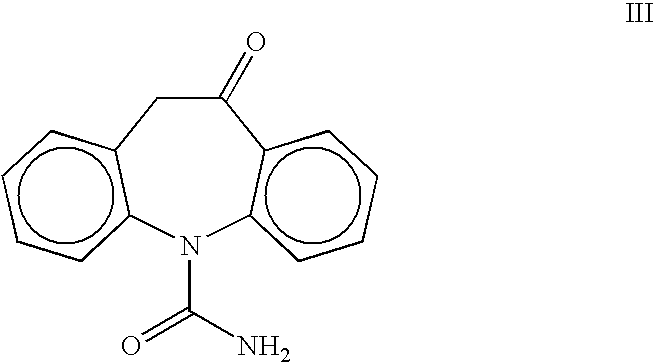
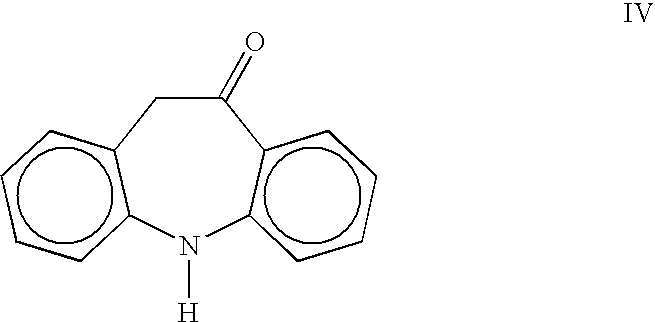
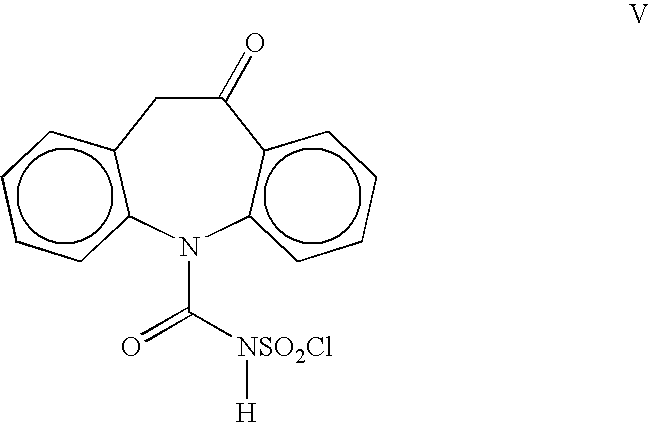
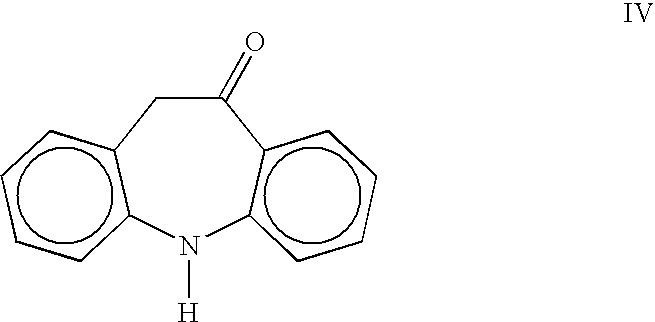
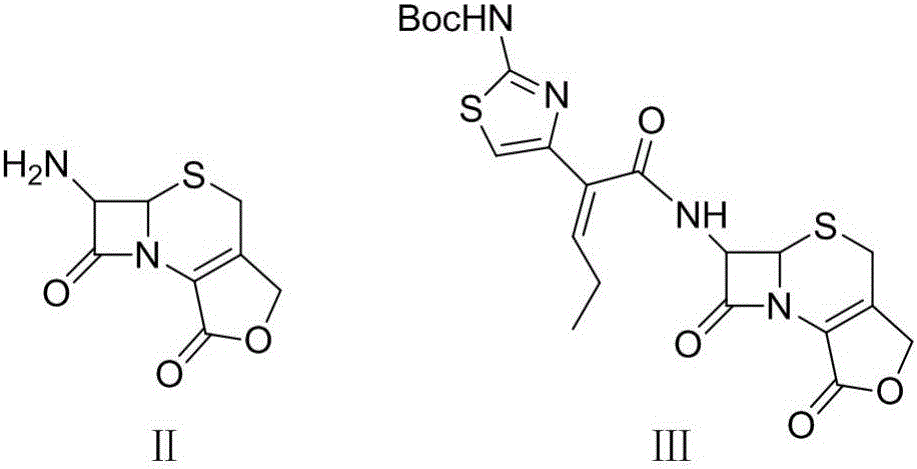
Process for the preparation of oxcarbazepine and related intermediates
InactiveUS20050282797A1High yieldSimple methodBiocideOrganic chemistryOrganic solventChlorosulfonyl isocyanate
A process for preparing Oxcarbazepine III comprising: a) reacting oximinostilbene IV with chlorosulfonyl isocyanate in an inert organic solvent and isolating compound V b) hydrolyzing compound V to form crude Oxcarbazepine III c) purifying oxcarbazepine.
Owner:APOTEX PHARMACHEN INC
Preparation method of cefoxitin sodium
The invention discloses a preparation method of cefoxitin sodium. The preparation method comprises the following steps: (1) bromizing, for example, the site 7 of a main nucleus of cefalotin by using an NBS (N-bromosuccinimide) reagent to form a bromination compound; performing nucleophilic substitution at the site 5 by using a methoxyl group to generate an intermediate IV; (2) performing acyl group hydrolysis on the site 3 of the intermediate IV to obtain an intermediate V; (3) substituting hydrogen atoms on the hydroxyl group by using chloriosulfonyl isocyanate, and then hydrolyzing to obtain the cefoxitin sodium. The method has the advantages of simple process, high product yield, high purity and high reaction selectivity; no special equipment is used in the production; the preparation method is suitable for industrial production.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
Method for preparing cefuroxime acid
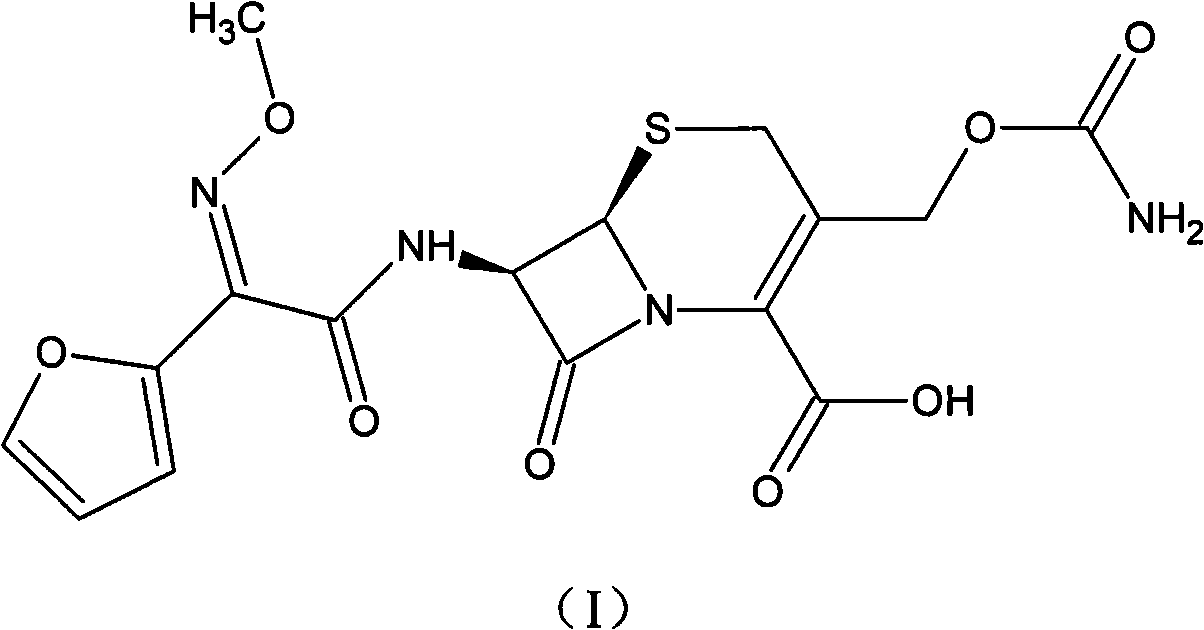
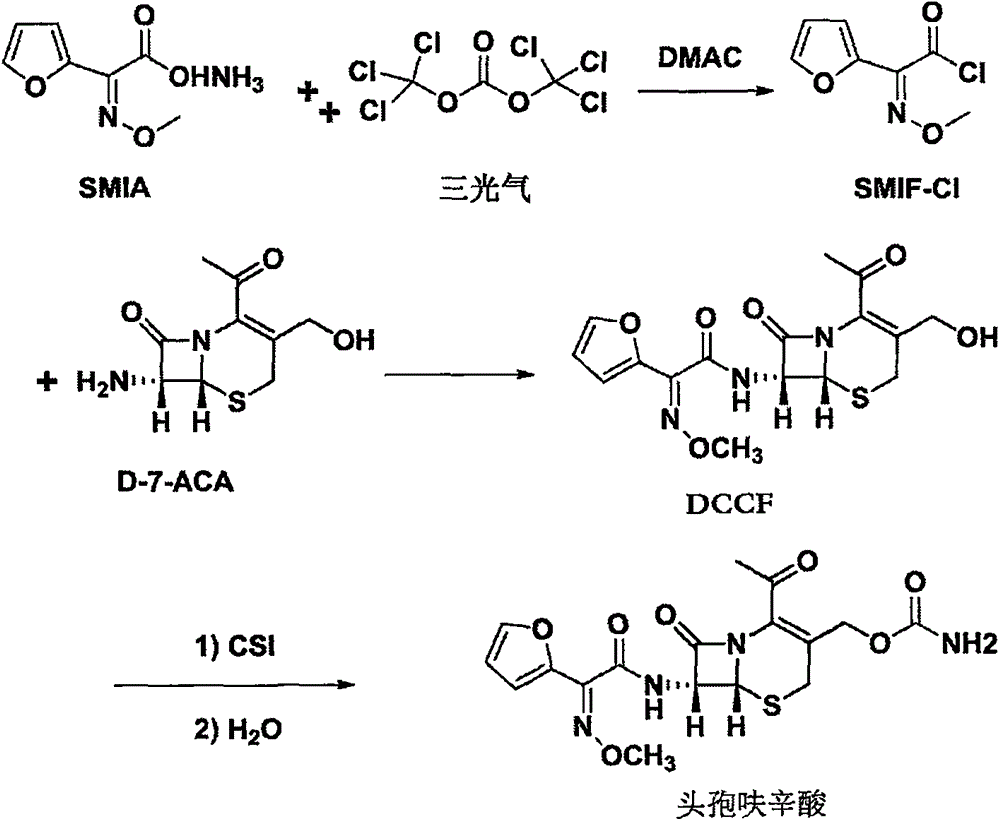
The invention discloses a method for preparing cefuroxime acid. The method comprises the following steps of: performing a chloride acylation reaction on 7-aminocephalosporanic acid (7-ACA) and methoxyiminofuran acetate serving as raw materials; performing deacetylation to synthesize DCCF; performing nucleophilic addition on the DCCF and chlorosulfonyl isocyanate (CSI) serving as a strong carbamoyl reagent to obtain chlorosulfonyl cefuroxime; and hydrolyzing the chlorosulfonyl cefuroxime to obtain cefuroxime acid. In the preparation method, the preparation process is simple, and the cefuroximeacid is crystallized by adopting aqueous solution, so that the loss of organic solvents is reduced; simultaneously, aids are added selectively in the reaction process to improve the quality of products, so that finished products with high purity and yield and good colors are obtained. The purity of the cefuroxime acid prepared by the method is more than 98.5 percent, and the weight yield is approximately 100 percent.
Owner:国药集团致君(苏州)制药有限公司
Preparation method of lithium bis (fluorosulfonyl) imide
The invention discloses a preparation method of lithium bis (fluorosulfonyl) imide. The preparation method comprises the steps of 1 by using chlorosulfonyl isocyanate and chlorosulfonic acid as reaction raw materials, dropwisely adding to react for 5 hours, and keeping the temperature to react for 10 hours to obtain bis (chlorosulfonyl) imide; 2 putting bis (chlorosulfonyl) imide serving as a reaction raw material into a tetrafluoro reaction bottle, reacting with anhydrous hydrogen fluoride under the action of a catalyst, and generating bis (fluorosulfonyl) imide after reacting for 20 hours; and 3 putting the bis (fluorosulfonyl) imide into a reaction bottle, and reacting with lithium hydroxide or lithium carbonate under the action of a catalyst to obtain a product containing lithium bis (fluorosulfonyl) imide. The preparation method disclosed by the invention is simple to operate, easy to control and small in environmental pollution, and meanwhile, inert gas is used as a reaction environment to remove water by low-temperature drying, so that the reaction of the bis (fluorosulfonyl) imide and the final product lithium bis (fluorosulfonyl) imide with water in air is avoided, the quality of the product is ensured, and the preparation method is suitable for industrial production of the lithium bis (fluorosulfonyl) imide.
Owner:泰兴华盛精细化工有限公司
New method for preparing cefuroxime sodium compound
ActiveCN101671349AImprove responseSuitable for large-scale industrial productionOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsCephalosporanic AcidsTriphenylphosphine oxide
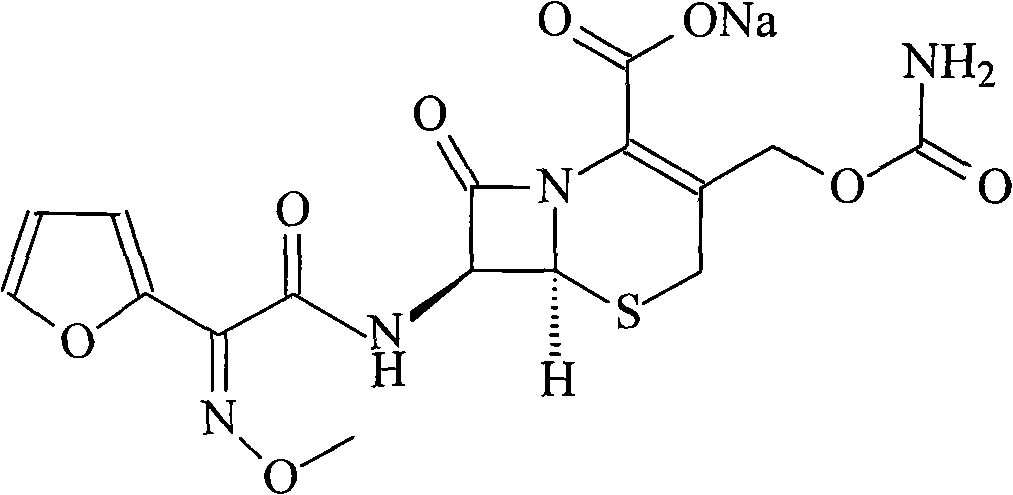
The invention relates to a new method for preparing a cefuroxime sodium compound. A target product is prepared by using triphosgene and triphenylphosphine oxide as catalysts, reacting 7-amino-cephalosporanic acid with (Z)-methoxyl imido furylacetic acid ammonium salt and sequentially adding chlorosulfonyl isocyanate and sodium iso-octoate for reaction. The cefuroxime sodium compound prepared by the method is greatly enhanced in purity and yield coefficient and has advantages of inexpensive using materials, simple synthesizing technology and equipment and easy production separation and purification.
Owner:灵康药业集团股份有限公司
Cefoxitin acid preparation method
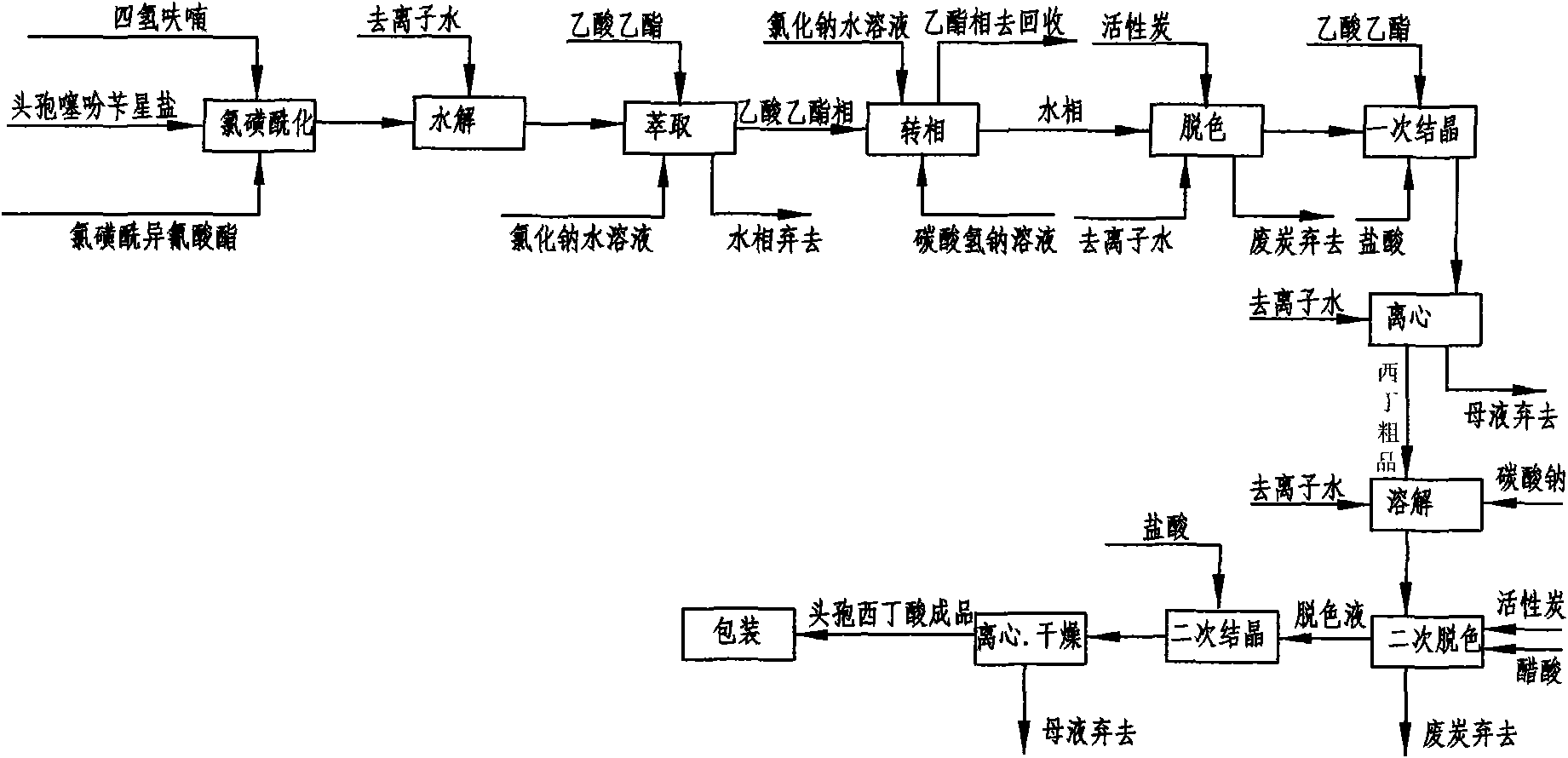
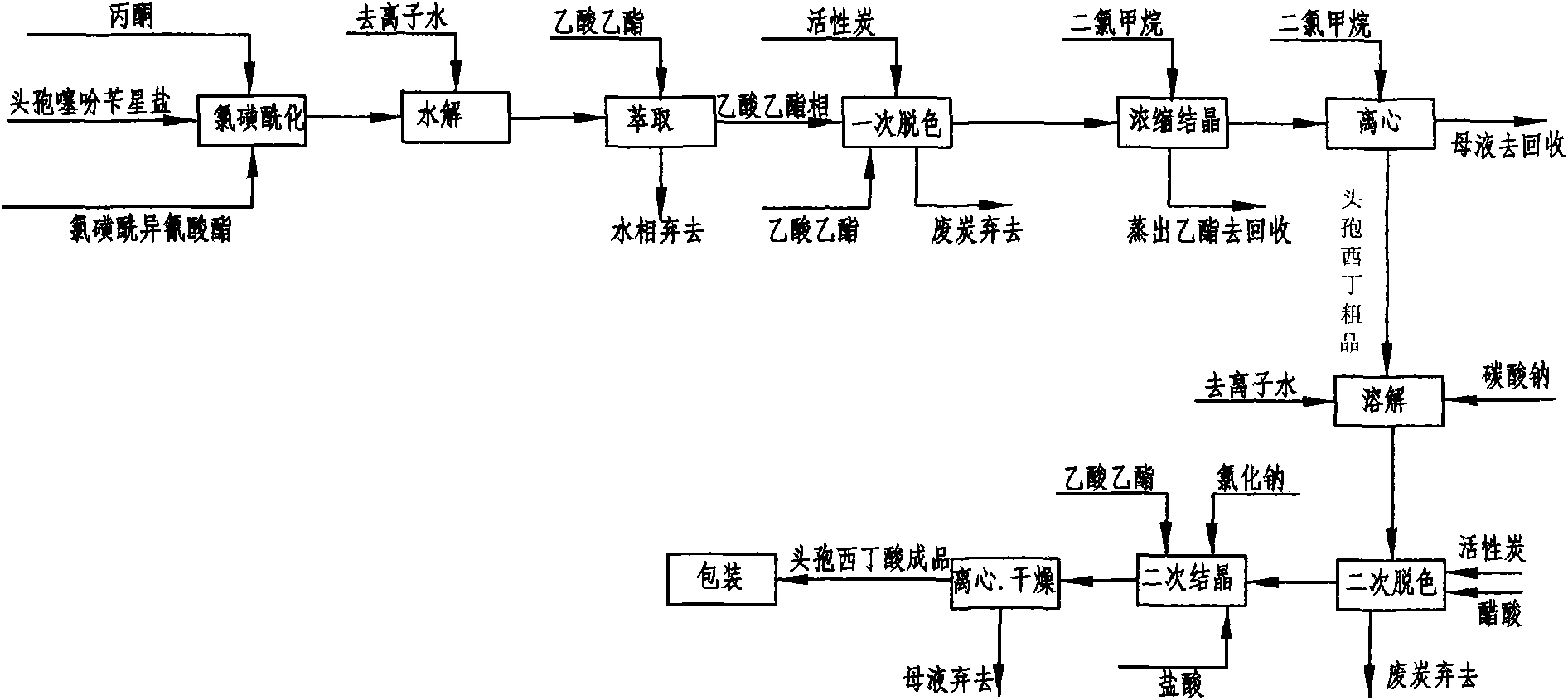
The invention discloses a cefoxitin acid preparation method, comprising the following steps: adding 7-alpha-methoxyl-3-deacetylcefalotin benzathine salt in acetone at room temperature, cooling the solution and adding chlorosulfonyl isocyanate to react and then producing the finished product by hydrolysis, extraction, decoloration, concentrated crystallization, filtration, dissolution and secondary crystallization. In the invention, acetone with a consumption of 2.6kg / kg which is easy to recycle is used instead of tetrahydrofuran which is used in the prior art with a consumption of 13kg / kg and the production cost is reduced by 120 Yuan / Kg. In the invention water phase decarburization is changed to organic phase decarburization, thus eliminating the step of phase inversion in the original process and avoiding the yield loss in phase inversion process; water phase crystallization is changed to the crystallization method which adopts organic phase for concentrating and adds dichloromethane for crystallizing so that the crystallization is realized fully, the yield is higher and the total yield of cefoxitin is increased from 58% to 67%. The material cost of the cefoxitin acid can be reduced by 240 Yuan / Kg, the total reduced cost can be about 360 Yuan / Kg and the product prepared by the method has stronger market competitiveness and remarkable economic benefit.
Owner:河北九派制药股份有限公司
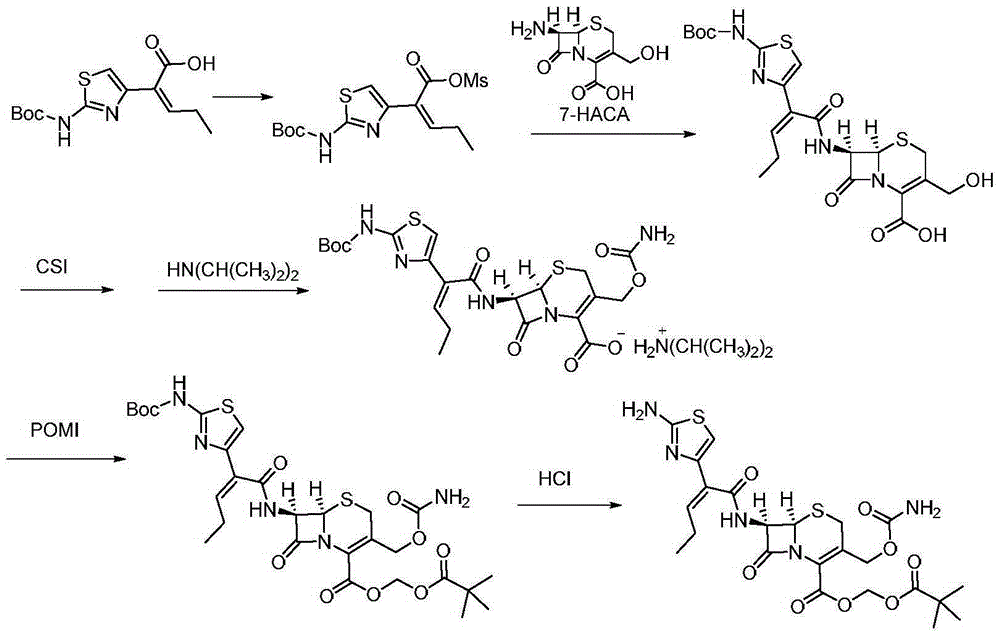
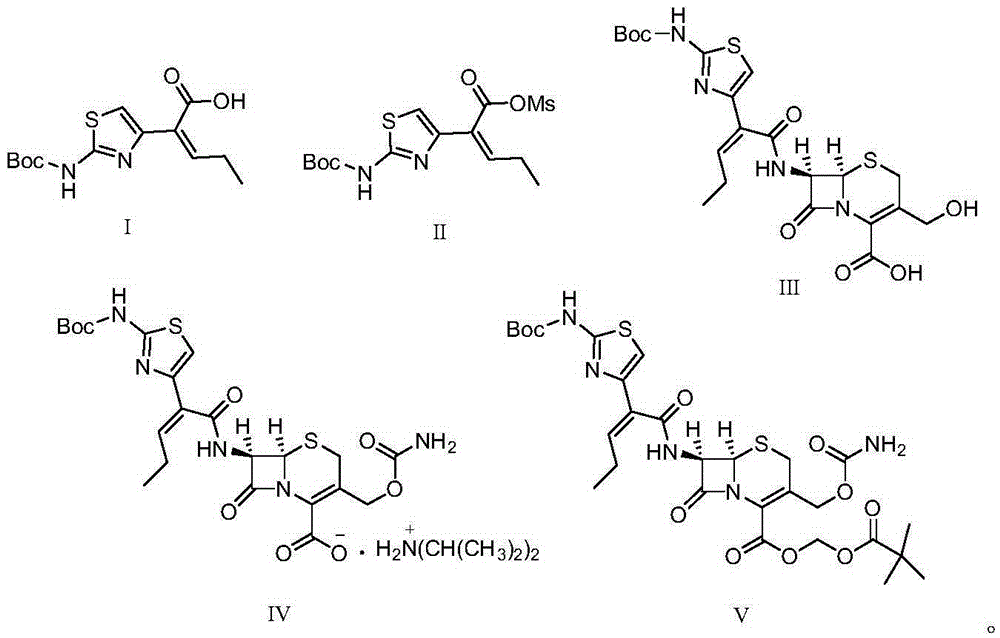
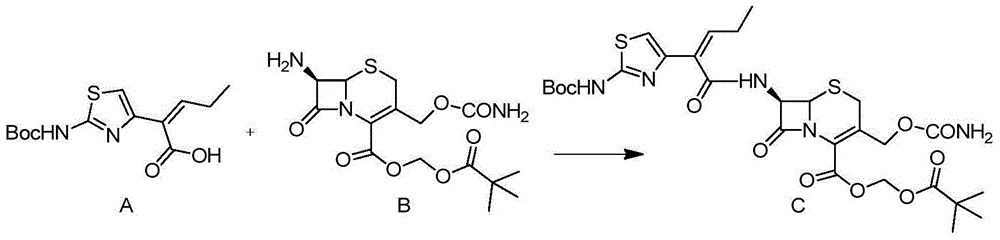
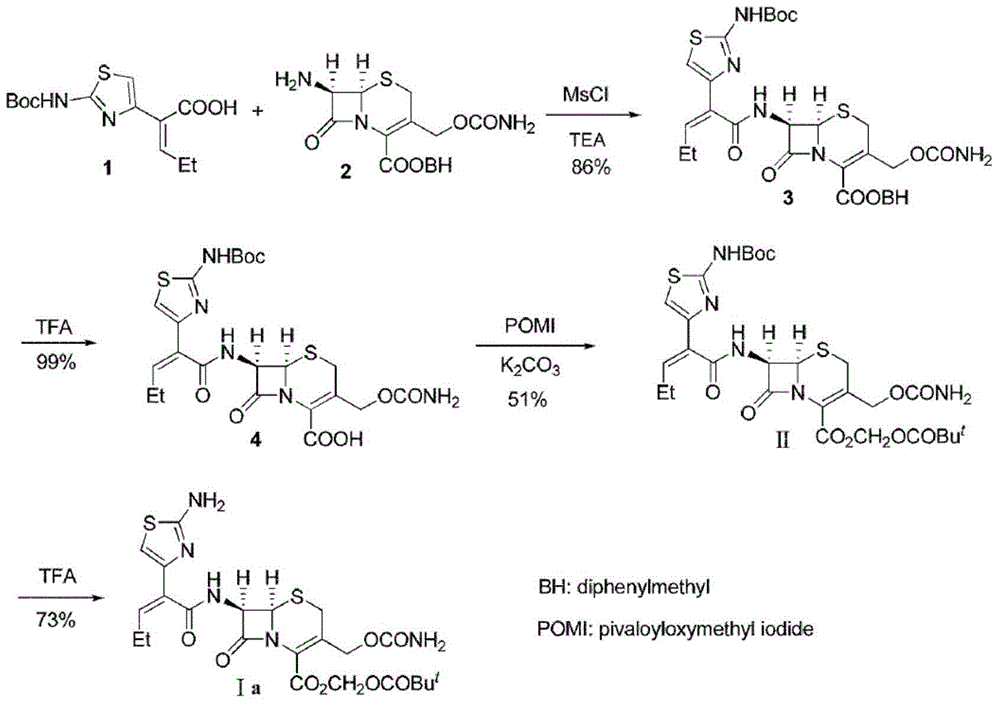
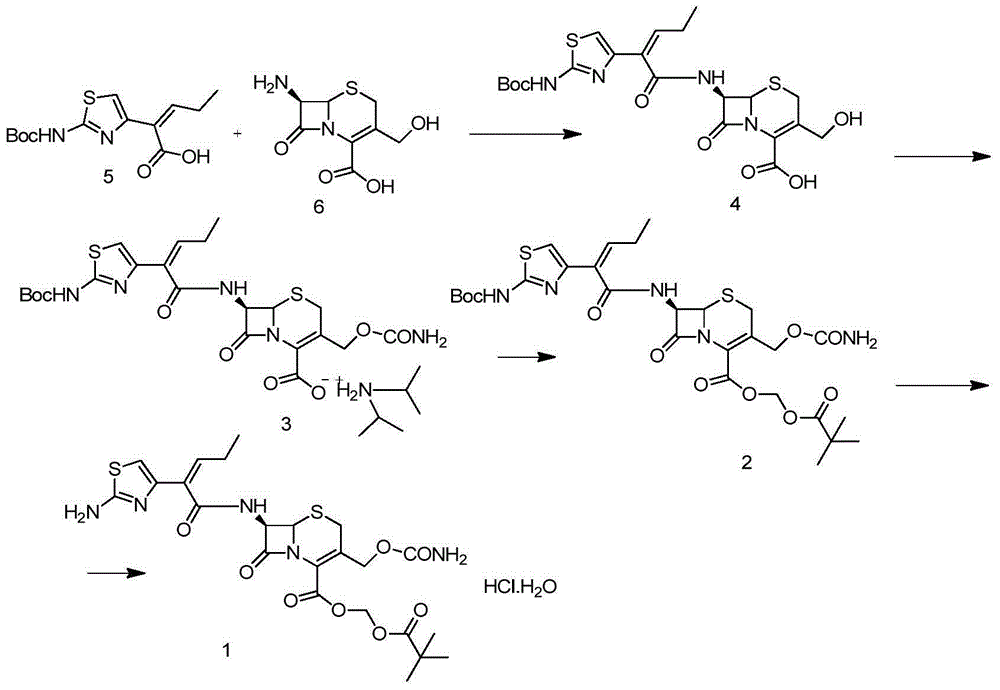
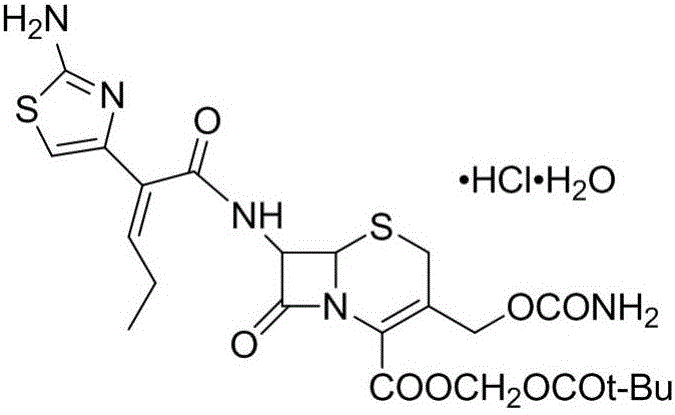
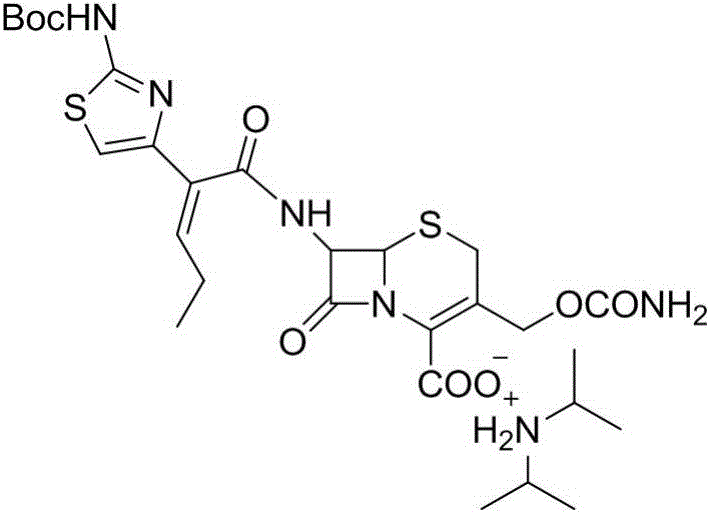
Preparation method for cefcapene pivoxil hydrochloride
ActiveCN105254649ASimple and fast operationConducive to follow-up reactionsOrganic chemistryBulk chemical productionMethanesulfonyl chlorideCefcapene pivoxil hydrochloride
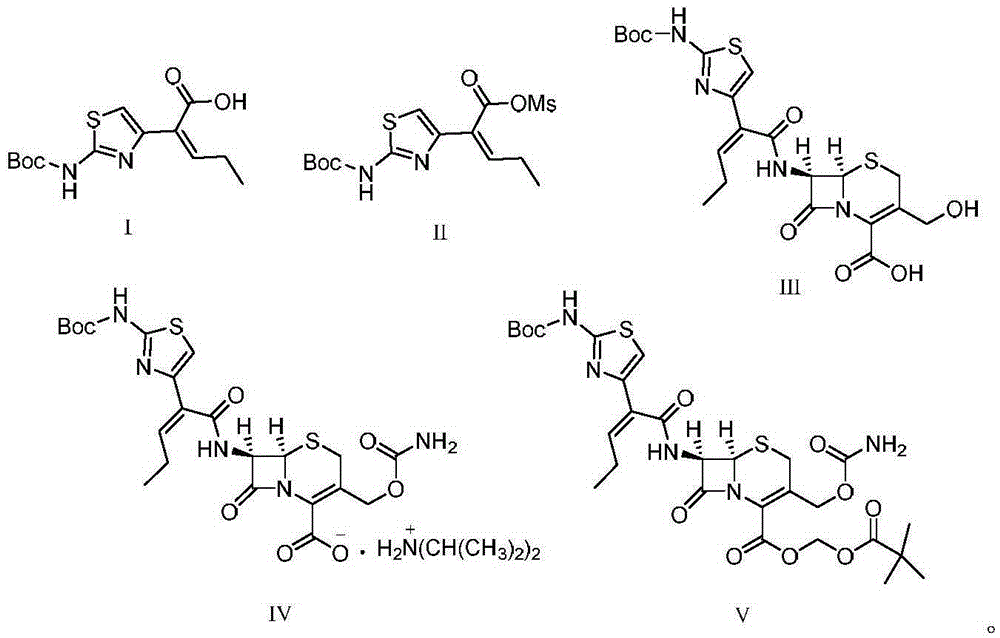
The invention discloses a preparation method for cefcapene pivoxil hydrochloride. The method comprises the following steps: (1) stirring and dissolving a compound which is shown as formula (I) in pyridine, adding methylsufonyl chloride to react to obtain a liquid which contains the compound shown as formula (II), placing the liquid at -15 DEG C to 0 DEG C for later use; (2) in the existence of proline and diisopropylamine, enabling 7-HACA and the liquid which contains the compound shown as the formula (II) to react in methyl alcohol to obtain the compound which is shown as formula (III); (3) adding diisopropylamine, enabling the compound which is shown as the formula (III) and chlorosulfonyl isocyanate to react, regulating the pH to 4 to 5, cooling the organic phase, and adding the diisopropylamine to obtain the compound which is shown as formula (IV); (4) in the existence of potassium phosphate and copper acetate, enabling the compound which is shown as the formula (IV) and iodomethyl pivalate to react in DMF (Dimethyl Formamide) to obtain the compound which is shown as formula (V); (5) removing protecting groups from the compound which is shown as the formula (V) in the methanol solution of hydrochloric acid to obtain the cefcapene pivoxil hydrochloride. According to the method, the product yield is greatly improved, and the method is suitable for industrial production.
Owner:湖北凌晟药业股份有限公司
Preparation method of cefoxitin
InactiveCN102633819AOvercoming the drawbacks of 101613361Avoid condensation side reactionsOrganic chemistrySodium methoxideHypochlorite
The invention relates to a preparation method of cefoxitin. The method comprises the following steps: adding a solvent A into a compound aqueous solution with the structure shown in the formula (IV), adding N,N'-dibenzylethylenediamine diacetate or aqueous solution thereof, filtering to obtain a compound with an intermediate structure shown in the formula (III); adding the compound with the intermediate structure shown in the formula (III) into acetone or tetrahydrofuran, adding chloriosulfonyl isocyanate to react, and hydrolyzing; adding acetic ether, filtering, extracting, decoloring, salting out, and filtering to obtain a compound with an intermediate structure shown in the formula (II); adding the compound with the intermediate structure shown in the formula (II) into an organic solvent B, adding organic acid to dissolve, adding a methanol solution with sodium methoxide and tert-butyl hypochlorite to perform methylation reaction, adding sodium pyrosulfite and acetic acid for neutralizing, adding water for extracting, acidifying water phase, filtering to obtain cefoxitin. According to the preparation method, deacetyled cefoxitin always exists in a mode of sodium salt or amine salt, so that the condensation side reaction easily caused by low pH can be avoided, and the product quality and yield can be well controlled.
Owner:山东安弘制药有限公司
Preparation method for cefcapene pivoxil hydrochloride
The invention belongs to the technical field of antibiotic synthesis, and relates to a preparation method for cefcapene pivoxil hydrochloride. The preparation method comprises the following steps: (1) reacting 7-ACA with sodium hydroxide in a solution with quaternary ammonium salt under the temperature of (-5 DEG C)-5 DEG C to generate 7-DACA; (2) adding (cefcapene pivoxil side chain acid, compound 5) into the solution containing 7-DACA, diisopropylamine and phenyltriethylammonium chloride under the temperature of 0-10 DEG C to react with a methylsufonyl chloride reaction solution under the temperature of (-15 DEG C)-0 DEG C to obtain a compound (4); reacting the compound 4 and chlorosulfonyl isocyanate to obtain a compound (3); further reacting the compound (3) with iodomethyl pivalate to obtain a compound (2); removing the protection base of the compound (2) in a hydrochloric acid methanol solution to obtain the cefcapene pivoxil hydrochloride (compound 1).
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
A kind of preparation method of bisfluorosulfonimide lithium salt
The invention relates to the field of chemical synthesizing, in particular to a preparation method of imidodisulfuryl fluoride lithium salt. The preparation method includes the steps of firstly, allowing chlorosulfonic acid and chlorosulfonyl isocyanate to react in the presence of catalyst to obtain dichlorosulfimide; secondly, allowing dichlorosulfimide and hydrogen fluoride to react in the presence of catalyst to obtain imidodisulfuryl fluoride; thirdly, allowing imidodisulfuryl fluoride to react with compound containing lithium to obtain the imidodisulfuryl fluoride lithium salt. The preparation method has the advantages that the chlorosulfonic acid and chlorosulfonyl isocyanate are used as the raw materials in the first-step reaction, the generation of waste gases such as SO2 and HCl is avoided, and environment protection requirements are satisfied.
Owner:SHANGHAI CHEMSPEC CORP +1
Cefuroxime sodium synthesizing method
The invention relates to the technical field of pharmaceutical and chemical industries and particularly discloses a cefuroxime sodium synthesizing method. The synthesizing method comprises the steps:dropwise adding alkali solution into 7-aminocephalosporanic acid aqueous solution for hydrolysis reaction, and then subjecting the mixture and (Z)-2-furyl-2-methoxyimino acetic acid p-toluene sulfonicanhydride to amidation to obtain 7-[(Z)-2-furyl-2-methoxyiminoacetamido]-3-hydroxymethyl-4-cephalosporanic acid; dissolving the 7-[(Z)-2-furyl-2-methoxyiminoacetamido]-3-hydroxymethyl-4-cephalosporanic acid into organic solvents to sequentially perform nucleophilic addition and hydrolysis reaction with chlorosulfonyl isocyanate, then adding sodium iso-octoate solution, and performing devitrification, filtering, washing and drying to obtain cefuroxime sodium. According to the synthesizing method, raw materials are wide and easy to obtain, the cost is low, the steps are simple, operation is simple, and side reaction is less.
Owner:湖北凌晟药业股份有限公司 +1
Method for preparing cefuroxime acid
ActiveCN102093390AEasy to operateReduce the discharge of three wastesOrganic chemistryAcetic acid7-ACA
The invention provides a method for preparing cefuroxime acid. The method comprises the following steps: (1) selectively hydrolyzing 7-aminocephalosporanic acid (7-ACA) with aqueous alkali so as to obtain 3-deacetylation-7-aminocephalosporanic acid (7-DACA); (2) condensing 2-(2-furyl)-2-(methoxyimino)acetic acid-(2,5-dioxo-pyrrolidyl)-1-ester and 7-DACA so as to obtain 3-decarbamyl-cefuroxime acid (DCCF); and (3) modifying 3-hydroxymethyl of DCCF with chlorosulfonyl isocyanate so as to obtain the cefuroxime acid. In the method, low-temperature selective hydrolysis is carried out by using inorganic base to remove the 3-ester group of 7-ACA so as to prepare 7-DACA; C7-amino modification is carried out by an active ester method so as to obtain DCCF; and the 3-hydroxymethyl of DCCF is modified into carbamoyl methoxyl so as to obtain the cefuroxime acid. The method has the advantages of less emission of three wastes and high yield, is simple and convenient to operate, and is suitable for industrial production.
Owner:蚌埠丰原涂山制药有限公司
Method for preparing cefuroxime acid
InactiveCN106432267AThe synthetic method is green and environmentally friendlyHigh yieldOrganic chemistryCefuroximeOrganic layer
The invention provides a method for preparing cefuroxime acid. The method is characterized by comprising the following steps: (1) mixing deammonized formyl cefuroxime in liquid ester, adding a strong amino carbamylation reagent sulfonylisocynate, and performing a temperature-controlled reaction; (2) adding purified water after the reaction is completed, and performing hydrolysis; (3) adding the liquid ester after hydrolysis is completed, adjusting the pH value to be 1.9-2.0 by using hydrochloric acid, standing for layering, and performing vacuum concentration on an organic layer so as to obtain an organic layer concentrated solution; (4) adding purified water into the organic layer concentrated solution, performing crystallization, and filtering so as to obtain cefuroxime acid, wherein the liquid ester used in the step (1) and the step (3) is selected from methyl acetate, acetic ether, n-butyl acetate, methyl formate, ethyl formate and propyl formate. The method for preparing cefuroxime acid, which is provided by the invention, is green and environmental-friendly, low in cost, high in yield and good in quality.
Owner:珠海保税区丽珠合成制药有限公司
Manufacturing method of chlorosulfonyl isocyanate
ActiveCN101891657AReduce consumptionReduce manufacturing costSulfonic acid amide preparationSodium cyanideSolvent
The invention relates to a manufacturing method of chlorosulfonyl isocyanate, in particular to a method of taking cyanogen chloride, sulfuric anhydride and a sodium hydride water solution as raw materials to produce chlorosulfonyl isocyanate with high efficiency and low consumption. The manufacturing method of chlorosulfonyl isocyanate comprises the following steps: simultaneously adding liquid chlorine and a sodium cyanide water solution to a chlorination reactor for chlorination reaction; then preparing pure liquid cyanogen chloride by cooling, drying and refining; then adding the cyanogen chloride and the sulfuric anhydride which are respectively dissolved in inert solvents in equal mole ratio to the reactor; preparing the crude product of chlorosulfonyl isocyanate by reaction at low temperature; and preparing the finished product of chlorosulfonyl isocyanate by desolventizing, thermal decomposition and rectifying. The invention has simple method and easy implementation, the traditional production process is changed, and the liquid chlorine is not gasified and directly reacts with the sodium cyanide water solution to prepare the cyanogen chloride, thereby simplifying the process, greatly improving the yield and quality of the product, reducing the energy consumption, reducing the production cost of enterprises, and being an ideal manufacturing method of the chlorosulfonyl isocyanate.
Owner:营口昌成新材料科技有限公司
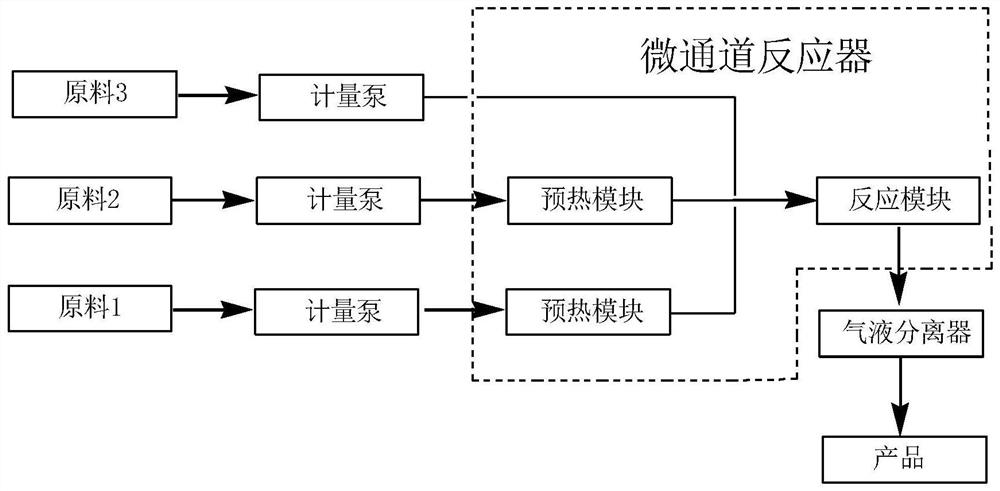
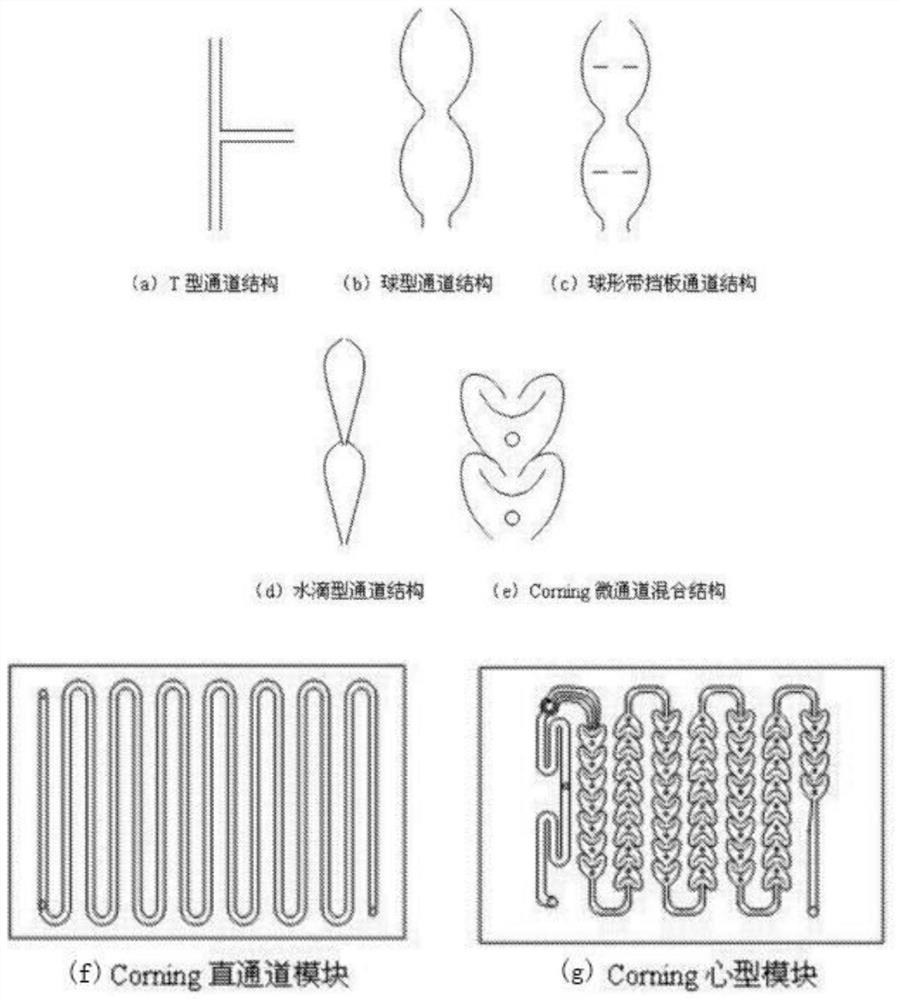
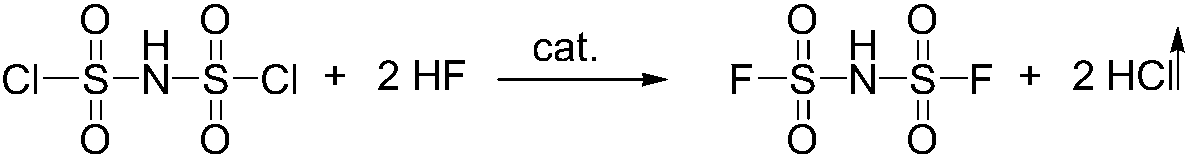
Continuous preparation method of bis(fluorosulfonyl)imide
The invention discloses a continuous preparation method of bis(fluorosulfonyl)imide. The continuous preparation of bis(fluorosulfonyl)imide is carried out in a microchannel reactor, and comprises the following steps: A1, preheating a first raw material chlorosulfonic acid and a second raw material chlorosulfonyl isocyanate in a preheating module at the preheating temperature of 30-150 DEG C; A2, feeding a third raw material hydrogen fluoride, the preheated first raw material chlorosulfonic acid and the preheated second raw material chlorosulfonyl isocyanate into a reaction module for mixed reaction, wherein the molar ratio of the hydrogen fluoride to the chlorosulfonic acid to the chlorosulfonyl isocyanate is (2-2.5):1:1, the flow rate of the chlorosulfonic acid is 1-100g / min, the flow rate of the chlorosulfonyl isocyanate is 1-100g / min, the reaction temperature is 30-150 DEG C, the reaction pressure is 0-0.5 MPa, and the retention time is 1-100s; and A3, carrying out gas-liquid separation on the product at the outlet of the reaction module to obtain bis(fluorosulfonyl)imide. The method has the advantages of simple process, low equipment loss, high yield and the like.
Owner:ZHEJIANG LANTIAN ENVIRONMENTAL PROTECTION HI TECH +1
Synthesis and purification method of t-butyloxycarboryl cefcapene diisopropylammonium
ActiveCN106220648AAvoid generatingInhibit growthOrganic chemistryChemical synthesisPurification methods
The invention belongs to the field of chemical synthesis of antibiotics, and in particular discloses a synthesis and purification method of t-butyloxycarboryl cefcapene diisopropylammonium which is used as a key intermediate of cefcapene pivoxil hydrochloride hydrate. The method comprises the following steps: carrying out an acylation reaction on D-7ACA and side chain acid under the actions of triethylamine and phosphorus oxychloride, purifying to obtain an acylation reaction feed liquid to be directly reacted with chlorosulfonyl isocyanate, carrying out water washing, drying and dropwise adding diisopropylamine to form salt and concentrating after the reaction is quenched, and performing crystallization by using a poor solvent of a target product to ensure that the pH of the feed liquid is weak acidic or neutral in the whole post-processing process so as to improve the stability of the feed liquid, thereby solving the problem of impurity increase caused by the prolonged subsequent processing time in large-scale production, and further improve the yield, thereby being especially suitable for industrialized mass production.
Owner:青岛睿森生物科技有限公司
A kind of preparation method of bisfluorosulfonimide potassium
The invention relates to the field of chemical synthesis, in particular to a preparation method of potassium bis(fluorosulfonyl)imide. The preparation method comprises the following steps: 1) carrying out a reaction between chlorosulfonic acid and chlorosulfonyl isocyanate to obtain bis(chlorosulfonyl)imide in the presence of a catalyst; 2) carrying out a reaction between the bis(chlorosulfonyl)imide and hydrogen fluoride in the presence of a catalyst to obtain bis(fluorosulfonyl)imide; 3) carrying out a reaction between the bis(fluorosulfonyl)imide and an alkaline potassium compound to obtain the potassium bis(fluorosulfonyl)imide. According to the preparation method provided by the invention, the chlorosulfonic acid and the chlorosulfonyl isocyanate can be used as raw materials in the first step, so that generation of waste gases such as SO2, HCl and the like is avoided, and thus a requirement on environment friendliness can be met better.
Owner:SHANGHAI CHEMSPEC CORP +1
Method for the preparation of bis(fluorosulfonyl)-imide and of its salts
InactiveUS20180362343A1Nitrosyl chlorideImidodisulfonic/nitrilotrisulfonic acidImideChlorosulfonyl isocyanate
The invention relates to a method for the preparation of bis(fluorosulfonyl)-imide, the method starts from bis(chlorosulfonyl)-imide or its respective derivatives, which is reacted with HF in the presence of chlorosulfonyl isocyanate, and uses a certain extraction step for extraction of bis(fluorosulfonyl)-imide from an aqueous solution; the invention is also useful for the preparation of certain salts of bis(fluorosulfonyl)-imide and its derivatives.
Owner:LONZA LTD
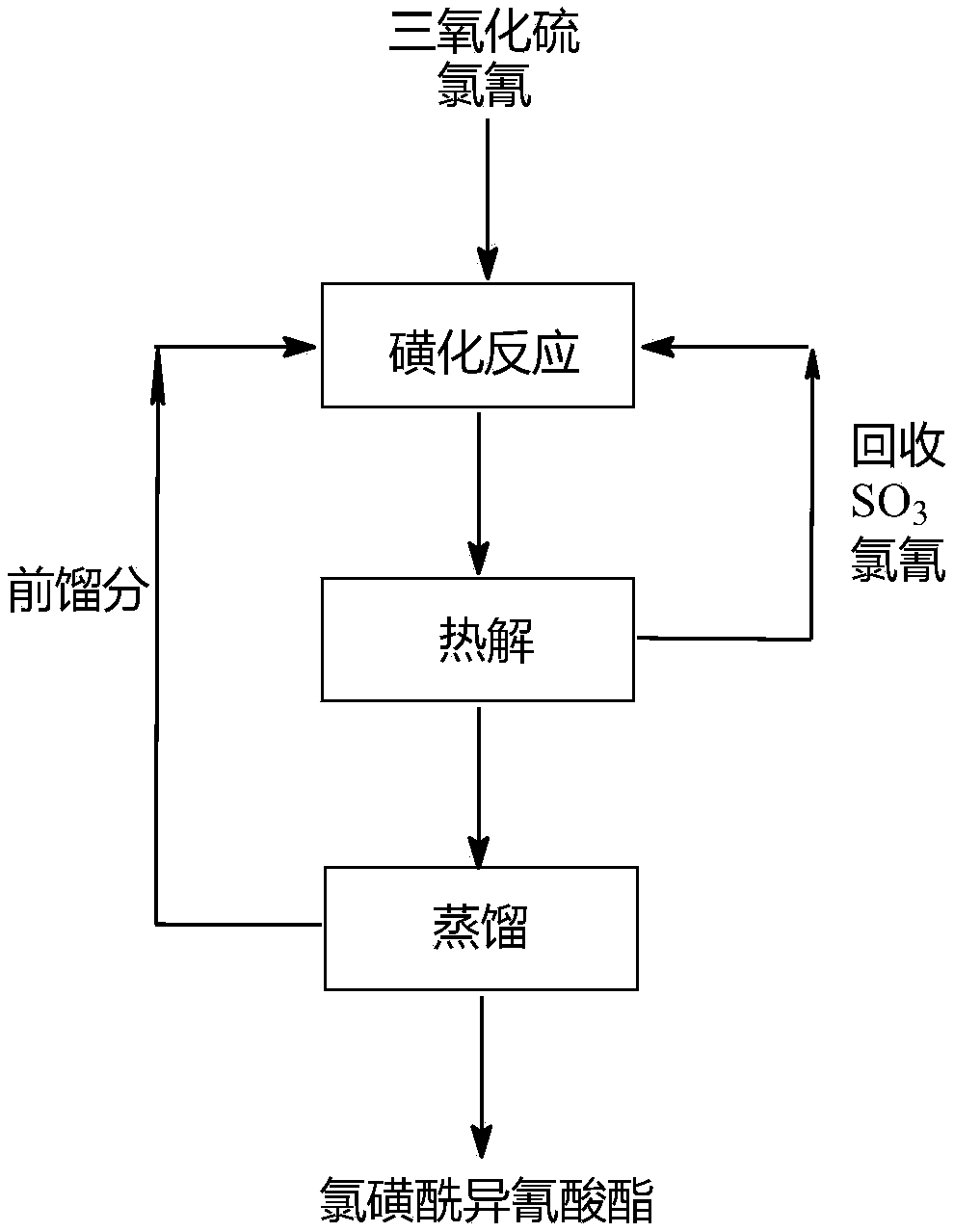
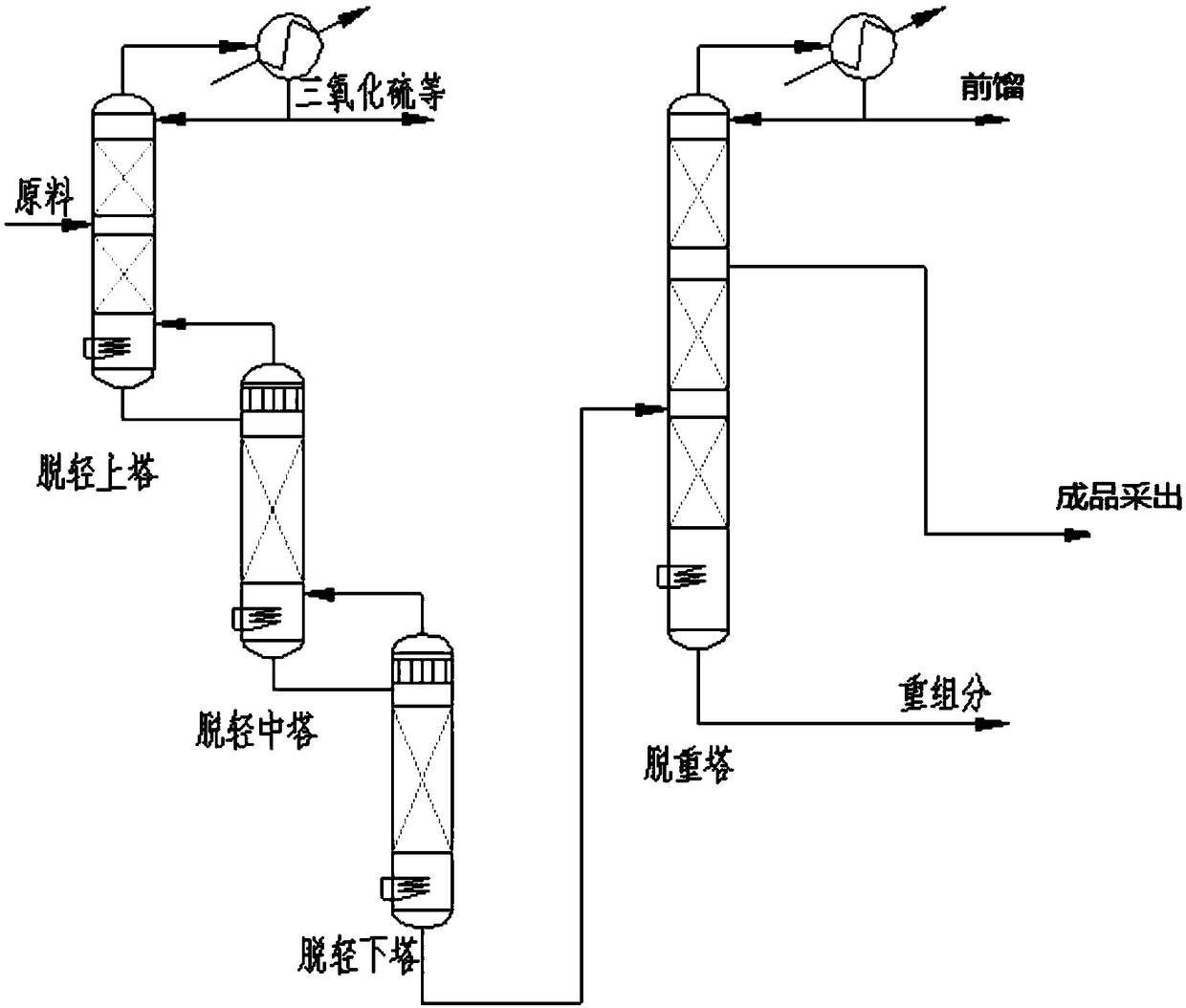
Synthesis method of high-purity chlorosulfonyl isocyanate
ActiveCN109400506AIncrease profitReduce usageSulfonic acid amide preparationPositive pressureSynthesis methods
The invention provides a synthesis method of high-purity chlorosulfonyl isocyanate. The synthesis method includes the steps that (A) under the condition of a diluent, sulfur trioxide and cyanogen chloride are subjected to sulfonation reaction to obtain a reaction solution; (B) the reaction solution is subjected to thermal decomposition reaction to obtain a reaction solution after thermal decomposition; (C) the reaction solution after thermal decomposition is subjected to positive pressure continuous distillation to obtain the high-purity chlorosulfonyl isocyanate and a distillate before distillation; and the diluent is selected from the chlorosulfonyl isocyanate or the distillate before distillation. According to the synthesis method of the high-purity chlorosulfonyl isocyanate, the totalyield of the process is increased; the distillate before distillation is used as a reaction solvent, use of a high boiling point solvent is avoided, in the pyrolysis process, the cyanogen chloride andthe sulfur trioxide are partially recovered, thus the utilization rate of raw materials is increased, and the production cost is lowered; and meanwhile, the rectification process is conducted throughthe mode of positive pressure distillation and continuous feeding, the yield of products is greatly increased, the distillation time is shortened, energy consumption and labor costs are lowered, andthe synthesis method of the high-purity chlorosulfonyl isocyanate is suitable for industrial mass production.
Owner:四平市精细化学品有限公司
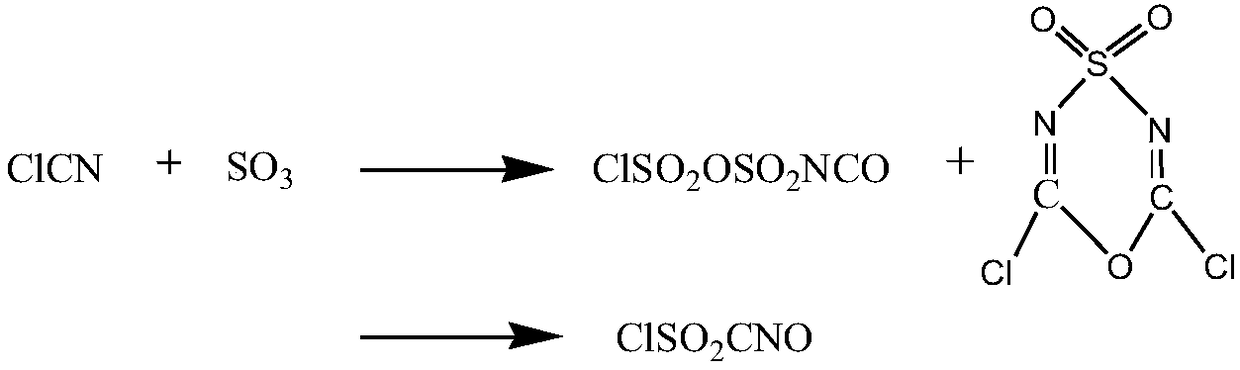
Method for producing chlorosulfonyl isocyanate by taking cyanogen chloride and sulfur trioxide as raw materials
InactiveCN104447438AUniform and controllable residence timeEnhanced mass transferOrganic compound preparationSulfonic acid amide preparationCyanogen halideImpurity
The invention relates to a method for producing chlorosulfonyl isocyanate by taking cyanogen chloride and sulfur trioxide as raw materials, belonging to the methods for producing chlorosulfonyl isocyanate. The method comprises the following steps: feeding the raw materials into a tubular reactor at a theoretical molar ratio, enabling the raw materials to react continuously in the tubular reactor, feeding the by-products of reaction into a tubular thermal decomposition reactor of by-products, continuously converting the by-products of reaction to the desired product, separating the crude product, and purifying to obtain the high-purity chlorosulfonyl isocyanate. The method provided by the invention can realize the production of the chlorosulfonyl isocyanate through continuous reaction in the tubular reactor, and the method has the advantages of low investment in devices, high production efficiency and strong production capacity. The advanced production process is adopted to produce the chlorosulfonyl isocyanate, a few by-products are generated during reaction in the tubular reactor, and the by-products can be completely converted into the target product through thermal decomposition reaction. No by-product is sent out of the bound in addition to the impurities involved into the raw materials in the production process, the utilization rate of the raw materials is high, the product has high quality, the production process is stable, and the method is easy to operate and has large operating flexibility.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY +1
Original quality cefuroxime acid and drug preparation thereof
ActiveCN105440055AReduce contentIncrease fat solubilityAntibacterial agentsOrganic active ingredientsTriethylphosphiteCefuroxime
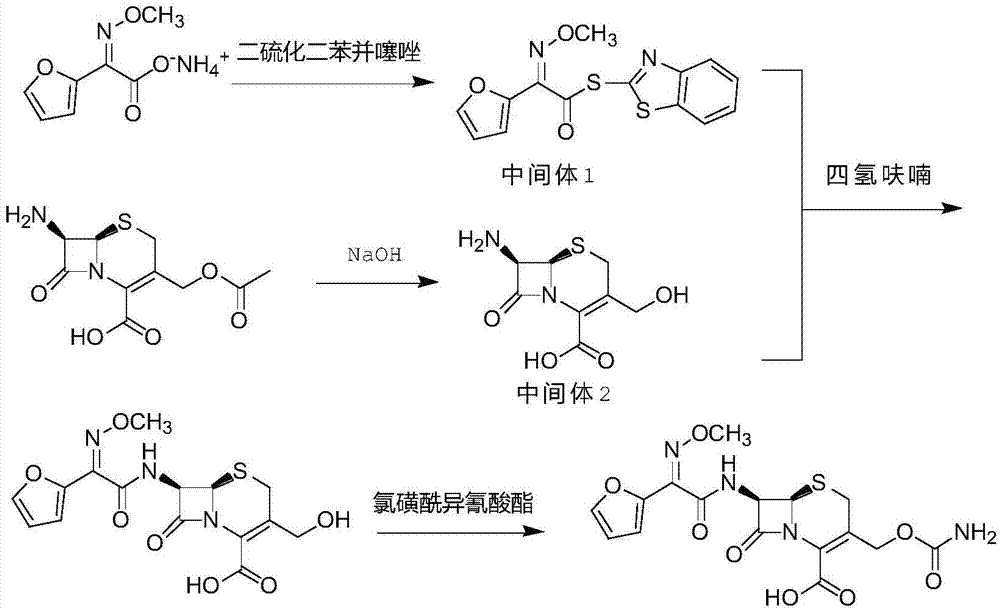
The invention discloses original quality cefuroxime acid and a drug preparation thereof. A preparation method of original quality cefuroxime acid comprises the following steps: (1), adding SMIA, dibenzothiazyl disulfide, phenylamine, dichloromethane, acetonitrile and triethylamine into a reaction bottle, stirring and increasing the temperature, dropwise adding triethyl phosphate, carrying out thermal reaction, cooling for crystallization, carrying out suction filtration, and drying to obtain an intermediate 1; (2), after hydrolyzing, crystallizing 7-ACA, filtering, and drying to obtain an intermediate 2; (3), in a tetrahydrofuran solvent, enabling the intermediate 1 and the intermediate 2 to be subjected to N-acylation reaction, after reaction, dropwise adding chlorosulfonyl isocyanate, and hydrolyzing to obtain cefuroxime acid reaction liquid; (4), purifying the cefuroxime acid reaction liquid to obtain cefuroxime acid products. The preparation method can effectively reduce the content of isomers and solve the residue problem of an accelerator M, is easy to operate, reliable in quality, and suitable for large-scale industrial production.
Owner:广东金城金素制药有限公司 +1
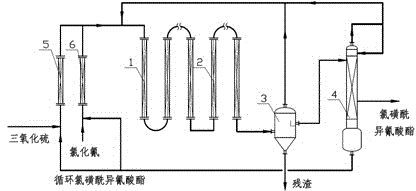
Method for Producing Chlorosulfonyl Isocyanate
ActiveUS20070286789A1Easy to controlSulfonic acid amide preparationCyanic/isocyanic acidSulfur trioxideChlorosulfonyl isocyanate
A method for producing chlorosulfonyl isocyanate by reaction of sulfur trioxide with cyanogen chloride, wherein chlorosulfonyl isocyanate or a solution including chlorosulfonyl isocyanate is used as a reaction solvent, sulfur trioxide and cyanogen chloride which are respectively diluted with the chlorosulfonyl isocyanate or the solution including chlorosulfonyl isocyanate are added at the same time to a reaction system in an almost equimolar amount under reflux. By the production method of present invention, chlorosulfonyl isocyanate can be produced from sulfur trioxide and cyanogen chloride in which the yield of the chlorosulfonyl isocyanate is high, the method has excellent operability, number of equipments is reduced, and time for controlling the temperature is saved.
Owner:NIPPON SODA CO LTD
Synthesis method of 3-aza-4-oxo-tricyclo[4.2.1.0(2,5)]non-7-ene
A synthesis method of 3-aza-4-oxo-tricyclo[4.2.1.0(2,5)]non-7-ene relates to a synthesis process of an important intermediate of 3-aminocarbonylbicycloheptenylpyridinediamine compound with anti-tumor active molecules. The method comprises the following steps: measuring off 2,5-norbornadiene, chlorosulfonyl isocyanate and weak-acid salt at a ratio of 1:1-2:1-5 (mol ratio); dissolving the 2,5-norbornadiene in an organic solvent, and adding the chlorosulfonyl isocyanate dropwise at the temperature of -10-25 DEG C; allowing reacting for 0.5-10h and then adding water of the same volume and the weak-acid salt; allowing reacting at the temperature of 0-50 DEG C for 5-48h; filtrating, separating, drying and eliminating the solvent by depressurized rotary evaporation to obtain a white solid; and recrystallizing with petroleum ether and ethyl acetate at a ratio of 1:5-1:2 to obtain the net product 3-aza-4-oxo-tricyclo[4.2.1.0(2,5)]non-7-ene. The synthesis method has simple operation, high product yield up to 77-91% which is 15-30% higher than that of the existing method and purity above 98%, and can be widely used for synthesizing natural products.
Owner:EAST CHINA NORMAL UNIV
Process for the preparation of oxcarbazepine and related intermediates
InactiveUS7125987B2High yieldSimple methodBiocideOrganic chemistryOrganic solventChlorosulfonyl isocyanate
A process for preparing Oxcarbazepine IIIcomprising:a) reacting oximinostilbene IV with chlorosulfonyl isocyanate in an inert organic solvent and isolating compound Vb) hydrolyzing compound V to form crude Oxcarbazepine IIIc) purifying oxcarbazepine.
Owner:APOTEX PHARMACHEN INC
Method for synthesizing cefuroxime sodium
The invention relates to a method for synthesizing cefuroxime sodium, which comprises the following steps: 1, performing the N- acylation reaction of 3-deacetyl-7aminocephalosporanic acid and methoxyaminofuranyl ammonium salt which serve as raw materials and adjusting the pH value with hydrochloric acid to less than 7 in a mixed solvent phase to precipitate crystals to obtain 3-deoxyformyl cefuroxime acid; 2, performing the addition reaction of the 3-deoxyformyl cefuroxime acid and chlorosulfonyl isocyanate serving as a strong ammonia formylating agent in an organic solvent to obtain chlorosulfonyl cefuroxime acid, dehydrating the chlorosulfonyl cefuroxime acid to obtain the cefuroxime acid, decarburizing and concentrating the cefuroxime acid, crystallizing the cefuroxime acid in a solvent phase, and drying the crystals under vacuum to obtain a solid product of cefuroxime acid; and 3, dissolving the cefuroxime acid in alkaline solution, decarburizing the resulting product, crystallizing the resulting product in a mixed solvent phase, filtering crystals, and drying the crystals under vacuum to obtain the cefuroxime sodium.
Owner:哈药集团股份有限公司 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com










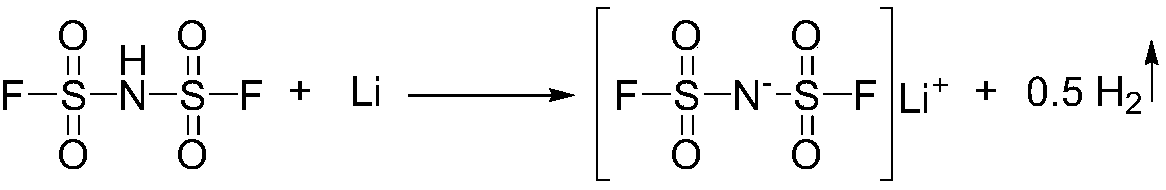


![Synthesis method of 3-aza-4-oxo-tricyclo[4.2.1.0(2,5)]non-7-ene Synthesis method of 3-aza-4-oxo-tricyclo[4.2.1.0(2,5)]non-7-ene](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/453c83d9-bc48-49d6-93b0-64b6b671da57/A20091004890900031.PNG)
![Synthesis method of 3-aza-4-oxo-tricyclo[4.2.1.0(2,5)]non-7-ene Synthesis method of 3-aza-4-oxo-tricyclo[4.2.1.0(2,5)]non-7-ene](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/453c83d9-bc48-49d6-93b0-64b6b671da57/A20091004890900041.PNG)