Preparation method of imidodisulfuryl fluoride lithium salt
A technology of bisfluorosulfonimide lithium salt and bisfluorosulfonimide is applied in the field of preparation of bisfluorosulfonimide lithium salt, which can solve the problem that it is not suitable for industrial production, affects practical application, and is not easy for LiF and LiFSI. separation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
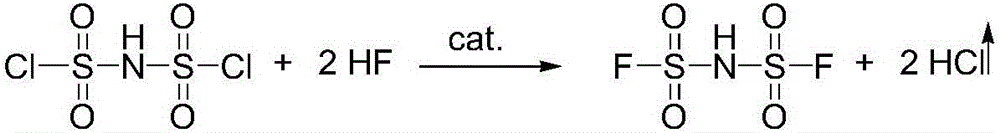
[0051] One aspect of the present invention provides a method for preparing lithium salt of bisfluorosulfonyl imide. The method for preparing lithium salt of bisfluorosulfonyl imide may include: reacting chlorosulfonic acid and chlorosulfonyl isocyanate in the presence of a catalyst Obtain two chlorosulfonimides (HClSI), and the specific reaction equation is as follows:
[0052]
[0053] In the above reaction process, the catalyst may be an acid, more specifically, the catalyst may be a protonic acid and / or a Lewis acid. The Lewis acid is usually based on the Lewis acid-base theory, and generally refers to a substance that can accept electron pairs. Examples of specific Lewis acids that can be used include but are not limited to NiCl 2 , FeCl 2 , FeCl 3 、CoCl 3 , ZnCl 2 , MnCl 2 The combination of one or more of them; the protonic acid is usually based on the Bronster-Lowry acid-base theory, usually refers to the proton (hydrogen ion, H + ) molecules or ions, examples ...
Embodiment 1
[0084] Preparation of bischlorosulfonimide:
[0085] In the 1000mL reaction flask, add 478.6g of chlorosulfonic acid, NiCl 2 2.1g, start stirring, raise the temperature to 105-115°C, and add 679.2g of chlorosulfonyl isocyanate dropwise. After the dropwise addition, gradually raise the temperature to 130-140°C, and continue stirring for 20 hours. Distilled under reduced pressure, 738 g of stable fractions were collected, with a yield of 83.9%. The product is a white solid with a melting point of about 36°C.
Embodiment 2
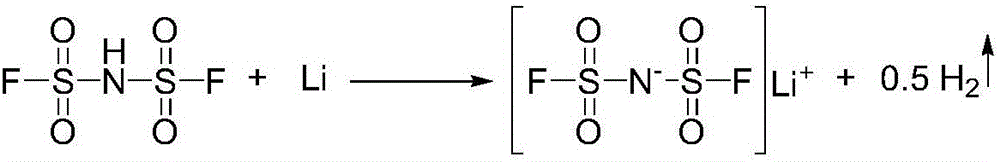
[0087] Preparation of bisfluorosulfonimide:
[0088] In the 1000mL tetrafluoro reaction bottle, add the HClSI 738g that embodiment 1 makes, MoCl 5 0.48g, heat up to 100-105°C, slowly introduce about 130g of HF gas under stirring, cool down to room temperature after 12 hours of reaction, and blow nitrogen for 16 hours to obtain about 612g of crude product, short-distilled to obtain 516g of product, yield 82.6%. The product is a colorless liquid with a melting point of about 17°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com