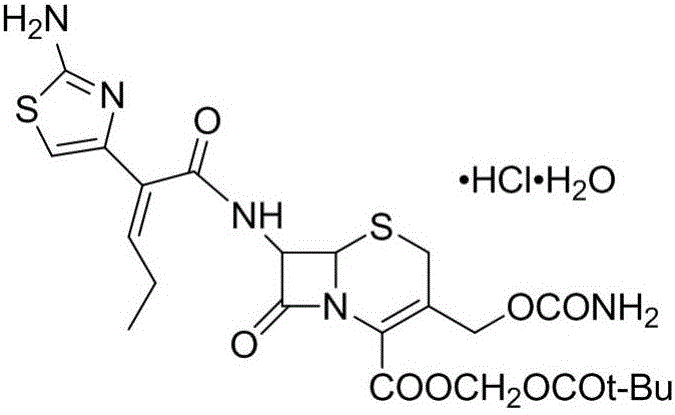
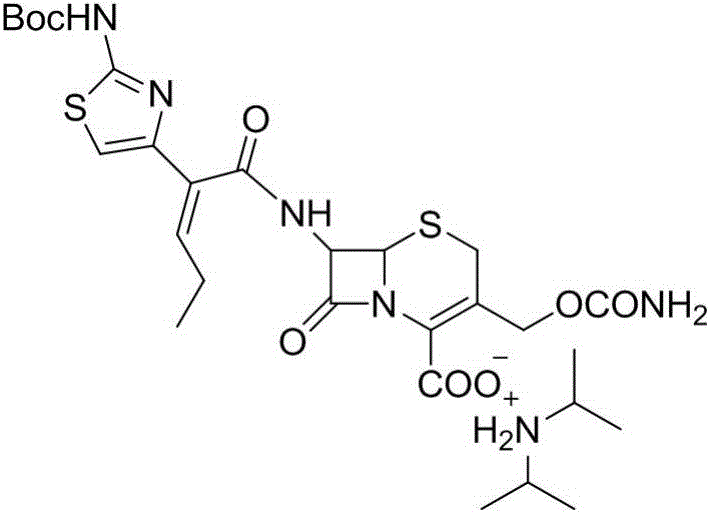
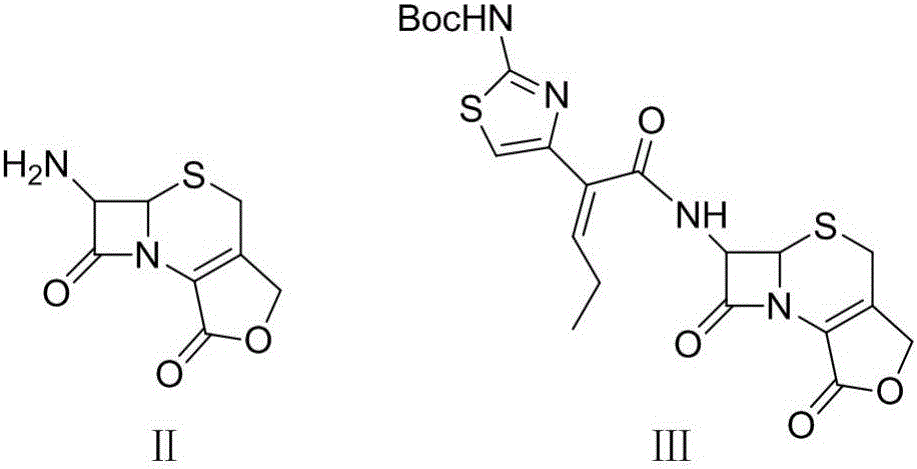
Synthesis and purification method of t-butyloxycarboryl cefcapene diisopropylammonium
A technology of carpine acid diisopropylamine salt and tert-butoxycarbonyl head, which is applied in the field of medicine and chemical industry, can solve the problems of extended production cycle, cumbersome operation, and a large number of by-products, and achieve energy saving and environmental protection. Growth of ester impurities, effects of avoiding production cycles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] (1)(6R,7R)-7-[(2Z)-(2-tert-butoxycarbonylamino-thiazol-4-yl)pent-2-enamido)-3-hydroxymethyl-8-oxo Synthesis and Purification of -5-thio-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (Compound Ⅰ)
[0046] Into a 5L reaction flask, add 1500mL of acetonitrile, 76g (0.33mol) of D-7ACA, 107.4g (0.36mol) of side chain acid, cool to -15°C, add 133.6g (1.32mol) of triethylamine dropwise, continue to drop after dropping Add 55.2g (0.36mol) of phosphorus oxychloride, after dripping, the material solution is heated to 0℃ to react for 2h, add 3000mL of dichloromethane, 1000mL of purified water, separate, collect the organic phase, and wash the organic phase twice with 1000mL of purified water , Add 50g of anhydrous magnesium sulfate, stir and dry for 1h, filter with suction to obtain the filtrate, refrigerate for later use;
[0047] (2) Synthesis and purification of tert-butyloxycarbonyl cefcapene acid diisopropylamine salt
[0048] Put the filtrate in step (1) into a 5L reaction flask...
Embodiment 2
[0050] (1)(6R,7R)-7-[(2Z)-(2-tert-butoxycarbonylamino-thiazol-4-yl)pent-2-enamido)-3-hydroxymethyl-8-oxo Synthesis and purification of -5-thio-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (compound Ⅰ)
[0051] Into a 5L reaction flask, add 1500mL of acetone, 76g (0.33mol) of D-7ACA, 119.3g (0.40mol) of side chain acid, reduce the temperature to -12°C, add 202.4g (2.0mol) of triethylamine dropwise, control the temperature -3 Continue to add phosphorus oxychloride (0.50mol) dropwise at ℃, keep the temperature at -3℃ for 2h, add 3000mL of dichloromethane, 1000mL of purified water, separate, collect the organic phase, wash the organic phase twice with 1000mL of purified water, add 50g anhydrous magnesium sulfate, stir and dry for 1h, filter with suction to obtain the filtrate, refrigerate for later use;
[0052] (2) Synthesis and purification of tert-butoxycarbonyl cefcapinic acid diisopropylamine salt
[0053] Put the filtrate in step (1) in a 5L reaction flask, lower the temperatur...
Embodiment 3
[0055] (1)(6R,7R)-7-[(2Z)-(2-tert-butoxycarbonylamino-thiazol-4-yl)pent-2-enamido)-3-hydroxymethyl-8-oxo -5-
[0056] Synthesis and Purification of Sulfur-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (Compound Ⅰ)
[0057] Into a 5L reaction flask, add 1500mL of acetonitrile, 76g (0.33mol) of D-7ACA, 107.4g (0.36mol) of side chain acid, cool to -13°C, add 267.1g (2.64mol) of triethylamine dropwise, continue to drop after dropping Add 76.7g (0.50mol) of phosphorus oxychloride, after dripping, the material solution is heated to -5℃ and react for 2h, add 3000mL of dichloromethane, 1000mL of purified water, separate, collect the organic phase, and wash the organic phase with 1000mL of purified water 2 Add 50g of anhydrous magnesium sulfate, stir and dry for 1 hour, filter with suction to obtain the filtrate, refrigerate for later use;
[0058] (2) Synthesis and purification of tert-butyloxycarbonyl cefcapene acid diisopropylamine salt
[0059] Put the filtrate in step (1) in a 5L react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com