Original quality cefuroxime acid and drug preparation thereof
A technology of cefuroxime acid and cefuroxime sodium, applied in drug delivery, medical preparations containing active ingredients, antibacterial drugs, etc. Increased fat solubility, less impurities, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
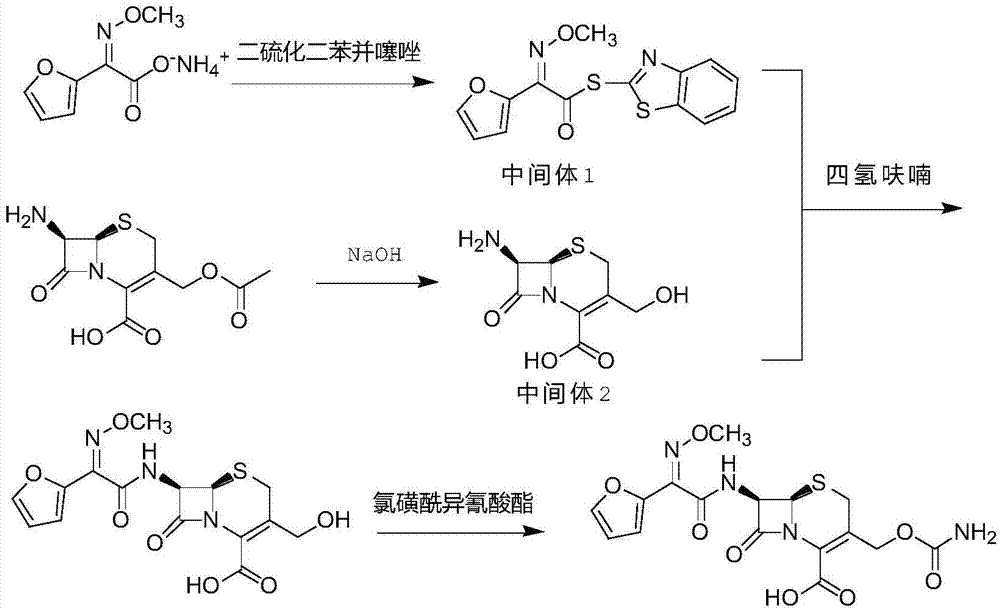
[0019] A kind of preparation method of original research quality cefuroxime acid, the synthetic route is as follows:
[0020]
Embodiment 1
[0022] Preparation of Intermediate 1
[0023] In a 500ml three-necked reaction flask, add 18.6 grams of furan ammonium salt, 36.5 grams of dibenzothiazole disulfide, 5 ml of aniline, 200 ml of dichloromethane, and 100 ml of acetonitrile, stir evenly, then add 10.2 g of triethylamine, stir and heat up to 30~ 35°C, put 19.9 grams of triethyl phosphite into the dropping funnel, drop it within 1.0-2.0 hours, continue the heat preservation reaction for 1.0 hours, when the remaining ammonium furan salt is less than 1% as detected by HPLC, cool down to 0-5°C and analyze After crystallization for 1.0 hour, 29.7 g of intermediate 1 was obtained by suction filtration and drying, with a yield of 93.4% and a purity of 99.4% by HPLC.
[0024] Preparation of Intermediate 2
[0025] Add 27.2g of 7-ACA into a 500ml three-necked bottle, then add 100ml of purified water and 150ml of ethanol, stir evenly, cool down to below -20°C, use 10% aqueous sodium hydroxide solution to adjust the pH to 11...
Embodiment 2
[0031] The preparation of intermediate 1, with embodiment 1
[0032] The preparation of intermediate 2, with embodiment 1
[0033] Preparation of Cefuroxime Acid
[0034] In a 1000ml three-necked reaction flask, add 150ml tetrahydrofuran, add 29.7g of intermediate 1 and 21.0g of intermediate 2 in turn, and stir at room temperature for 2.0 hours; after the completion of the reaction of intermediate 2 by HPLC, cool the reaction solution to below -30°C , add 32.3 g of chlorosulfonyl isocyanate (n=2.5) dropwise, and after insulated and stirred for 1.0 hour, add 200 ml of purified water dropwise, and naturally heat up to 0-5° C. to obtain a cefuroxime acid reaction solution;
[0035] Refining Treatment of Cefuroxime Acid
[0036] Add 350ml of dichloromethane to the cefuroxime acid reaction solution, stir and separate at 0-5°C, wash the organic phase with 100ml of 1.0mol / L hydrochloric acid aqueous solution and 100ml of saturated saline twice, wash the organic phase with 10 400ml...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com