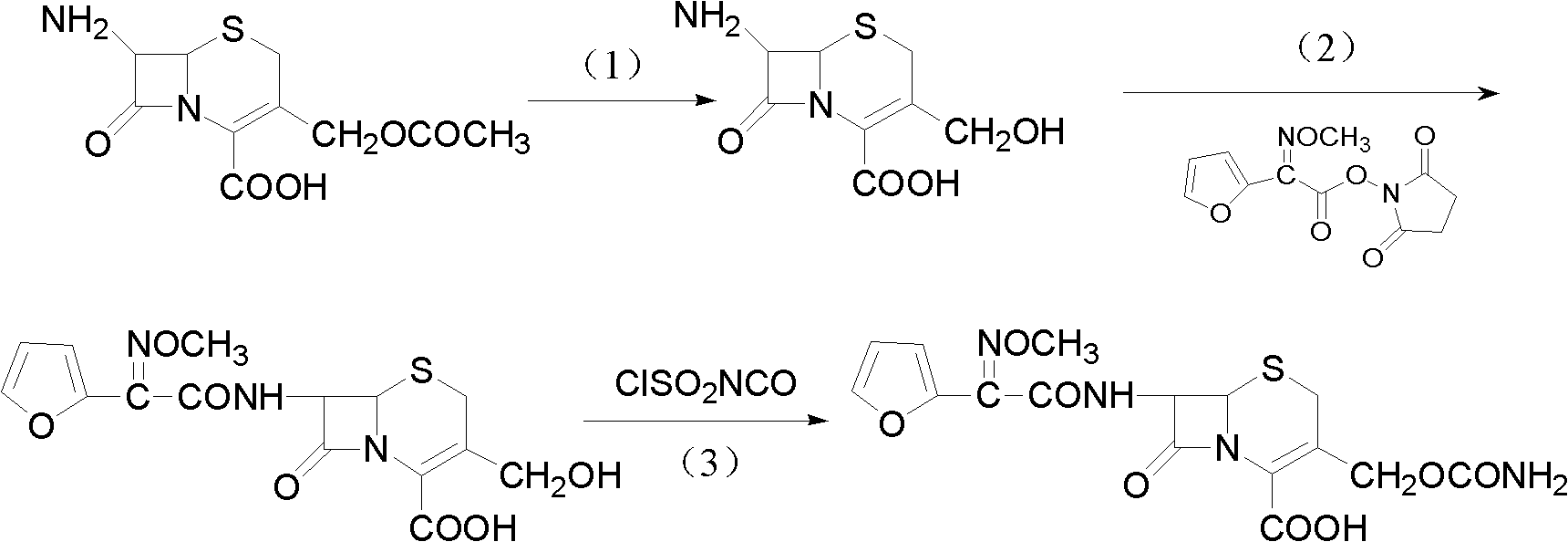
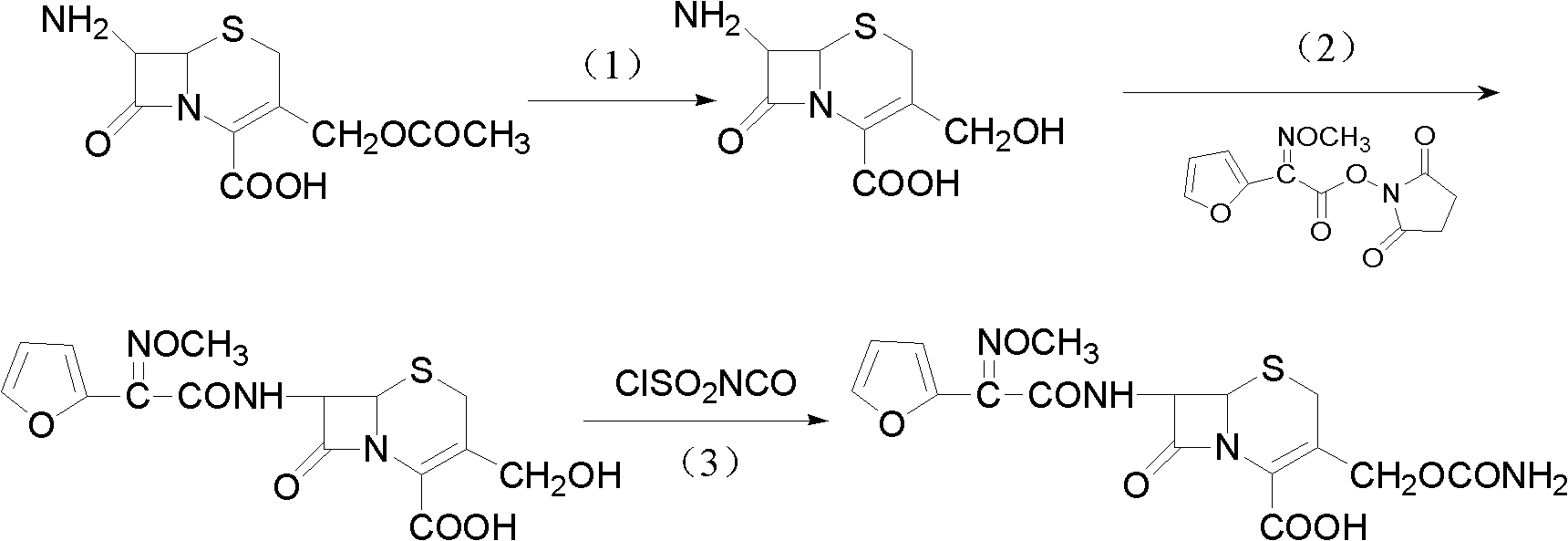
Method for preparing cefuroxime acid
A technology of cefuroxime acid and cephalosporanic acid, which is applied in the direction of organic chemistry, can solve the problems of restricting industrial production, and achieve the effects of less discharge of three wastes, simple process operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 550ml of methanol and 550ml of purified water into the reaction flask to form a mixed solvent, then add 100g of 7-ACA, stir and cool down to -15°C, add 15% sodium hydroxide purified aqueous solution dropwise, control the temperature at -15°C, and pH at 11 Under the condition of ~12, carry out selective hydrolysis reaction; HPLC detects that 7-ACA peak completely disappears as the reaction end point, and the reaction time is about 1 hour; DACA solution is kept at low temperature for use;
[0031] Add 80 ml of Et to the above 7-DACA solution 3 N, 118.4g 2-[2-furyl]-2-[methoxyimino]acetic acid-(2,5-dioxo-pyrrolidinyl)-1-ester, reaction under ice-water bath conditions, HPLC detection 7 - DACA residual area < 1% is the reaction end point; after the reaction is completed, separate the water phase, add 6g of activated carbon and stir for half an hour to decolorize; then filter, wash with water, cool down to 5°C, add 2mol / L hydrochloric acid dropwise to pH = 2, keep warm ...
Embodiment 2
[0034] Add 300ml of ethanol, 300ml of methanol, and 600ml of purified water into the reaction flask to form a mixed solvent, then add 100g of 7-ACA, stir and cool down to 0°C, add 15% potassium hydroxide purified aqueous solution dropwise, control the temperature to 0°C, and the pH to 11-12, carry out selective hydrolysis reaction; HPLC detects that the 7-ACA peak completely disappears as the reaction end point, and the reaction time is about 1 hour; after the reaction is completed, add 2mol / L hydrochloric acid dropwise to adjust the pH to 7.0-8.0 to obtain a 7-DACA solution low temperature standby;
[0035] Add 100 ml of Et to the above 7-DACA solution 3 N, 146.5g 2-[2-furyl]-2-[methoxyimino]acetic acid-(2,5-dioxo-pyrrolidinyl)-1-ester, reaction under ice-water bath conditions, HPLC detection 7 - DACA residual area < 1% is the reaction end point; after the reaction is completed, separate the water phase, add 6g of activated carbon and stir for half an hour to decolorize; the...
Embodiment 3
[0038] Add 600ml of isopropanol and 600ml of purified water into the reaction flask to form a mixed solution, then add 100g of 7-ACA, stir and cool down to -15°C, add 30% sodium hydroxide purified aqueous solution dropwise, control the temperature at -15°C, pH The value is 11~12, carry out selective hydrolysis reaction; HPLC detects that 7-ACA peak completely disappears as the reaction end point, and the reaction time is about 1 hour; DACA solution is kept at low temperature for use;
[0039] Add 110 ml of Et to the above 7-DACA solution 3 N, 160.5g 2-[2-furyl]-2-[methoxyimino]acetic acid-(2,5-dioxo-pyrrolidinyl)-1-ester, reaction under ice-water bath conditions, HPLC detection 7 - DACA residual area < 1% is the reaction end point; after the reaction is completed, separate the water phase, add 6g of activated carbon and stir for half an hour to decolorize; then filter, wash with water, cool down to 5°C, add 2mol / L hydrochloric acid dropwise to pH = 2, keep warm Stir for 2 ho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific rotation | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com