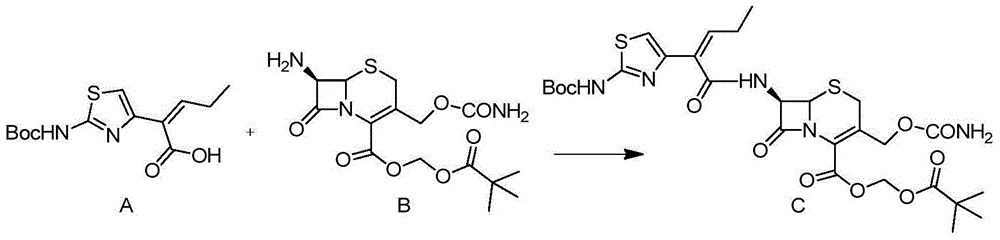
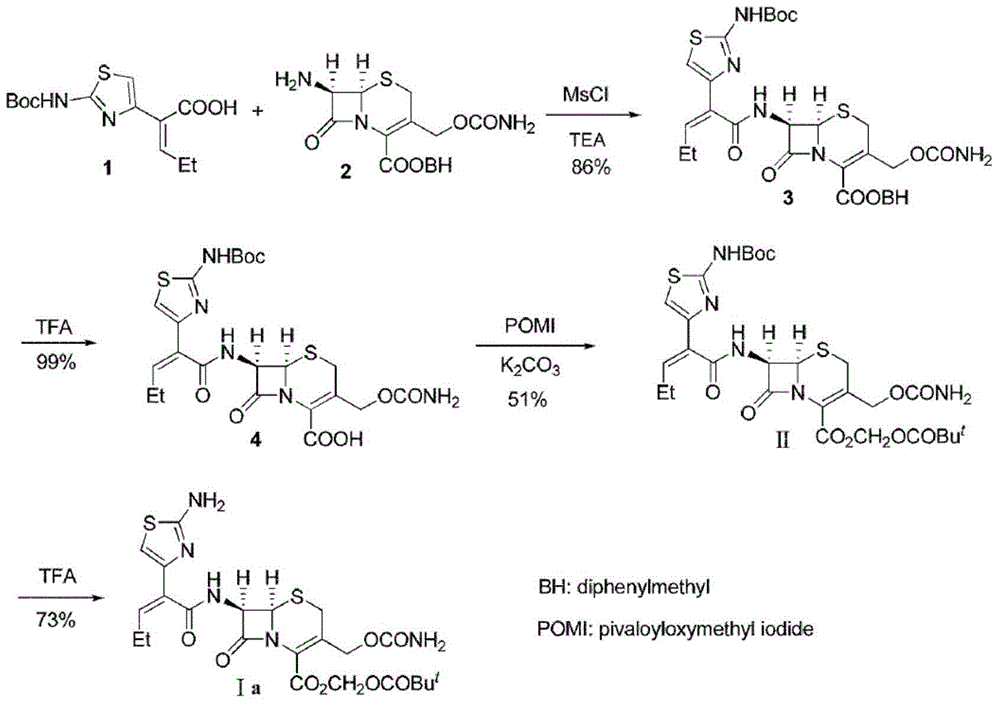
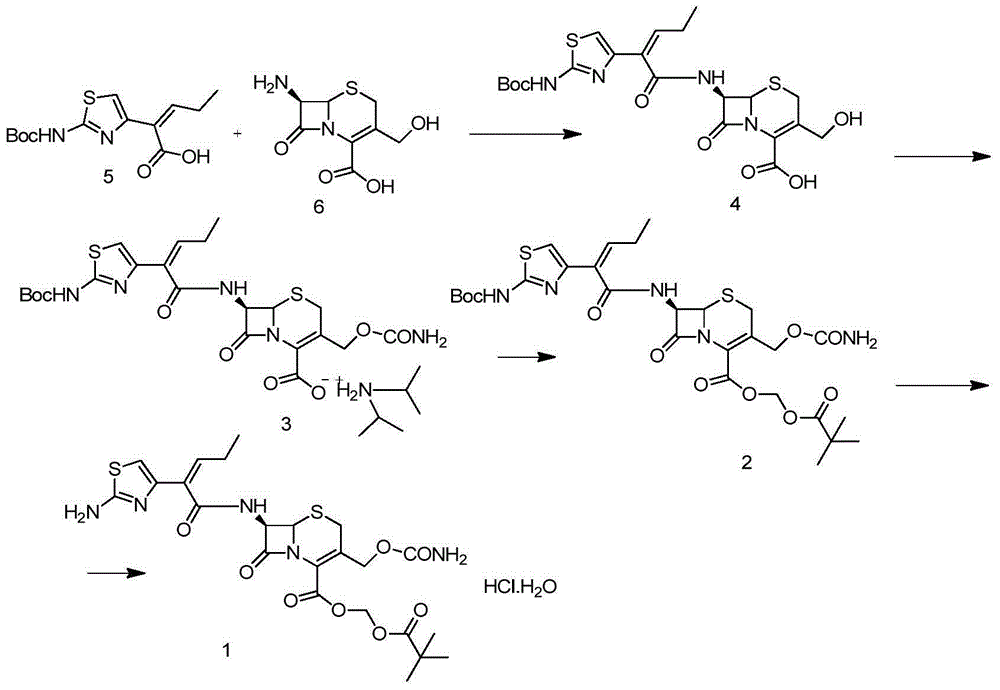
Preparation method for cefcapene pivoxil hydrochloride
A technology of cefcapene hydrochloride and carboxylic acid, applied in the direction of organic chemistry, etc., can solve the problems of cefcapene carboxylic acid core structure damage, easy production of by-products and impurities, affecting yield and quality, etc., to avoid ring opening Effect of destruction, reduction of dissolution time, improvement of yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the synthesis of 7-amino-3-hydroxymethyl cephalosporanic acid (7-DACA, compound 6)
[0042] Weigh 27.2g (0.1mol) of 7-ACA, add 50mL methanol and 50mL water, add 3.0g tetra-n-butylammonium chloride and stir to dissolve at -5-5°C, and add 2mol / L hydrogen dropwise at this temperature Sodium oxide solution 105mL, after dripping, keep the temperature and continue to stir for 0.5-1 hour, then add 30% hydrochloric acid to adjust the pH to neutral, precipitate a solid, suction filter, wash with absolute ethanol, dry to obtain 20.7g of off-white solid, and collect The yield was 90.1%, the HPLC purity analysis was 97.35% (area normalization method), and the lactone impurity was 0.33%.
Embodiment 2
[0043] Embodiment 2: the synthesis of 7-amino-3-hydroxymethyl cephalosporanic acid (7-DACA, compound 6)
[0044] Weigh 27.2g (0.1mol) of 7-ACA, add 50mL methanol and 50mL water, add 1.5g tetra-n-butylammonium chloride and stir to dissolve at -5-5°C, and add 2mol / L hydrogen dropwise at this temperature Sodium oxide solution 105mL, after dripping, keep the temperature and continue to stir for 0.5-1 hour, then add 30% hydrochloric acid to adjust the pH to neutral, precipitate a solid, suction filter, wash with absolute ethanol, dry to obtain 19.6g of off-white solid, and collect The yield was 85.2%, the HPLC purity analysis was 95.58% (area normalization method), and the lactone impurity was 0.56%.
Embodiment 3
[0045] Embodiment 3: the synthesis of 7-amino-3-hydroxymethyl cephalosporanic acid (7-DACA, compound 6)
[0046] Weigh 27.2g (0.1mol) of 7-ACA, add 50mL of methanol and 50mL of water, add 1.5g of benzyltrimethylammonium chloride and stir to dissolve at -5-5°C, and add 2mol / L of Sodium hydroxide solution 105mL, after dropping, keep the temperature and continue to stir for 0.5-1 hour, then add 30% hydrochloric acid to adjust the pH to neutral, a solid is precipitated, suction filtered, washed with absolute ethanol, and dried to obtain 18.6g of off-white solid, Yield 80.5%, HPLC purity analysis 93.8% (area normalization method), lactone impurity 0.88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com