Oxcarbazepine and synthesizing process of its intermediate
A synthesis method and intermediate technology, applied in the field of reaction to obtain OCBZ, can solve the problem of high cost, achieve the effects of increased yield, increased productivity, and shortened process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
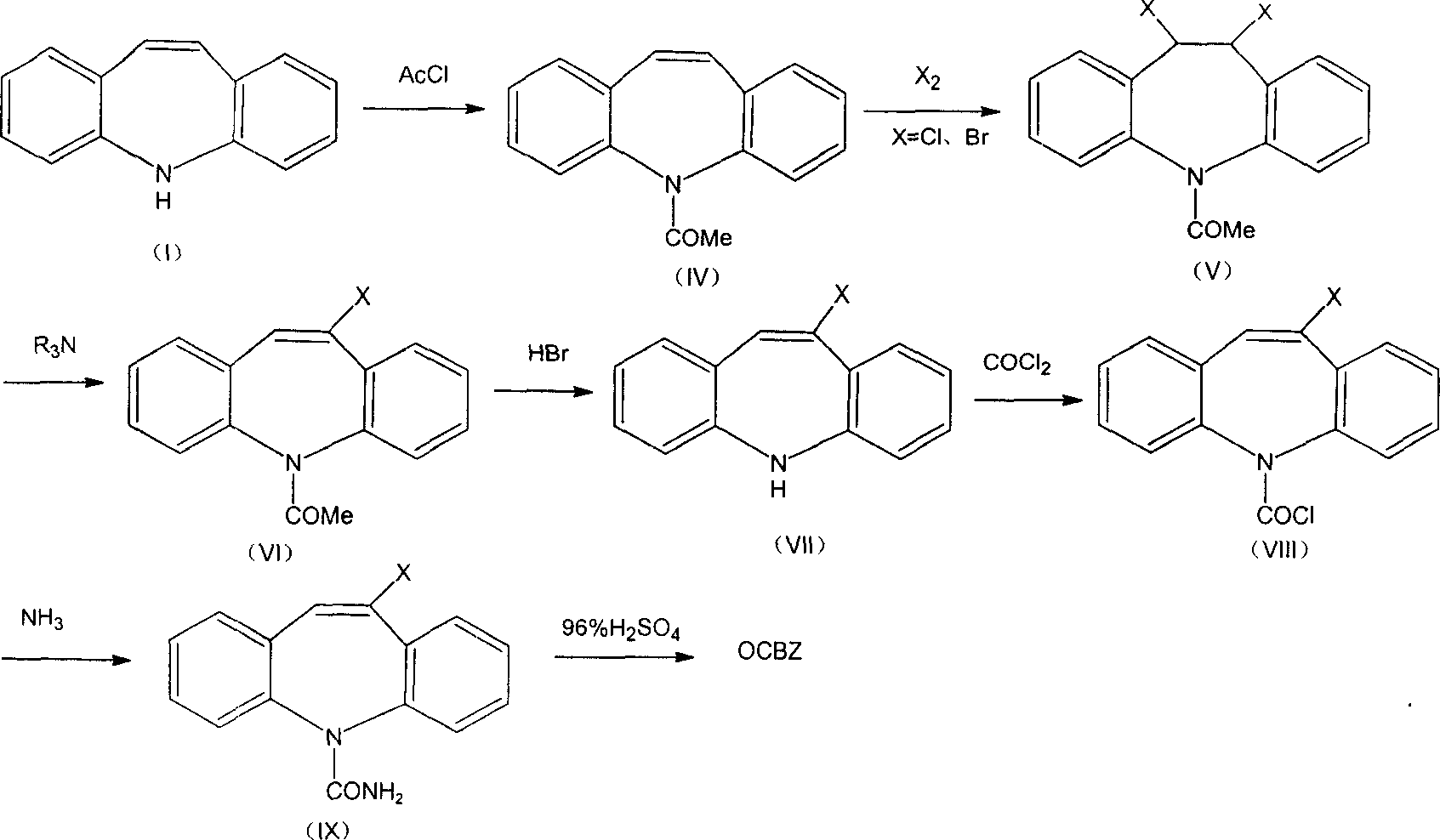
[0045] Add 300mL of chloroform and 15.3g (20mmol) of 5H-dibenzo[b,f]azepine-5-carbonyl chloride into a 500mL four-neck flask, then add 4.8g (20mmol) of bromine dropwise within 10min below 30°C , then stirred at 20-30°C for 3h, concentrated at 0.02MPa to remove 70mL of chloroform, cooled to 5-10°C and filtered to obtain brown crystal 10,11-dibromo-5H-dibenzo[b,f]azepine 23.2g of azo-5-formyl chloride (XI), melting point 163-165°C; HPLC content 98.6%, yield 93%;
[0046]Add chloroform 200mL in 500mL autoclave, above-mentioned 10,11-dibromo-5H-dibenzo[b,f]azepine-5-formyl chloride 23.2g, pass into liquid ammonia 3.8g ( 224mmol) and the pressure is about 0.5MPa, and the reaction is stirred until the pressure does not drop, about 30h. Then emit ammonia gas, and feed nitrogen to drive away the ammonia gas, then take out the reaction solution and filter to remove the ammonium chloride and ammonium bromide solids, and wash the solids twice with 50mL chloroform respectively, then comb...
Embodiment 2
[0049] Through ammonia 4.7g, dichloromethane consumption is 330mL, ammonolysis reaction initial pressure is about 1.2MPa, normal temperature reaction is about 22h, other is the same as Example 1, obtains (IX) 18.8g, HPLC content 96.8%, yield 95%.
[0050] After the same treatment as in Example 1, 9.5 g of OCBZ crystals were obtained, with a melting point of 219-221° C., an HPLC content of 96.8%, and a yield of 63%.
Embodiment 3
[0052] Through ammonia 1.0g, the amount of tetrachloromethane is 33mL, the initial pressure of ammonolysis reaction is about 0.2MPa, and the reaction is about 50h at -10°C. Others are the same as in Example 1, and 19.8g of (IX) is obtained. The HPLC content is 97.5%, and the yield is 97%. .
[0053] After the same treatment as in Example 1, 9.5 g of OCBZ crystals were obtained, with a melting point of 220-222° C., an HPLC content of 98.2%, and a yield of 64%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com