Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
158 results about "Carbonyl chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Rhodium carbonyl chloride is an organorhodium compound with the formula Rh2Cl2(CO)4. It is a red-brown volatile solid that is soluble in nonpolar organic solvents. It is a precursor to other rhodium carbonyl complexes, some of which are useful in homogeneous catalysis.
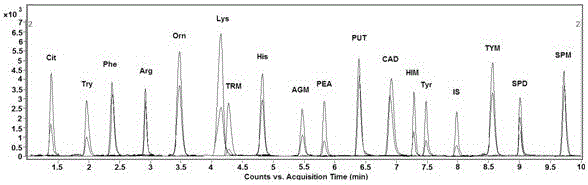
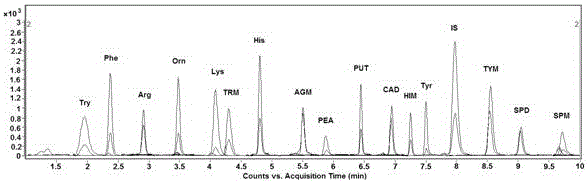
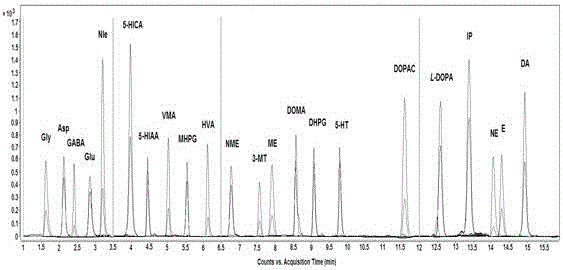
Analysis method for simultaneous detection of amino acids and biogenic amines in foods
ActiveCN105866316AEasy to separateHigh detection sensitivityComponent separationDerivatizationUltrasound assisted
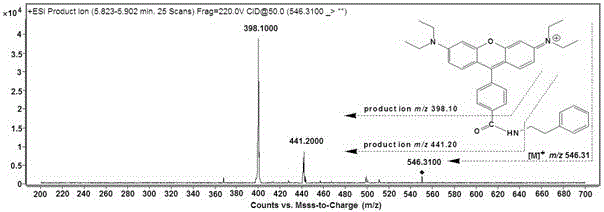
The invention belongs to the field of analytical chemistry and particularly relates to an analysis method for simultaneous detection of amino acids and biogenic amines in foods. The method is characterized in that for the first time, 4'-carbonyl chloride-rhodamine is used as a derivatization reagent to realize simultaneous derivatization of the amino acids and the biogenic amines in the food by means of ultrasonic assistance, in-situ derivation and dispersive liquid-liquid microextraction, and qualitative and quantitative detection and analysis are performed by means of ultra-high performance liquid chromatography coupled with triple quadrupole tandem mass spectrometry in multi-reaction monitoring mode. The method can be used for simultaneous analysis and detection of eight amino acids and nine biogenic amines; by adoption of 1,7-diamino heptane as an internal standard substance, contents of the amino acids and the biogenic amines in different foods can be obtained accurately according to an internal standard method for quantification. The method has advantages of simplicity, quickness, high sensitivity, high selectivity and the like, and high recovery rate is achieved. In addition, by the analysis method, accurate and reliable technical means can be provided for food quality assessment and supervision.
Owner:食药环检验研究院(山东)集团有限公司
Metal porphyrin langmuir blodgett film optical fiber gas sensor
InactiveCN101059422AGood repeatabilityShort response timeMaterial analysis by optical meansCarbonyl chloridePorphyrin structure
A metal porphyrin lange mull bulogit film optical fiber gas sensor relates to a sensor for checking harmful gas device in industrial condition. The inventive sensor comprises a dual-arm optical fiber, a reaction chamber, and a metal porphyrin LB film array board. The invention is characterized in low check limit, high sensitivity, repeat application, and wide check air range, and the invention can be used to check various volatile harmful gas, more particularly check the discharge and leaked harmful gas as NO2, SO2, NO, CO, H2S and carbonyl chloride.
Owner:CHONGQING UNIV
Preparation of isocyanato silanes
A process for the preparation of alkoxysilyl group-containing isocyanate compounds which includes the step of reacting carbonyl chloride ClC(═O)Cl with an alkoxysilyl group-containing amine in the presence of a high boiling point base, and optionally in the presence of an inert organic solvent, in order to minimize by product production and to simplify product purification, is disclosed.
Owner:MOMENTIVE-PERFORMANCE MATERIALS GMBH
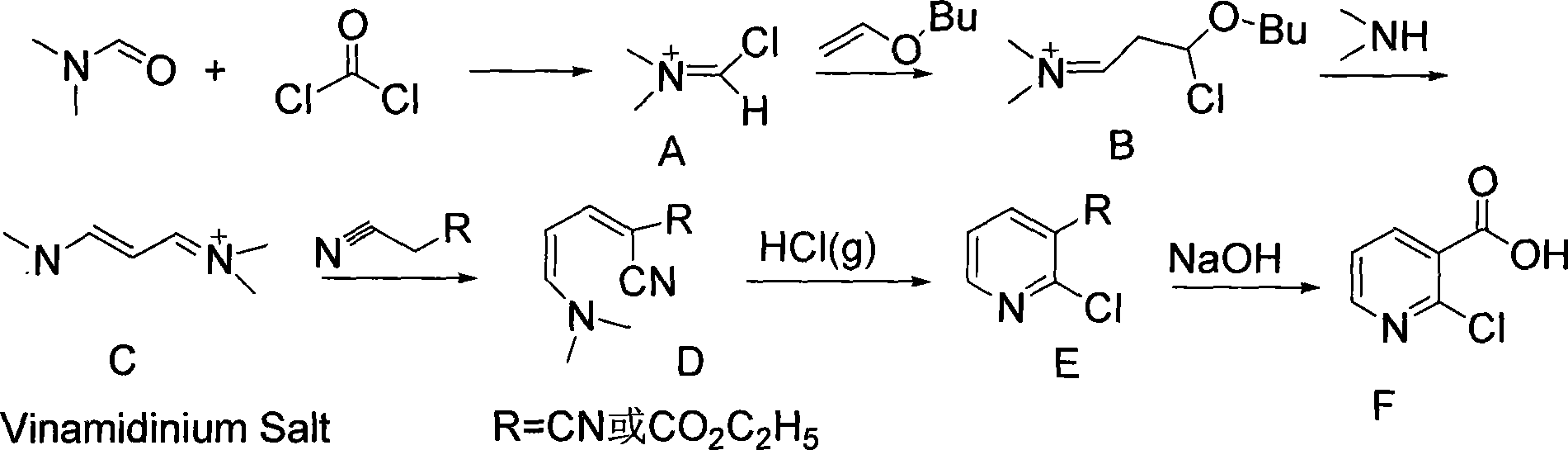
Synthesis of 2-chlorine apellagrin
The present invention relates to a chemical synthesis method of an important intermediate 2-chlorine nicotine acid in medicine and pesticides, and a production process thereof. The route of synthesis reaction comprises: solid carbonyl chloride reacts with N, N-dimethyl formamide, then reacts with ethenyl n-butyl ether, added into dimethylamine aqueous solution, and reacts with cyano ethyl acetate after being dehydrated, the HCl gas is added, and the prepared compound is dissolved in alkaline solution. The raw materials, namely, the solid carbonyl chloride, the N, N-dimethyl formamide, the dimethylamine and the cyano ethyl acetate can be easily acquired. Only one step of distillation and purification is needed from the raw materials to the product; no harsh conditions are required in all the steps, the operation is simple and conducive to the environmental protection, and the present invention has remarkable social benefits and economic benefits, and is suitable for industrial production.
Owner:NANKAI UNIV
Detecting method for determining various neurotransmitters on the basis of in situ derivation
ActiveCN105866303AHigh detection sensitivityHigh selectivityComponent separationChemical structureCarboxylic acid
Owner:山东住融物业有限公司
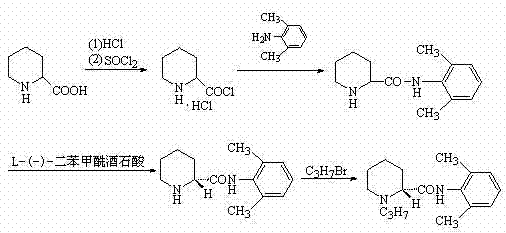
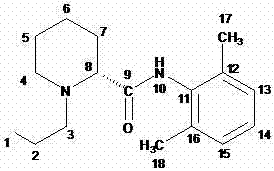
Method for preparing ropivacaine
InactiveCN103086954AAvoid separationSimple processOrganic chemistryDimethylaniline N-oxideCarboxylic acid
The invention relates to a one-pot method for preparing ropivacaine. According to the invention, L-piperidine-2-carbonyl chloride is prepared from L-piperidine-2-carboxylic acid; intermediate separation is not needed, and the material is directly subjected to a reaction with 2,6-dimethylaniline, and (S)-N-(2,6-dimethylphenyl)piperidine-2-carboxamide is prepared; intermediate separation is not needed, and the material is directly subjected to a reaction with bromopropane, such that ropivacaine is prepared. According to the invention, when the intermediate is prepared, no separation is needed, and reactions can be directly carried out. The method is green and environment-friendly. With the method, process operation is simplified, cost is reduced, and yield is improved. The method is more suitable for large-scale industrialized productions.
Owner:SHANDONG INST OF PHARMA IND
Method for preparing 3-(3,4-dichlorophenyl)-1-methoxy-1-methyl urea
ActiveCN101781236ANothing producedEasy to recycleUrea derivatives preparationOrganic compound preparationCarbonyl chlorideHydroxylamine sulfate
The invention discloses a method for preparing 3-(3,4-dichlorophenyl)-1-methoxy-1-methyl urea, which comprises the following steps: taking carbonyl chloride as an acylating agent to carry out an acylation reaction with 3,4-dichlorophenyl; obtaining 3,4-dichlorophenyl isocyanate; generating 1-hydroxy-3-(3,4-dichlorophenyl) urea by the reaction of 3,4-dichlorophenyl isocyanate and hydroxylamine sulfate or hydroxylamine hydrochloride under an alkaline condition; neutralizing 1-hydroxy-3-(3,4-dichlorophenyl) urea and alkali; and obtaining 3-(3,4-dichlorophenyl)-1-methoxy-1-methyl urea by a methylation reaction of 1-hydroxy-3-(3,4-dichlorophenyl) urea and dimethyl carbonate under the condition with a catalyst and a solution. The invention has less quantity of three wastes, high yield, good quality and complete reaction, and can reduce pollution and the cost.
Owner:JIANGSU KUAIDA AGROCHEM
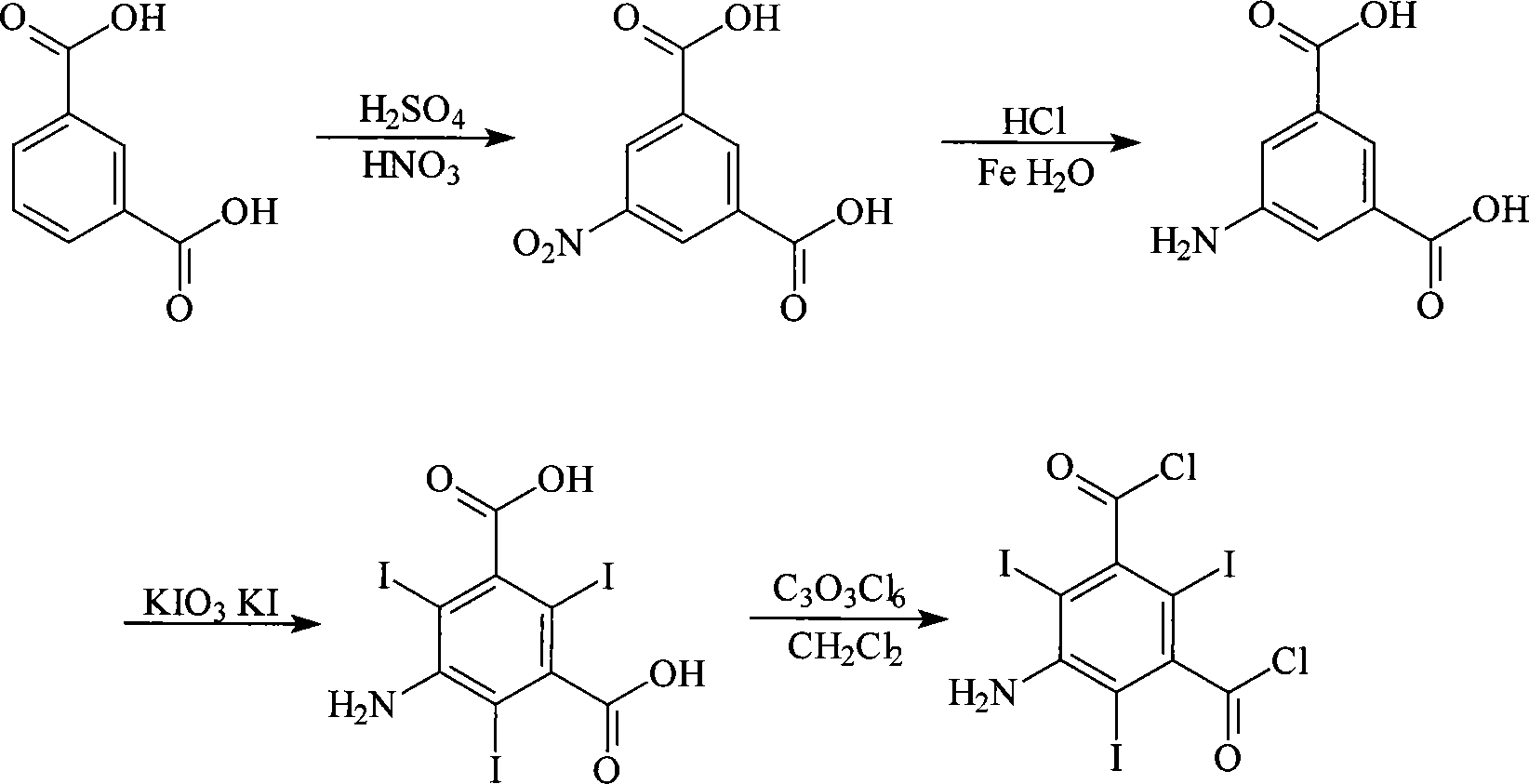
Method for synthesizing 5-amino-2,4,6-triiodoisophthaloyl dichloride
ActiveCN101215244AEasy to operateNot easy to recycleOrganic compound preparationAmino-carboxyl compound preparationCarbonyl chlorideNitration
A synthesis process of 5-amino-2, 4, 6-triiodoisophthalic acid acyl chloride, which comprises using m-phthalic acid as starting material, preparing 5-amino isophthalic acid through nitration and reduction, preparing 5-amino-2, 4, 6-triiodoisophthalic acid through iodobenzene reaction, using solid carbonyl chloride as acyl chloride agent and addling initiating agent, conducting acyl chloride reaction towards 5-amino-2, 4, 6-triiodoisophthalic acid to prepare 5-amino-2, 4, 6-triiodoisophthalic acid acyl chloride. The 5-amino-2, 4, 6-triiodoisophthalic acid acyl chloride which is prepared by the invention is mainly used to synthesize intermediate compound of ionic contrast agent.
Owner:山西新天源药业有限公司
Preparation method for flucloxacillin sodium
The invention relates to a preparation method for flucloxacillin sodium. The method comprises the following steps of: (1) weighing 6-aminopenicillanic acid, 3-(2-chloro-6-fluorophenyl)-5-methyl isoxazole-4-phosgene and sodium iso-octoate; (2) adding water into 6-aminopenicillanic acid, cooling in an ice bath, and adding acetone to obtain a solution A; (3) preparing 3-(2-chloro-6-fluorophenyl)-5-methyl isoxazole-4-carbonyl chloride and an organic solvent into a solution B; (4) adding the solution B into the solution A, and performing an acylation reaction; (5) after reacting, acidifying, and adding an organic solvent for extracting; (6) slowly adding a part of organic solution of sodium iso-octoate into the an extracting solution obtained in the step (5), performing a salt-forming reaction, and growing a crystal after the crystal is precipitated out; and (7) after growing the crystal, continually adding the residual sodium iso-octoate solution, preserving heat, stirring, leaching, soaking, washing and drying in vacuum. The method has the advantages of short process route, high process operability and capability of effectively increasing product quality and yield.
Owner:河北华日药业有限公司
Process for preparing 1,3-dibenzyl imidazoline-2-ketone-cis-4, 5-dicarboxylic acid (Ôàá)
InactiveCN1434039AImprove responseSave raw materialsOrganic compound preparationAmino-carboxyl compound preparationElectrophilic additionPotassium hydroxide
The present invention provides a method for preparing 1,3-dibenzylimidazoline-2-ketone-cis-4,5- dicarboxylic acid, and is characterized by that it utilizes chlorine gas to make fumaric acid produce electrophilic addition reaction to obtain meso-2,3-dichlorosuccinic acid, in the pressence of phase transfer catalyst the meso-2,3-dichlorosuccinic acid and benzylamine are undergone the processes of benzylamination reaction in the solvent so as to synthesize cis-2,3-dibenzylamine succinic acid, the latter and solid carbonyl chloride and undergone the process of phase transfer catalytic ring-closing reaction in the potassium hydroxide solution so as to obtain the invented product. Its total yield rate is up to 74%.
Owner:FUDAN UNIV
Treatment technology for tail gas generated from production process of cis-Z-3-(2-chloro-3, 3, 3-triflulo-1-propenyl)-2, 2-dimethyl cyclopropane carbonyl chloride
The invention discloses an improved treatment technology for tail gas from production of cis-Z-3-(2-chloro-3, 3, 3-triflulo-1-propenyl)-2, 2-dimethyl cyclopropane carbonyl chloride. The improved treatment technology is performed by adopting a four-stage tail gas absorption system to respectively absorb chlorine hydride and sulfur dioxide by utilizing the different solubilities of chlorine hydride gas and sulfur dioxide gas in water. The improved treatment technology disclosed by the invention has the advantages of low investment, fast onset of action and effect of changing waste into valuable things, and can enable products generated by absorption to re-enter into a production link or a selling link, not only reduce environmental pollution, but also save production cost and increase economic benefits.
Owner:安徽新北卡化学有限公司
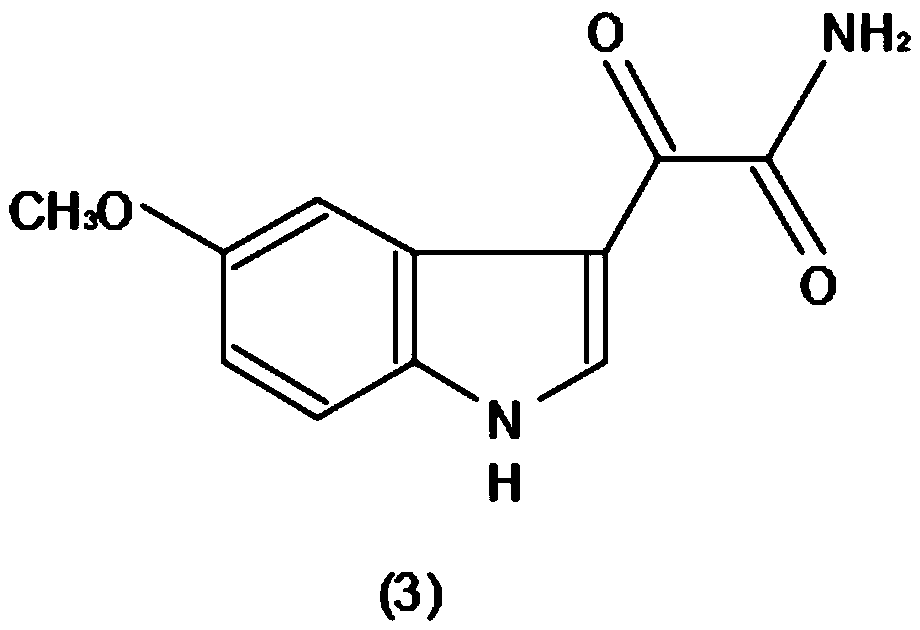
Method for efficiently producing melatonin
InactiveCN110229092AHigh total extraction rateShort operating cycleOrganic chemistryMelatonin synthesisState of art
The invention discloses a method for efficiently producing melatonin, relates to the technical field of melatonin synthesis, and aims to solve the problems that purity and an extraction rate of melatonin are relatively low and preparation cost is relatively high in the prior art. According to the method, 5-methoxy indole is taken as 100% of a raw material, carbonyl chloride accounts for 98% of theraw material, ammonium hydroxide accounts for 57% of a generation compound, and lithium aluminum hydride accounts for 80% of the generated compound. The 5-methoxy indole (1) is taken as a raw material, and a carbonyl chloride reaction solvent is added to acylate the 5-methoxy indole, so that a reaction is carried out to generate a compound (2); the generated compound (2) is stranded, and then anammonium hydroxide solvent is mixed with the generated compound (2) according to a certain usage amount to generate a compound (3); and after the compound (3) is generated, a lithium aluminum hydridereagent is added to carry out a reduction reaction on the compound (3) to generate a compound (4).
Owner:罗田县新普生药业有限公司
Preparation method and applications of oriented immobilized PEGA composite resin
ActiveCN104844710ADoes not affect conformationDoes not affect activityCarrier-bound/immobilised peptides4-nitrobenzyl alcoholTert-Butyloxycarbonyl protecting group
The invention discloses a preparation method and applications of an oriented immobilized PEGA composite resin. The preparation method comprises following steps: 2-amino-6-chloropurine and 4-nitrobenzyl alcohol are taken as raw materials for Williamson ether synthesis so as to obtain 4-nitryl-O6-benzylguanine; 4-nitryl- O6-benzylguanine is subjected to reduction reaction with protection of t-butyloxycarboryl; coupling reaction of an obtained compound with gamma-aminobutyric acid protected by carbobenzoxy chloride is carried out in the presence of ethyl dimethyl carbodiimide and 1-hydroxybenzotriazole; O<6>-benzylguanine derivative modified PEGA resin is obtained via reduction, separation and purification, reaction with PEGA, and deprotection; and transalkylation reaction of the O<6>-benzylguanine derivative modified PEGA resin with a protein containing MGMT label is carried out, so that oriented immobilization on PEGA resin surface via thioether covalent bonds is realized. Reaction of the preparation method is stable; the steps are simple; a stable uniform protein coating with uniform orientation can be formed on solid material surface by a prepared product; and the preparation method is used for preparing reagent kit with specific recognition effects.
Owner:NORTHWEST UNIV
Filgotinib synthetic method
ActiveCN104987333AWon't happenLess impuritiesOrganic chemistryChemical synthesisTert-Butyloxycarbonyl protecting group
The invention discloses a filgotinib synthetic method and belongs to the technical field of chemical synthesis of medicines. 6-chloro-2-aminopyridine and di-tert-butyl dicarbonate ester are subjected to condensation reaction to obtain 6-chloro-2-tert-butyloxycarbonyl aminopyridine; hydrolysis reaction is performed; the obtained 6-chloro-2-tert-butyloxycarbonyl aminopyridine and trifluorinated methyl sulfonic anhydride are subjected to trifluoromethanesulfonic acid esterification reaction; the obtained 2-tert-butyloxycarbonylamino-6-pyridyltrifluoromethanesulfonate and [(1,1-dioxo-4-thiomorpholinyl)methyl]benzo-4-boronic acid pinacol ester are subjected to condensation reaction to obtain a tert-butyl ester derivative; the tert-butyl ester derivative is treated by trifluoroacetic acid and subjected to de-protection; the obtained intermediate and ethoxycarbonyl isothiocyanate are subjected to isothiocyanate reaction; the obtained intermediate and hydroxylamine hydrochloride are subjected to ring closing reaction; the obtained intermediate and cyclopropane carbonyl chloride are subjected to amidation reaction to obtain the finished product. Operation is simplified; reagents are available; the concept of environment friendliness and environment protection is embodied.
Owner:SUZHOU FUSHILAI PHARMA CO LTD
Process for preparing butyl isocyanate
ActiveCN1844091AReduce side effectsImprove conversion rateIsocyanic acid derivatives preparationOrganic compound preparationCarbonyl chlorideDistillation
The invention discloses a Method for preparation of butylisocyanate, using n-butylamine as raw materal, dimethylbenzene as dissolvant, reacting with excess carbonyl chloride, getting the product which the purity is higher than 99% after two steps photo aerification reaction of low-temperature, high-temperature and distillation. Adopting two steps photo aerification reaction of low-temperature, high-temperature can reduce occurrence of secondary reaction effectly. In n-butylamine terms, the total yield of this invention is more than 95%.
Owner:JIANGSU ANPON ELECTROCHEM
Preparation method of 4-(nitrobenzophenone)-3-morpholone and method for preparing rivaroxaban by using 4-(nitrobenzophenone)-3-morpholone
ActiveCN103980221ALow priceSimple operation processOrganic chemistryMorpholineTert-Butyloxycarbonyl protecting group
The invention relates to the technical field of preparation of rivaroxaban and an intermediate thereof and particularly relates to a preparation method of 4-(nitrobenzophenone)-3-morpholone which is prepared from halogenated nitrobenzene, ethanolamine and chloroacetyl chloride through a one-pot method. The method for preparing rivaroxaban comprises the steps of reducing 4-(nitrobenzophenone)-3-morpholone into 4-(aminophenyl)-3-morpholone; enabling 4-(aminophenyl)-3-morpholone to react with R-epichlorohydrin to obtain a product; enabling the product to react with N, N-carbonyldiimidazole to obtain a product; enabling the product to react with tert-butyl iminodicarboxylate; preparing hydrochloride; enabling hydrochloride to react with 5-penphene-2-carbonyl chloride. The preparation method of 4-(nitrobenzophenone)-3-morpholone is capable of realizing one-pot production and free of purifying intermediate products in the process, so that the operation process is simplified, the time is saved, and the labor cost is reduced; the preparation method of 4-(nitrobenzophenone)-3-morpholone is low in raw material price, high in obtained product yield and easy to realize large-scale industrial production; in addition, the method for preparing rivaroxaban is cheap, nontoxic and harmless in raw material, simple in process and high in product yield.
Owner:山东康美乐医药科技有限公司
Efficient cyhalothrin
InactiveCN101434562AShort process routeImprove reaction efficiencyCarboxylic acid nitrile preparationOrganic compound preparation4-pentenoic acidFiltration
The invention relates to an efficient cyhalothrin and a manufacturing technology thereof, the manufacturing technology is as follows: (1) layer addition-reaction materials are 3, 3-dimethyl-4-pentenoic acid methyl ester and trichlorotrifluoroethane; (2) cyclization- reaction materials are tertiary butyl alcohol, Bifenthrin and sodium tert-butoxide; (3) elimination of hydrolysis-reaction materials are potassium hydroxide and cyclic Bifenthrin, and by exsolution, hydrolysis, separation and filtration 2-cis-3-(2-chloro-3, 3, 3-trifluro-1-propenyl)-2, 2-dimethyl cyclopropane carboxylic acid can be prepared; (4) chloroformylation-reaction materials are thionyl chloride, methylbenzene and 2-cis-3-(2-chloro-3, 3, 3-trifluro-1-propenyl)-2, 2-dimethyl cyclopropane carboxylic acid, and by exsolution and distillation, 3-(2-chloro-3, 3, 3-trifluoro-1-propenyl)-2, 2-dimethylcyclopropane carbonyl chloride is obtained; (5) esterification- reaction materials are phenylate aldehyde, sodium cyanide, PTC and 3-(2-chloro-3, 3, 3-trifluoro-1-propenyl)-2, 2-dimethylcyclopropane carbonyl chloride, by water rinse, exsolution and distillation, the cyhalothrin is prepared; and (7) epimerization-inoculating crystal is added for carrying out an epimerization reaction, and after the reaction, an end product-the efficient cyhalothrin is obtained by filtration and drying. As the invention adopts the technical proposal, the efficient cyhalothrin prepared by the invention greatly shortens the technological route and obviously improves the reaction efficiency through the improvement of technical skills, thereby reducing the cost of industrialization.
Owner:JIANGSU HUANGMA AGROCHEM
Octadecanoyl tributyl citrate plasticizer and preparation thereof
InactiveCN101255240AIncrease the relative molecular massHigh plasticizing efficiencyCarbonyl chlorideStearic acid
The invention provides octade carbonyl tributyl citrate plasticizer and preparation method thereof. The composition of octade carbonyl tributyl citrate plasticizer (in mass percent): tributyl citrate 43.5-53.8%, octade carbonyl chloride 45.2-55.0%, catalyst 1.0-1.5%. The preparation method comprises: (1) adding citric acid, butylalcohol, water-carrying agent and catalyst into reaction kettle, stirring, heating up until reflowing to obtain tributyl citrate; (2) adding stearic acid into reaction kettle, stirring, heating up to 50 degree and then adding phosphorous trichloride and heating up to 75 degree to perform thermal insulating reaction for 3 hours and layering to obtain octade carbonyl chloride; (3) adding tributyl citrate and catalyst in the reaction kettle, stirring, heating up to 70 degree and then adding octade carbonyl chloride to obtain chlorine hydride and the chlorine hydride being absorbed in water containing soda in drier, when the pH of the water containing soda is constant, the reaction being stopped and the product being subjected to neutralizing, cleaning, pump filtering under reduced pressure to obtain octade carbonyl tributyl citrate.
Owner:LANZHOU UNIVERSITY OF TECHNOLOGY
Recovery method for rhodium from deactivated catalyst used in formylation of propenyl hydrogen
ActiveCN103509061AHigh yieldSimple recycling processGroup 8/9/10/18 element organic compoundsRecovery methodNitrogen gas
The invention discloses a recovery method for rhodium from a deactivated catalyst used in formylation of propenyl hydrogen. The method realizes high efficiency recovery of rhodium by directly preparing RhCl(CO)(TPP)2 with a rhodium phosphine complex-containing waste liquid of a catalyst used in formylation of propenyl hydrogen as a starting raw material. The method comprises the following steps: subjecting the catalyst waste liquid to oxidation treatment under an acidic condition so as to break a rhodium cluster; synthesizing rhodium triphenylphosphine carbonyl chloride in a nitrogen atmosphere; and carrying out filtering, washing and drying so as to obtain rhodium. According to the invention, process conditions are mild, equipment used in the method is simple, burning or high temperature treatment is not needed, and little pollution is posed to the environment; the method has the advantages of high yield, simple process and mild conditions, substantially simplifies process flow from catalyst waste liquid to catalyst and reduces treating procedures generating high environmental pollution, e.g., burning, so the method has considerable economic benefits and social benefits.
Owner:CHINA PETROLEUM & CHEM CORP +1
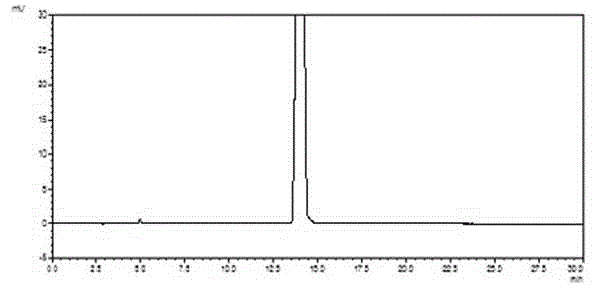
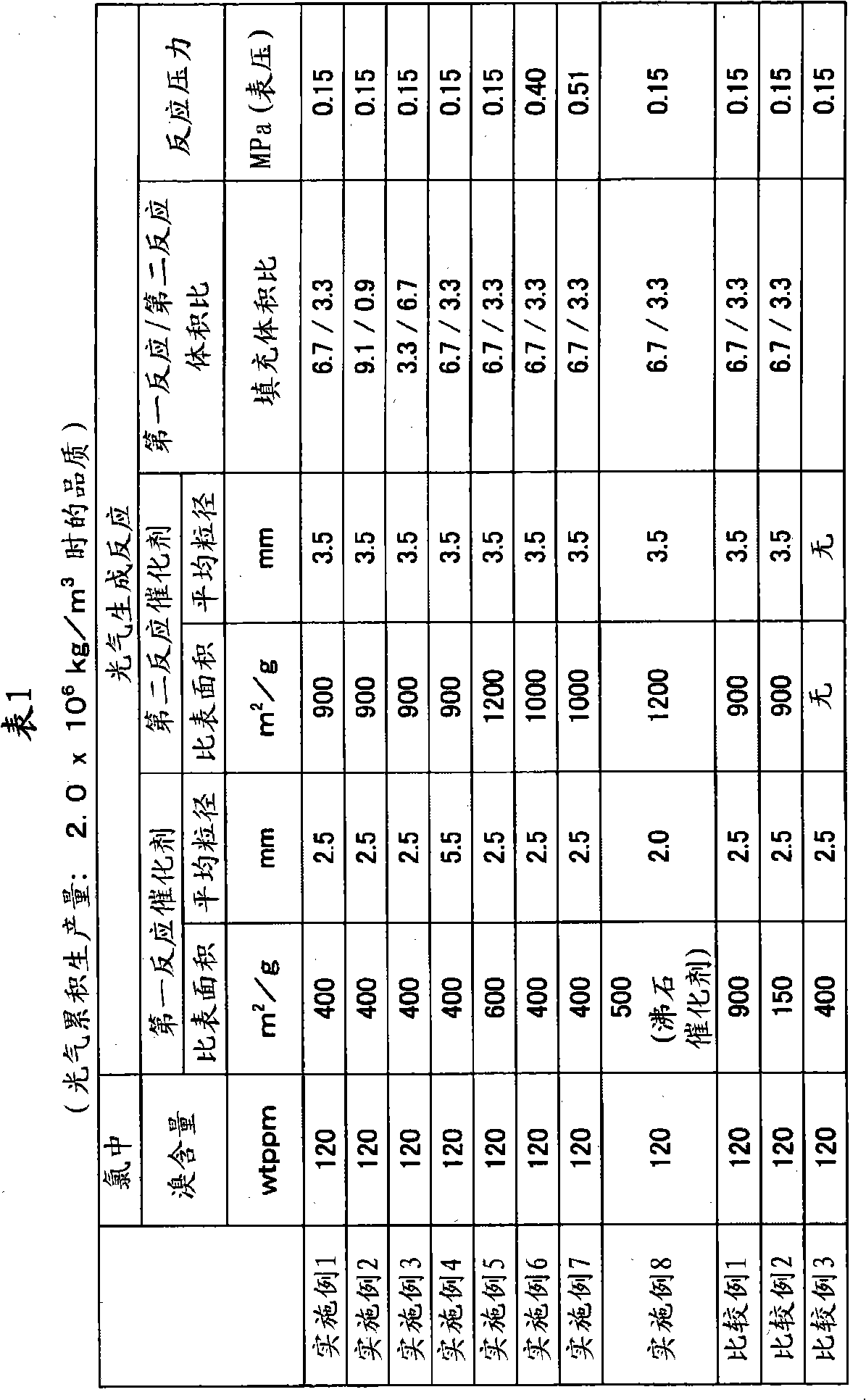
Method for producing carbonyl chloride
Provided are a method for producing carbonyl chloride which has few impurities and a good hue, and a method for producing polycarbonate resins which contain little metal-corroding material, have an excellent hue, and have excellent thermal stability when moulded. The method for producing carbonyl chloride containing small amounts of carbon tetrachloride, molecular chlorine and molecular bromine includes a process (i) wherein chlorine and carbon monoxide are brought into contact with a first catalyst having a specific surface area of 200 to 800 m2 / g and a second process (ii) wherein the product obtained in (i) is brought into contact with a second catalyst having a specific surface area of 800 to 1,500 m2 / g. A method for producing polycarbonate resin using the carbonyl chloride obtained is also included.
Owner:TEIJIN KASEK KK
Method for preparing alpha-alkylated ketone by coupling alcohol with alpha-methyl/methylene ketone
ActiveCN103613487AHigh activityHigh catalytic efficiencyOrganic compound preparationCarbonyl compound preparationPtru catalystKetone
The invention relates to a method for preparing alpha-alkylated ketone by coupling alcohol with alpha-methyl / methylene ketone. The method comprises the following steps: sequentially adding alpha-methyl / methylene ketone compounds and alcohol compounds into a reaction solvent, then, adding 2mol% of catalyst bistriphenylphosphine rhodium carbonyl chloride (RhCl (CO) (PPh3) 2), finally adding an inorganic alkali, and reacting at 50-100 DEG C to obtain reaction liquid after the reaction is completed; and filtering, concentrating and column chromatography separating the reaction liquid according to a conventional method to obtain the alpha-alkylated ketone. The method provided by the invention is efficient, rapid and environmental-friendly.
Owner:盐城盐西幸福产业发展有限公司
Preparation method of isepamicin sulfate (I)
InactiveCN105254688ALower synthesis costHigh puritySugar derivativesSugar derivatives preparationIsepamicin sulfateEthanol
The invention discloses a preparation method of isepamicin sulfate (I). The method comprises the following steps that (1) gentamicin gentamycin B (II) serves as a starting raw material, and after a reaction reagent and trimethyl silicone ethoxy carbonyl chloride protect, an intermediate (III) is obtained; (2) the intermediate (III) reacts with N,N-dicyclohexylamine and is coupled with N-phthalic anhydride-(S)-isoserine, and the product is subjected to column chromatography isolation to obtain an intermediate (IV); (3) the intermediate (IV) is de-protected and acidified by vitriol to obtain a crude product, and the crude product is re-crystallized with anhydrous alcohol to obtain isepamicin sulfate (I). The preparation method of the isepamicin sulfate (I) disclosed by the invention has the advantages of low synthesizing cost, short synthesizing period, and high product purity and yield, and is suitable for industrial production.
Owner:WUXI FORTUNE PHARMA
Method for synthesizing 2,4-Dichlorobenzoyl chloride
InactiveCN101037385AEasy to produceTimely distillateCarboxylic acid halides preparationCarbonyl chlorideBoiling point
A method for synthesizing the 2,4-dichlorobenzoyl chloride belongs to a field of intermediate synthesis of the compound, including catalyzing the 2,4-dichlorobenzotrichloride and carboxylic acid to produce single or compound coarse acyl chloride in the effect of the catalyzer; then rectifying to get corresponding acyl chloride. The advantages of the invention is that environment of the production is clean; the catalyzer can be recycled; carbonyl chloride with low boiling point may be drived off in time so as to get a high empty yield.
Owner:江苏强盛功能化学股份有限公司
Radix stemonae alkaloid analogue near-infrared fluorescent probe and preparation method thereof
ActiveCN111393430ALow costRaw materials are easy to getOrganic chemistryLuminescence/biological staining preparationFluoProbesCarboxylic acid
The invention relates to the field of optical imaging, in particular to a radix stemonae alkaloid analogue near-infrared fluorescent probe and a preparation method thereof. The near-infrared fluorescent probe is applied to rapid detection of biological mercaptan, and is applied to imaging of the biological mercaptan in cells and living bodies. Existing reported fluorescent probes for detecting thebiological mercaptan have certain limitations, such as complex synthetic routes, long response time, short emission wavelength and the like. The preparation method of the near-infrared fluorescent probe comprises the following steps: 5-(hydroxymethyl)-7-methyl-3, 3 a-dihydrocyclopenta [ b ] chroman cyclo-1 (2H)-one and 3, 7-bis (dimethylamino)-10H-benzothiazine-10-carbonyl chloride react under the action of 4-dimethylamino pyridine and pyridine to generate (7-methyl-1-oxy-1, 2, 3, 3 a-tetrahydrocyclopenta [ b ] chroman cyclo-5-yl)-methyl-3, 7-bis (dimethylamino)-10H-benzothiazine-10-carboxylate.
Owner:SHANXI UNIV
Photoluminescent room-temperature ionic liquid preparation method
ActiveCN104927843AImprove thermal stabilityNovel structureOrganic chemistryLuminescent compositionsSolubilityHalohydrocarbon
The invention relates to a photoluminescent room-temperature ionic liquid preparation method. The photoluminescent room-temperature ionic liquid preparation method includes that under an action of thionyl chloride, carboxyl-functionalized naphthaline (pyrene) is converted into naphthyl (pyrenyl) carbonyl chloride, using the naphthyl (pyrenyl) carbonyl chloride to interact with amino imidazole (pyridine) to generate intermediate naphthoyl (pyrene acid) aminoimidazole (pyridylamine); in the presence of triphenylphosphine and carbon tetrabromide, branched alkyl alcohol is converted into branched alkyl halohydrocarbon, and the intermediate naphthoyl (pyrene acid) aminoimidazole (pyridylamine) and the branched alkyl halohydrocarbon are subjected to quaterisation reaction to generate naphthaline-ring-contained (pyrene-ring-contained) photoluminescent room-temperature ionic liquid. The generate naphthaline-ring-contained (pyrene-ring-contained) photoluminescent room-temperature ionic liquid is novel in structure, high in heat stability, low in melting point and adjustable in viscosity and solubility, has photoluminescent characteristics in a solution or in a solvent-free condition and is applicable to the field of fluorescent labels, photoelectric devices and the like.
Owner:SHANDONG UNIV
Method for producing polycarbonate
The invention relates to a preparation method of polycarbonate having good hues. The method is characterized in that, in the presence of organic solvents and sodium hydrosulfite, the content of formate in the hydrosulfite is below 0.3wt%, during the preparation of polycarbonate using bisphenol compounds and carbonyl chloride.
Owner:MITSUBISHI GAS CHEM CO INC
Preparation method of buprofezin
PendingCN108530388AReduce generationImprove protectionOrganic chemistrySodium bicarbonateChlorobenzene
The invention provides a preparation method of buprofezin, comprising the steps of 1), adding sodium thiocyanate and water into an esterification kettle until dissolution; adding tertiary butanol andhydrochloric acid to obtain a mixed ester; allowing transposition and catalytic reaction to obtain t-butyl isothiocyanate; 2), adding the t-butyl isothiocyanate into chlorobenzene, stirring, dropwiseadding isopropyl amine to obtain 1-isopropyl-3-tert-butylthiourea solution; 3), adding N-methylaniline into chlorobenzene; introducing carbonyl chloride and chlorine gas in sequence to obtain N-chloromethyl-N-benzenecarbamoylchloride solution; 4), adding sodium bicarbonate into water, and adding the resultant into chlorobenzene; adding the 1-isopropyl-3-tert-butylthiourea solution; dropwise addingthe N-chloromethyl-N-benzenecarbamoylchloride solution, filtering, allowing layering, performing high-vacuum steaming to remove the chlorobenzene, crystallizing, centrifuging, and drying to obtain buprofezin. The substitutive average yield of the preparation process of buprofezin reaches 90% and above; the content of finished buprofezin reaches 98.0% and above; the preparation process is free ofammonia nitrogen wastewater.
Owner:JIANGSU ANPON ELECTROCHEM
Method for preparing bifenthrin
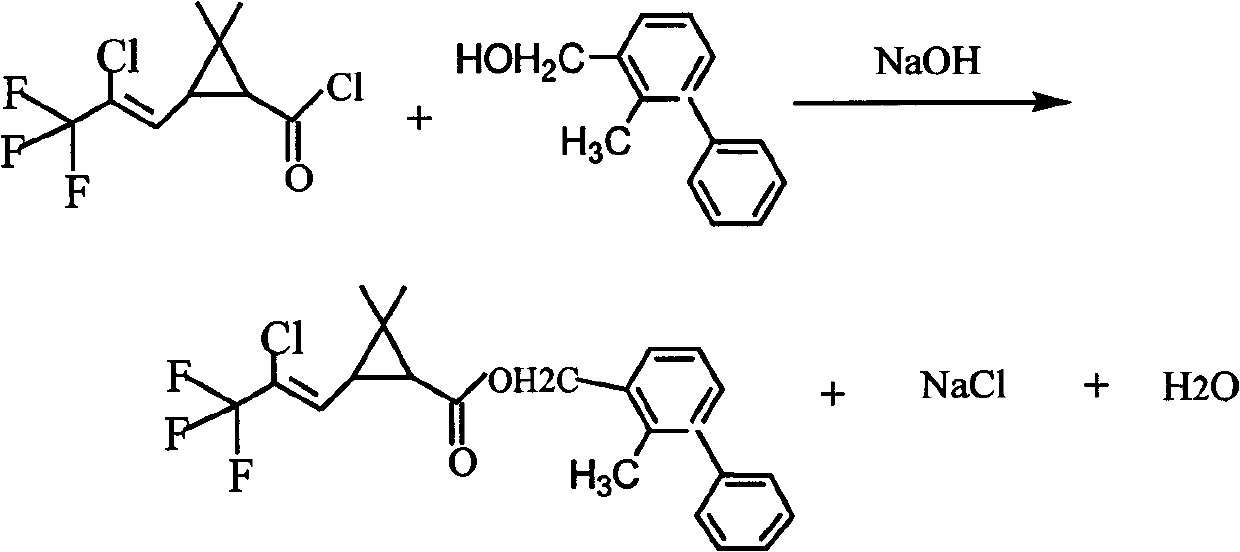
InactiveCN103319345AHarm reductionReduce consumptionPreparation from carboxylic acid halidesAlkaline earth metalCarbonyl chloride
The invention discloses a method for preparing bifenthrin. The method comprises: a condensation reaction of cis-Z-3-(2-chloro-3,3,3-triflulo-1-propenyl)-2,2-dimethyl cyclopropane carbonyl chloride and 2-methyl-3-biphenylmethyanol is carried out under a condition of an alkali metal compound or an alkaline earth metal compound as an acid-binding agent, and bifenthrin is obtained. Bifenthrin prepared by the method has high yield and high content, wherein the yield reaches 92.0-95.0%, and the content reaches 95.0-98.5%. At the same time, the alkali metal compound or the alkaline earth metal compound instead of pyridine is used as the acid-binding agent, and thus the harm of pyridine to human and environment can be effectively reduced, the consumption of raw materials is reduced, and the labor strength of operating personnel is relieved.
Owner:YINGDE GREATCHEM CHEM
Novel process for preparation of 10-oxo-10, 11-dihydro-5h-dibenz [b,f] azepine-5-carbox- amide (oxcarbazepine) via intermediate, 10-methoxy-5h-debenz[b,f] azepine-5-carbonyl- chloride
InactiveUS20070032647A1Inhibition formationReduce the temperatureOrganic chemistryOrganic baseBis(trichloromethyl) carbonate
A process for preparation of 10-oxo-10,11-dihydro-5H-dibenz[b,f]azepine-5-carboxamide (oxcarbazepine) via intermediate 10-methoxy-5H-dibenz[b,f]azepine-5-carbonyl chloride, comprising the steps: a) Preparation of an intermediate 10-methoxy-5H-dibenz[b,f]azepine-5 carbonyl, chloride from 10-methoxyiminostillbene using bis (trichloromethyl) carbonate (BTC) with organic base such as aliphatic or aromatic tertiary amines in organic solvent; b) Conversion of the intermediate to 10-methoxy-5H-dibenz[b,f]azepine-5-carboxamide using ammonia in organic solvent; c) Formation of oxcarbazepine from step (b) using Bronsted acid in an organic solvent at a temperature between 25° C.-80° C., preferably at 50° C. to 70° C.; and d) Isolation of oxcarbazepine.
Owner:AMOLI ORGANICS LTD
Method for preparing di-tert-butyl dicarbonate
ActiveCN102557952AHigh yield of catalytic reactionMild reaction conditionsProductsReagentsSatiationsCarbonyl chloride
The invention discloses a method for preparing di-tert-butyl dicarbonate. The method comprises the steps of: adding sodium tert-butoxide and normal hexane in a reaction kettle, heating until the sodium tert-butoxide and the normal hexane resolve completely under the protection of N2, stirring and cooling to 0 to 50 DEG C; after introducing carbon dioxide into the reaction kettle until satiation, adding a three way catalyst into the liquid reactant; controlling the internal temperature of the reaction kettle to a range from minus 12 DEG C to 20 DEG C; adding solid carbonyl chloride into the reaction kettle for 10 times every 0.5 hour, and reacting for 3 hours after adding is finished; after reacting, adding water and washing, and vaporizing the normal hexane solvent off under normal pressure to obtain a crude product of di-tert-butyl dicarbonate; and crystallizing at the temperature of below 0 DEG C, and centrifugalizing the mother liquid after crystallizing is finished completely so as to obtain the required di-tert-butyl dicarbonate.
Owner:GENCHEM & GENPHARM CHANGZHOU CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com









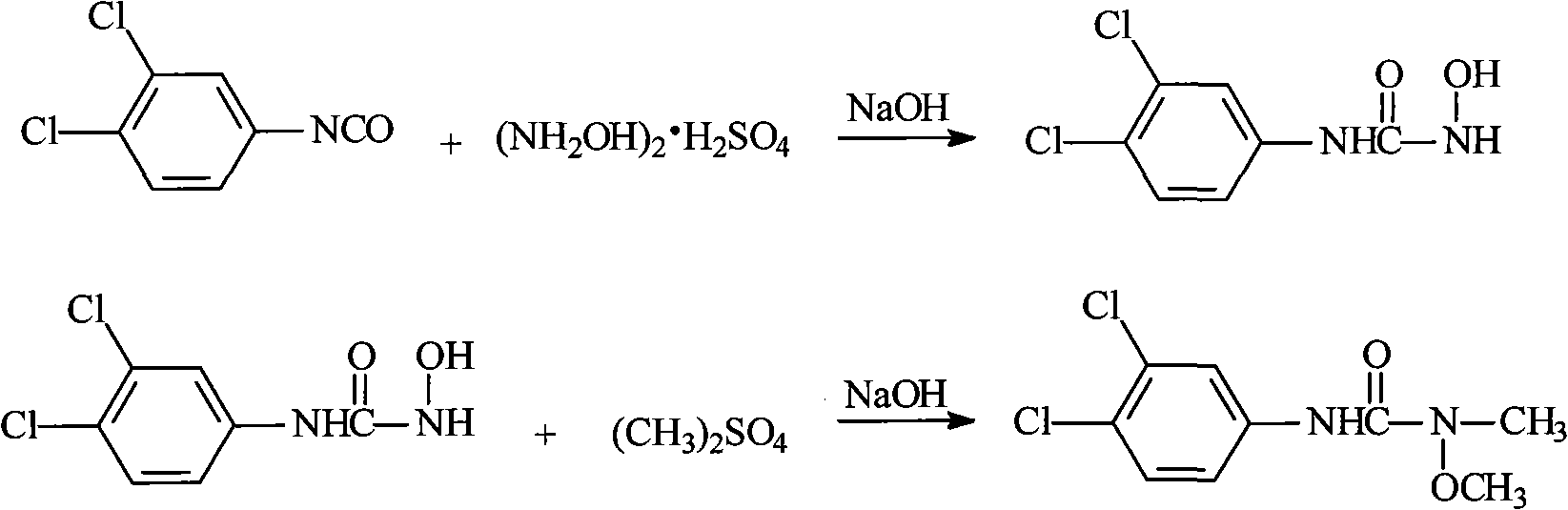















![Novel process for preparation of 10-oxo-10, 11-dihydro-5h-dibenz [b,f] azepine-5-carbox- amide (oxcarbazepine) via intermediate, 10-methoxy-5h-debenz[b,f] azepine-5-carbonyl- chloride Novel process for preparation of 10-oxo-10, 11-dihydro-5h-dibenz [b,f] azepine-5-carbox- amide (oxcarbazepine) via intermediate, 10-methoxy-5h-debenz[b,f] azepine-5-carbonyl- chloride](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/eb90b6a6-a1f7-4a40-9209-d51f74866cc8/US20070032647A1-20070208-C00001.png)
![Novel process for preparation of 10-oxo-10, 11-dihydro-5h-dibenz [b,f] azepine-5-carbox- amide (oxcarbazepine) via intermediate, 10-methoxy-5h-debenz[b,f] azepine-5-carbonyl- chloride Novel process for preparation of 10-oxo-10, 11-dihydro-5h-dibenz [b,f] azepine-5-carbox- amide (oxcarbazepine) via intermediate, 10-methoxy-5h-debenz[b,f] azepine-5-carbonyl- chloride](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/eb90b6a6-a1f7-4a40-9209-d51f74866cc8/US20070032647A1-20070208-C00002.png)
![Novel process for preparation of 10-oxo-10, 11-dihydro-5h-dibenz [b,f] azepine-5-carbox- amide (oxcarbazepine) via intermediate, 10-methoxy-5h-debenz[b,f] azepine-5-carbonyl- chloride Novel process for preparation of 10-oxo-10, 11-dihydro-5h-dibenz [b,f] azepine-5-carbox- amide (oxcarbazepine) via intermediate, 10-methoxy-5h-debenz[b,f] azepine-5-carbonyl- chloride](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/eb90b6a6-a1f7-4a40-9209-d51f74866cc8/US20070032647A1-20070208-C00003.png)