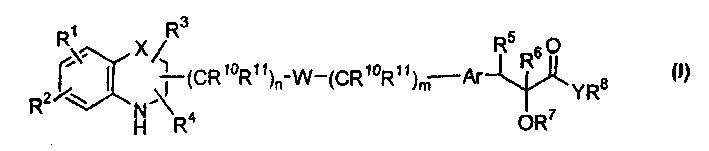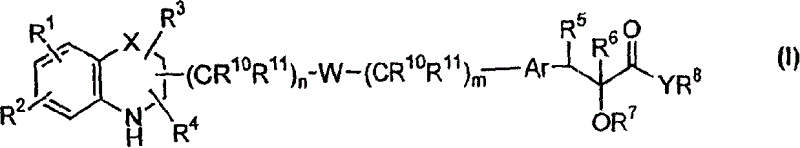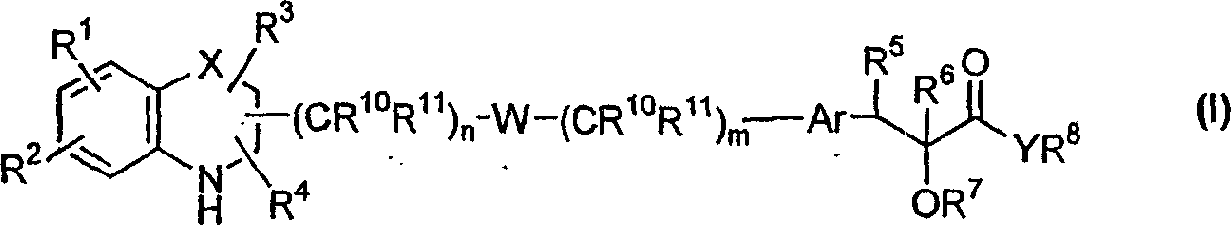Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
126 results about "Benzothiazine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Benzothiazine is a heterocyclic compound consisting of a benzene ring attached to the 6-membered heterocycle thiazine. The name is applied to both the 2H- and 4H-isomers of the molecule. 2,1-Benzothiazine, a type of benzothiazines was first reported in the 1960s. Subsequently, their preparation and intensive biological and physiological studies have been reported. In recent years, 2,1-benzothiazines have been of enormous interest to synthetic chemists. An enantioselective synthesis of such benzothiazines has been developed by Harmata and Hong who have formulated transformations of these compounds designed to target chiral, non-racemic building blocks as well as natural products.
Benzothiazinone and benzoxazinone compounds
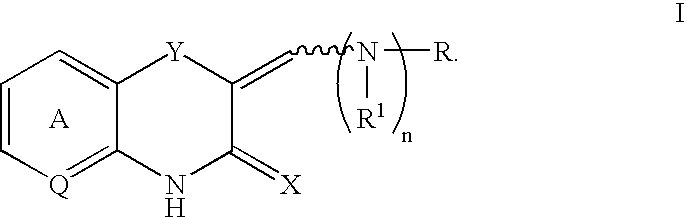
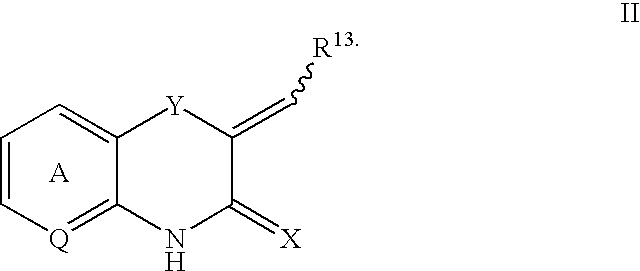
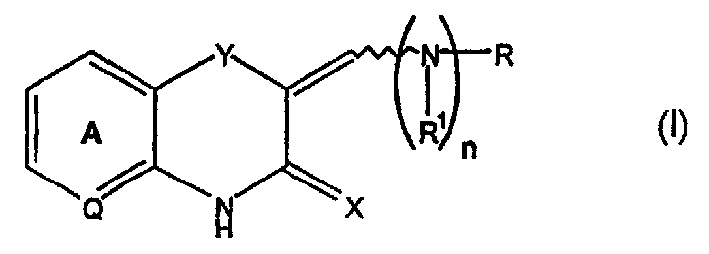
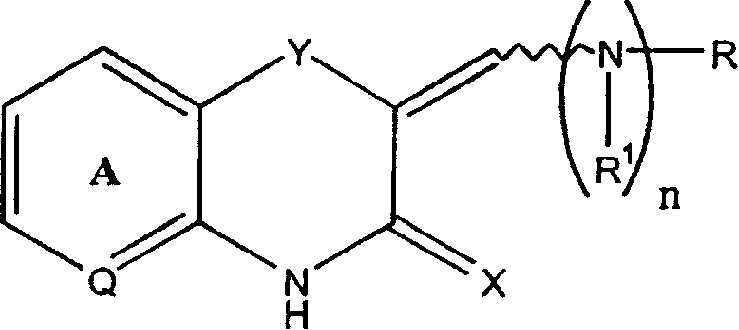
Q is —N=or CR2 X is S, O or NOR3 Y is —O—, —S—, —SO— or —SO2—R and R1 are each, independently, H, a substituted or unsubstituted aliphatic, aromatic, heteroaromatic or aralkyl groupR2 is H or a substituentR3 is H, or —C(O)R4 R4 is a substituted or unsubstituted aliphatic or aromatic groupn is an integer from 0 to 1Chemical compounds having structural formula I and physiologically acceptable salts thereof, are inhibitors of serine / threonine and tyrosine kinase activity. Several of the tyrosine kinases, whose activity is inhibited by these chemical compounds, are involved in angiogenic processes. Thus, these chemical compounds can ameliorate disease states where angiogenesis or endothelial cell hyperproliferation is a factor. These compounds can be used to treat cancer and hyperproliferative disorders.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
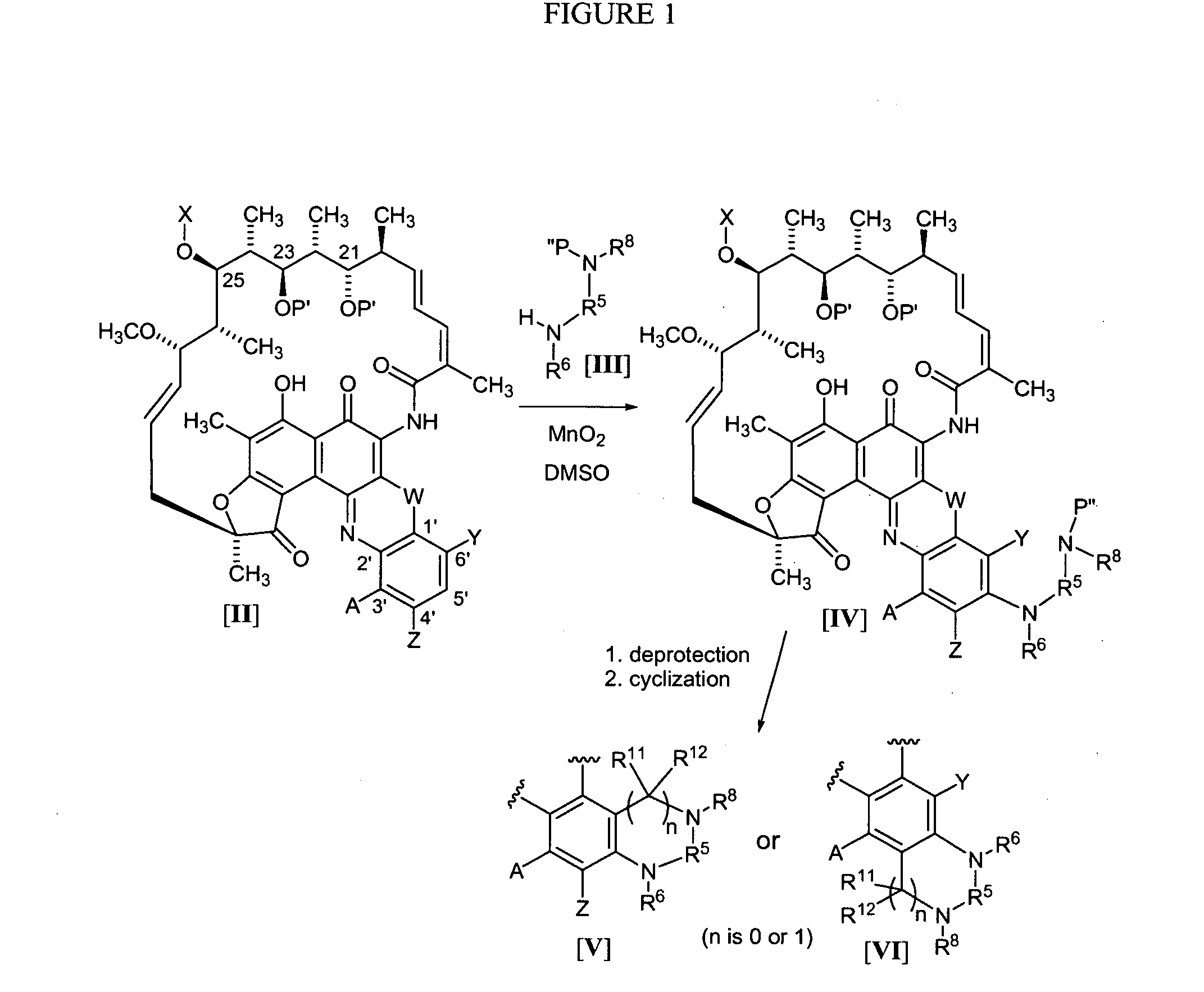
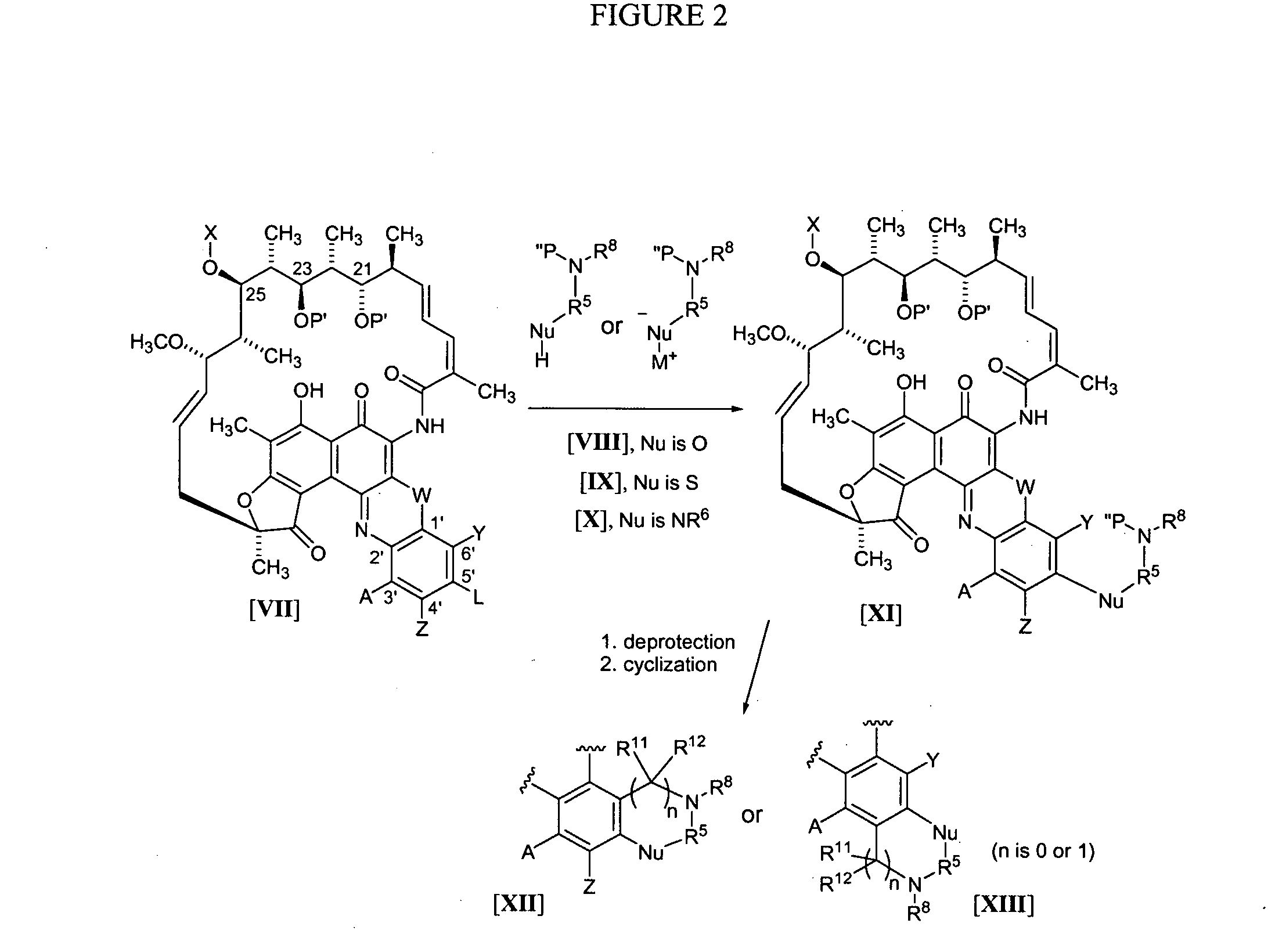
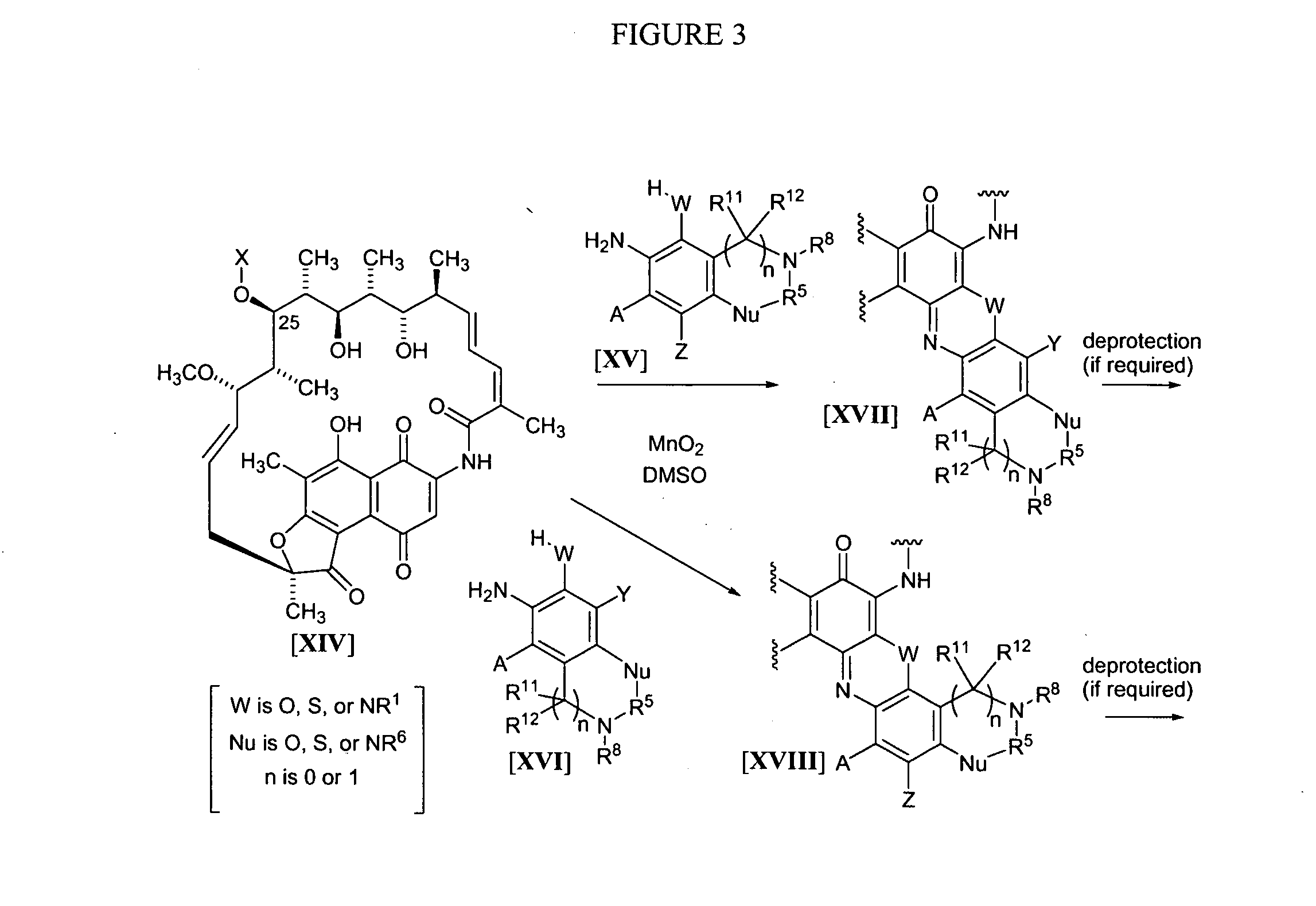
Rifamycin analogs and uses thereof
InactiveUS20050043298A1Improve permeabilityGood curative effectAntibacterial agentsBiocideMycinamicinsPharmacology
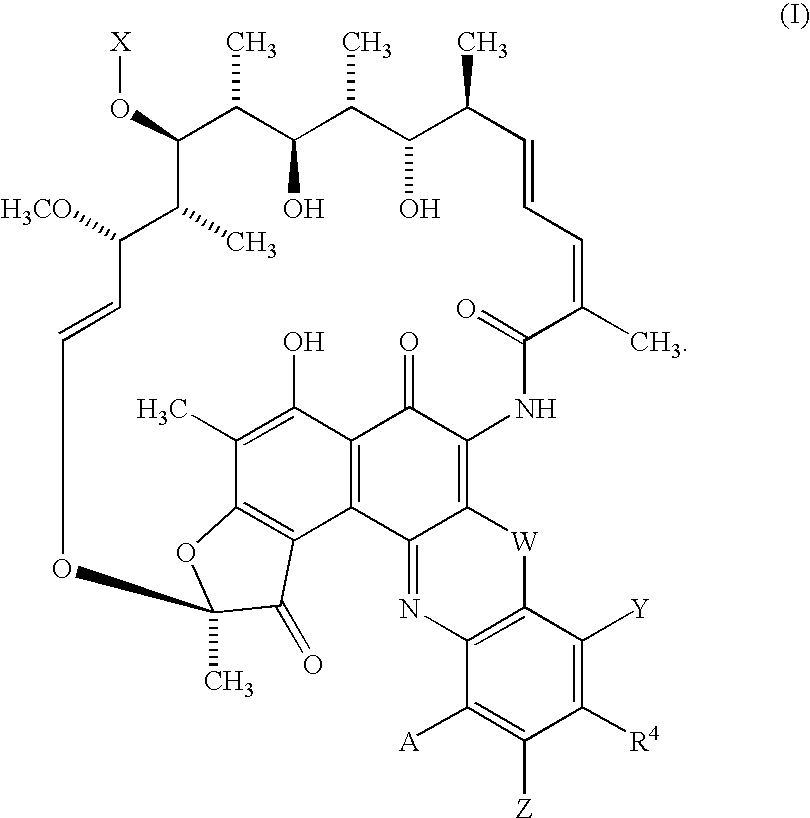
The present invention features rifamycin analogs that can be used as therapeutics for treating or preventing a variety of microbial infections. In one form, the analogs are acetylated at the 25-position, as is rifamycin. In another form, the analogs are deacetylated at the 25-position. In yet other forms, benzoxazinorifamycin, benzthiazinorifamycin, and benzdiazinorifamycin analogs are derivatized at various positions of the benzene ring, including 3′-hydroxy analogs, 4′-and / or 6′ halo and / or alkoxy analogs, and various 5′ substituents that incorporate a cyclic amine moiety.
Owner:ACTIVBIOTICS PHARMA
Haloxyfop-r-methyl-containing herbicide composition
InactiveCN102349527AGood effectEffective controlBiocideAnimal repellantsBristlegrassesAlopecurus aequalis
The invention discloses a haloxyfop-r-methyl-containing herbicide composition. The haloxyfop-r-methyl-containing herbicide composition is utilized for controlling gramineous weeds, broad-leaved weeds and sedge weeds in broad-leaved crop fields. The haloxyfop-r-methyl-containing herbicide composition comprises active components of 1, a compound A, wherein the compound A is haloxyfop-r-methyl, and 2, a herbicide mixed synergistic active component B, wherein the herbicide mixed synergistic active component B is one or more of diphenyl ether herbicides, sulfonylurea herbicides or benzothiazine dione herbicides. A weight ratio of haloxyfop-r-methyl to the herbicide mixed synergistic active component B is (1 to 30%): (0.5 to 50%). The haloxyfop-r-methyl-containing herbicide composition can be processed into all realizable dosage forms. The haloxyfop-r-methyl-containing herbicide composition can be widely utilized for controlling gramineous weeds such as barnyard grass, green bristlegrass, alopecurus aequalis, crabgrass, annual bluegrass and moleplant seed, broad-leaved weeds such as purslane, solanum nigrum, cocklebur, bindweed, chenopodium album and goosegrass herb, and sedge weeds in soybean and peanut fields, wherein the broad-leaved weeds are difficult to be controlled. The haloxyfop-r-methyl-containing herbicide composition has the characteristics of wide weed control spectrum, safety on crops, and high efficiency.
Owner:GAUNGXI TIANYUAN BIOCHEM
Rifamycin analogs and uses thereof
InactiveUS7342011B2Good curative effectReduce the amount requiredAntibacterial agentsBiocideBenzenePolymicrobial Infections
The present invention features rifamycin analogs that can be used as therapeutics for treating or preventing a variety of microbial infections. In one form, the analogs are acetylated at the 25-position, as is rifamycin. In another form, the analogs are deacetylated at the 25-position. In yet other forms, benzoxazinorifamycin, benzthiazinorifamycin, and benzdiazinorifamycin analogs are derivatized at various positions of the benzene ring, including 3′-hydroxy analogs, 4′- and / or 6′ halo and / or alkoxy analogs, and various 5′ substituents that incorporate a cyclic amine moiety.
Owner:ACTIVBIOTICS PHARMA
Rifamycin analogs and uses thereof
InactiveUS7271165B2Good curative effectReduce the amount requiredAntibacterial agentsAntimycoticsBenzenePolymicrobial Infections
The present invention features rifamycin analogs that can be used as therapeutics for treating or preventing a variety of microbial infections. In one form, the analogs are acetylated at the 25-position, as is rifamycin. In another form, the analogs are deacetylated at the 25-position. In yet other forms, benzoxazinorifamycin, benzthiazinorifamycin, and benzdiazinorifamycin analogs are derivatized at various positions of the benzene ring, including 3′-hydroxy analogs, 4′- and / or 6′ halo and / or alkoxy analogs, and various 5′ substituents that incorporate a cyclic amine moiety.
Owner:ACTIVBIOTICS PHARMA
Rifamycin analogs and uses thereof
InactiveUS20050137189A1Improve permeabilityGood curative effectAntibacterial agentsBiocideBenzeneAcetylation
The present invention features rifamycin analogs that can be used as therapeutics for treating or preventing a variety of microbial infections. In one form, the analogs are acetylated at the 25-position, as is rifamycin. In another form, the analogs are deacetylated at the 25-position. In yet other forms, benzoxazinorifamycin, benzthiazinorifamycin, and benzdiazinorifamycin analogs are derivatized at various positions of the benzene ring, including 3′-hydroxy analogs, and / or various fused ring systems with the benzene ring at the 4′,5′ or 5′,6′ positions.
Owner:ACTIVBIOTICS PHARMA
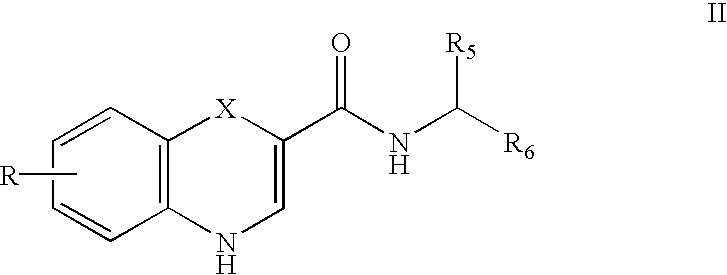
Substituted 4H-1,4-benzothiazine-2-carboxamide: GABA brain receptor ligands
Disclosed are 4H-1,4-Benzothiazine-2-carboxamides. These compounds are highly selective agonists, antagonists or inverse agonists for GABAa brain receptors or prodrugs of agonists, antagonists or inverse agonists for GABAa brain receptors. These compounds are useful in the diagnosis and treatment of anxiety, depression, sleep, cognitive and seizure disorders, and overdose with benzodiazepine drugs and for enhancement of alertness. Pharmaceutical compositions, including packaged pharmaceutical compositions, are further provided. Compounds of the invention are also useful as probes for the localization of GABAA receptors in tissue samples.
Owner:NEUROGEN
Compounds for the Treatment of Inflammation of the Central Nervous System
KATP channel openers (KCOs) are useful for the prophylactic and / or therapeutic treatment of CNS chronic inflammation associated with a disease or state in a mammal, including a human. The administration of KCOs, including the groups of benzopirans, cyanoguanidines, thioformamides, benzothiadiazines, pyridyl nitrates, pyrimidine sulfates, cyclobutenediones, DHP-related compounds, tertiary carbinols, 6-sulfonil-chromenes, 1,2,3-triazoles, pyridothiadiazines, benzothiazines, halogenquinazolins and phenylbenzimidazoles, and in particular, the compound diazoxide, result in a reduction of reactive microglial response in various CNS pathologies such as axonal injury, brain tumors, traumatic damage, neurodegeneration, spinal cord injury, infectious and autoimmune diseases. KCOs, isotopically modified, are also useful for the preparation of diagnostic agents for detection and follow-up of CNS chronic inflammation.
Owner:NEUROTEC PHARMA
Benzothiazinethione derivatives and their preparation and use
ActiveCN102276598AHas anti-Mycobacterium tuberculosis activityAntibacterial agentsOrganic active ingredientsIsoniazidAntituberculous drugs
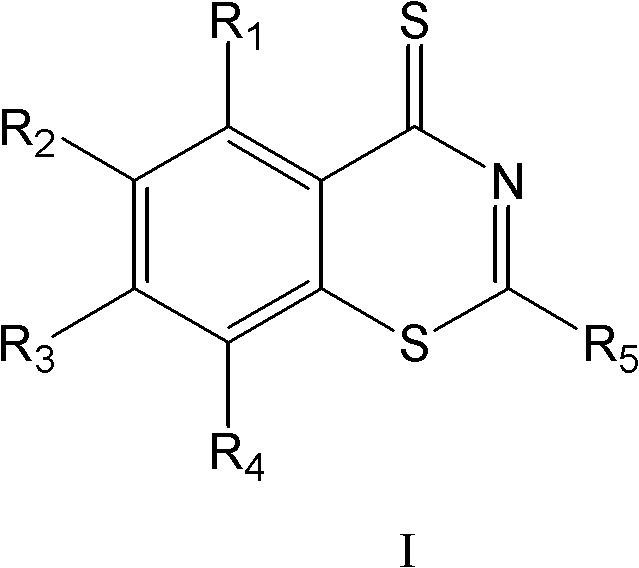
The invention belongs to the medicine field, and particularly relates to benzothiazinethione derivatives and preparation methods and uses thereof. In the aspect of the present invention, novel benzothiazinethione derivatives of formula I are provided, the benzothiazinethione derivatives of the invention are new compounds obtained based on extensive screening. Experimental results show that the benzothiazinethione derivatives of formula I have obvious inhibitory effects on mycobacterium tuberculosis, with effects equivalent to or even better than that of isoniazide (MIC 90 =0.8µM). The benzothiazinethione derivatives of formula I have anti-mycobacterium tuberculosis activities, and provide new choices for the development and application of antitubercular agents.
Owner:SICHUAN UNIV
Fluorescent conjugated polymer containing phenothiazine group, and synthesis method and application thereof
InactiveCN104151533AImprove solubilityImprove thermal stabilityLuminescent compositionsSolubilitySynthesis methods
The invention belongs to the field of conjugated polymers, and discloses a fluorescent conjugated polymer containing a phenothiazine group, and a synthesis method and an application thereof. The synthesis method comprises the following steps: firstly, synthesizing an N-alkyl-3,6-bi(acetenyl) phenothiazine monomer, then completely dissolving the N-alkyl-3,6-bi(acetenyl) phenothiazine monomer and an N-octyl-3,6-bi(acetenyl) carbazole monomer or a 4,7-diacetenyl-2,1,3-benzothiazine oxazole monomer into orthodichlorobenzene, and carrying out reaction under stirring in a catalytic system containing CuCl and TMEDA; precipitating and filtering the mixture in a mixed solution of methyl alcohol and concentrated hydrochloric acid after reaction is ended, and carrying out vacuum drying, so as to obtain the fluorescent conjugated polymer containing the phenothiazine group. According to the fluorescent conjugated polymer, the solubility of the polymer is improved by introducing an alkyl chain into the phenothiazine group, the structure is improved by introducing groups with different electron donating and withdrawing effects, and the conjugated polymer with excellent photoelectric property, solubility and heat stability is provided.
Owner:SOUTH CHINA UNIV OF TECH
Benzothiazine-4-ketone compounds containing basic nitrogen heterocyclic fragments and preparing methods of benzothiazine-4-ketone compounds
ActiveCN105669664AHigh activityGood curative effectAntibacterial agentsOrganic active ingredientsBenzeneNitrogen
The invention relates to benzothiazine-4-ketone compounds (shown in the formula I and formula I' in the description) containing basic nitrogen heterocyclic fragments, preparing methods and medical application of the benzothiazine-4-ketone compounds and antituberculosis drug compositions with the benzothiazine-4-ketone compounds as effective constituents, in particular to a 6-trifluoromethyl-8-nitryl-4H-benzo[e][1,3] thiazine-4-ketone compound. A 2-substituent group is 1-nitrogen heterocyclic alkyl or dinitrogen heterocyclic alkyl. R represents H, an alkyl group of 1-4 C atoms, heterocyclic alkyl of 4-7 C atoms, a phenyl group and a substituted phenyl; R1 represents H, an alkyl group of 1-3 C atoms and heterocyclic alkyl of 3-6 C atoms; R2 represents H, an alkyl group of 1-4 C atoms, heterocyclic alkyl of 4-7 C atoms, a phenyl group and substituted phenyl; n1 represents 0-1; n2 represents 1-3; n3 represents 1 and 3.
Owner:ZHEJIANG STARRY PHARMA +1
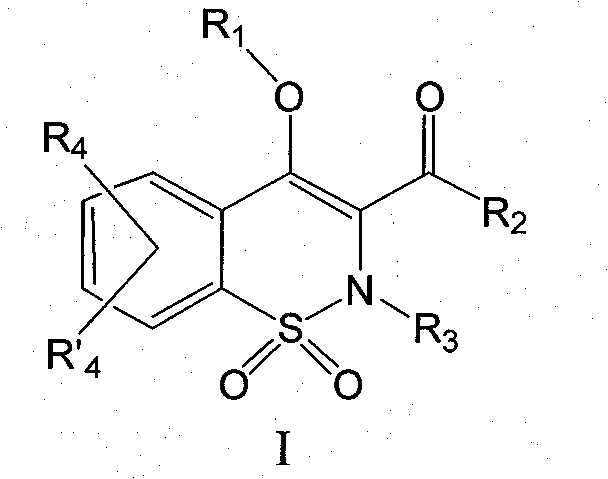
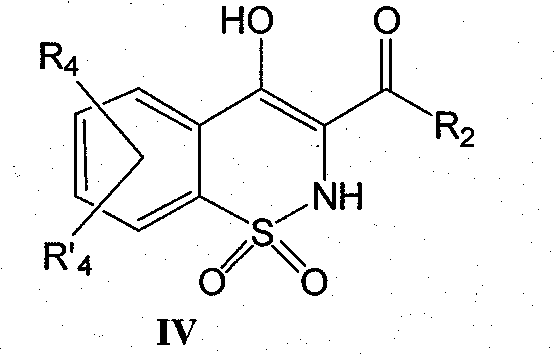
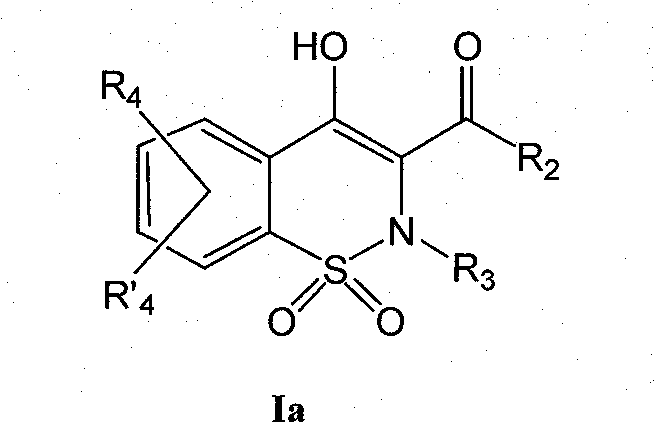
Derivatives of benzothiazines, preparation thereof and application thereof as drugs
The object of the present invention is benzothiazine derivatives having the capability of inhibiting 11[beta]-HSD1 not only at an enzymatic level but also at a cell level. The compounds of the present invention are of general formula (I). Wherein notably R1 represents a hydrogen or OR1 represents an ester or an ether. R2 represents a naphthyl or a 1, 2, 3, 4-tetrahydro-naphthalene or a biphenyl or phenyl pyridine or a substituted phenyl. R3 represents a methyl or ethyl; R4 and R'4 represent a hydrogen.
Owner:PIERRE FABRE MEDICAMENT SAS
Radix stemonae alkaloid analogue near-infrared fluorescent probe and preparation method thereof
ActiveCN111393430ALow costRaw materials are easy to getOrganic chemistryLuminescence/biological staining preparationFluoProbesCarboxylic acid
The invention relates to the field of optical imaging, in particular to a radix stemonae alkaloid analogue near-infrared fluorescent probe and a preparation method thereof. The near-infrared fluorescent probe is applied to rapid detection of biological mercaptan, and is applied to imaging of the biological mercaptan in cells and living bodies. Existing reported fluorescent probes for detecting thebiological mercaptan have certain limitations, such as complex synthetic routes, long response time, short emission wavelength and the like. The preparation method of the near-infrared fluorescent probe comprises the following steps: 5-(hydroxymethyl)-7-methyl-3, 3 a-dihydrocyclopenta [ b ] chroman cyclo-1 (2H)-one and 3, 7-bis (dimethylamino)-10H-benzothiazine-10-carbonyl chloride react under the action of 4-dimethylamino pyridine and pyridine to generate (7-methyl-1-oxy-1, 2, 3, 3 a-tetrahydrocyclopenta [ b ] chroman cyclo-5-yl)-methyl-3, 7-bis (dimethylamino)-10H-benzothiazine-10-carboxylate.
Owner:SHANXI UNIV
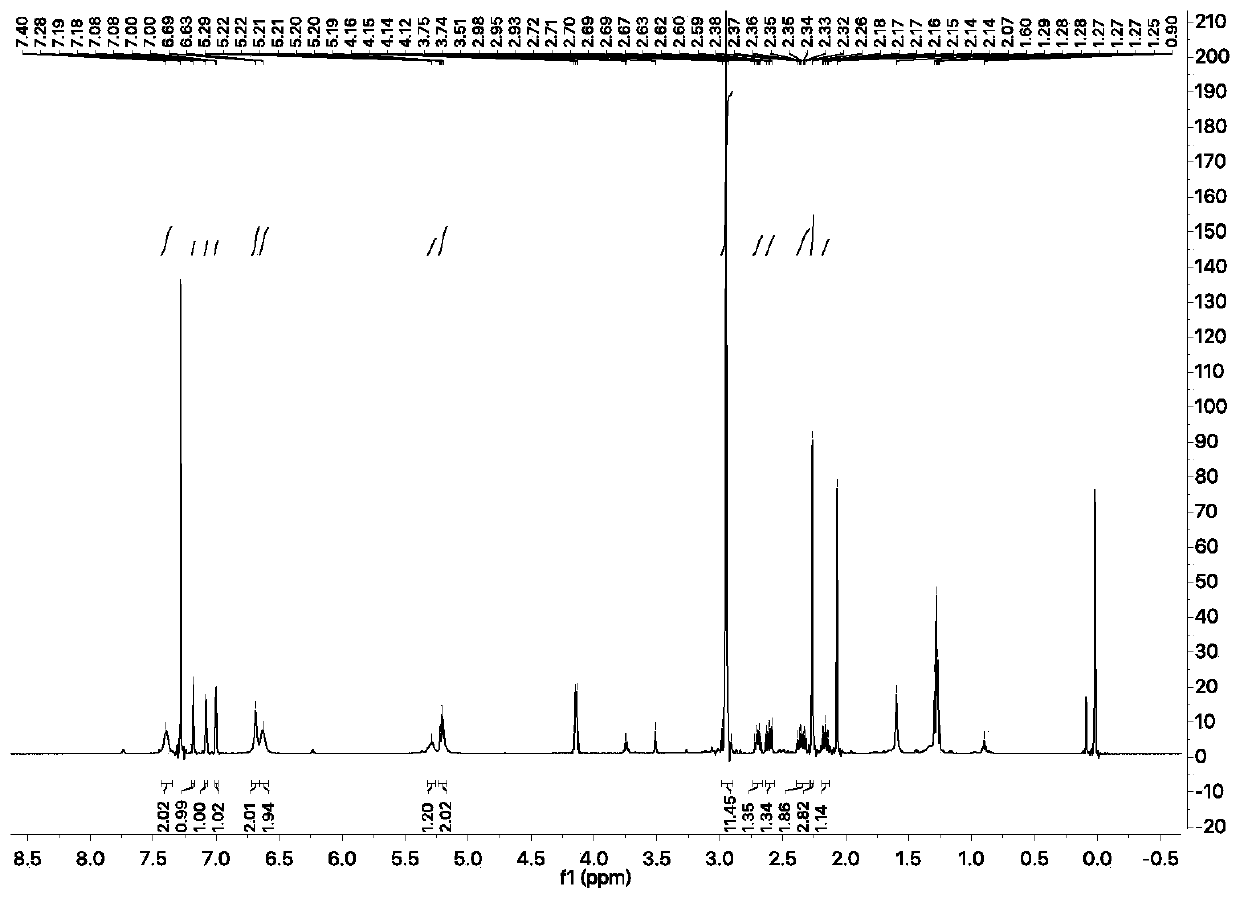
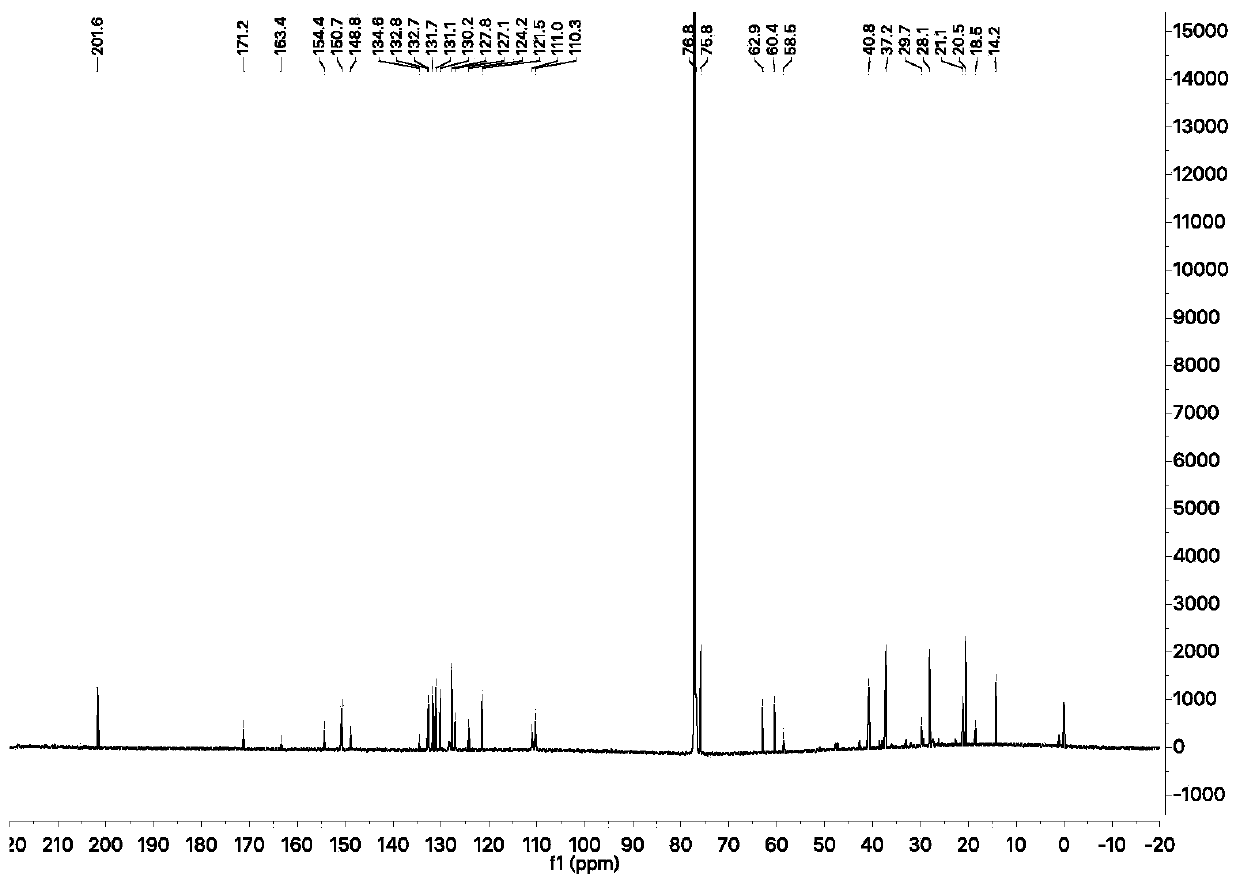
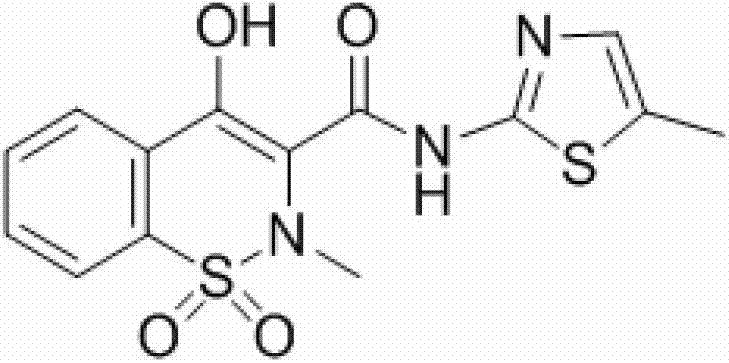
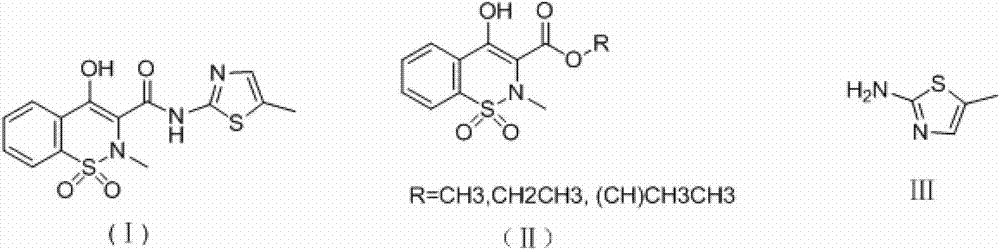
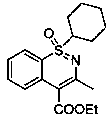
Synthesis method of meloxicam
The invention relates to a synthesis method of meloxicam, belonging to the field of medicine synthesis. The method comprises the following steps of: adding 4-hydroxyl-2-methyl-2H-1,2-benzothiazine-3-ethyl formate-1,1-dioxide and 2-amino-5-methylthiazole into dimethyl sulfoxide; reacting at a certain temperature while steaming out ethanol; after the reaction, adding a certain amount of ethanol into the reaction liquid; cooling and filtering to obtain a crude product of meloxicam; and recrystallizing the crude product to obtain a high-purity purified product of meloxicam.
Owner:QINGDAO AGRI UNIV +2
Benzothiazinone and benzoxazinone compounds
The present invention provides a compound of formula (I), wherein Q is -N= or CR2; X is S, O or NOR3; Y is -O-, S-, -SO- or -SO2-; R and R1 are independently is H, substituted or unsubstituted aliphatic, aromatic, heteroaromatic or aralkyl-yl; group; R2 is H or a substituent; R3 is H, or -C(O)R4; R4 is substituted or unsubstituted Substituted aliphatic or aromatic groups; n is an integer 0-1. Compounds of formula (I) and their physiologically acceptable salts are inhibitors of serine / threonine and tyrosine kinase activity. Several tyrosine kinases whose activity can be inhibited by these compounds are involved in the angiogenic process. Thus, these chemical compounds are able to ameliorate disease states in which angiogenesis or endothelial cell proliferation is a factor. These compounds are useful in the treatment of cancer and hyperproliferative diseases.
Owner:KNOLL GMBH
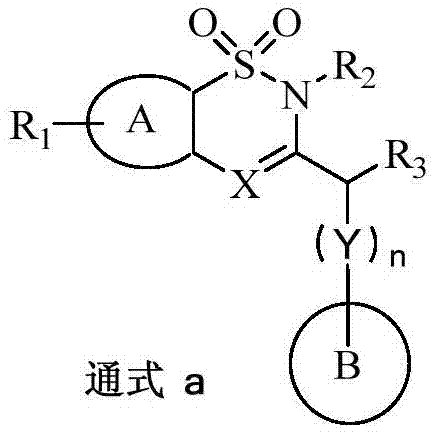
Benzothiazine and benzothiadiazine compounds, preparation and application
ActiveCN107033145AHas inhibitory effectEnhanced inhibitory effectOrganic active ingredientsSenses disorderBenzothiadiazinesDisease
The invention provides benzothiazine and benzothiadiazine compounds as well as analogues, pharmaceutically acceptable salts, stereoisomers and solvates of the compounds having a structure shown in a general formula a. Repeated experiments prove that the compounds have an inhibition action on PI3Kdelta, wherein most compounds selectively have a remarkableinhibition action on PI3Kdelta, the compounds can be applied to preparation of anti-inflammation and anti-tumor drugs, the range of involved tumor and inflammatory diseases is wide, and the compounds particularly have a better inhibition effect on FL (follicular B-cell non-Hodgkin lymphoma) relapsing in a treatment period, recurrent CLL (chronic lymphocytic leukemia) and recurrent SLL (small lymphocytic lymphoma). Prepared drugs can be combined with other anti-tumor drugs for use and have an obvious effect, and novel treatment drugs are provided for clinically resisting tumors and inflammation diseases.
Owner:ZHEJIANG UNIV
Method for preparing 2-amino benzothiazine
InactiveCN103232410AHigh yieldHigh chemoselectivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsChemical structureEthyl cinnamate
The invention discloses a method for preparing 2-amino benzothiazine. The method comprises the steps of preparing the 2-amino benzothiazine by addition reaction of catalytic o-amino ethyl cinnamate and isothiocyanate under the catalysis of trifluoromethanesulfonic acid rare-earth compound Ln(OTf)3 serving as a catalyst, wherein Ln represents a triad rate-earth metal ion and is one of lanthanum, neodymium, samarium or ytterbium, OTf represents trifluoromethanesulfonic acid radical and the chemical structural formula of o-amino ethyl cinnamate is shown in the specification; and the general formula of the chemical structure of isothiocyanate is RNCS. In the method, the catalyst is good in chemical selectivity, high in reactivity, mild in reaction condition, and short in reaction time; the reaction has high atom economy and does need a solvent; and the yield of a target product can be up to 99%.
Owner:SUZHOU UNIV
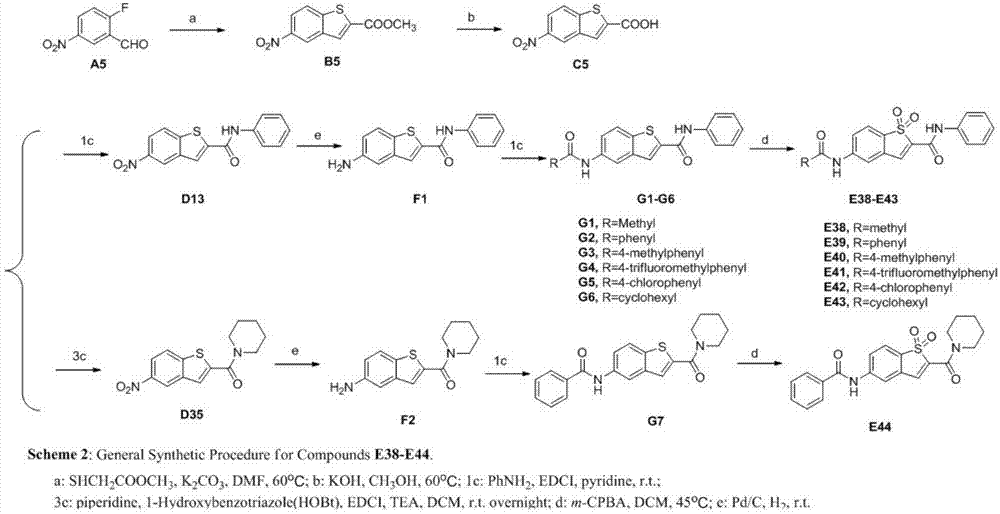
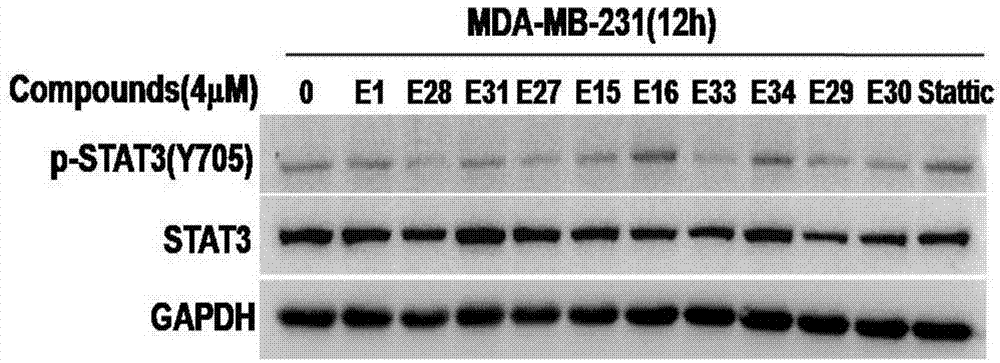
Targeting STAT3 inhibitor and application thereof
PendingCN104844563AGood antitumor activityGood physical and chemical propertiesOrganic chemistryAntineoplastic agentsStat3 inhibitorBenzothiazine
The invention discloses a benzothiazine-1,1-dioxides having a structure of Formula I as a new type of highly efficient STAT3 small molecule inhibitor, which is used for developing antineoplastic drugs.
Owner:SUZHOU UNIV
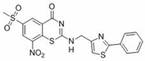
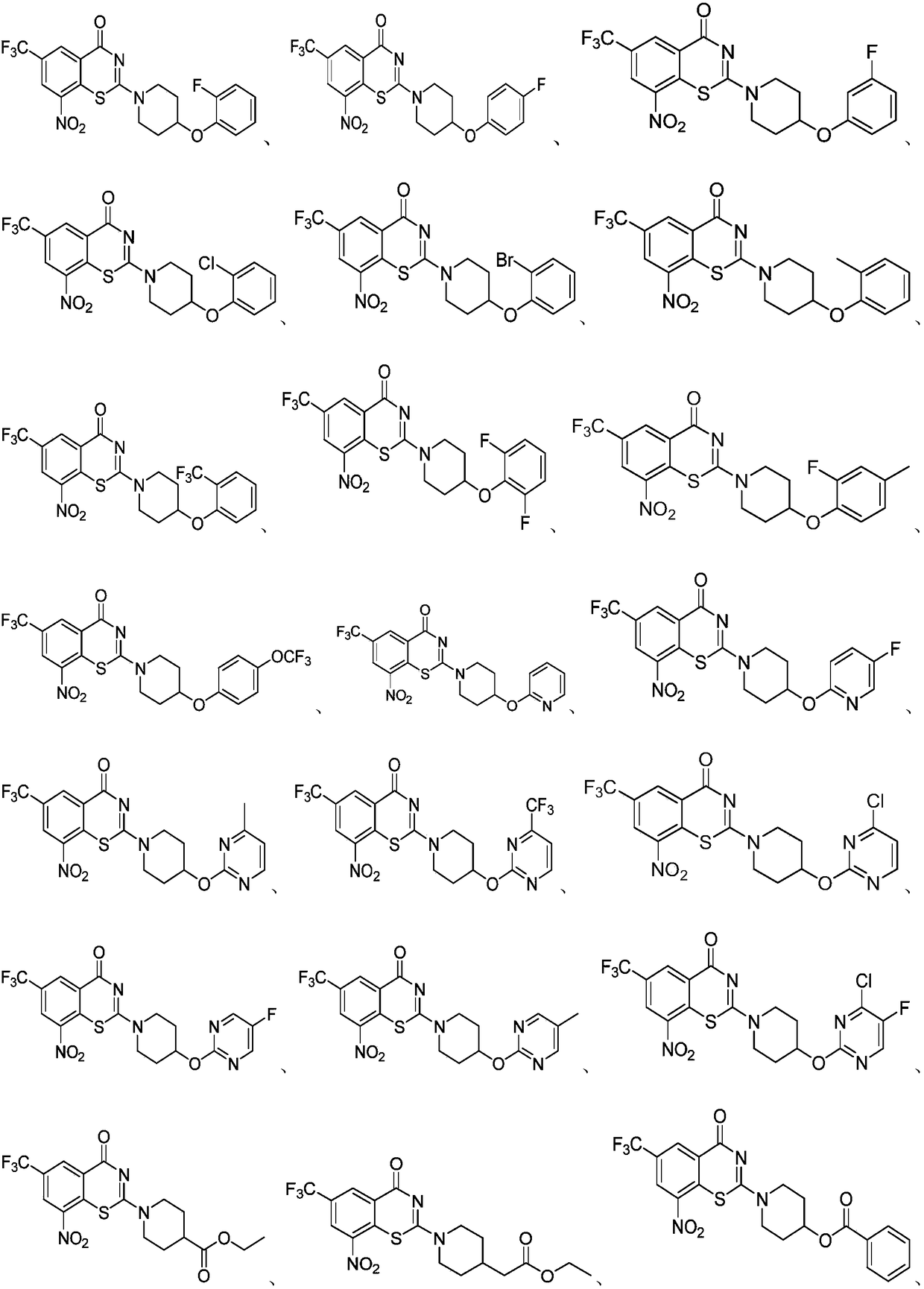
Benzothiazinone compound, preparation method thereof and application of benzothiazinone compound as antituberculosis drug
ActiveCN112409293AGood antibacterial effectWork around the bug with high cLogP valuesAntibacterial agentsOrganic active ingredientsAntituberculosis drugAntituberculous drugs
The invention discloses a benzothiazinone compound and a preparation method and application of the benzothiazinone compound as an antituberculosis drug, and particularly relates to a novel compound with a benzothiazinone skeleton. The compound has an inhibition effect on tubercle bacillus, especially tubercle bacillus with clinical drug resistance. Results show that the compound shows an obvious antibacterial effect, the antibacterial effect far exceeds that of a positive control isoniazide, and particularly, compared with a positive control pBTZ169, the compound has an obvious and good cLogPvalue.
Owner:SUZHOU UNIV
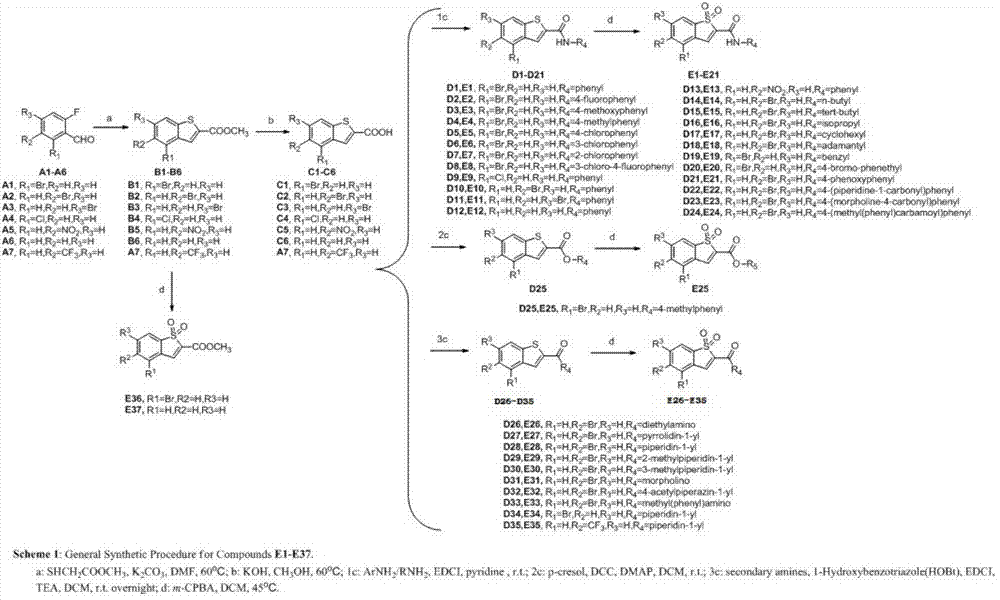
Synthetic method of 2,3-dihydro-[1,4]-benzothiazine compound
The invention discloses a synthetic method of 2,3-dihydro-[1,4]-benzothiazine compound. The method comprises the steps of: by taking 2-iodine-N-(2-iodoethyl) aniline derivant as a reaction material and taking Na2S2O3 as a vulcanization reagent, reacting in a reaction solvent in the presence of a metal palladium catalyst to obtain multisubstituted 2,3-dihydro-[1,4]-benzothiazine compound. The synthetic method is mild in reaction condition, available in material, cheap in material, simple to react and operate and high in yield, provides a key skeleton structure for synthesis of a plurality of natural products and medicines, and can be widely applied to industrial mass production.
Owner:EAST CHINA NORMAL UNIVERSITY
Benzothiazine derivative, a preparation method and uses thereof
The invention belongs to the field of chemical medicine, particularly relates to a benzothiazine derivative, a preparation method and uses thereof, and provides a benzothiazine derivative, a preparation method and uses thereof, wherein the structure is represented by a formula I. The invention further provides a preparation method and uses of the benzothiazine derivative. The formula I is definedin the specification.
Owner:SICHUAN UNIV
Method for ammonolyzing and synthesizing meloxicam under catalysis of Lewis acid
The invention relates to a method for ammonolyzing and synthesizing meloxicam under the catalysis of Lewis acid. Under the catalysis of silicate Lewis acid, the meloxicam is synthesized from 4-hydroxy-2-methyl-2H-1,2-benzothiazine-3-carboxylic acid ethyl ester 1,1-dioxide and 2-amino-5methylthiazol. Compared with the direct ammonolysis method, the method has the advantages that: (1) the reaction time is about 4 to 6 hours which is shortened by four times and ammonolysis is complete; and (2) the ammonolysis yield is high which is more than or equal to 85 percent; decomposers and carbides are avoided; the product is easy to purify; the method for synthesizing the meloxicam is suitable for mass production; the product purity reaches 99 percent through HPLC detection; the product is light yellow green; and the product quality meets Chinese pharmacopoeia 2005.
Owner:SHANDONG FANGMING PHARMACEUTICAL CO LTD
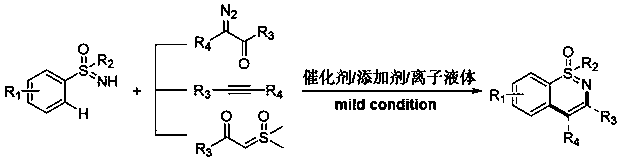
Novel green synthetic method for 1,2-benzothiazine derivative through transition metal catalyzed C-H activation/cyclization
ActiveCN110105305AAvoid pollutionReduce production finished productOrganic chemistryChemical recyclingPtru catalystAlkyne
The invention relates to a novel green synthetic method. According to the method, N1,N3-disubstituted imidazole type ionic liquid is taken as a solvent, NH-sulfoximine is taken as a raw material, a diazo compound, alkyne and sulfur ylide are taken as coupling reagents, and a 1,2-benzothiazine derivative is mildly and efficiently synthesized through a transition metal catalyzed C-H activation / cyclization reaction. Compared with other technologies, the condition is milder, the operation is simple, the method is safe, and the environmental friendliness is achieved; the catalytic activity is high,the reaction yield is high, and the application range of substrates is wide; the solvent and catalysts can be recycled, so that the reaction cost is reduced.
Owner:SICHUAN UNIV
Benzothiazinone compound, preparation method thereof and application of benzothiazinone compound as antituberculosis drug
InactiveCN111269197ALow cLogP valueGood medicineAntibacterial agentsOrganic active ingredientsAntituberculosis drugPharmaceutical drug
The invention discloses a benzothiazinone compound, a preparation method thereof and an application of the benzothiazinone compound as an antituberculosis drug, and particularly relates to a novel compound with a benzothiazinone skeleton. The compound has an inhibition effect on tubercle bacillus, especially tubercle bacillus with clinical drug resistance. Results show that the compound shows an obvious antibacterial effect, the antibacterial effect far exceeds that of a positive control isoniazide, and particularly, compared with a positive control pBTZ169, the compound has an obviously goodcLogP value.
Owner:SUZHOU UNIV
Method for producing benzo thiazides compounds
The invention belongs to the technical field of organic chemistry, in particular to a benzothiazole compounds and a preparation method for the compounds. The structures of the compounds are attributed and determined by the methods like <1>H NMR, <13>CNMR, IR, MS, elementary analysis, and a single-crystal X-ray diffraction, etc. The invention uses a silver triflate as a catalyst to prepare 4-methylene-1H-benzo[d] [1, 3] thiazine-2(4H)-imine framework compounds by mixing an ortho-alkynyl aniline and an isothiocyanate in a tetrahydrofuran to react at a room temperature. The method of the invention has the advantages of mild reaction condition, simple operation, lower cost, fewer side effects and high purity of the product; thereby having a great application prospect.
Owner:FUDAN UNIV
Method for preparing polysubstituted 3,4-dihydro-3-methyl-2H-1,4-benzoxazine-2-one
The invention discloses a method for producing multi-substituted 3,4-bihydrogen-3-methyl-2H-1,4- benzothiazine-2-ketones. The method comprises the following steps: all kinds of multi-substituted 3,4-bihydrogen-3-methyl-2H-1,4- benzothiazine-2-ketone are produced from multiple substituted o-nitryl phenol through one-pot synthesis of catalytic hydrogenation / reduction ammoniation / esterification with rare metals are used as the catalysts in a mixed solution of an alcohol and water. The method provided by the invention uses o-nitryl phenol as the norm to achieve a mole yield more than 25 percent of multi-substituted 3,4-bihydrogen-3-methyl-2H-1,4- benzothiazine-2-ketones. The cheap raw material for the reaction is cheap and the one-pot synthesis is used, so that the technology provided by the invention for producing multi-substituted 3,4-bihydrogen-3-methyl-2H-1,4- benzothiazine-2-ketones is an effective method and has a great prospect in the industrialized production.
Owner:EAST CHINA UNIV OF SCI & TECH
Squaraine dye containing benzothiazine structure and preparation method thereof
The invention discloses a squaraine dye containing a benzothiazine structure and a preparation method thereof. The preparation method comprises the following steps: mixing a thiazine cyclic compound with squaric acid based on the mol ratio of 2:1; adding a mixed solvent of benzene and n-butyl alcohol; and under catalysis of 3-5ml of quinoline or pyridine, stirring and refluxing for 12-48h for carrying out condensation reaction to obtain the squarylium cyanine dye. In the invention, absorption wavelength for near-infrared absorption is 700-1100nm. The squaraine dye has excellent photo-thermal property, excellent chemical stability, good compatibility with organic matrix materials and small side effect on human body; the squaraine dye is a new type dye with excellent optical property; and the squaraine dye has wide application prospect in fields such as near-infrared laser protection, biomolecular fluorescence labeling, photoelectric functional materials, laser electrostatic copying materials, a dye laser and the like.
Owner:NAT UNIV OF DEFENSE TECH
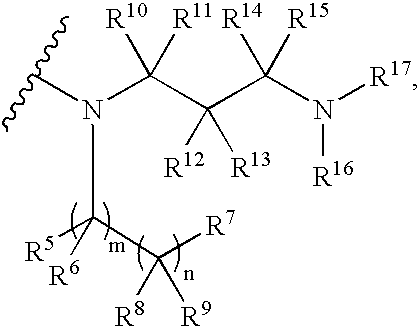
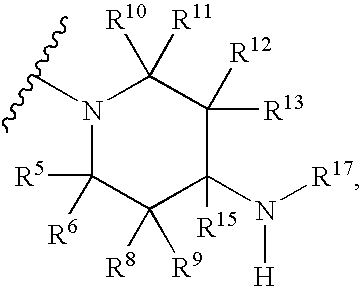
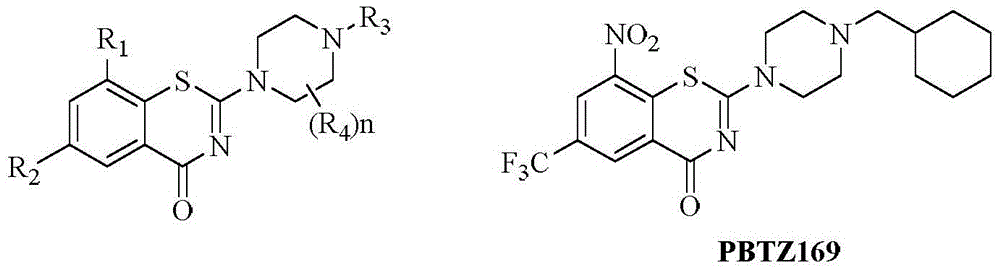
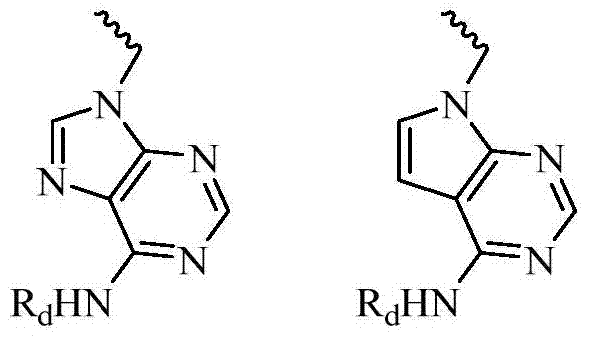
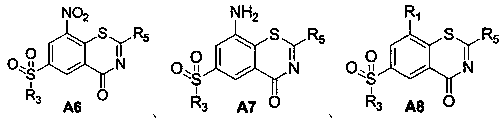
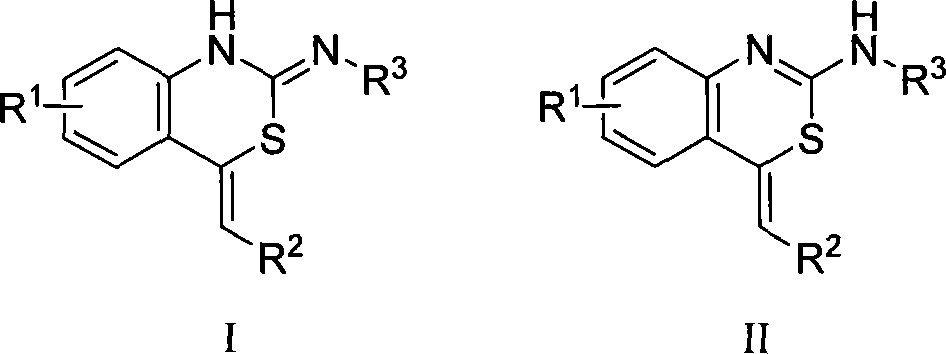
Benzothiazine-4-one compound containing 2,7-diazaspiro[3.5]nonane fragments and preparation method thereof
ActiveCN108530447AHigh activityGood curative effectAntibacterial agentsOrganic active ingredientsHydrogen atomBenzothiazine
The invention relates to a benzothiazine-4-one compound containing 2,7-diazaspiro[3.5]nonane fragments and a preparation method thereof and in particular relates to 6-trifluoromethyl-8-nitro-4H-benzo[e][1,3]thiazine-4-one compounds. The 2-position substituent group is 2,7-diazaspiro[3.5]nonane-7-yl, wherein Ar represents phenyl, pyridyl, naphthyl, quinolyl, pyrazinyl, pyrimidyl, pyrazolyl, imidazolyl, furyl or thienyl, and optionally, the hydrogen atom at any position of the groups can be substituted by the group R; the group R is selected from alkyl having 1-4 carbon atoms, alkoxy having 1-3carbon atoms, halogen, -CF3, -OCF3, -NO2 or -CN.
Owner:ZHEJIANG STARRY PHARMA +1
Methods for synthesizing benzothiazepine compounds
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
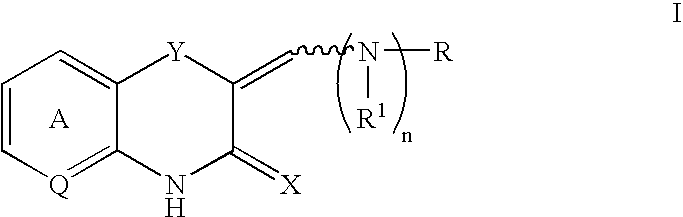
Benzoxazine and benzothiazine derivatives and parmaceutical compositions containing them
The present invention relates to novel antidiabetic, hypolipidemic, antiobesity and hypocholesterolemic compounds of formula (I) their derivatives, their analogs, their tautomeric forms, their stereoisomers, their polymorphs, their pharmaceutically acceptable salts, their pharmaceutically acceptable solvates and pharmaceutically acceptable compositions containing them, to a process for preparing such compounds. More particularly, the present invention relates to novel alkyl carboxylic acids of the general, their derivatives, their analogs, their tautomeric forms, their stereoisomers, their polymorphs, their pharmaceutically acceptable salts, their pharmaceutically acceptable solvates and pharmaceutically acceptable compositions containing them, to a process for preparing such compounds. The present invention also relates to processes for the preparation of the compounds of formula (I), novel intermediates, processes for their preparation, their use in the preparation of the above said compounds and their use as antidiabetic, hypolipidemic, antiobesity and hypocholesterolemic compounds.
Owner:DR REDDYS LAB LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com











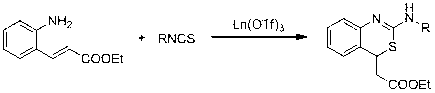
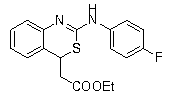


![Synthetic method of 2,3-dihydro-[1,4]-benzothiazine compound Synthetic method of 2,3-dihydro-[1,4]-benzothiazine compound](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2a00b7bf-cd46-46de-9064-12dfeb763d1c/FDA00002771201600011.png)
![Synthetic method of 2,3-dihydro-[1,4]-benzothiazine compound Synthetic method of 2,3-dihydro-[1,4]-benzothiazine compound](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2a00b7bf-cd46-46de-9064-12dfeb763d1c/BDA00002771201700011.png)
![Synthetic method of 2,3-dihydro-[1,4]-benzothiazine compound Synthetic method of 2,3-dihydro-[1,4]-benzothiazine compound](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2a00b7bf-cd46-46de-9064-12dfeb763d1c/BDA00002771201700021.png)









![Benzothiazine-4-one compound containing 2,7-diazaspiro[3.5]nonane fragments and preparation method thereof Benzothiazine-4-one compound containing 2,7-diazaspiro[3.5]nonane fragments and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/55acf9dc-b08b-4da5-a85a-64f4fc67539e/BDA0001237103160000021.png)
![Benzothiazine-4-one compound containing 2,7-diazaspiro[3.5]nonane fragments and preparation method thereof Benzothiazine-4-one compound containing 2,7-diazaspiro[3.5]nonane fragments and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/55acf9dc-b08b-4da5-a85a-64f4fc67539e/BDA0001237103160000022.png)
![Benzothiazine-4-one compound containing 2,7-diazaspiro[3.5]nonane fragments and preparation method thereof Benzothiazine-4-one compound containing 2,7-diazaspiro[3.5]nonane fragments and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/55acf9dc-b08b-4da5-a85a-64f4fc67539e/BDA0001237103160000031.png)