Fluorescent conjugated polymer containing phenothiazine group, and synthesis method and application thereof
A conjugated polymer, phenothiazine-based technology, used in the field of fluorescent conjugated polymers and their synthesis, can solve problems such as difficult testing and processing, insoluble and infusible polymers, and achieve improved solubility and good thermal stability. , the effect of good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
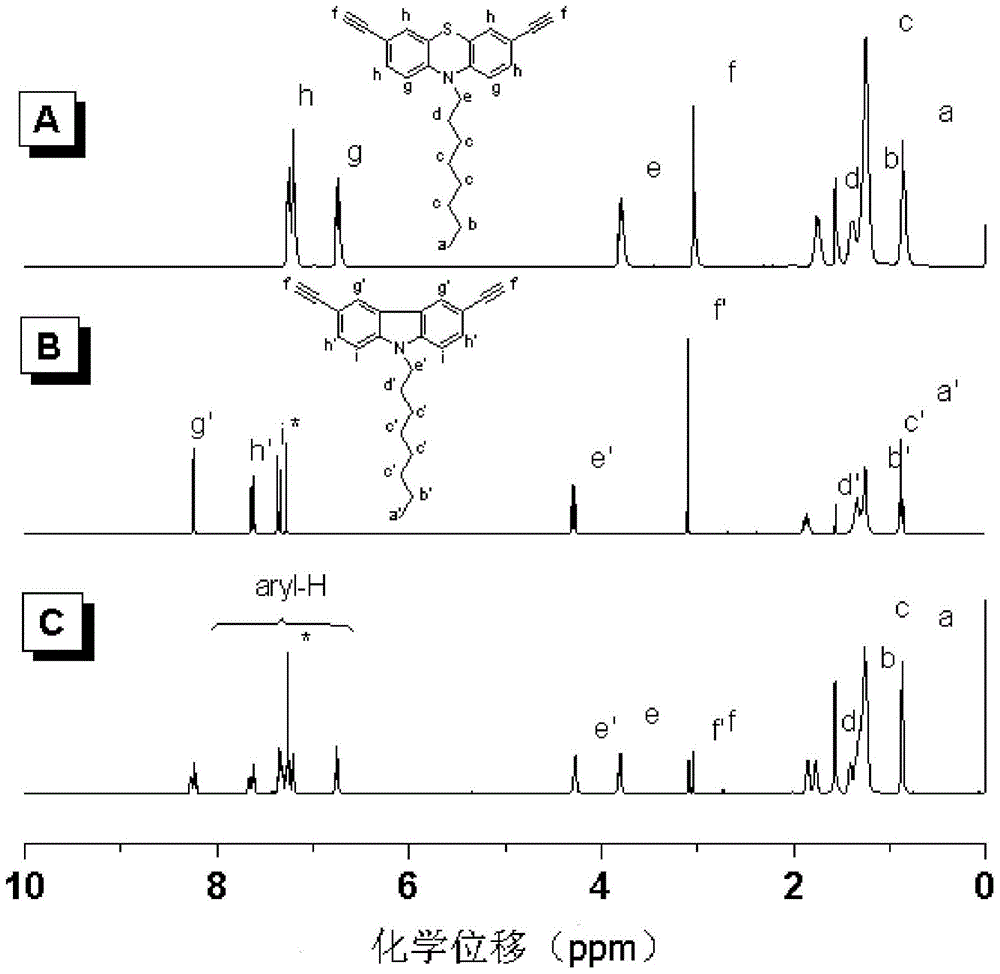
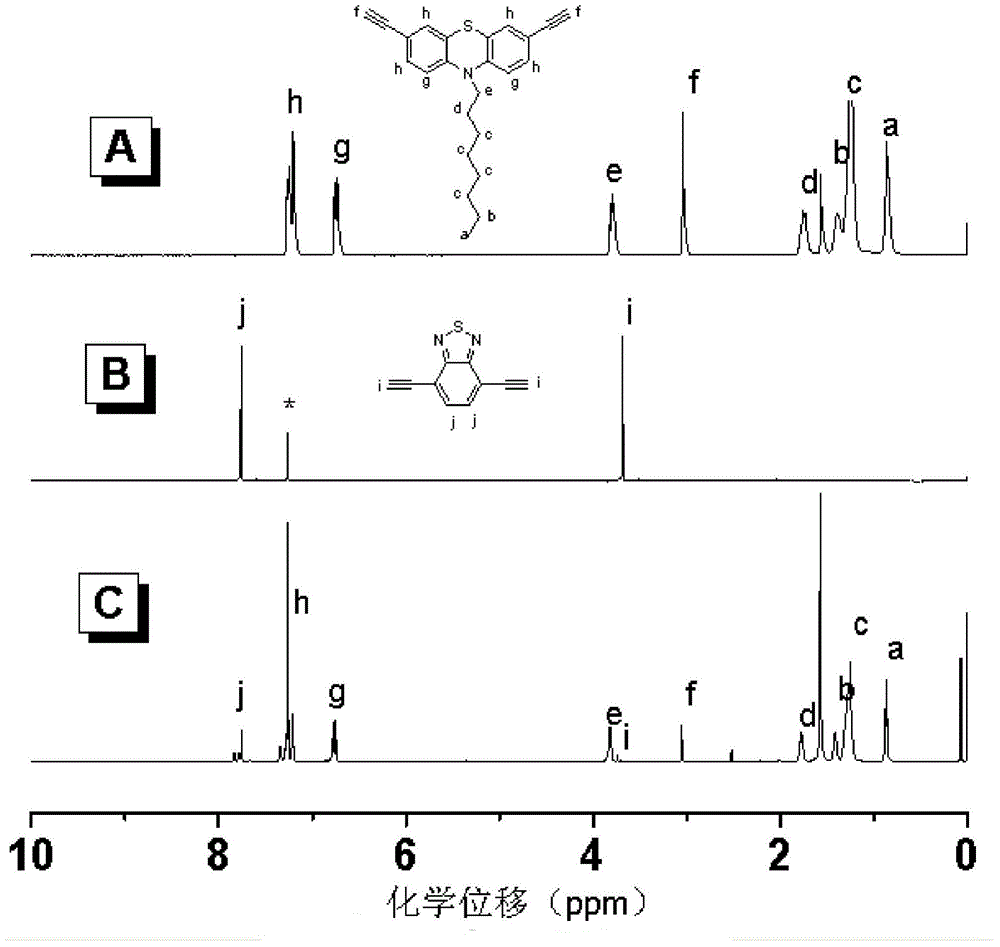
[0039] Embodiment 1, the synthesis of N-octyl-3,6-two (ethynyl) phenothiazines:
[0040] 2g of 3,6-dibromo-N-octylphenothiazine (4.26mmol), 2mg of CuI, 60mg of Pd(Ph 3 P) 2 Cl 2 Sequentially add to a 100ml (19#) two-neck flask, under nitrogen protection, add 30ml of toluene, 30ml of triethylamine and 1.25ml of 3,3'-dimethyl-3-hydroxypropyne, heat to 110°C, React for 12 hours; filter the reaction product, wash with anhydrous ether, evaporate the solvent to dryness, and purify by column chromatography. The developer uses a mixed solvent of petroleum ether and ethyl acetate, and the volume ratio of petroleum ether and ethyl acetate is 3:1 , and purified to obtain 1.384 g of yellow solid, with a yield of 68%;
[0041] Take 0.35g of the yellow solid (0.73mmol) obtained in the above steps and 30mg of NaOH (0.73mmol) into a 100ml (19#) two-neck flask, under the protection of nitrogen, add 15ml of toluene, heat to 110°C to condense and reflux, and react 4h; the reaction mixture wa...
Embodiment 2
[0046] Embodiment 2, the synthesis of N-octyl-3,6-two (ethynyl) carbazoles:
[0047] 1 g of 3,6-diiodo-N-octylcarbazole (1.9 mmol), 1 mg of CuI (0.005 mmol), 30 mg of Pd(Ph 3 P) 2 Cl 2 Add to 100ml (19#) single-necked round bottom flask successively, under N 2 Under atmosphere, add 15ml of triethylamine, then add 0.6ml of 3,3'-dimethyl-3-hydroxypropyne, stir at room temperature for 16h; after the reaction stops, filter the reaction mixture and wash with anhydrous ether Atmospheric pressure distillation removes solvent, carries out column chromatography purification, developing agent adopts the mixed solvent of sherwood oil and ethyl acetate, and the volume ratio of sherwood oil and ethyl acetate is 3: 1, purifies and obtains light yellow solid 0.706g, productive rate 84.5%;
[0048] Take 0.5g of the light yellow solid (1.18mmol) obtained in the above steps and 0.047g of NaOH (1.18mmol) into a 100ml (19#) two-necked round bottom flask, add 15ml of toluene under nitrogen pro...
Embodiment 3
[0053] Embodiment 3, the synthesis of 4,7-diethynyl-2,1,3-benzothiaxazole:
[0054] 1g of 4,7-dibromobenzothiaxazole (3.4mmol), a small amount of CuI and 48mg of Pd(Ph 3 P) 2 Cl 2 (0.068mmol) was added to 100ml (19#) two-necked round bottom flask successively, under N 2 Under the atmosphere, add 15ml of toluene, 15ml of triethylamine and 1ml of 3,3'-dimethyl-3-hydroxypropyne, raise the temperature to 110°C, condense and reflux, and react for 16h; after the reaction is stopped, filter the reaction mixture and use Washing with anhydrous ether; distilled at normal pressure to remove the solvent, then purified by column chromatography, using a mixed solvent of petroleum ether and ethyl acetate as the developer, the volume ratio of petroleum ether and ethyl acetate was 2:1, and purified to obtain a light yellow solid of 0.761 g, yield 74.6%;
[0055] Take 0.5 g of the light yellow solid (1.67 mmol) obtained in the above steps and 0.067 g of NaOH (1.67 mmol) into a 100 ml (19#) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com