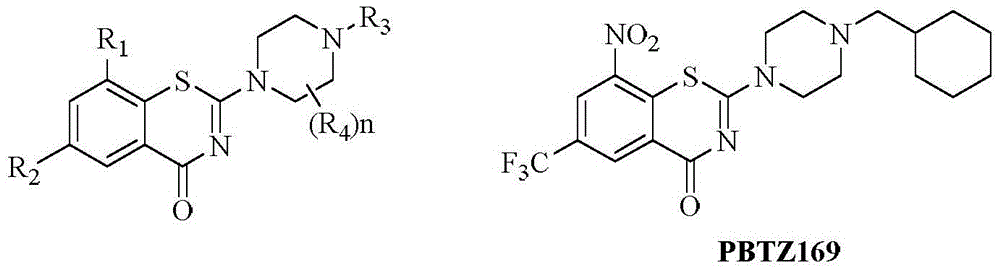
Benzothiazine-4-ketone compounds containing basic nitrogen heterocyclic fragments and preparing methods of benzothiazine-4-ketone compounds
A compound, the technology of thiazide, which is applied in the field of medicinal chemistry, can solve the problems of poor water solubility and the in vivo activity of BTZ043, which is not as expected
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0068] Example 12-[3-(cyclohexylmethyl)(methyl)aminoazetidin-1-yl]-6-trifluoromethyl-8-nitro-4H-benzo[e][1, 3] Thiazin-4-one
[0069] 2-Methylthio-6-trifluoromethyl-8-nitro-4H-benzo[e][1,3]thiazin-4-one (0.16g, 0.5mmol) was dissolved in absolute ethanol ( 8mL), added triethylamine (0.10g, 1.0mmol), added dropwise a solution of 3-(cyclohexylmethyl)(methyl)aminoazetidine (0.18g, 1.0mmol) in absolute ethanol (10mL), The reaction was stirred at 60° C. for 3 h, filtered, and the filtrate was concentrated under reduced pressure. The residue was separated by column chromatography to obtain a yellow solid (51% yield).
[0070] 1 HNMR (500MHz, CDCl 3 )δ9.12(s,1H),8.78(s,1H),3.73–3.36(m,5H),2.26(s,3H),2.18-2.09(m,2H),1.90–1.56(m,5H) ,1.35–1.13(m,6H).
[0071] MS-ESI(m / z):457(M+H) + .
Embodiment 22-
[0072] Example 22-[3-(p-methoxybenzyl)(ethyl)aminoazetidin-1-yl]-6-trifluoromethyl-8-nitro-4H-benzo[e] [1,3]thiazin-4-one
[0073] With the preparation method of the compound of Example 1, 3-(p-methoxybenzyl) (ethyl) amino azetidine and 2-methylthio-6-trifluoromethyl-8-nitro-4H- Benzo[e][1,3]thiazin-4-one was condensed to give a yellow solid (55% yield).
[0074] 1 HNMR (500MHz, CDCl 3 )δ9.12(s,1H),8.78(s,1H),7.25-7.02(m,4H),3.92(s,3H),3.75–3.41(m,7H),2.64(q,2H),1.02 (t,3H).
[0075] MS-ESI(m / z):495(M+H) + .
Embodiment 32
[0076] Example 32-[3-(cyclohexylmethyl)(methyl)aminopyrrolidin-1-yl]-6-trifluoromethyl-8-nitro-4H-benzo[e][1,3] Thiazin-4-one
[0077] With the preparation method of the compound of Example 1, 3-(cyclohexylmethyl)(methyl)aminopyrrolidine and 2-methylthio-6-trifluoromethyl-8-nitro-4H-benzo[e] [1,3] Thiazin-4-one was condensed to give a yellow solid (53% yield), mp: 126-129°C.
[0078] 1 HNMR (500MHz, CDCl 3 )δ9.17(s,1H),8.78(s,1H),4.36–4.18(m,4H),4.15–4.03(m,1H),3.91(s,3H),3.80–3.61(m,2H) ,1.89–1.45(m,7H),1.33–1.13(m,6H).
[0079] MS-ESI(m / z):471(M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com