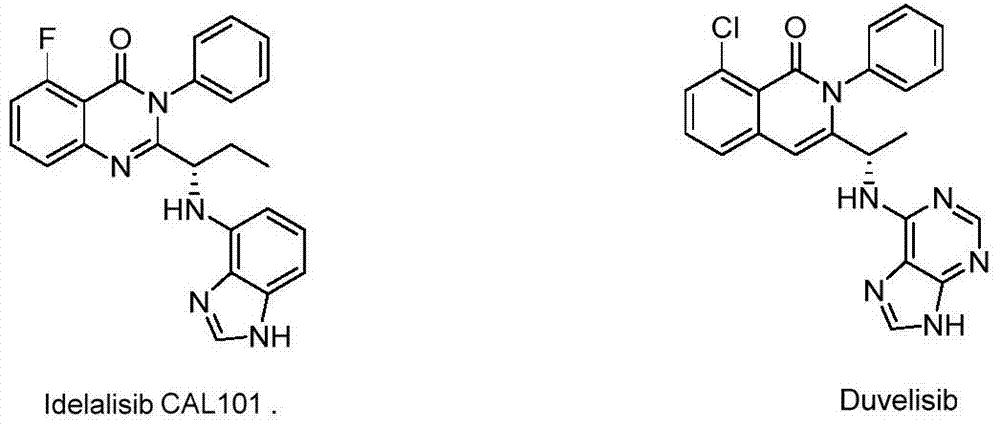
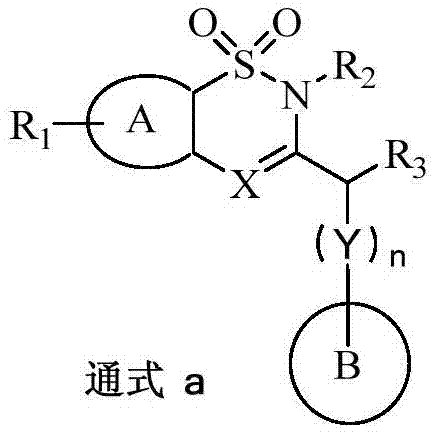
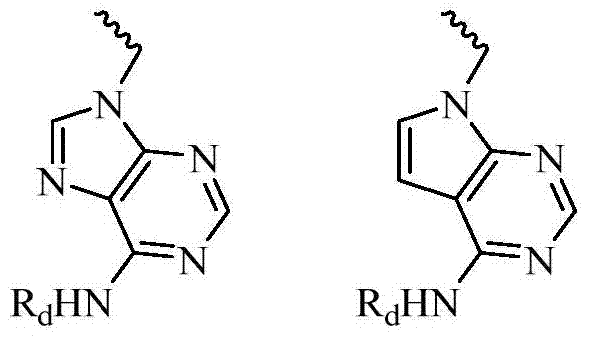
Benzothiazine and benzothiadiazine compounds, preparation and application
A technology of benzothiadiazine and benzothiazine, which is applied in the field of medicine and can solve problems such as toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Embodiment 1: the synthetic route of class I compound (benzothiadiazines)
[0083]
[0084] Step 1: Benzyl(2-fluoro-6-nitrophenyl)sulfide (1a)
[0085] Dissolve 2,3-difluoronitrobenzene (5.00g, 31.3mmol), benzylthiol (3.7mL, 31.3mmol) in anhydrous DMF (30mL), add anhydrous K 2 CO 3 (4.51g, 32.7mmol), stirred overnight at room temperature. Add an appropriate amount of ethyl acetate to the system, wash with water and saturated brine successively, and wash the organic layer with anhydrous Na 2 SO 4 Drying and concentration gave a light yellow solid, which was directly used in the next reaction. Yield 95%; mp 60-61 °C.
[0086] Benzyl(2-chloro-6-nitrophenyl)sulfide (1b)
[0087] The preparation of intermediate 1b refers to the synthesis of 1a, replacing 2,3-difluoronitrobenzene with 2,3-dichloronitrobenzene. Pale yellow solid. Yield 93%; mp 55-57°C.
[0088] Step 2: 2-fluoro-6-nitrobenzenesulfonyl chloride (2a)
[0089] Dissolve 1a (7.82g, 29.7mmol) in dichloro...
Embodiment 2
[0248] Embodiment 2: the synthetic route of class I compound (pyridothiadiazines) compound
[0249]
[0250] 2-Phenyl-3-(1-((9H-purine-6-)amino)ethyl)-2H-pyridin[2,3-e][1,2]thiadiazine-1,1-dioxide Things (I-22)
[0251] Using 2-chloro-3-nitropyridine as raw material, according to the method of Example 1, the target product was prepared. ESI-MS: m / z=421[M+H] + .
[0252] 2-Phenyl-3-(1-((4-amino-5-cyano-pyrimidine-6-)amino)ethyl)-2H-pyridin[2,3-e][1,2]thiadiazine -1,1-dioxide (I-23)
[0253] Using 2-chloro-3-nitropyridine as raw material, replacing 6-chloropurine with 4-amino-5-cyano-6-chloropyrimidine, according to the method of Example 1, the target product was prepared. ESI-MS: m / z=421[M+H] + .
[0254] 2-Phenyl-3-(1-((9H-purine-6-)amino)ethyl)-2H-pyridin[4,3-e][1,2]thiadiazine-1,1-dioxide Object (I-24)
[0255] According to the method of Example 1, using 3-chloro-4-nitropyridine as raw material, the target product was prepared. ESI-MS: m / z=420[M+H] + .
[0256...
Embodiment 3
[0258] Embodiment 3: the synthetic route of class I compound (pyrazinothiadiazines) compound
[0259]
[0260] 2-Phenyl-3-(1-((9H-purine-6-)amino)ethyl)-2H-pyrazine[2,3-e][1,2]thiadiazine-1,1-di Oxide (I-26)
[0261] Using 2-chloro-3-nitropyrazine as raw material, according to the method of Example 1, the target product was prepared. ESI-MS:m / z=422[M+H] + .
[0262] 2-Phenyl-3-(1-((9H-purine-6-)amino)ethyl)-2H-pyrazine[2,3-e][1,2]thiadiazine-1,1-di Oxide (I-27)
[0263] According to the method of Example 1, 2-chloro-3-nitropyrazine was used as raw material, and 6-chloropurine was replaced with 4-amino-5-cyano-6-chloropyrimidine to prepare the target product. Yield: ESI-MS: m / z=421 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com