Enterococcus faecium microcapsule formulation and method for preparing same
An Enterococcus faecium and microcapsule technology, applied in the field of microorganisms, can solve the problems of limited large-scale application, large loss of active ingredients, short product shelf life, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Screening of Enterococcus faecium and preparation of seed solution
[0032] 1. Method:
[0033] Feces from newborn infants were obtained by aseptic operation, diluted to a certain number of times, spread on a plate made of a specific medium, and cultured for 24 hours to obtain a certain number of colonies, and each colony was regarded as a selected target strain. After the growth curve, metabolic process determination and pathogenic bacteria inhibition experiment, the strains with high growth vigor and lactic acid production and significant antibacterial effect were selected as the final target strains and seed strains for coating.
[0034] 2. Enterococcus faecium culture and its culture conditions:
[0035] The optimal culture medium for Enterococcus faecium screened by single factor and orthogonal experiment is: glucose 10g / l, soybean peptone 5g / l, yeast extract 2g / l, ammonium citrate 2g / l, sodium acetate 5g / l , K2HPO 4 2g / l, Tween 801ml, MgSO 4 ·7H 2 ...
Embodiment 2
[0038] Embodiment 2: biological activity experiment
[0039] 1. Method
[0040] The growth, metabolism and bacteriostasis of Enterococcus faecium isolated in this laboratory were compared with those of imported Enterococcus faecalis and Enterococcus faecalis.
[0041] in:
[0042] MLK: Enterococcos from improt probiotics, reference strain, screened from Micola cream in the United States.
[0043] Baby-1: Enterococcos faecalis (Obtaied by our lab), Enterococcus faecalis Baby-1.
[0044] Baby-2: Enterococcos faecium, Enterococcus faecalis Baby-2.
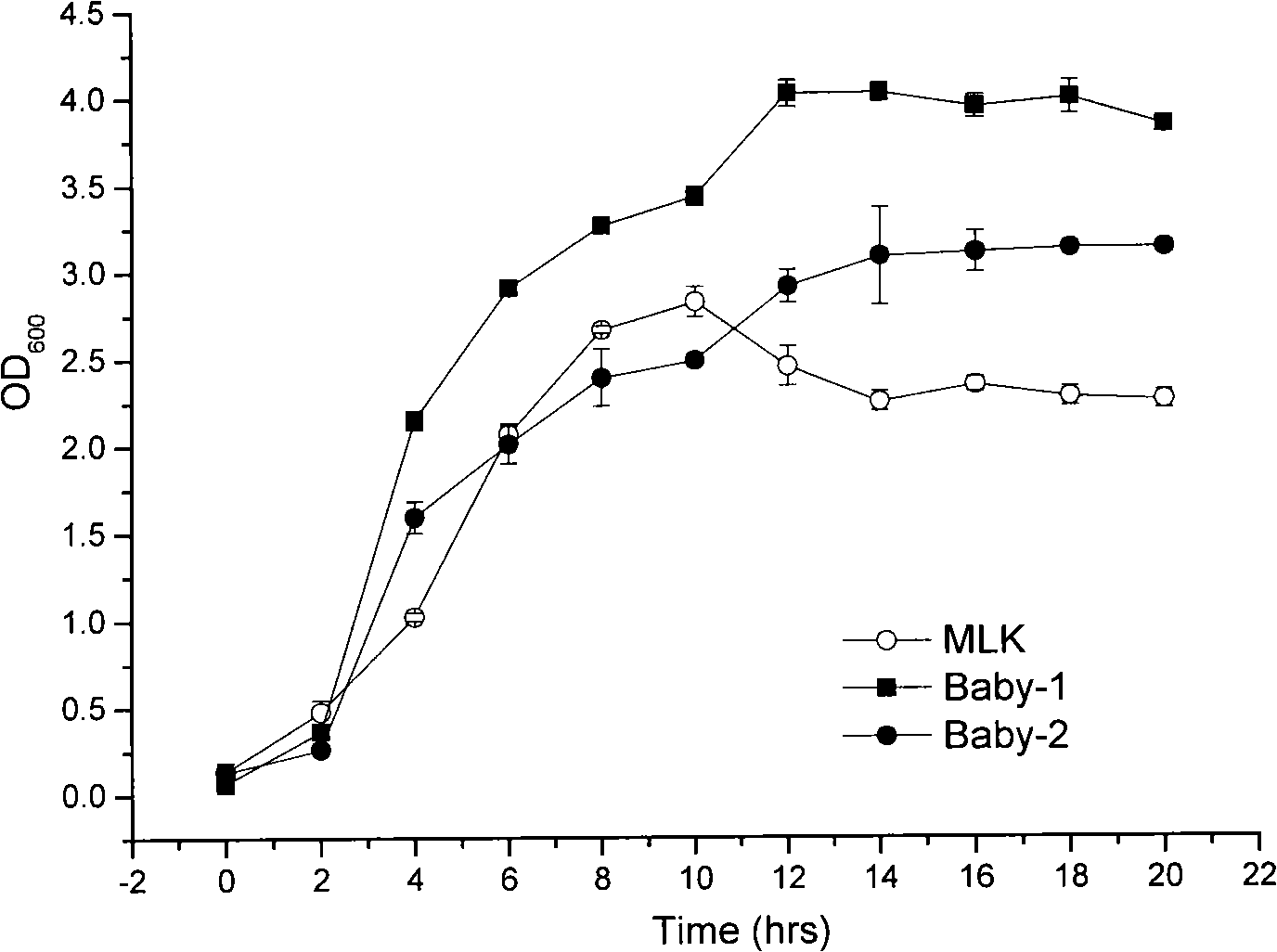
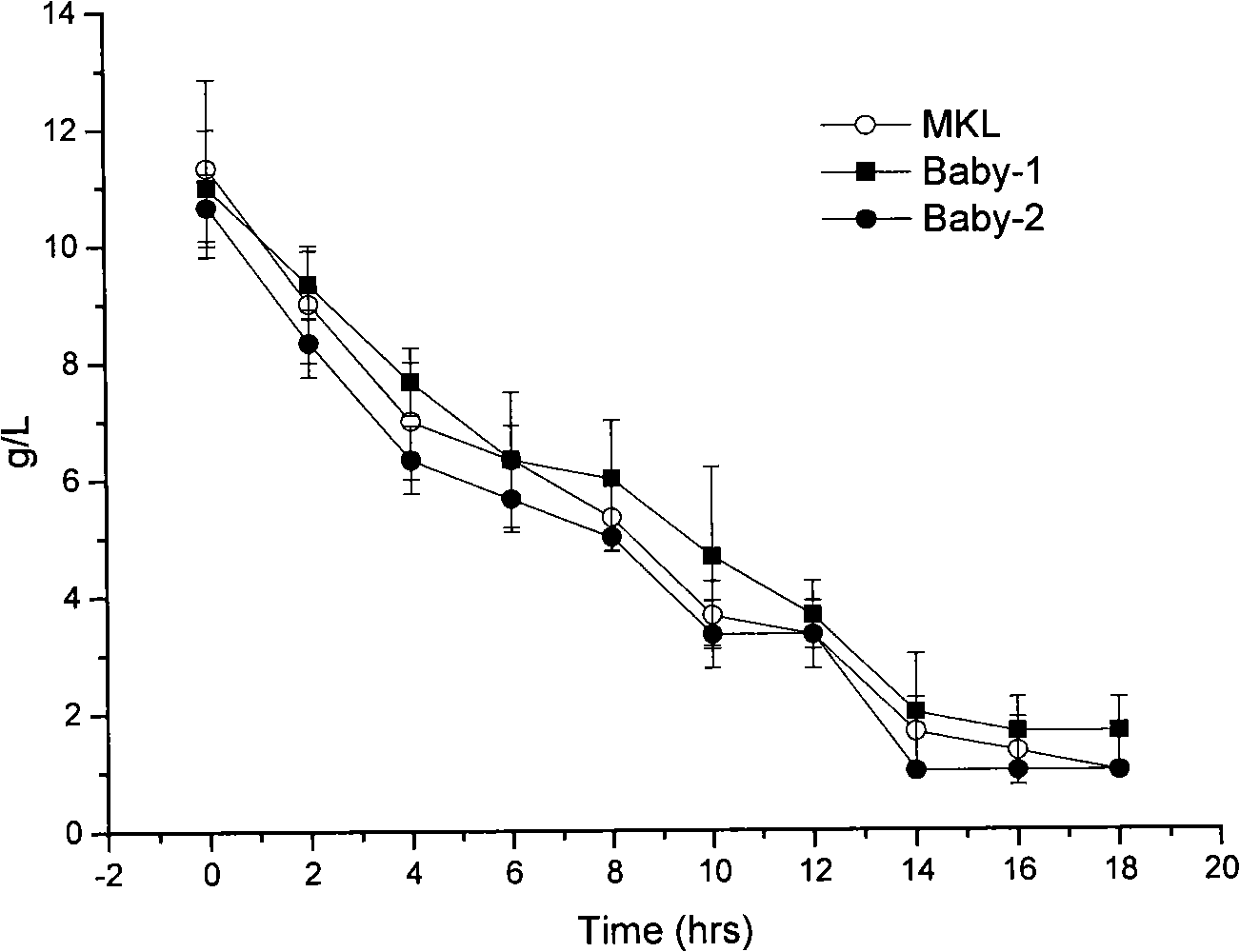
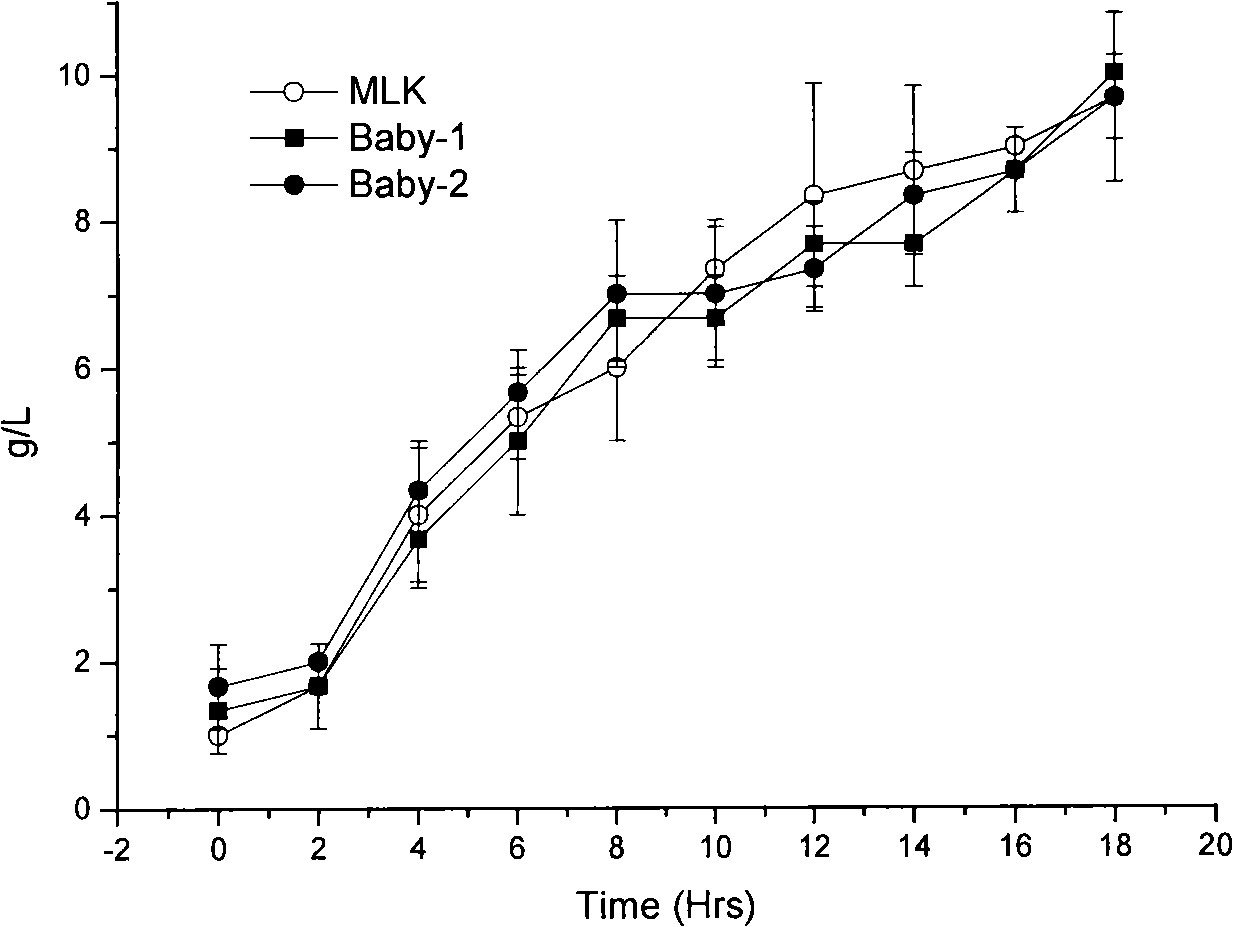
[0045] 1. Comparison of growth, metabolism and antibacterial conditions:
[0046] Activate two generations of tested strains with 1% inoculum size (about 2×10 6 cfu / mL) were inoculated in a 300mL Erlenmeyer flask containing 100mL of MRS medium, cultured at 37°C and 150rpm, samples were taken every 2 hours, and the absorbance at 600nm was measured. Take the culture time as the abscissa and the corresponding absorbance value as th...
Embodiment 3
[0056] Embodiment 3: preparation microcapsule preparation
[0057] 1. Materials:
[0058] The capsule material is sodium alginate solution with a concentration of 1.5g / l (1.5%w / v).
[0059] The curing agent is calcium chloride (CaCl 2 ) aqueous solution with a concentration of 0.1 mol / l.
[0060] Enterococcus faecium, the preservation number is CGMCC No.2516.
[0061] 2. Microencapsulation process of Enterococcus faecium:
[0062] 1. Preparation and sterilization of capsule material solution: Add sodium alginate to distilled water in batches under continuous stirring, stir to make a transparent glue solution, sterilize at 115°C for 20 minutes, and set aside.
[0063] 2. Preparation and sterilization of curing agent: make a 0.1mol / l solution with distilled water, sterilize at 115°C for 20 minutes, and set aside.
[0064] 3. Enterococcus faecium seed liquid culture: using the culture medium and culture conditions described in Example 1, the bacteria in the logarithmic growt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com