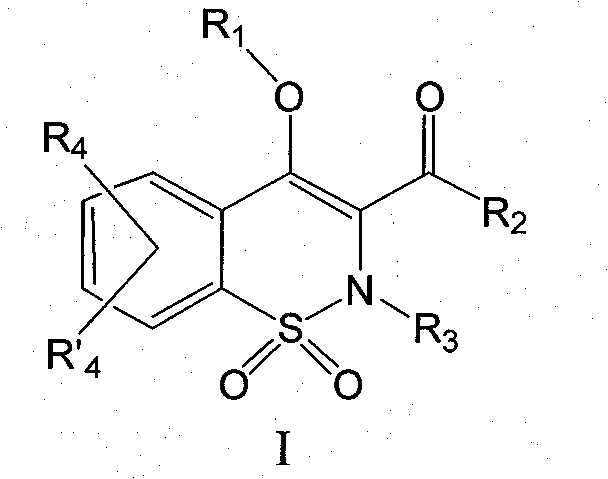
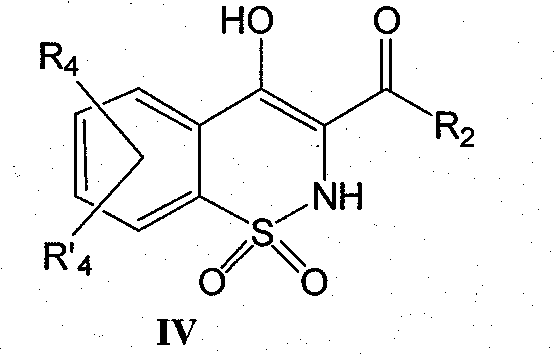
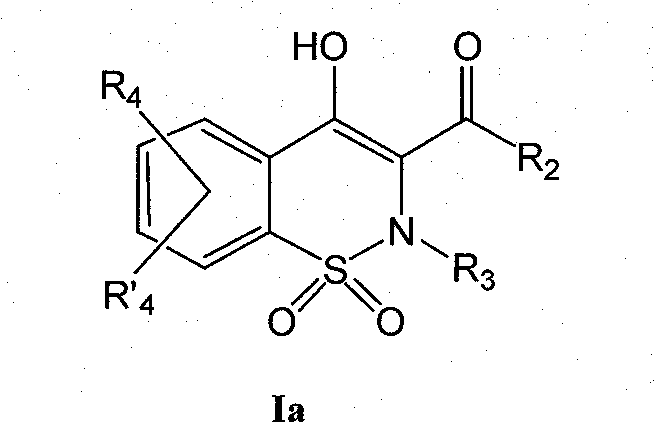
Derivatives of benzothiazines, preparation thereof and application thereof as drugs
A benzo, thiazine technology, applied in the field of benzothiazine derivatives, can solve problems such as failure to reach the blood sugar target value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] (4-Hydroxy-2-methyl-1,1-dioxide-2H-benzo[e][1,2]thiazin-3-yl)(naphthalene-2-yl)methanone
[0111]
Embodiment 1A
[0112] Example 1A -2-(2-(naphthalene-2-yl)-2-oxoethyl)benzo[d]isothiazol-3(2H)-one-1,1-dioxide
[0113] Add saccharin (25 g, 136 mmol) and DMF (350 mL) into a three-necked flask equipped with a thermometer and a condenser. The medium was inerted by successive exchanges of vacuum / nitrogen (3 times). Sodium hydride (6 g, 150 mmol) was added slowly, followed by 2-bromo-1-(naphthalen-2-yl)ethanone (37.4 g, 150 mmol). The reaction medium is heated to 65° C. for 4 hours and subsequently cooled to room temperature. The resulting precipitate was filtered, rinsed with water and dried to constant weight to obtain the product 1A 37 g of milky white solid (HPLC: RT = 4.97 min, 100%). A second crop of product was obtained by adding water to the filtrate. The resulting precipitate was filtered, rinsed with water and then a minimum amount of ethyl to obtain 10 g of product after drying (HPLC: RT = 4.97 min, 93%). The overall yield of the reaction was 96%.
[0114] 1 H NMR, dmso-d 6...
Embodiment 1B
[0116] Example 1B -(4-Hydroxy-1,1-dioxide-2H-benzo[e][1,2]thiazin-3-yl)(naphthalen-2-yl)methanone
[0117] Under an inert atmosphere, ethanol (165 mL) was added to a two-necked flask fitted with a condenser, followed by slow addition of sodium sliced and rinsed with heptane (8 g, 347 mmol). After the addition is complete, the reaction medium is heated to 70° C. until the sodium has completely reacted. The reaction was then cooled to room temperature and compound was added rapidly 1A (47 g, 131 mmol). Intense vermilion and subsequent blood red coloration occurs in addition to the dense precipitate. The reaction medium is heated briefly to its solidification temperature of 60°C. It was then cooled to room temperature and diluted with 500 mL of ethyl acetate. Then 1N aqueous HCl was added until a pale yellow suspension was obtained. The precipitate was filtered and rinsed with water and a minimum of 50 / 50 water / ethanol mixture. Subsequent vacuum drying to constant weigh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com