Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
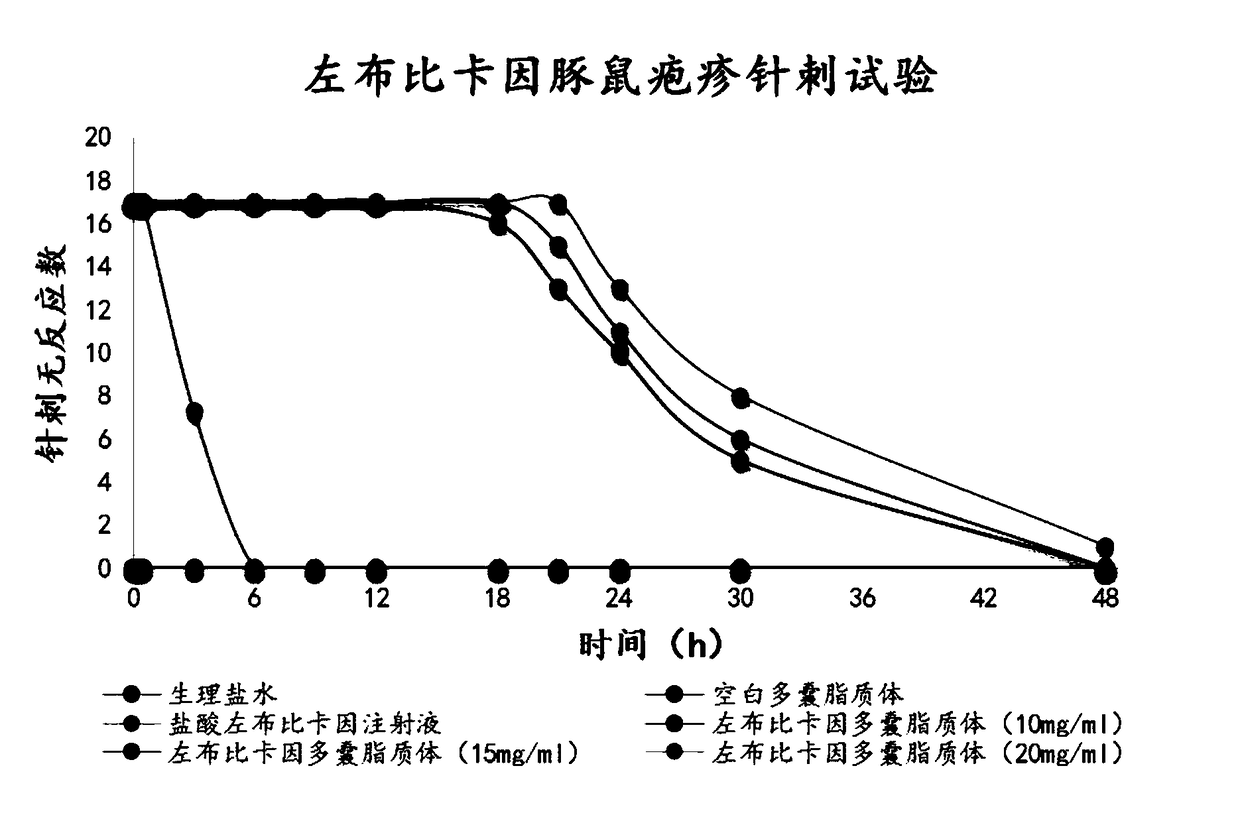
81 results about "Ropivacaine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ropivacaine (rINN) /roʊˈpɪvəkeɪn/ is a local anaesthetic drug belonging to the amino amide group. The name ropivacaine refers to both the racemate and the marketed S-enantiomer. Ropivacaine hydrochloride is commonly marketed by AstraZeneca under the trade name Naropin.
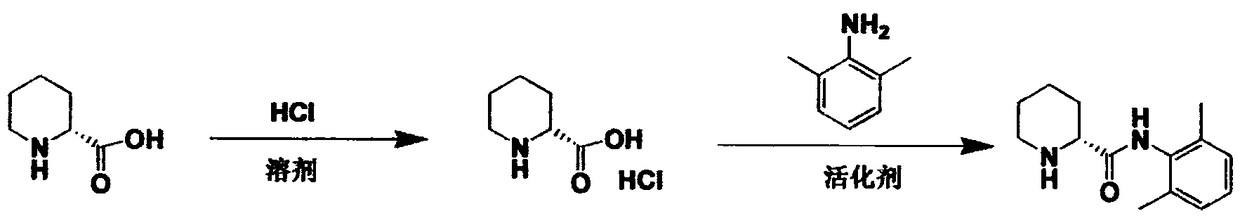
Anesthetic methods and compositions
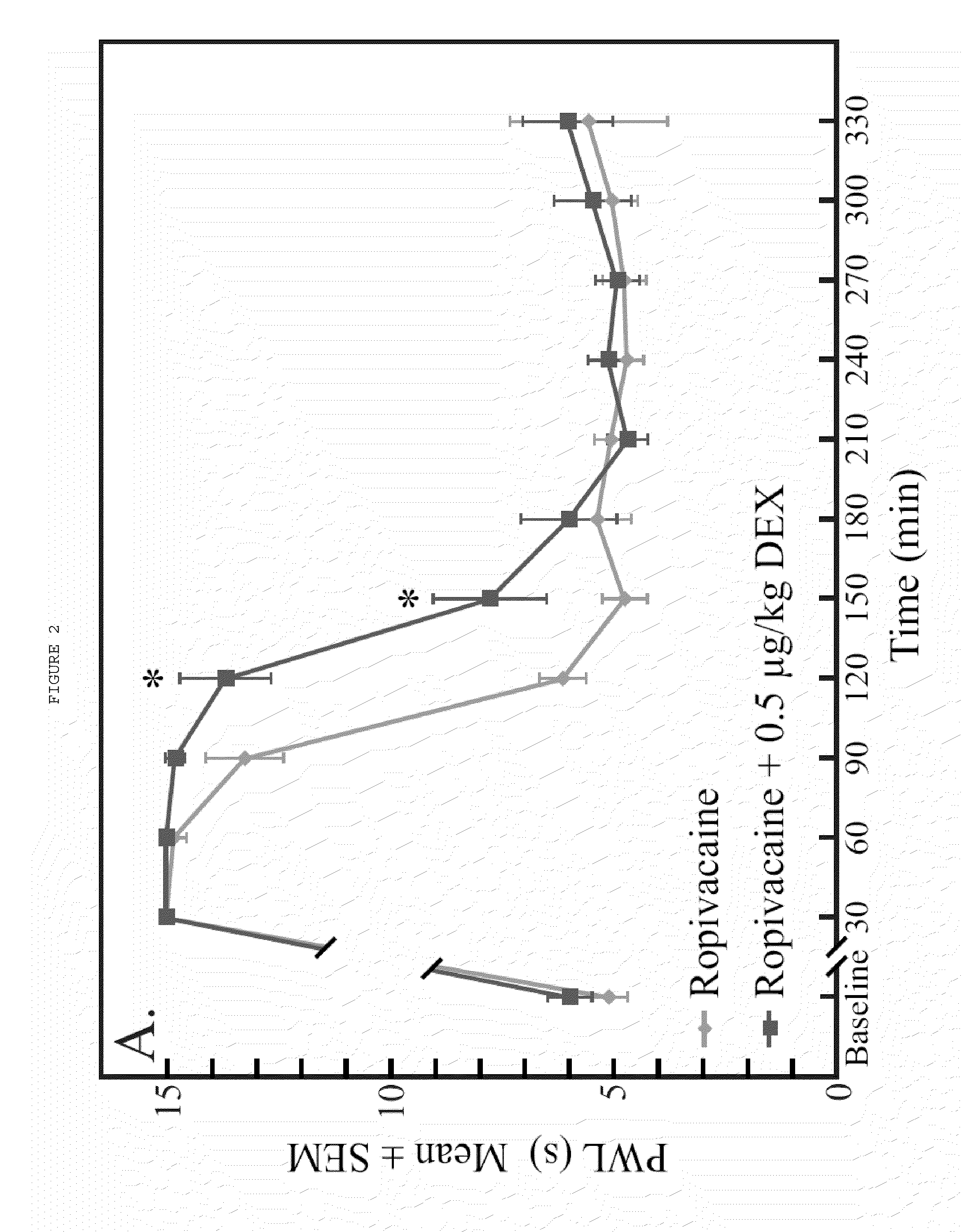
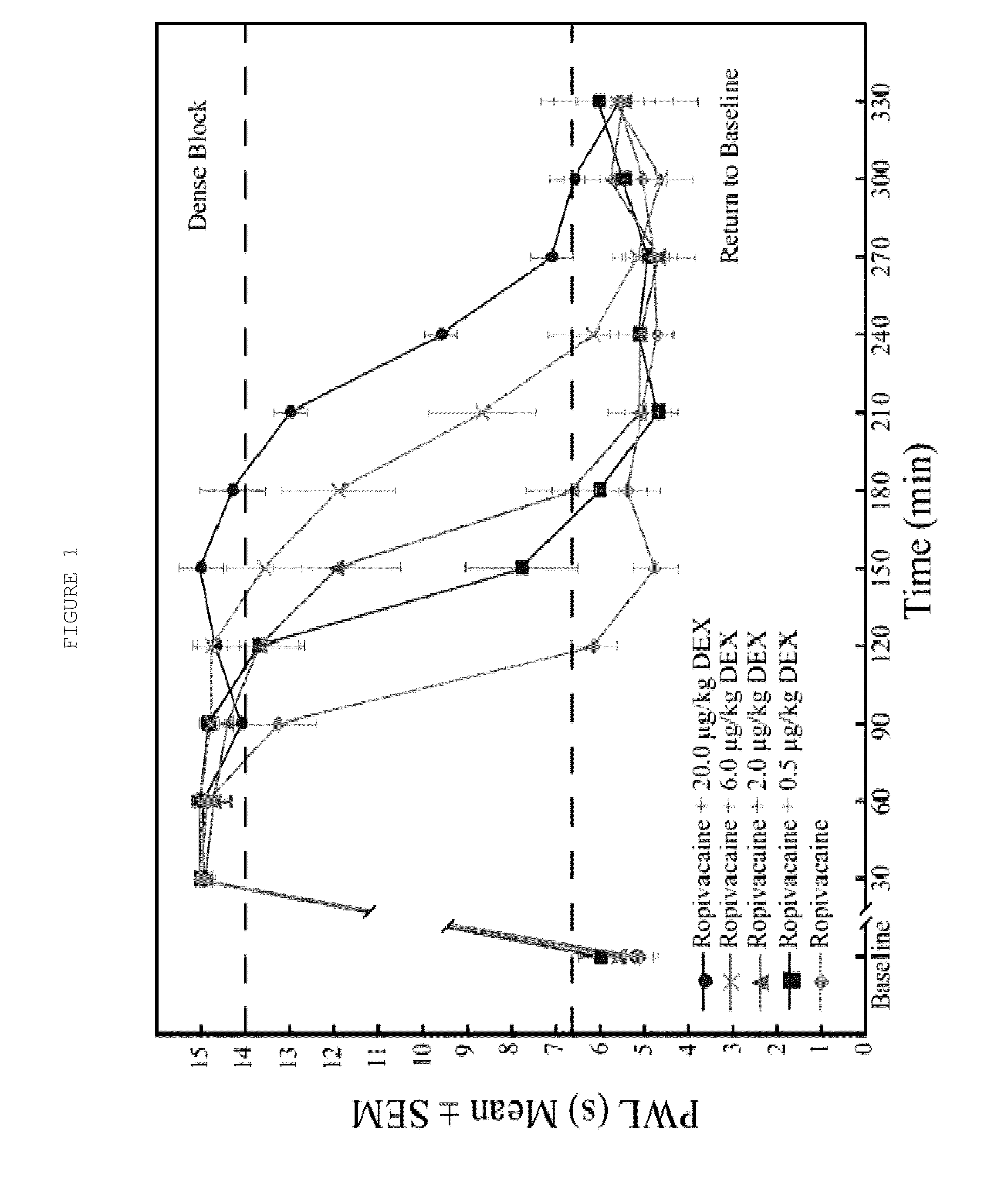
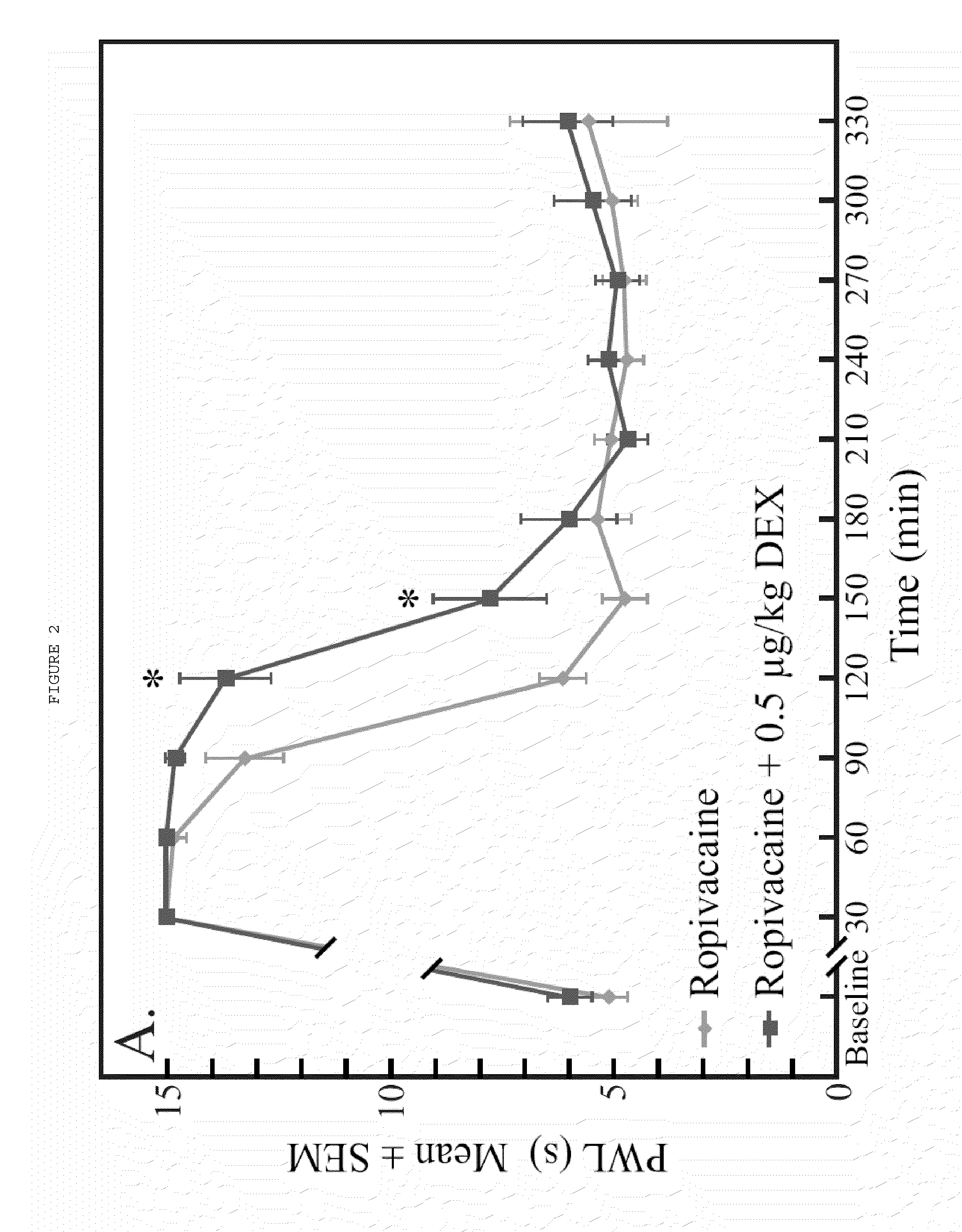
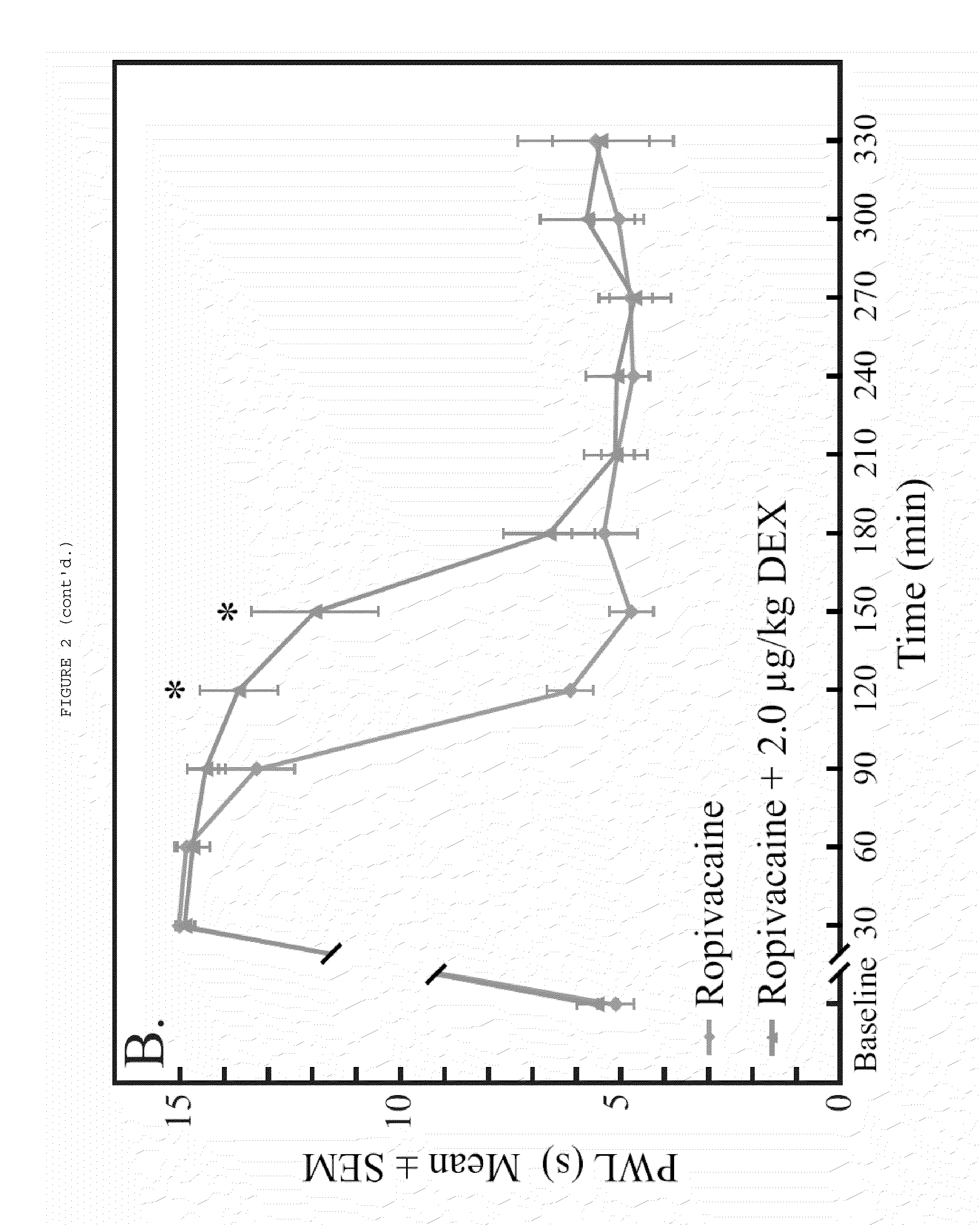
The present invention relates to compositions and methods use in pain reduction, including but not limited to, peripheral nerve blocks. In particular, the present invention relates to compositions and methods for the administration of perineural dexmedetomidine and ropivacaine in combination for increased antinociception in peripheral nerve blocks. In addition, this invention relates to any use of dexmedetomidine alone or in combination with other agents for the purpose of decreasing inflammation around peripheral nerves, thereby decreasing the potential for peripheral nerve injury. Further, the invention relates to the use of dexmedetomidine to reduce inflammation in the muscle to lessen or prevent muscle damage.
Owner:RGT UNIV OF MICHIGAN
Local anesthetic phospholipid-mixed solvent-oil sustained-release drug delivery system and preparation method thereof
ActiveCN108743952AExtended release timeLess irritatingAntipyreticAnalgesicsVitamin E AcetatePhospholipid
The invention relates to a local anesthetic sustained-release preparation with phospholipid-mixed solvent-oil as a carrier, and a preparation method thereof. The sustained-release preparation is prepared from local anesthetic as an active ingredient and a mixture of phospholipid-mixed solvent-oil as a sustained-release drug delivery system, and selectively is prepared from an antioxidant, whereinthe local anesthetic is selected from one of bupivacaine or ropivacaine free base or a mixture thereof; the mixed solvent is a mixture of benzyl benzoate and one or two of benzyl alcohol, ethanol; theantioxidant is vitamin E acetate, lipoic acid and the like; in the phospholipid-mixed solvent-oil mixture, the content of phospholipid does not exceed 50%, the content of benzyl benzoate does not exceed 10%, and the rest is oil.
Owner:XIAN LIBANG PHARMA TECH
Dermal compositions of substituted amides and the use thereof as medication for pain and pruritus
Dermal compositions comprising topical formulations of bupivacaine or ropivacaine, characterized by effective dermal absorption and long duration of dermal anesthetic activity, and intended for use in patients suffering from pruritus and dermal pain, including neuropathic pain, are provided. Compositions containing both bupivacaine and capsaicin are provided. Methods of alleviating pain by the topically administration of these compounds are also provided.
Owner:BRIDGE PHARMA INC
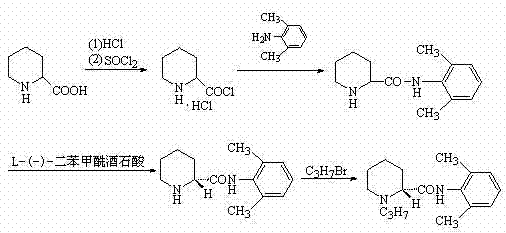
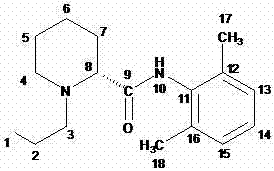
Method for preparing ropivacaine
InactiveCN103086954AAvoid separationSimple processOrganic chemistryDimethylaniline N-oxideCarboxylic acid
The invention relates to a one-pot method for preparing ropivacaine. According to the invention, L-piperidine-2-carbonyl chloride is prepared from L-piperidine-2-carboxylic acid; intermediate separation is not needed, and the material is directly subjected to a reaction with 2,6-dimethylaniline, and (S)-N-(2,6-dimethylphenyl)piperidine-2-carboxamide is prepared; intermediate separation is not needed, and the material is directly subjected to a reaction with bromopropane, such that ropivacaine is prepared. According to the invention, when the intermediate is prepared, no separation is needed, and reactions can be directly carried out. The method is green and environment-friendly. With the method, process operation is simplified, cost is reduced, and yield is improved. The method is more suitable for large-scale industrialized productions.
Owner:SHANDONG INST OF PHARMA IND
Ropivacaine nanometer lipid carrier temperature-sensitive in-situ gel and preparation method thereof
InactiveCN103816111AImprove solubilityReduce releaseAerosol deliveryOintment deliveryLipid formationBiocompatibility Testing
The invention belongs to the technical field of medicinal preparations and specifically to a ropivacaine nanometer lipid carrier temperature-sensitive in-situ gel and a preparation method thereof. The ropivacaine nanometer lipid carrier temperature-sensitive in-situ gel disclosed by the invention mainly comprises bulk drug ropivacaine, a solid lipid material, a liquid lipid material, a surfactant, a cosurfactant, a gel matrix and injection water. The preparation method is a high-temperature emulsification low-temperature setting and cold melt method. The ropivacaine nanometer lipid carrier temperature-sensitive in-situ gel prepared in the invention is applied through transdermal drug delivery and has the advantages of a slow release function, a high transdermal permeation rate, a high entrapment rate, high drug loading capacity, good biocompatibility and good stability.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Long acting drug delivery system for treating breast cancer and preparation method and applications thereof
InactiveCN108159055AEnsure safetyIncrease concentrationOrganic active ingredientsPharmaceutical delivery mechanismProcainePhospholipid
The invention provides a long acting drug delivery system, which comprises fulvestrant or derivatives thereof and takes phospholipid as a sustained release material. The invention mainly discloses a high concentration formula of fulvestrant or derivatives thereof. The main component is fulvestrant or derivatives thereof. The sustained release material is different phospholipids or a mixture of phospholipids and different kinds of plant oil. The solvent is at least one of ethanol, ethyl lactate, 1,2-propylene glycol, and ethyl acetate. The analgesic is benzyl alcohol, lidocaine, procaine, or ropivacaine. The viscosity of the formula is 20-45 mPa.s, and the concentration of the formula is 60-300 mg / mL. The antioxidant of the formula is Ve, lipoic acid, and the like. The phospholipids can increase mutual solubility and has a sustained release function. The preparation is prepared in an aseptic filtration mode.
Owner:XIAN LIBANG PHARMA TECH
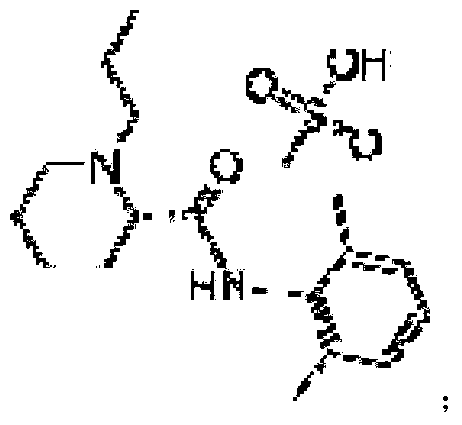
Preparation method of ropivacaine methanesulfonate and its compound and preparation
InactiveCN1517337ADoes not affect optical rotation measurementsImprove solubilityNervous disorderOrganic chemistryKetone solventsFormamide
A ropivacaine methanesulfonate as anesthetic or analgetic has a chemical formula: (-)-(S)-N-(2,6-dimethylphenyl)-1-propyl piperidine-2-formamide methanesulfonate, and is prepared through preparing raw materials, dissolving, adding, methane sulfonic acid, adding ketone solvent, filtering, baking and recrystallizing. Its advantages are high stability and output rate and low cost.
Owner:ZHEJIANG XIANJU PHARMA
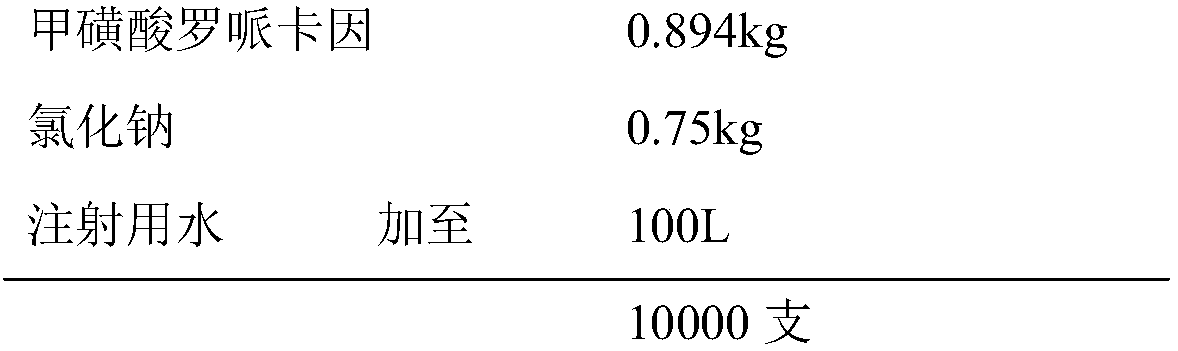
Ropivacaine mesylate freeze-dried powder injection
ActiveCN102038651AAvoid the risk of safety accidentsGood freeze-dried appearancePowder deliveryPharmaceutical product form changeFreeze-dryingPhysical chemistry
The invention relates to a ropivacaine mesylate freeze-dried powder injection which consists of ropivacaine mesylate and a PH regulator and is prepared by using the following freeze drying method: (1) a section quick-freezing stage: keeping bulked ropivacaine mesylate solution at the temperature of 0 DEG C for 10-30 minutes, and then keeping the bulked ropivacaine mesylate solution at the temperature of minus 35 DEG C-minus 45 DEG C for 1-2 hours; (2) a lyophilization stage: heating to 0 DEG C under the vacuum degree of 10-20 Pa and at the speed of 2-10 DEG C / h, and then keeping the temperature for 1-3 hours; and (3) a desorption drying stage: heating to 30 DEG C under the vacuum degree of 0-10 Pa and at the speed of 5-10 DEG C / h and keeping the temperature for 2-5 hours. The freeze-dried powder injection provided by the invention has the advantages of high yield, good re-dissolubility, more stable quality and the like.
Owner:鲁南新时代生物技术有限公司
Suspension preparation of temperature change painless nano sulfadiazine metallic compound hyaluronic acid
ActiveCN104013574AStrong penetrating powerImprove bioavailabilityOrganic active ingredientsSolution deliveryCelluloseChlorhexidine
The invention discloses a suspension preparation of temperature change painless nano sulfadiazine metallic compound hyaluronic acid. The formula comprises a sulfadiazine metallic compound, a hyaluronic acid substance, an analgetic, a dispersing agent aid, a suspension aid and water, wherein the sulfadiazine metallic compound comprises sulfadiazine silver and sulfadiazine zinc; the hyaluronic acid substance is sourced from biological fermentation and animal tissue extraction and the like and can be hyaluronic acid or hyaluronate or crosslinked hyaluronic acid or crosslinked hyaluronate; the analgetic comprises ropivacaine, bupivacaine, levobupivacaine, lidocaine and other local anesthetics or combination of the local anesthetics; the dispersing agent aid comprises chlorhexidine, Tween series, oleic acid or sodium oleate; the suspension aid can comprise glycerinum, celluloses, poloxamer series and hyaluronic acid substances. The suspension preparation is mainly used for burn, scald or the surface of a wound caused by explosion or anabrosis, has the main clinical characteristics that pain is rapidly relieved, the surface of the wound is subjected to rapid film formation and rapid healing of the wound is promoted, and the conventional main administration modes refer to spraying, smearing and coating.
Owner:LIPONT PHARMA
Local anesthesia pain relieving and slow-release medicine delivery system as well as preparation method and application thereof
InactiveCN108354903AHigh encapsulation efficiencyHigh drug loadingAnaestheticsPharmaceutical non-active ingredientsMultivesicular liposomesOil phase
The invention discloses a novel local anesthesia pain relieving and slow-release medicine delivery system, which comprises an inner water phase, an outer water phase, an oil phase, an organic solvent,an isotonic regulator and a pH regulator, wherein the inner phase comprises a pain relieving agent, a medicine solvent and a medicine solubilizing agent; the pain relieving agent is selected from onekind of materials from bupivacaine, levobupivacaine, ropivacaine, lidocaine, bupivacaine or mepivacaine; the pain relieving agent is in a free alkali form or acid salt form; the medicine solvent is selected from N or P containing inorganic acid; the medicine solubilizing agent is selected from one or several kinds of materials from sugar or annular organic acid. The prepared muhivescular liposomes have high encapsulation rate, high medicine carrying capacity, uniform particle diameter and good slow release effect.
Owner:XIAN LIBANG BIOMEDICAL TECH CO LTD
Ropivacaine mesylate compound, preparation process thereof and pharmaceutical composition thereof
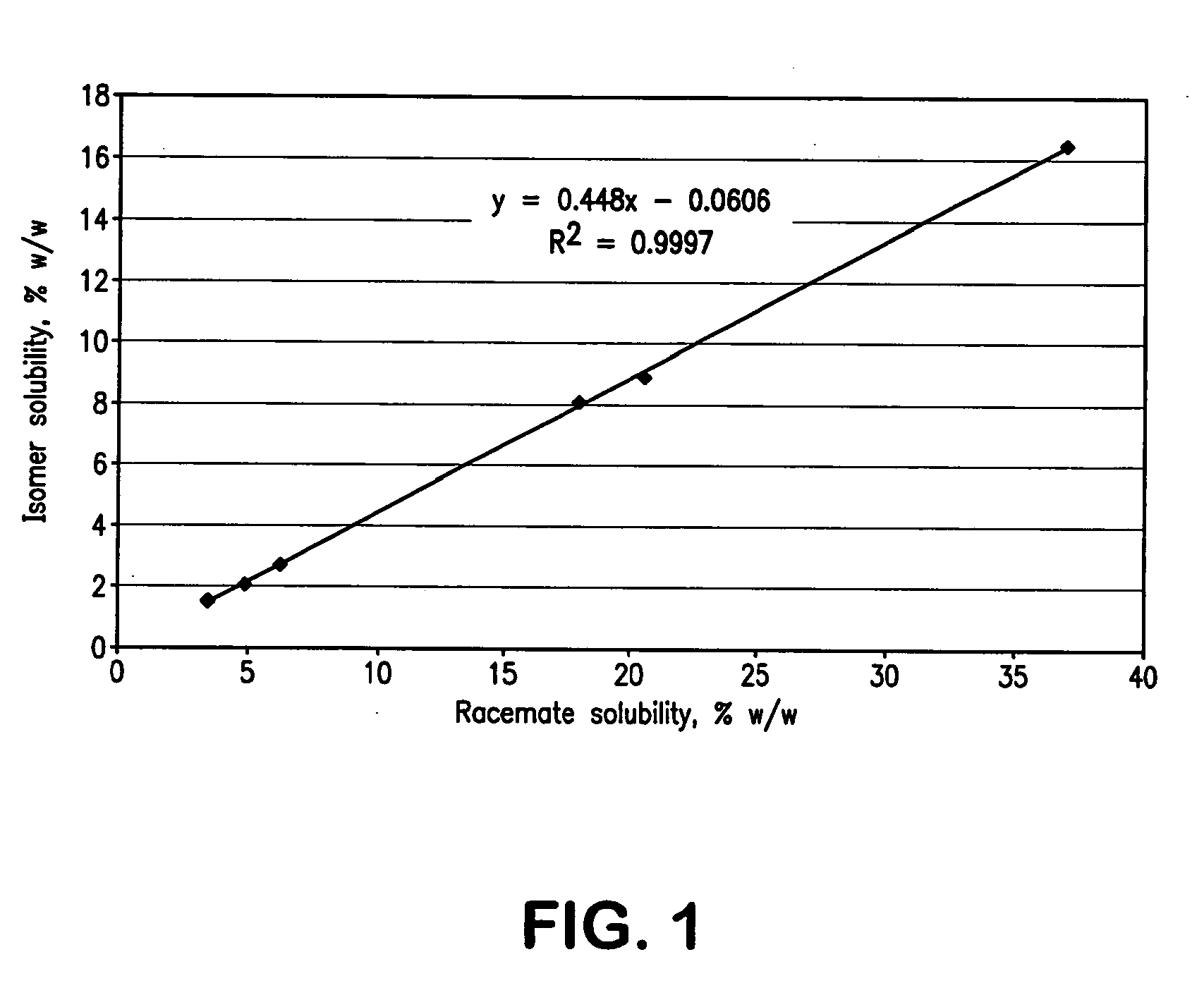
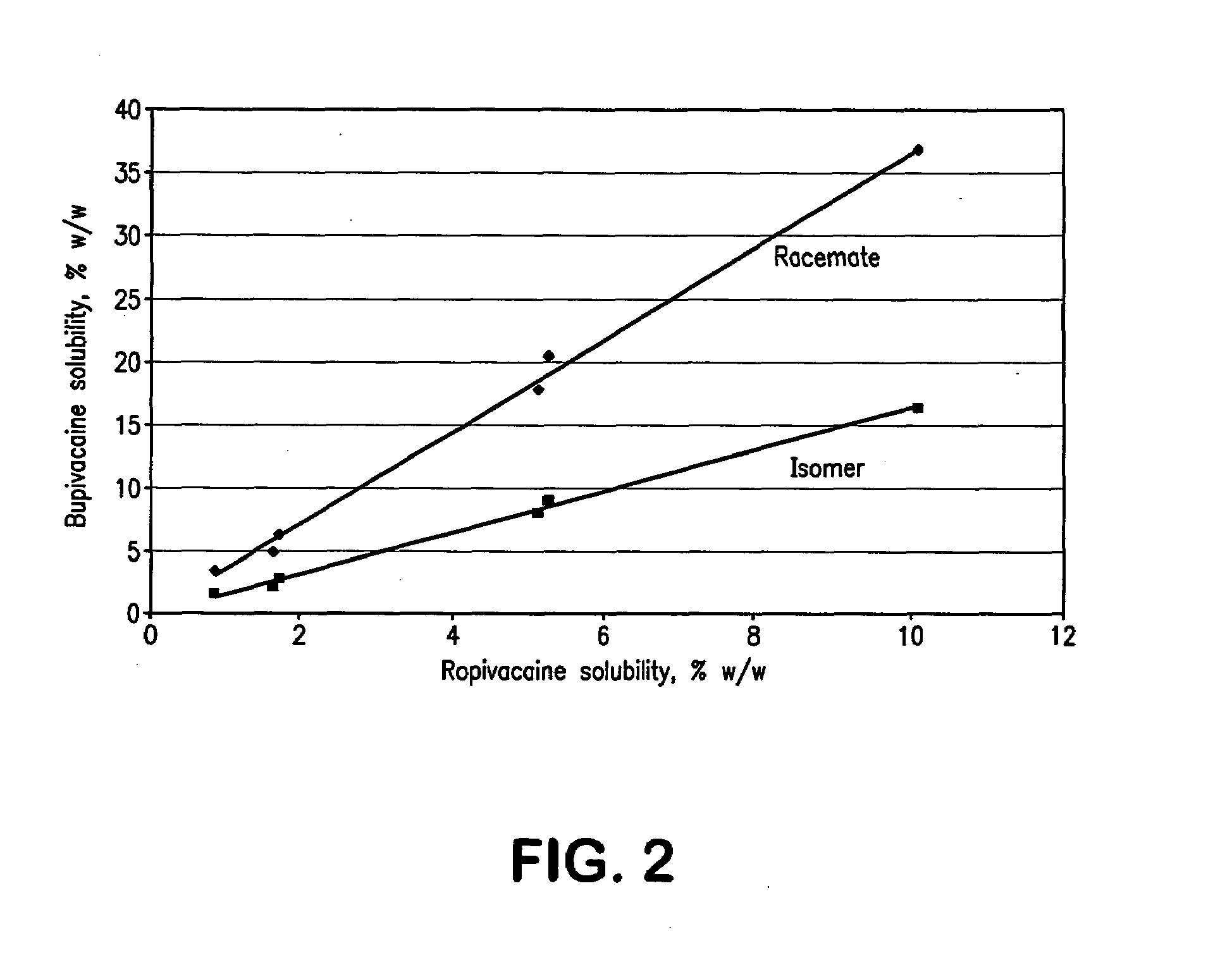
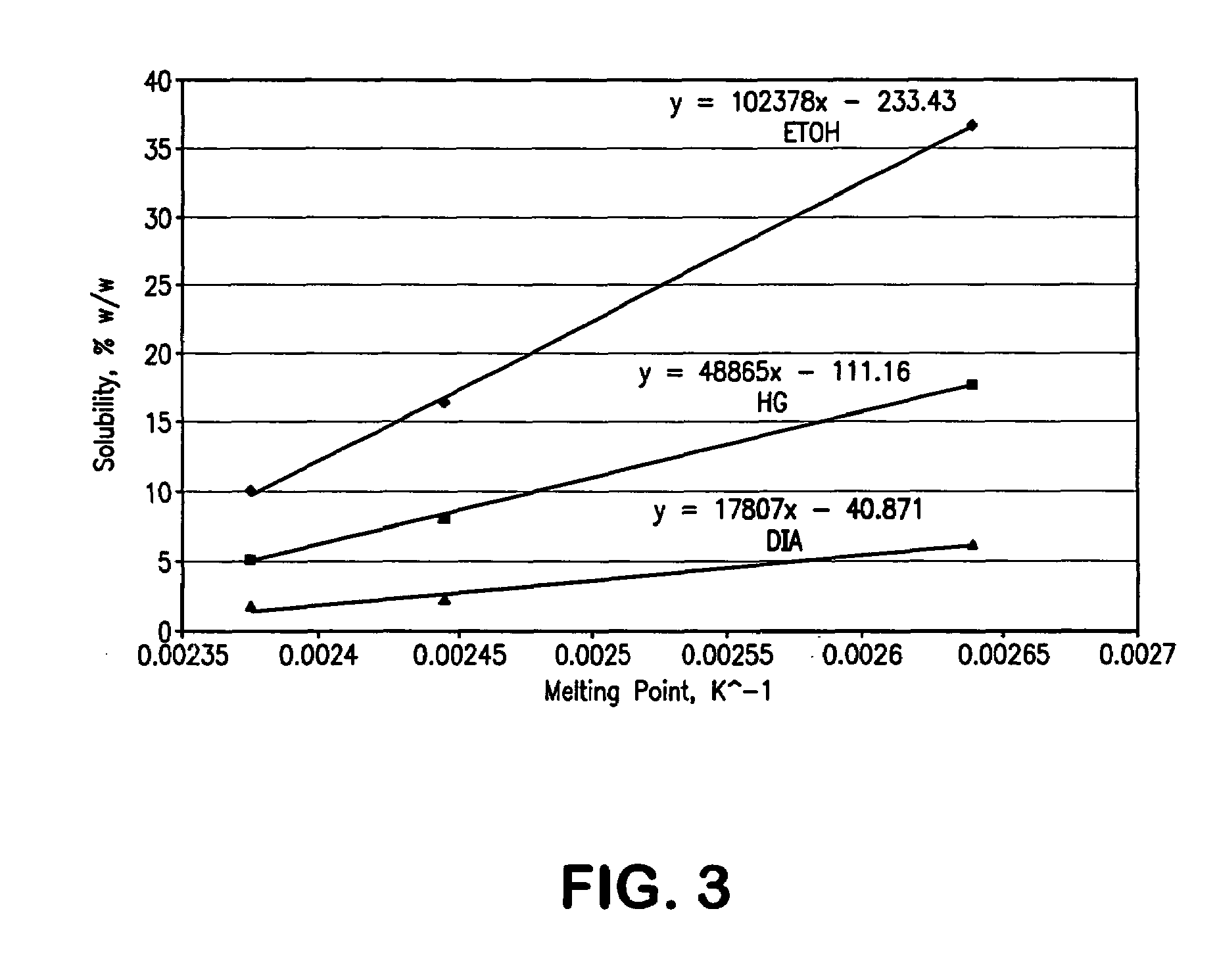
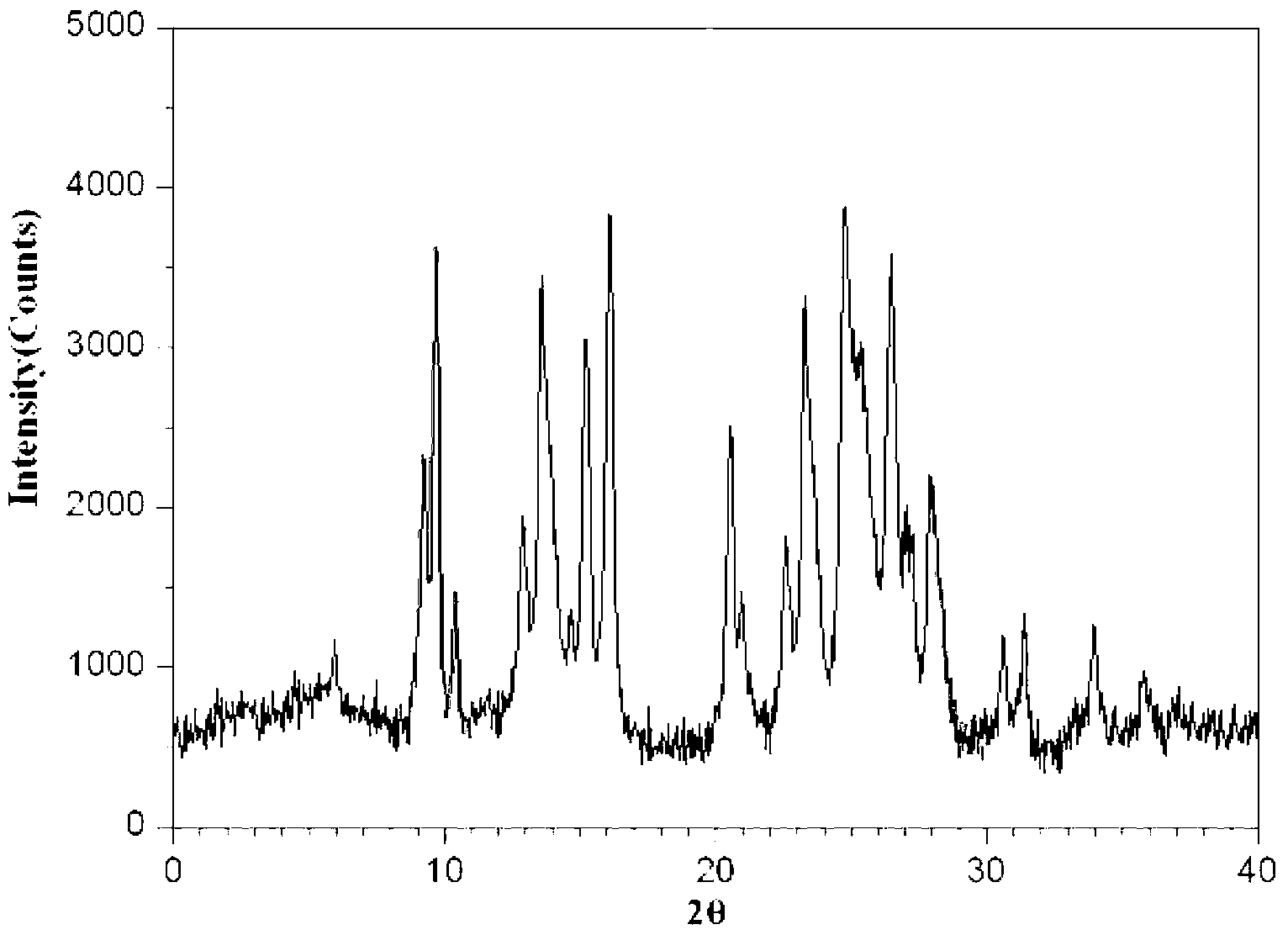
InactiveCN103304471AImprove solubilityFast dissolution rateSulfonic acids salts preparationHeterocyclic compound active ingredientsSolubilityX-ray
The invention belongs to the technical field of medicines and particularly relates to a ropivacaine mesylate compound. The structural formula of the ropivacaine mesylate compound is shown in the description, and an X-ray powder diffraction spectrogram obtained by measuring the ropivacaine mesylate compound with Cu-K alpha rays is shown in figure 1. The invention also provides a preparation process of the ropivacaine mesylate compound, a pharmaceutical composition containing the ropivacaine mesylate compound and a preparation process of the pharmaceutical composition. Dosage forms of the ropivacaine mesylate pharmaceutical composition are powder injections, small volume injections and large volume injections. The ropivacaine mesylate compound is high in solubility and high in dissolution rate, and the pharmaceutical composition of the ropivacaine mesylate compound is good in dissolubility, easy to dissolve and composite and high in bioavailability.
Owner:四川省惠达药业有限公司
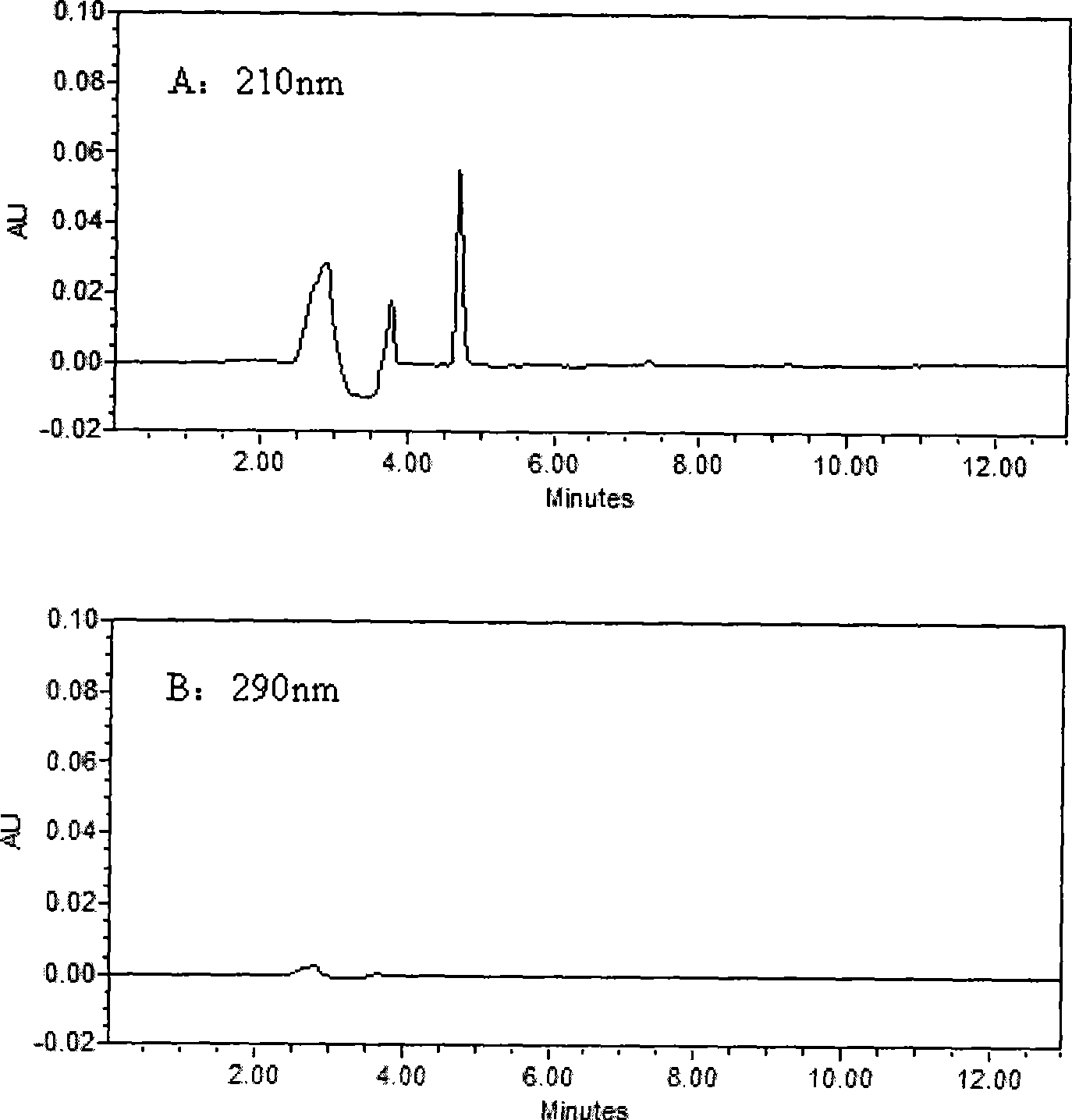
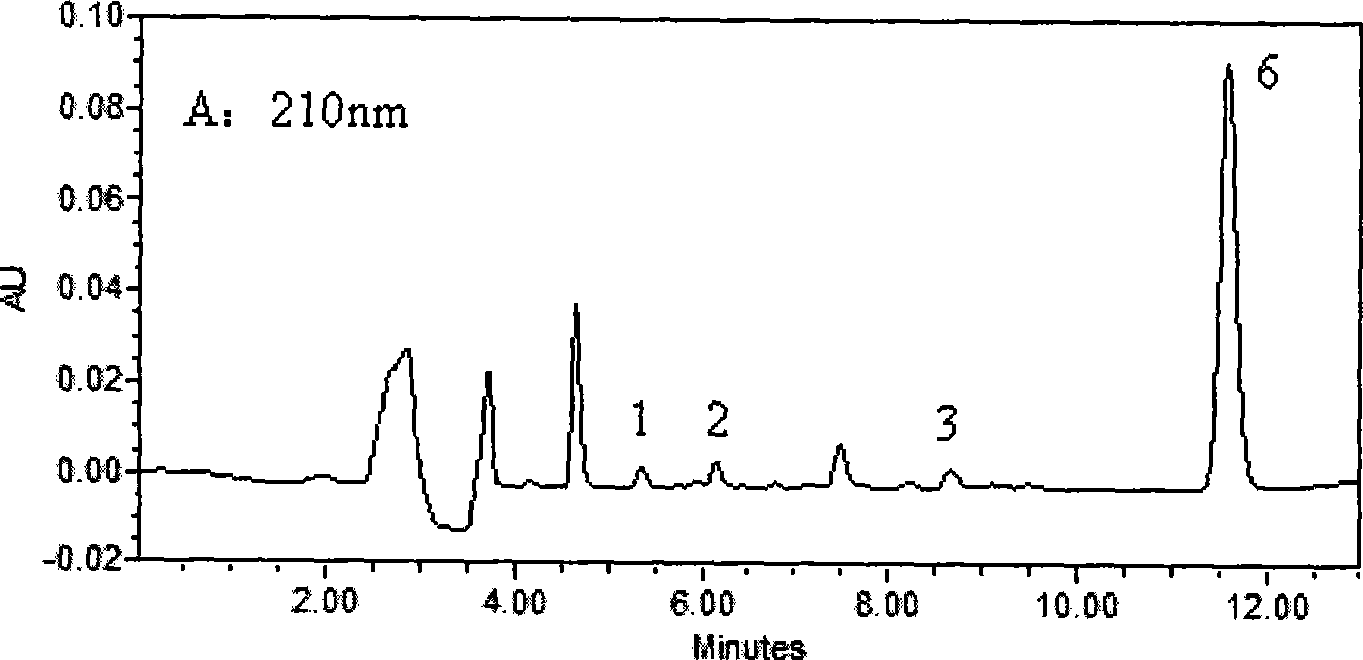
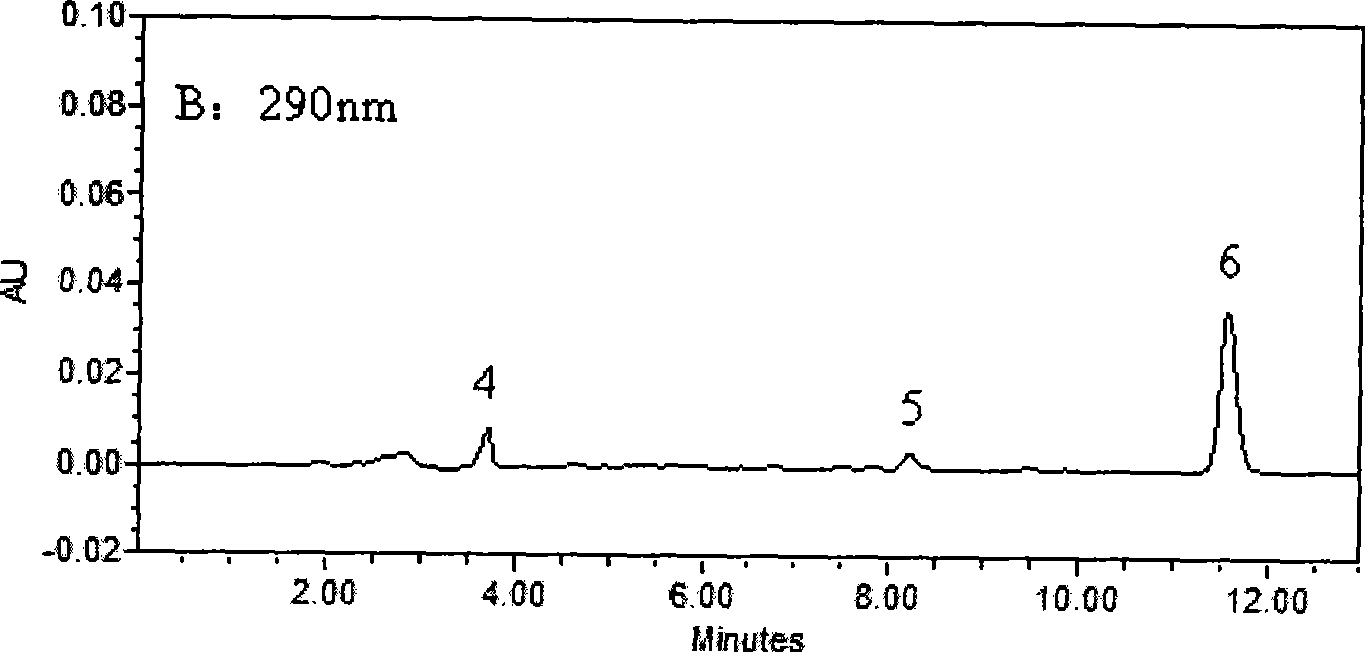
Method for simultaneously determining of concentration multi anesthesia medicament in blood plasma
InactiveCN101393196ASimple and fast operationEasy to operateComponent separationMaterial analysis by optical meansProcaineUltraviolet absorption
The invention belongs to the field of medical examination and relates to a method capable of synchronously determining the concentrations of a plurality of local anesthetic drugs in human plasma. The method adopts a plasma cholineesterase inhibitor to inhibit the activity of plasma cholinesterase under ice bath condition below 3 DEG C, which controls the hydrolysis of totokaine and assures the accuracy of the method; by utilizing characteristics that lidocaine, ropivacaine and bupivacaine have stronger characteristic of ultraviolet absorption at wavelength of 210nm, and procaine and the totokaine have stronger characteristic of ultraviolet absorption at wavelength of 290nm, an ultraviolet dual-wavelength method is used to detect after the separation of an acid mobile phase at a chromatographic column; and the method can ensure that the sensitivity of synchronous determination of the local anesthetic drugs is greatly improved. The method has less sampling from samples and simple, quickand sensitive pretreatment, does not need expensive equipment or reagents, has short analysis period and low cost, and is suitable for the monitoring of clinical conventional blood concentration of aplurality of drugs.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Microneedle devices and methods
A medical device, comprising: an array of microneedles, and a coating disposed on the microneedles, wherein the coating comprises: a local anesthetic selected from the group consisting of lidocaine, prilocaine, and a combination thereof; and a local anesthetic dose-extending component selected from the group consisting of tetracaine, ropivacaine, bupivacaine, procaine and a combination thereof; wherein the local anesthetic is present in an amount of at least 1 wt-% based upon total weight of solids in the coating, and wherein the local anesthetic and dose-extending component are in a non-eutectic weight ratio; a medical device, comprising an array of dissolvable microneedles, the microneedles comprising: a dissolvable matrix material; at least 1 wt-% of a local anesthetic selected from the group consisting of lidocaine, prilocaine, and a combination thereof; and a local anesthetic dose-extending component selected from the group consisting of tetracaine, ropivacaine, bupivacaine, procaine and a combination thereof; wherein the local anesthetic and dose-extending component are in a non-eutectic weight ratio, and wherein wt-% is based upon total weight of solids in all portions of the dissolvable microneedles which contain the local anesthetic; a method of extending a topically delivered local anesthetic dose in mammalian tissue using the devices; and methods of making the devices are provided.
Owner:3M INNOVATIVE PROPERTIES CO
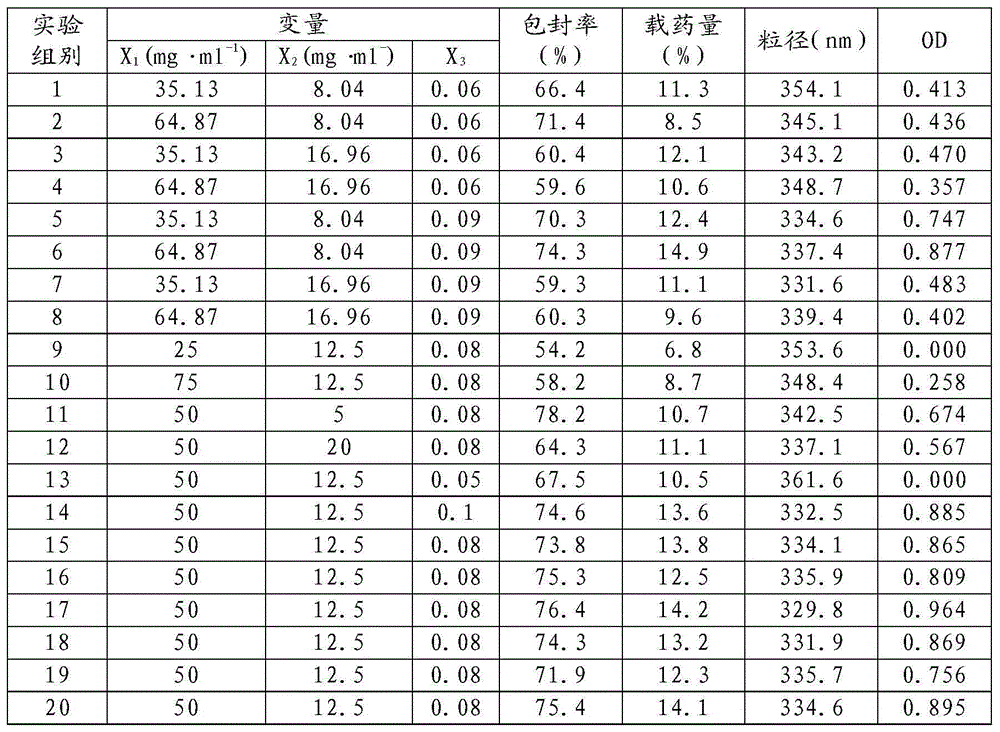
Ropivacaine nano particle, preparation method thereof and optimizing experimental method of effect of the ropivacaine nano particle
ActiveCN104146972AAvoid damageHigh encapsulation efficiencyPowder deliveryAnaestheticsFreeze-dryingPolyvinyl alcohol
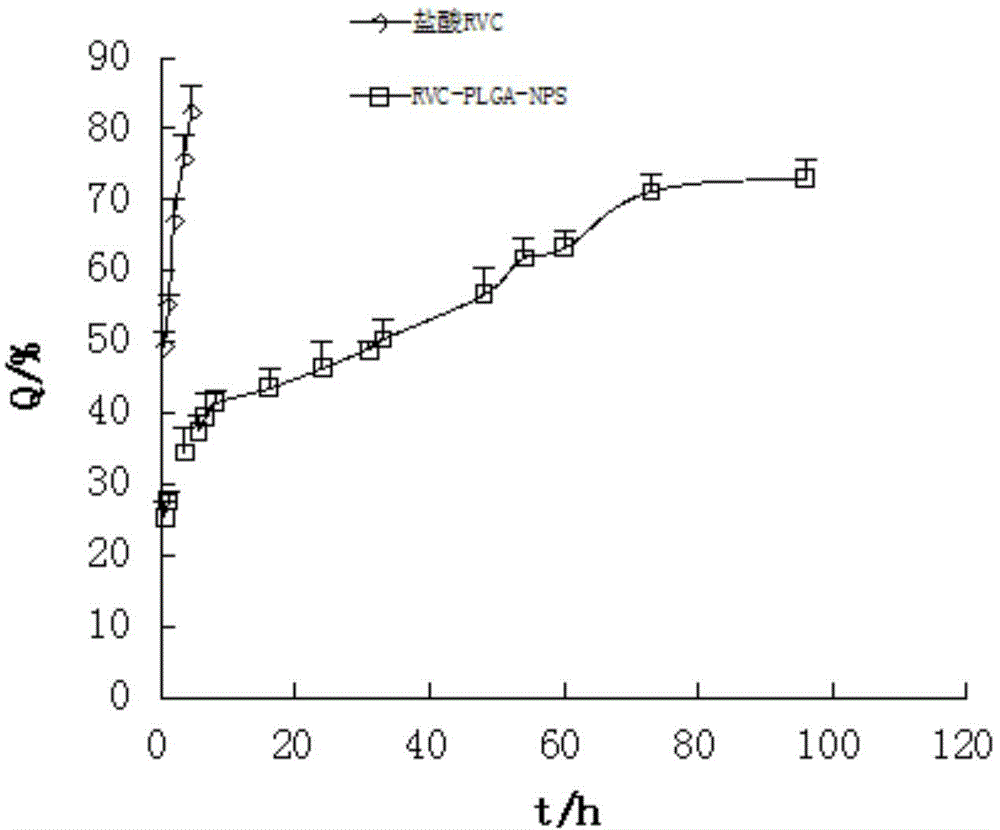
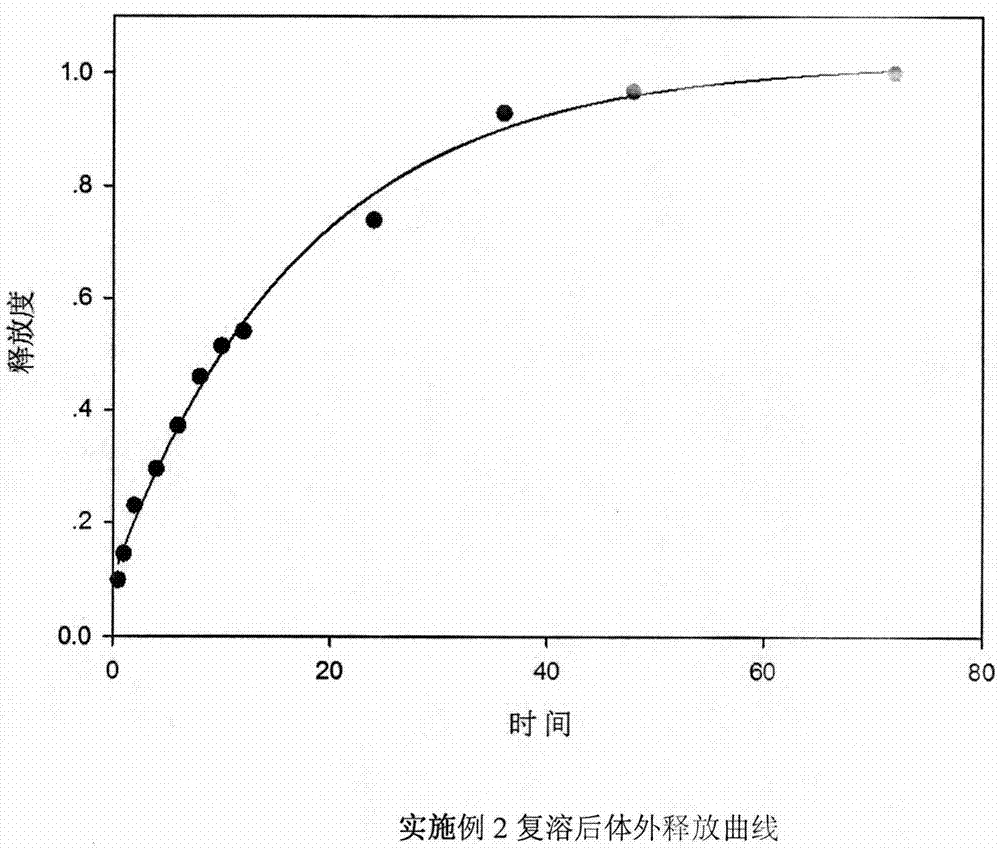
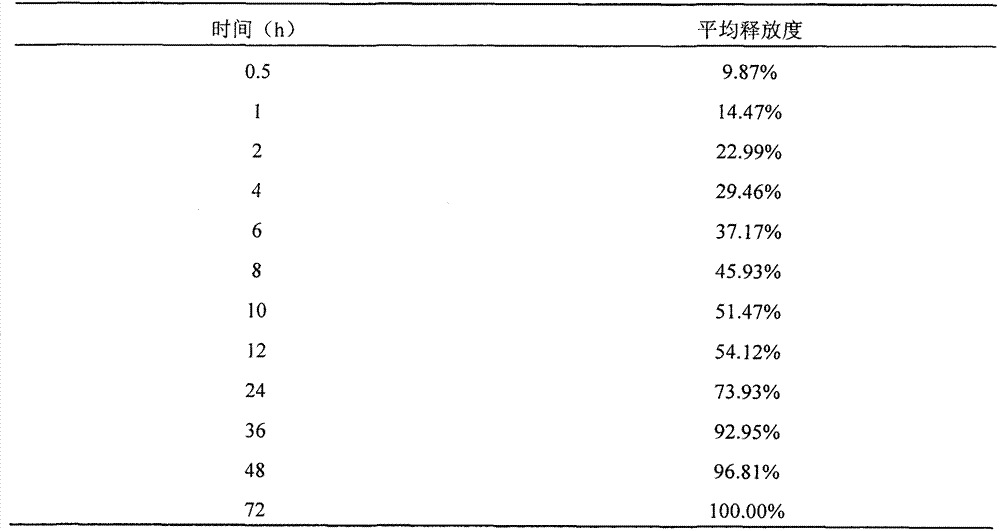
The invention relates to the field of preparation of medicines and particularly relates to ropivacaine nano particles, a preparation method thereof and an optimizing experimental method of effects of the ropivacaine nano particles. The preparation method comprises following steps: (A) dissolving ropivacaine free alkali and polylactic acid-polyglycollic acid segmented copolymer in dichloromethane to form an organic phase while a polyvinyl alcohol solution is employed as an aqueous phase; (B) performing evaporation to a white emulsion at 30-40 DEG C to remove the organic phase to obtain a pale blue opalescence suspension liquid; and (C) performing centrifugal separation to the pale blue opalescence suspension liquid to obtain a precipitate, washing the precipitate, and performing ultrasonic dispersion and a vacuum freeze-drying process to obtain the ropivacaine nano particles. By means of the prepration method of the ropivacaine nano particles, an in-vitro releasing research proves that the ropivacaine nano particles has a releasing rate being about 73% in 96 h, a slow-releasing effect is quite good and a pain-relieving requirement on acute pains, such as post-operation pain and the like, can be satisfied just through one-time dosing.
Owner:FUZHOU GENERAL HOSPITAL OF NANJING MILITARY COMMAND P L A
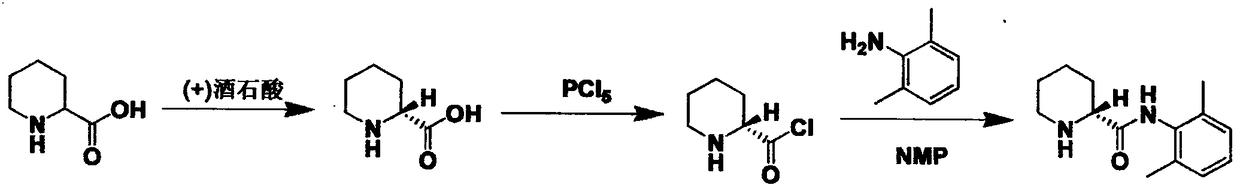
Method for enriching piperidine-2-formanilide optically active compound
The invention relates to a method for enriching a piperidine-2-formanilide optically active compound. The method comprises the following steps of: mixing R type ropivacaine and bupivacaine or an intermediate thereof with an inert solvent, an initiating agent and mercaptan and carrying out a racemization reaction to obtain a reaction solution containing RS configuration ropivacaine, bupivacaine orthe intermediate thereof; adding acid to the reaction solution to carrying out salifying reaction, bleaching and extracting through an organic solvent, drying and dewatering an organic phase, and carrying out spinning evaporating to obtain racemized ropivacaine, bupivacaine or the intermediate solid thereof; adding the obtained solid salt to acetone to separate out DBTA (Dibenzoyl Tartaric Acid) salt which is an S type enantiomer, wherein at the moment, a large quantity of white precipitates are generated in the solution, and bleaching to obtain white solid; and dissolving the solid into alkali, and then bleaching to obtain an enriched piperidine-2-formanilide optically active compound. The method provided by the invention shortens the time and reduces the temperature of the racemization reaction, reduces pollution to environment, caused by wastes and improves the production efficiency.
Owner:YICHANG HUMANWELL PHARMA
Composite local anesthetic and preparation method of composite local anesthetic injection
InactiveCN104146954AComposition is stableEasy to usePharmaceutical delivery mechanismAnaestheticsAnalgesia postoperativeLocal anaesthetic
The invention provides a composite local anesthetic which comprises lidocaine and ropivacaine, wherein the molar concentration ratio of lidocaine to ropivacaine is (1:1) to (1:3). The composite local anesthetic is clinically suitable for various local anesthesia and nerve blocking anesthesia. The invention further provides a preparation method of a composite local anesthetic injection. The composite local anesthetic has the advantages of stable component, convenience in use, quick action, long action time, good postoperative analgesia effect and small toxic and side effects.
Owner:广东埃纳生医疗投资发展有限公司
Compound isopropyl phenol injection contg. local anesthetic and prepn. method therefor
InactiveCN1903187ADefinite curative effectMature technologyHydroxy compound active ingredientsPharmaceutical delivery mechanismRopivacainePhenols
A compound isopropylphenol injection containing local anesthetic contains proportionally isopropylphenol, the local anesthetic chosen from procaine, lidocaine, etc, the refined plant oil for injection, emulsifier, isotonic regulator, antioxidant, pH regulator and the water for injection. Its preparing process is also disclosed.
Owner:SHENYANG PHARMA UNIVERSITY
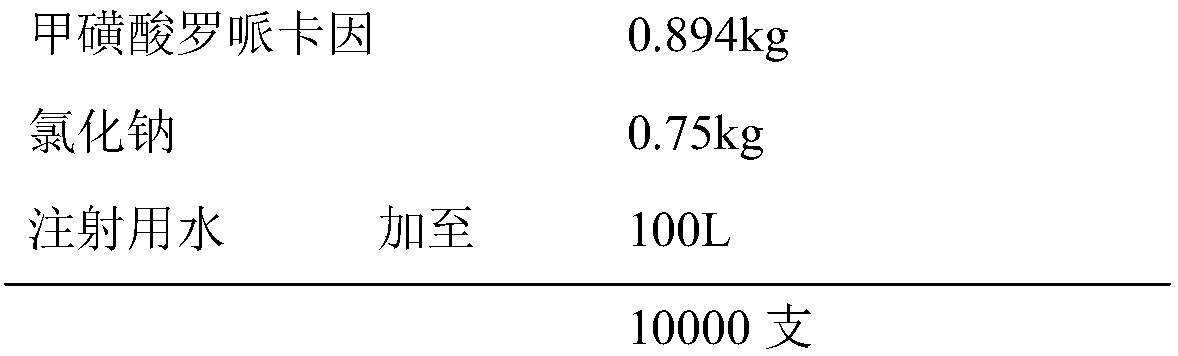
Ropivacaine mesylate freeze-dried powder injection
ActiveCN102038651BAvoid the risk of safety accidentsGood freeze-dried appearancePowder deliveryPharmaceutical product form changeDesorptionFreeze-drying
The invention relates to a ropivacaine mesylate freeze-dried powder injection which consists of ropivacaine mesylate and a pH regulator and is prepared by using the following freeze drying method: (1) a section quick-freezing stage: keeping bulked ropivacaine mesylate solution at the temperature of 0 DEG C for 10-30 minutes, and then keeping the bulked ropivacaine mesylate solution at the temperature of minus 35 DEG C-minus 45 DEG C for 1-2 hours; (2) a lyophilization stage: heating to 0 DEG C under the vacuum degree of 10-20 Pa and at the speed of 2-10 DEG C / h, and then keeping the temperature for 1-3 hours; and (3) a desorption drying stage: heating to 30 DEG C under the vacuum degree of 0-10 Pa and at the speed of 5-10 DEG C / h and keeping the temperature for 2-5 hours. The freeze-dried powder injection provided by the invention has the advantages of high yield, good re-dissolubility, more stable quality and the like.
Owner:鲁南新时代生物技术有限公司
Nerve block medicinal composition
InactiveCN103877099AAvoid damageReduce toxicityAnaestheticsHeterocyclic compound active ingredientsGlucocorticoidAllergy
The invention relates to a nerve block medicinal composition. The nerve block medicinal composition consists of the following components in parts by volume: 10 parts of 0.75 percent ropivacaine, 2 parts of glucocorticoid and 13 parts of 0.9 percent normal saline. The nerve block medicinal composition has the advantages that dexamethasone is added into a formula, so that injuries to nerve roots and toxic reactions of local anesthetics in a puncturing process can be reduced; dexamethasone has the effects of resisting allergy, resisting angioedema and reducing the toxic reactions of local anesthetics, so that the nerve block success rate is increased, and toxic reactions are reduced.
Owner:王寿世
Pharmaceutical composition, patch, and preparation methods and application of pharmaceutical composition and patch
PendingCN110038130AGuaranteed thicknessGet Continuous Pain ReliefNervous disorderAntipyreticProcaineBULK ACTIVE INGREDIENT
Owner:张洁
Transdermal and transmucosal local narcotic analgesic and preparation method of gel formulation thereof
InactiveCN101357116AQuick effectGood effectPharmaceutical delivery mechanismAnaestheticsSide effectRopivacaine
The invention relates to a transdermal transparent mucosa local anesthesia and analgesics and the preparation method of gelatinous formulation thereof. The technical problem to be solved by the invention is that how to provide the transdermal transparent mucosa local anesthesia and analgesics which takes effect quickly, has long drug action and can be used without injection as well as the preparation method of gelatinous formulation thereof. The transdermal transparent mucosa local anesthesia and analgesics comprises bupivacaine type compounds, azone type compounds and menthol. The bupivacaine type compounds contain Bupivacaine HCL, Levobupivacaine HCL, Ropivacaine and ramification thereof. The azone type compound contains azone, Laurocapram and ramification thereof. The transdermal transparent mucosa local anesthesia and analgesics has the advantages of rapid drug effect, strong action, maintenance for long time, fewer side effects, and so on. The transdermal transparent mucosa local anesthesia and analgesics is suitable for anesthesia and reliving pain during the surgery of human skin, mucosa and when in examination by apparatus, and also can be applied to symptomatic treatment of all kinds of chronic and acute pain of skin affection and mucosa affection.
Owner:珠海市横琴灵敏科技有限公司
Local analgesic drug entrapped near-infrared response lipid temperature-sensitive gel
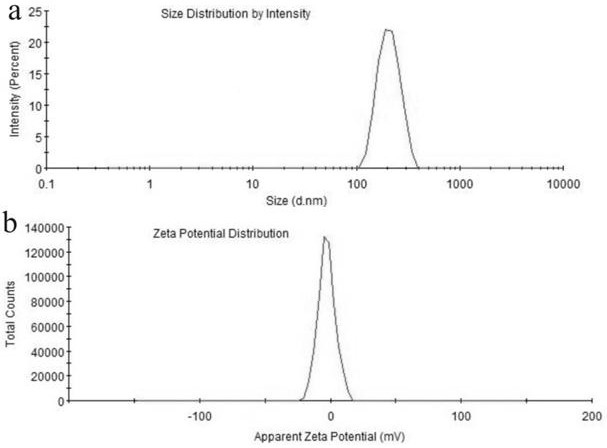
ActiveCN113663080AEliminate side effectsReduce complianceDrug photocleavageAerosol deliveryAnalgesics drugsSide effect
The invention discloses a local analgesic drug entrapped near-infrared response lipid temperature-sensitive gel, which can reduce and eliminate the toxic and side effects of the existing temperature-sensitive gel, prolong the slow release period, realize responsiveness and adjustable release, enable a patient to autonomously adjust the administration, greatly improve the treatment effect of pain and reduce the compliance of the patient. The composite drug delivery system is of a grid structure, the liposome is embedded in the system, the average particle size ranges from 150 nm to 200 nm, and the potential is about -2.88 mV; singlet oxygen is generated under the action of near infrared rays, so that a light response release behavior can be realized; the release behavior is first sudden release and then slow release, the slow release effect is obvious, meanwhile, near-infrared response can be achieved, the release rate is increased, and the analgesic concentration of ropivacaine is increased within a certain period of time.
Owner:JILIN UNIV
Composition containing bupivacaine for local anaesthesia and application thereof
InactiveCN103933041AImprove securityHigh precisionAnaestheticsHeterocyclic compound active ingredientsIndometacinTetrahydropalmatine
The invention relates to the field of medicines, and particularly relates to a pharmaceutical composition which has anesthetic action in operation. The composition is prepared from 1.0-3.0mg of bupivacaine, 1-5mu g of sufentanil, 15-30mu g of tetrahydropalmatine, 15-30 mu g of indometacin and 0.1-0.5mg of ropivacaine. The anesthetic composition is preferably prepared from 3.0mg of bupivacaine, 3mu g of sufentanil, 20mu g of tetrahydropalmatine, 20mu g of indometacin and 0.3mg of ropivacaine.
Owner:QINGDAO CENT HOSPITAL
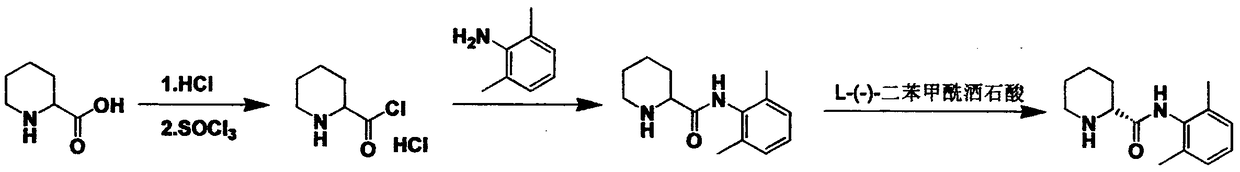
Method for preparing ropivacaine intermediate
The invention discloses a method for preparing a ropivacaine intermediate. The method includes: using L-piperidine-2-formic acid to prepare L-piperidine-2-formate, directly allowing L-piperidine-2-formate without separation to be in one-step reaction with 2, 6-dimethylaniline and phosphorus oxychloride to directly prepare S-N-(2, 6-dimethylphenyl) piperidine-2-formamide. By the method, process operation is simplified, cost is lowered, yield is increased, and the intermediate is more suitable for large-scale industrial production.
Owner:SHANDONG KEYUAN PHARMA
Dental ulcer pasting film containing chlorophyll and preparation method thereof
ActiveCN106474137AAddresses deficiencies that favor microbial growthImprove plasticityAntibacterial agentsDigestive systemDaucosterolDissolution
Owner:GUANGDONG COOWAY BIOTECH CO LTD
Ropivacaine freeze-drying agent capable of forming micelle
ActiveCN105434374AAvoid hydrolysisAvoid generatingPowder deliveryAnaestheticsFreeze-dryingRopivacaine
The invention discloses a ropivacaine freeze-drying agent. Ropivacaine is a widely used pain-relief drug, and the ropivacaine freeze-drying agent is provided for extending the action period. The ropivacaine freezing agent comprises a segmented copolymer of methoxy poly(ethylene glycol)-poly(lactic acid), and has the advantage that after redissolving, a micelle can be formed, so the action period of the drug is extended, and the release effect is realized.
Owner:CHINA PHARM UNIV
Liquid drug injection containing ropivacaine mesylate and preparation method thereof
InactiveCN109568259AImprove stabilityIsomer lessInorganic non-active ingredientsPharmaceutical delivery mechanismDrug injectionDrugs preparations
The invention belongs to the technical field of drug preparation, and relates to a liquid drug injection containing ropivacaine mesylate and a preparation method thereof. The preparation method comprises the following concrete steps that 1) 80 percent of theoretical preparation quantity of water for injection is added into a drug preparation tank; a prescription dosage of sodium chloride and ropivacaine mesylate is added; stirring is performed till dissolution is complete; wetted active carbon for needles is added; the stirring is performed for adsorption; (2) a pH value is regulated to 4.5 to5.5 by a pH value regulating agent; the water for injection is supplemented to the full dose; pull circulation is performed for 10 to 20 minutes; (3) bottle preparation by three-in-one equipment, N2filling for filling, opening sealing, sterilization and package are performed. Compared with the prior art, the ropivacaine mesylate injection liquid prepared by the method has the advantages of safeand reliable effects, high stability, and few isomers. In addition, compared with glass ampule package, the polypropylene ampule package of a bottle blowing, filling and opening sealing three-in-one sterile system has the advantages that in an opening process, fragments and particulate fine powder cannot be easily generated; the transportation is convenient; the safety and reliability are realized.
Owner:CISEN PHARMA
Sustained release local anesthetic hydrogel composition
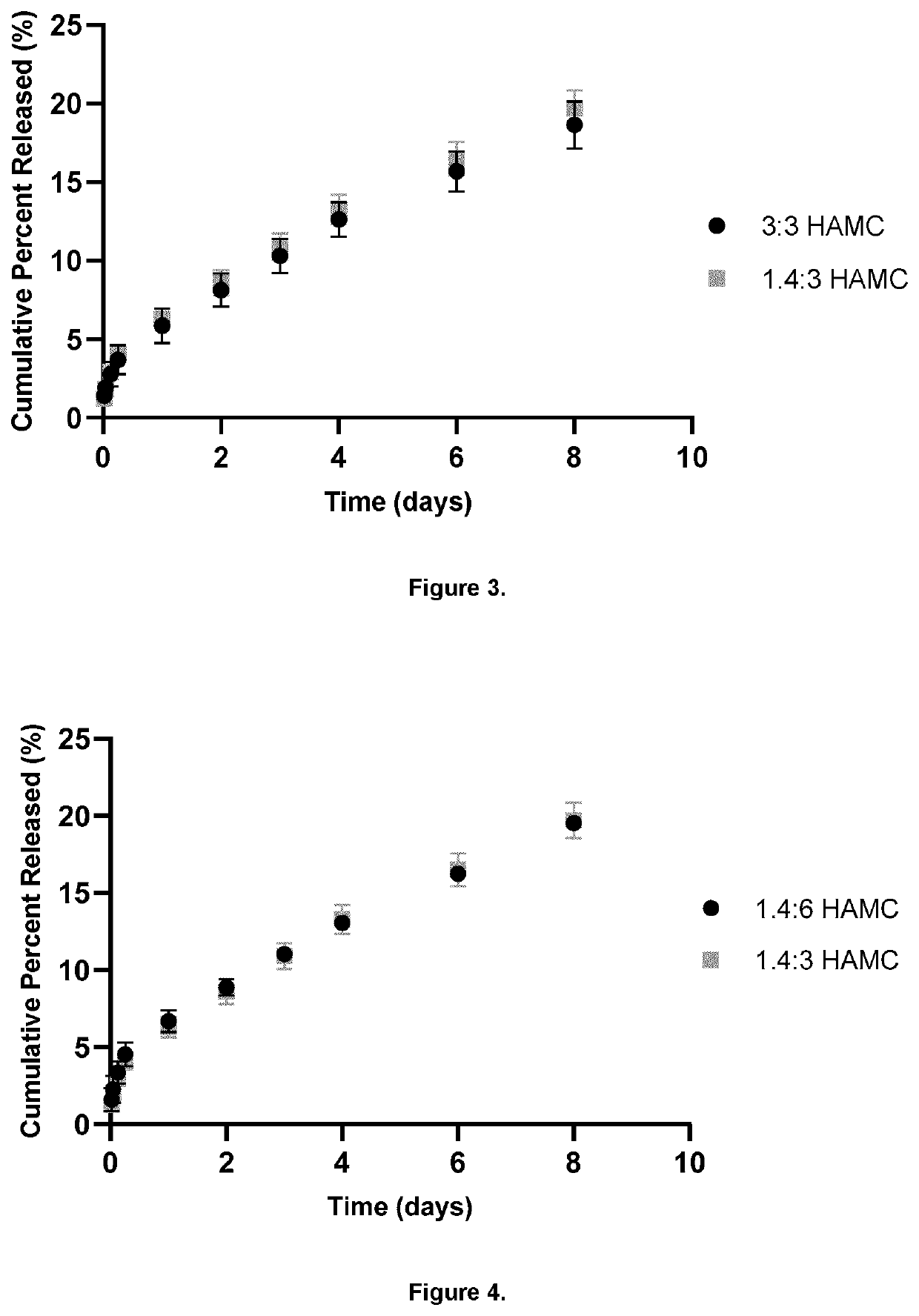
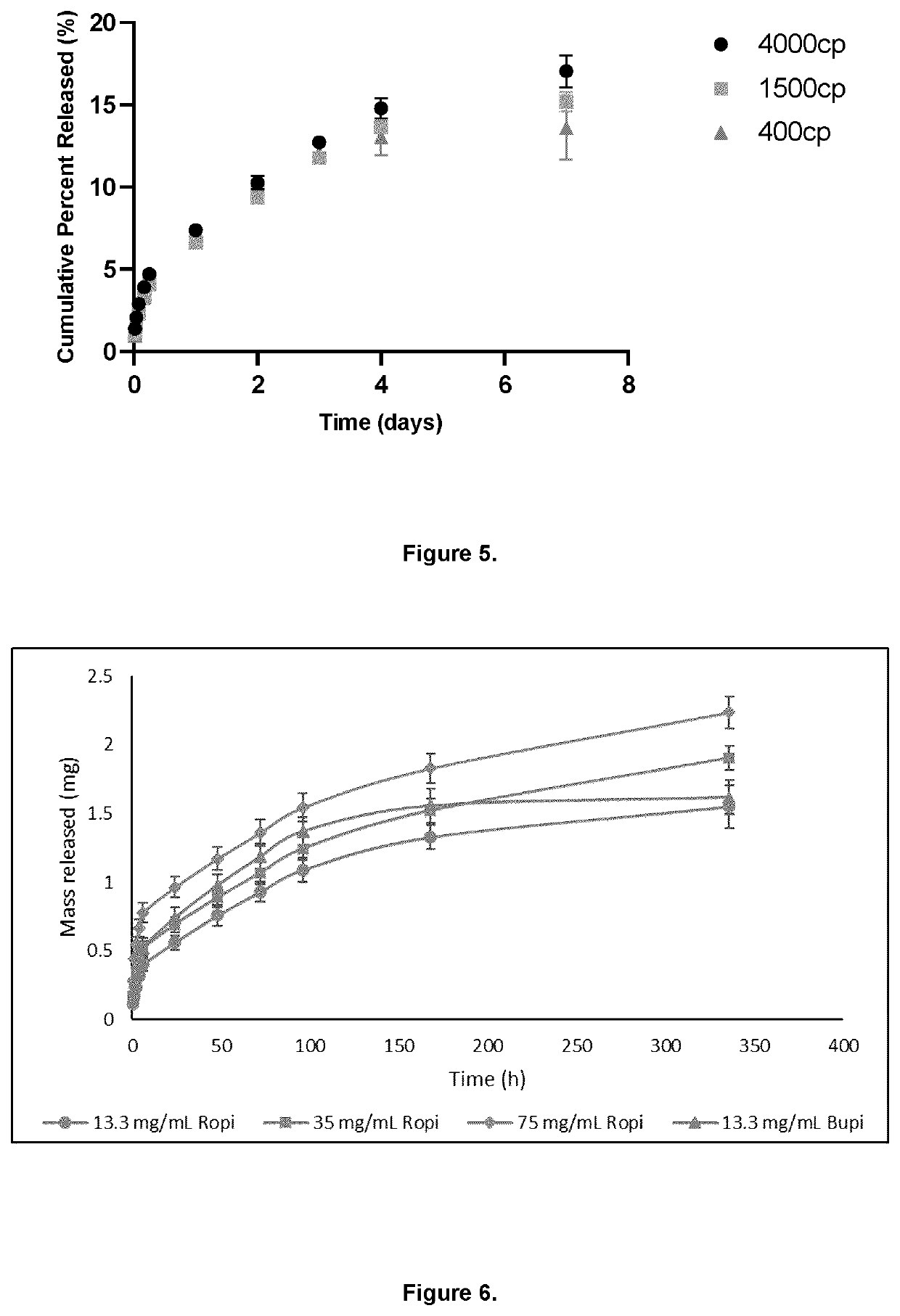
A bioresorbable, sustained release pharmaceutical composition comprising: 1.8 wt % to 3.0 wt % methylcellulose and 0.1 wt % to 3.0 wt % hyaluronan in the form of a gel polymer matrix, and at least one local anesthetic agent, suitably ropivacaine, which may be administered by injection.
Owner:THE GOVERNINIG COUNCIL OF THE UNIV OF TORANTO
Microneedle devices and methods
The present invention provides a medical device, which comprises: a microneedle array, and a coating disposed on the microneedles, wherein the coating comprises: a local anesthetic selected from lidocaine, Prilocaine and combinations thereof; and local anesthetic dose sustained-release components selected from tetracaine, ropivacaine, bupivacaine, procaine and combinations thereof; wherein based on the solids in the coating The total weight of the local anesthetic is present in an amount of at least 1% by weight, and wherein the weight ratio of the local anesthetic and the dose-extending component is a non-eutectic weight ratio; a medical device comprising An array of dissolving microneedles comprising: a dissolvable matrix material; at least 1% by weight of a local anesthetic selected from the group consisting of lidocaine, prilocaine, and combinations thereof; and a local anesthetic dose sustained release component , which is selected from tetracaine, ropivacaine, bupivacaine, procaine, and combinations thereof; wherein the weight ratio of the local anesthetic and the dose-sustaining component is a non-eutectic weight ratio, and wherein the weight % based on the total weight of solids in all portions of the dissolvable microneedles containing the local anesthetic; a method of using the device to provide sustained release of a locally delivered dose of local anesthetic in mammalian tissue; and a method of manufacturing method of the device.
Owner:3M INNOVATIVE PROPERTIES CO
Anesthetic methods and compositions
ActiveUS8410140B2Relieve painToxicity associated with the local anesthetic may be reduced or eliminatedBiocideAntipyreticDexmedetomidinePeripheral neuron
The present invention relates to compositions and methods use in pain reduction, including but not limited to, peripheral nerve blocks. In particular, the present invention relates to compositions and methods for the administration of perineural dexmedetomidine and ropivacaine in combination for increased antinociception in peripheral nerve blocks. In addition, this invention relates to any use of dexmedetomidine alone or in combination with other agents for the purpose of decreasing inflammation around peripheral nerves, thereby decreasing the potential for peripheral nerve injury. Further, the invention relates to the use of dexmedetomidine to reduce inflammation in the muscle to lessen or prevent muscle damage.
Owner:RGT UNIV OF MICHIGAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com