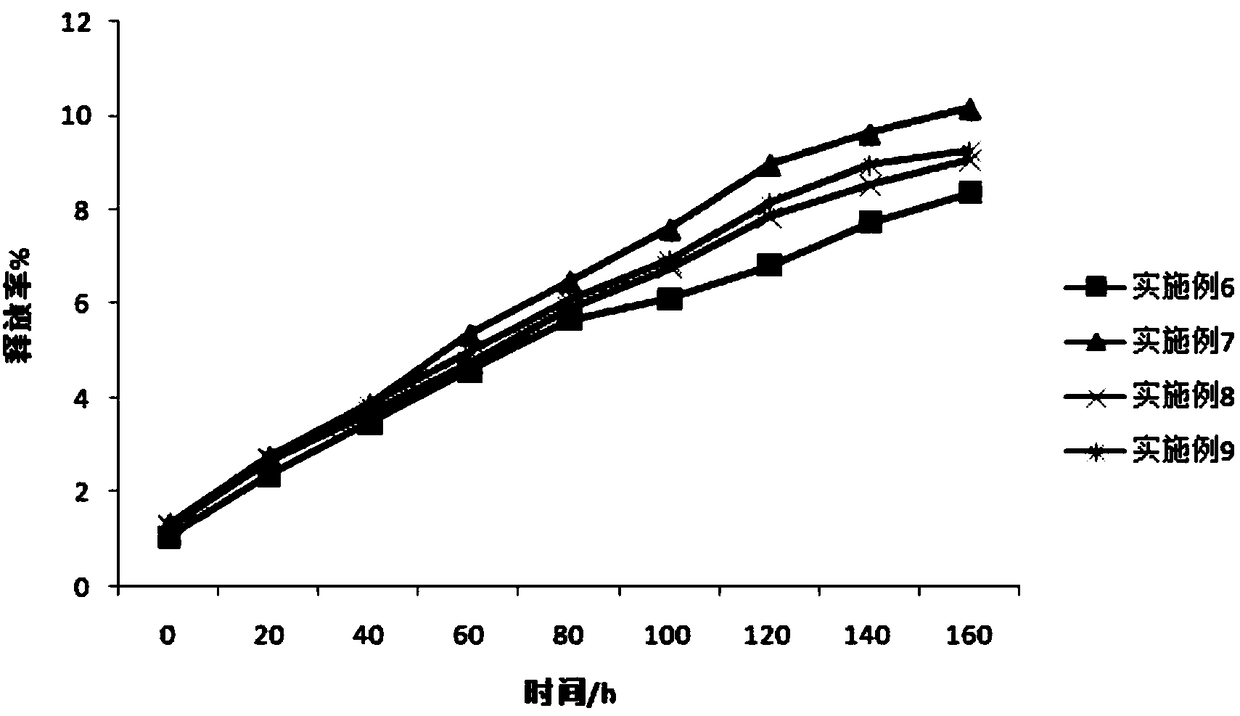
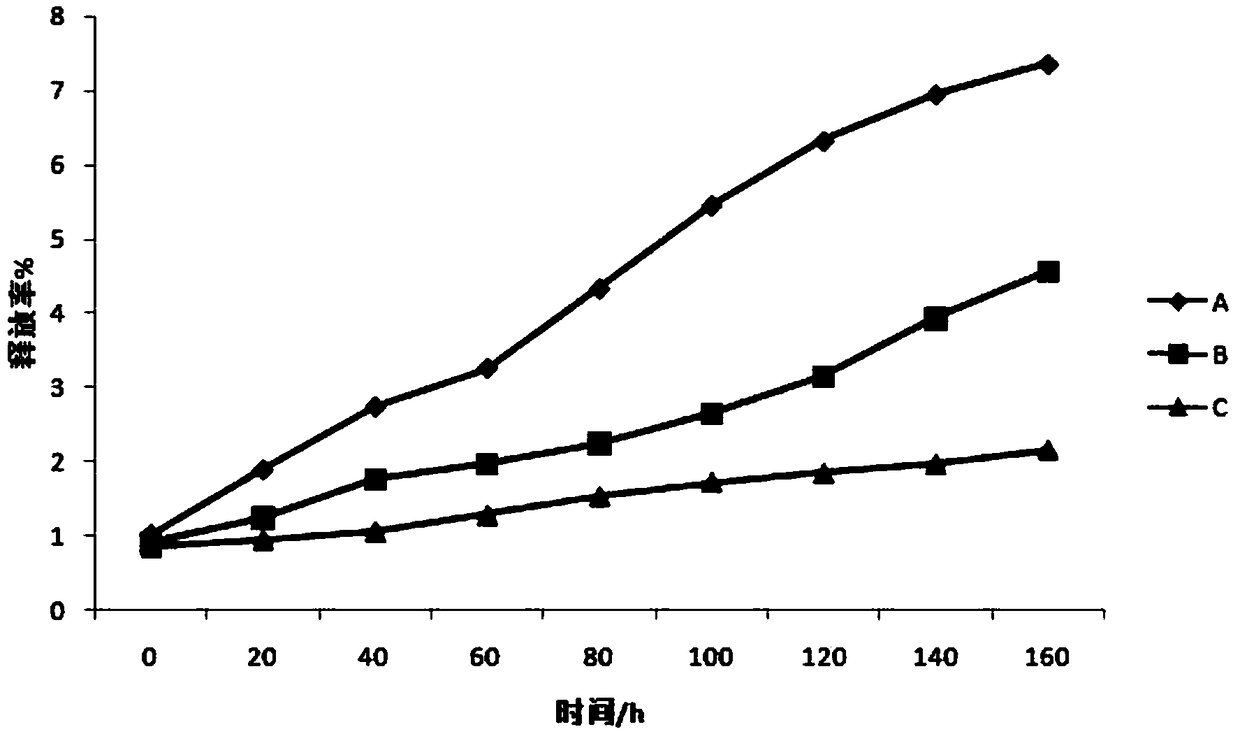
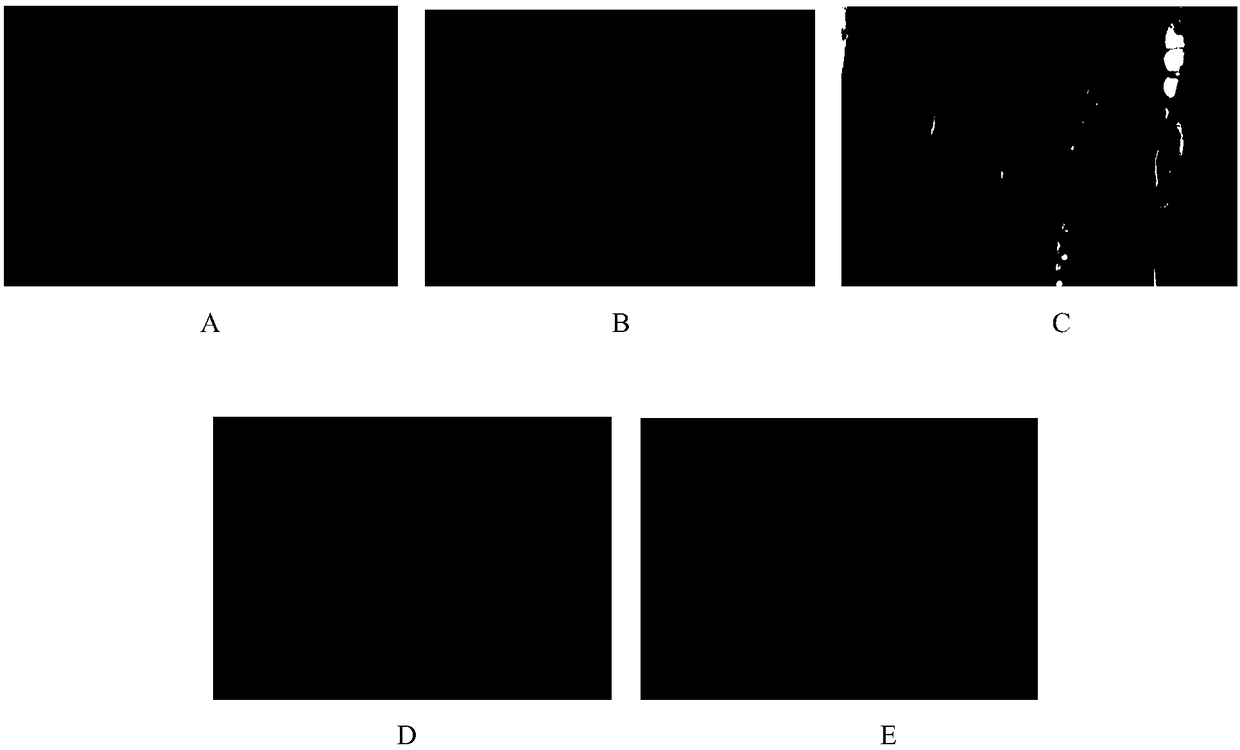
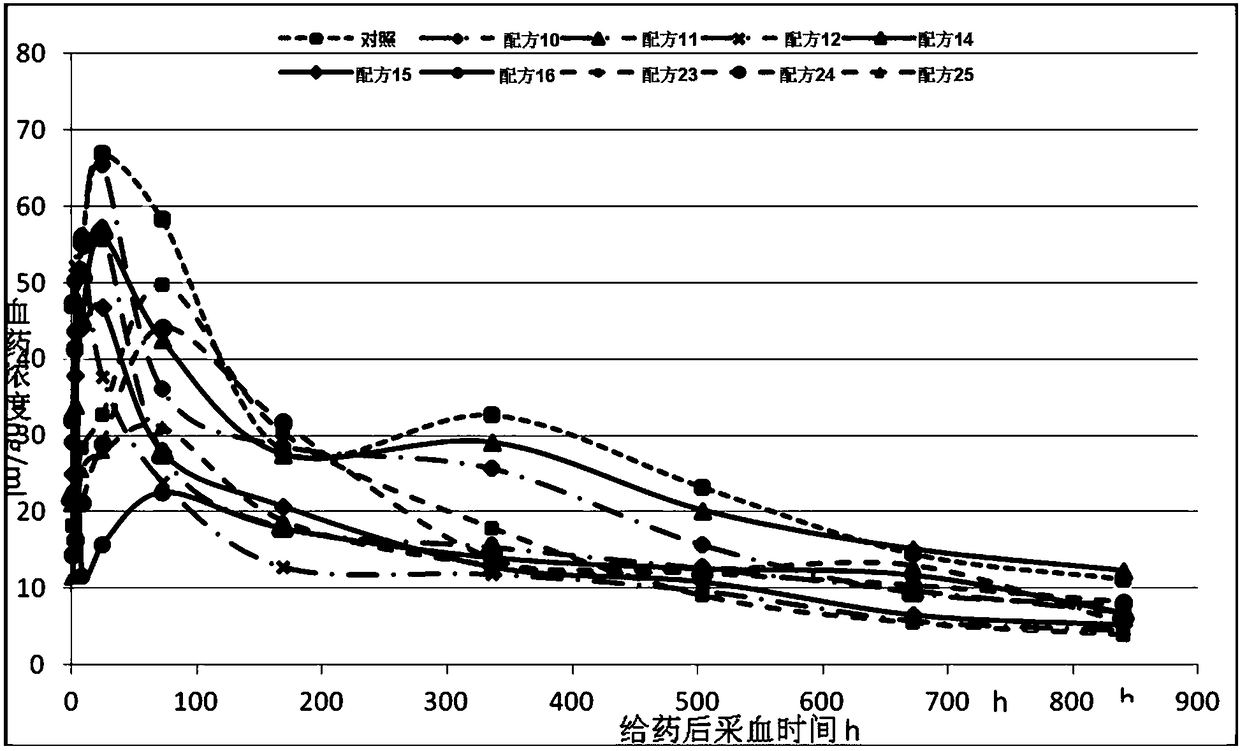
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
98 results about "Procaine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
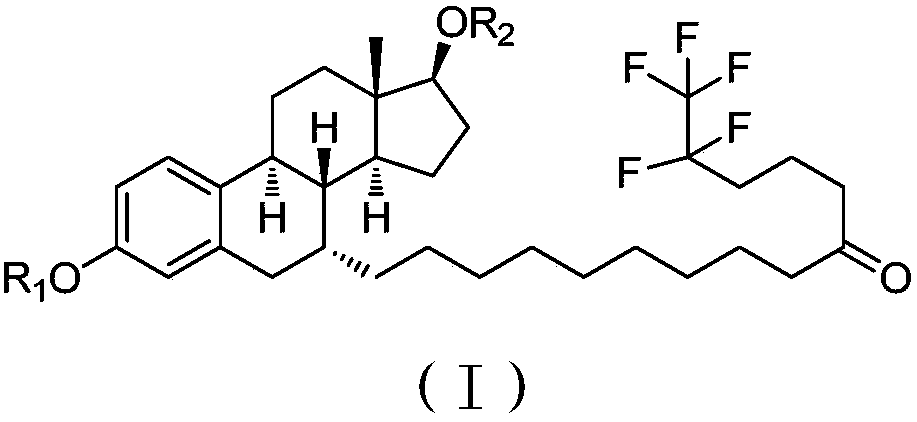
Procaine is a local anesthetic drug of the amino ester group. It is used primarily to reduce the pain of intramuscular injection of penicillin, and it is also used in dentistry. Owing to the ubiquity of the trade name Novocain, in some regions, procaine is referred to generically as novocaine. It acts mainly as a sodium channel blocker. Today it is used therapeutically in some countries due to its sympatholytic, anti-inflammatory, perfusion-enhancing, and mood-enhancing effects.
Antimicrobial therapeutic compositions and method of use
InactiveUS6921539B2Enhanced broad based antimicrobial activityEasy to useBiocideHydroxy compound active ingredientsMicroorganismProcaine
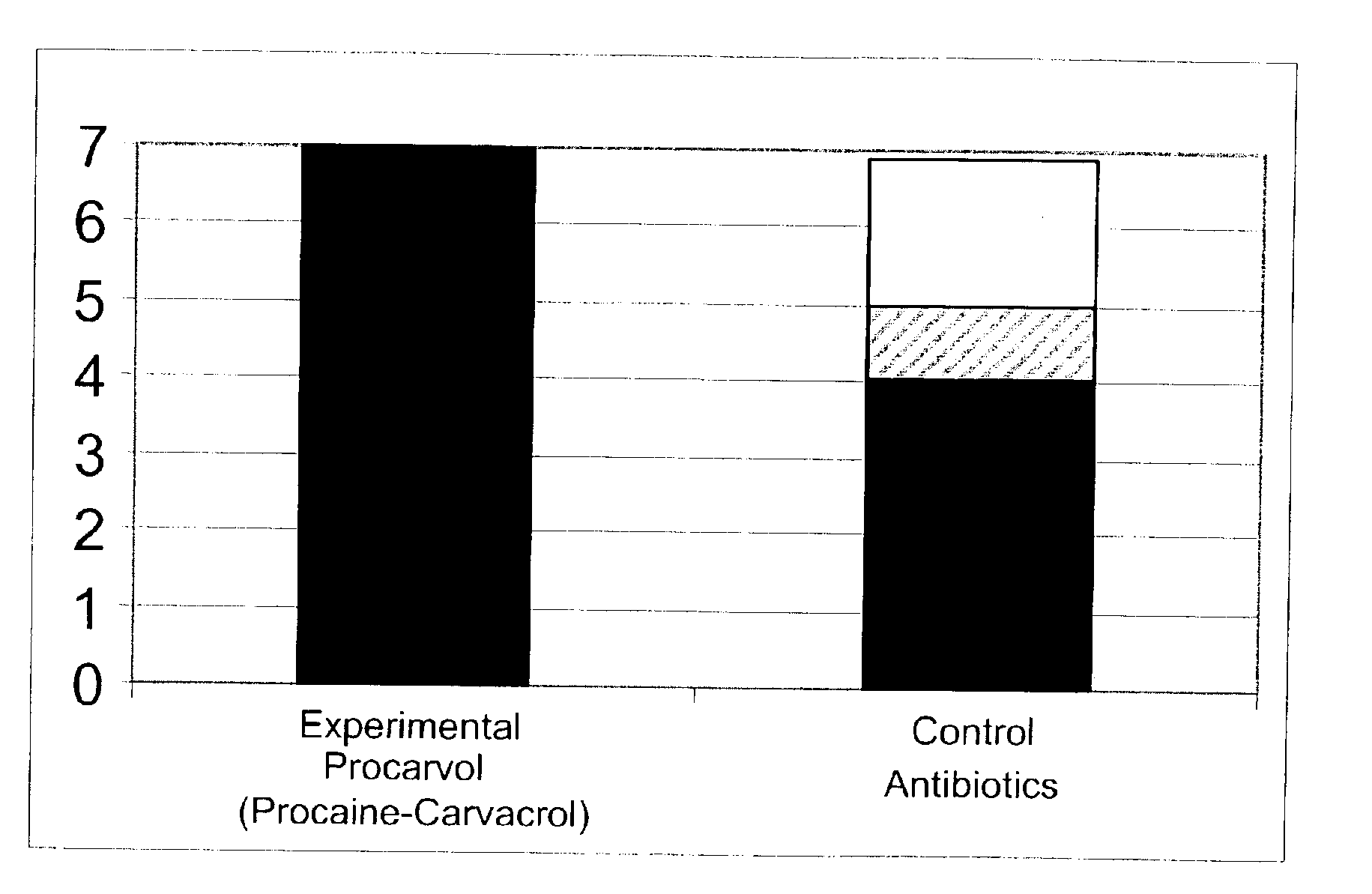
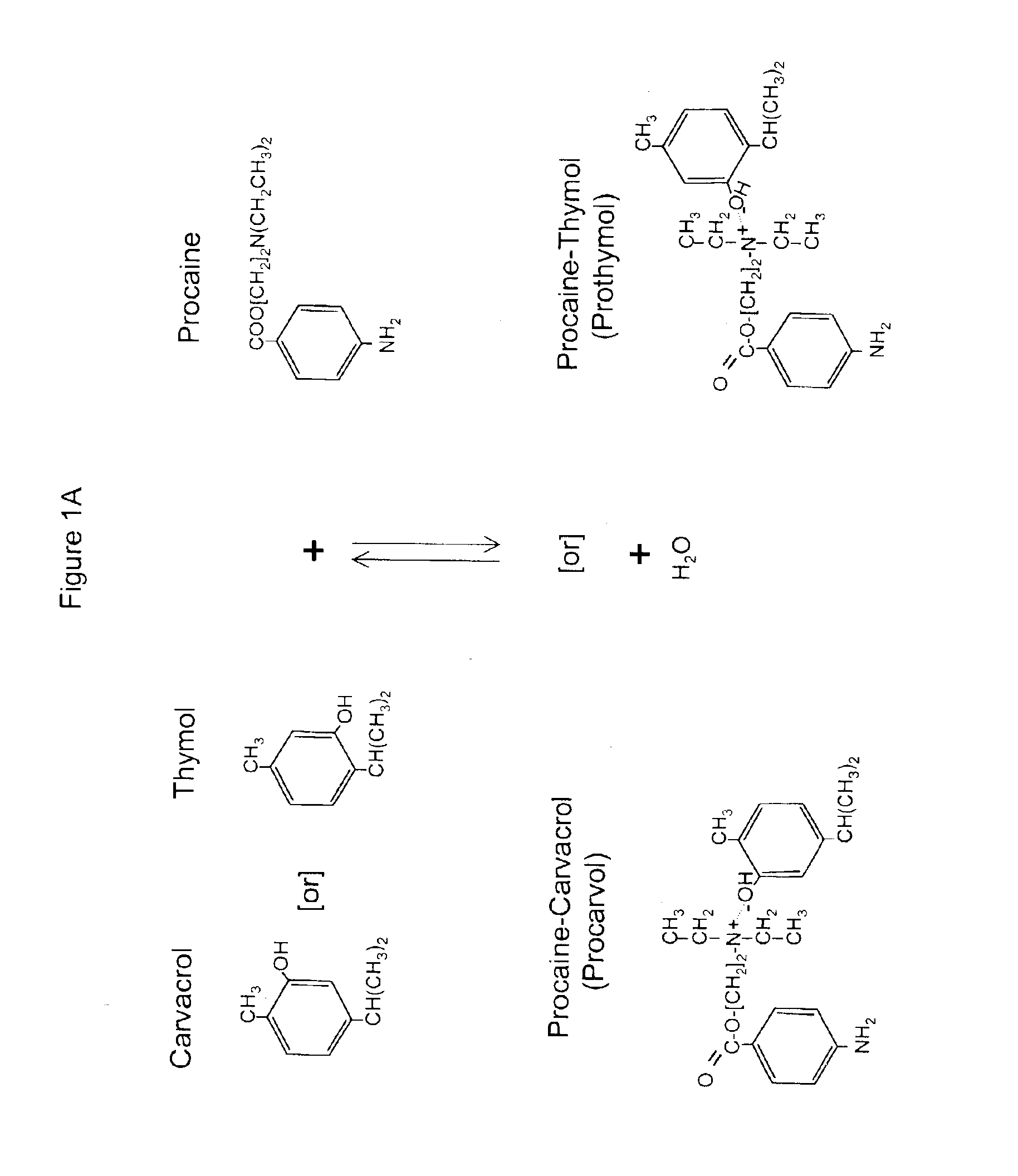
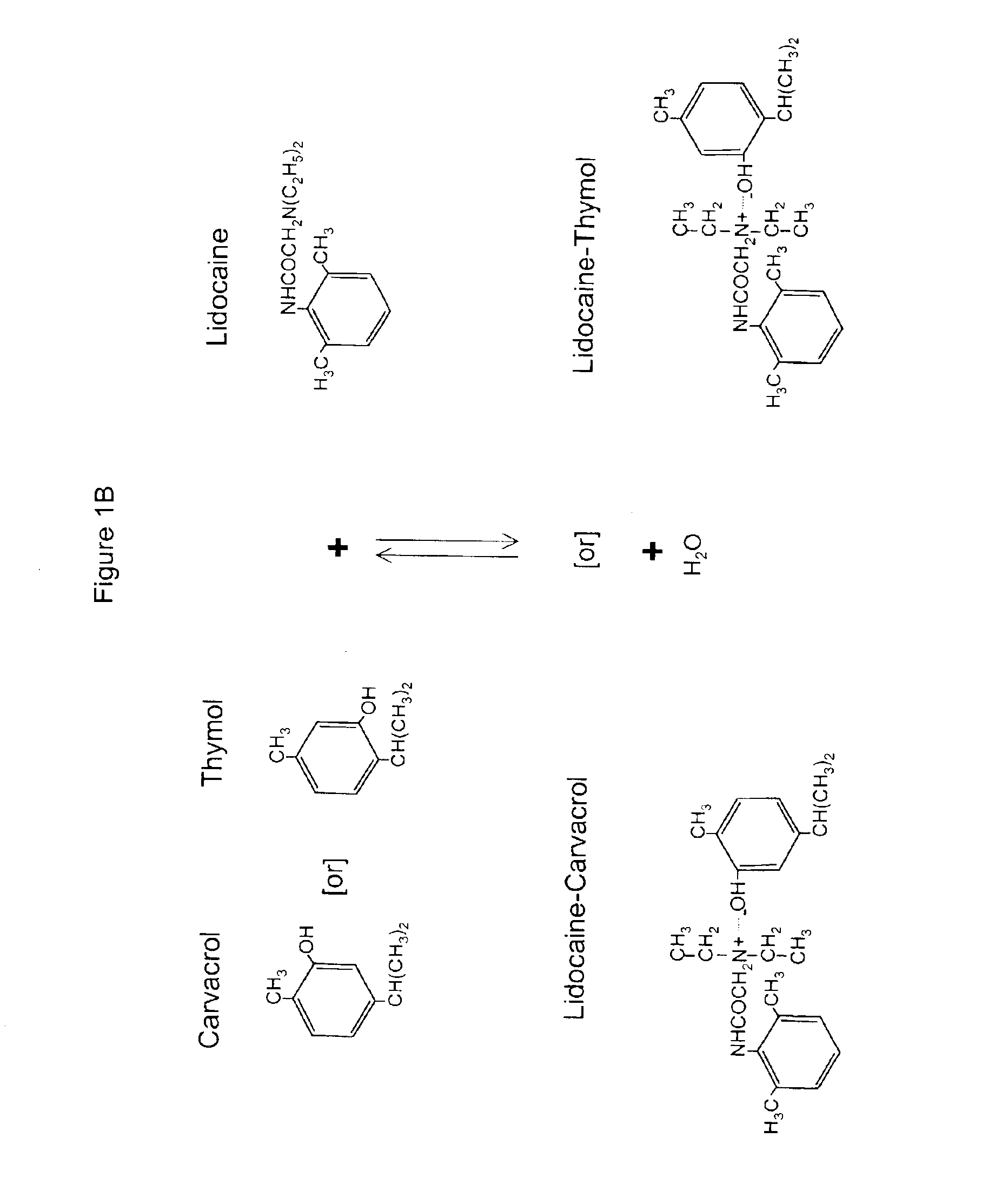
The invention provides therapeutic antimicrobial compositions and methods for their use based on natural organic phenolic compounds combined with pharmacological agents. The antimicrobial activities of each carvacrol and thymol are believed to be enhanced, while the pharmacological properties of procaine and related compounds are added to provide their unique properties to facilitate usefulness and effectiveness in humans. The therapeutic compositions are active against bacterial, fungal, and protozoan infections. The forms of the invention are intended to treat various internal infections through parenteral, subcutaneous, intradermal, intravenous, and intramuscular injections. They are also intended as useful agents to treat microbial infections that have become resistant to conventional anitibiotics as well as secondary opportunistic infections.
Owner:EUROVLOOT +2
Slow-release preparation for postoperation analgesia and preparation method of slow-release preparation
ActiveCN108379269AProlong the action timeImprove bioavailabilityAntipyreticAerosol deliveryProcaineSide effect
The invention discloses a slow-release preparation for postoperation analgesia and a preparation method of the slow-release preparation. The slow-release preparation is mainly prepared from the following raw materials: 10-15 parts of phospholipid, 4-7 parts of glyceride, 0.3-5 parts of poloxamer, 0.8-5 parts of a cosolvent and 0.5-2 parts of a local anesthetic mixture of cocaine, procaine and lidocaine, wherein the mass ratio of the cocaine to the procaine to the lidocaine in the local anesthetic mixture is 1:1:(4-7.5). The phospholipid is phosphatidylcholine, and preferably soybean phosphatidylcholine; the glyceride is dinolin; the cosolvent is ethanol; the slow-release preparation is easy to inject, and medicines can be slowly released in situ; the action time of the medicines is prolonged, the administration times are reduced, and the bioavailability of the medicines is improved; meanwhile, toxic and side effects caused by too high medicine concentrations can be avoided.
Owner:武汉百纳礼康生物制药有限公司
Long acting drug delivery system for treating breast cancer and preparation method and applications thereof
InactiveCN108159055AEnsure safetyIncrease concentrationOrganic active ingredientsPharmaceutical delivery mechanismProcainePhospholipid
The invention provides a long acting drug delivery system, which comprises fulvestrant or derivatives thereof and takes phospholipid as a sustained release material. The invention mainly discloses a high concentration formula of fulvestrant or derivatives thereof. The main component is fulvestrant or derivatives thereof. The sustained release material is different phospholipids or a mixture of phospholipids and different kinds of plant oil. The solvent is at least one of ethanol, ethyl lactate, 1,2-propylene glycol, and ethyl acetate. The analgesic is benzyl alcohol, lidocaine, procaine, or ropivacaine. The viscosity of the formula is 20-45 mPa.s, and the concentration of the formula is 60-300 mg / mL. The antioxidant of the formula is Ve, lipoic acid, and the like. The phospholipids can increase mutual solubility and has a sustained release function. The preparation is prepared in an aseptic filtration mode.
Owner:XIAN LIBANG PHARMA TECH
Bis-[6-oxa-(2-carboxylbenzenesulfonyl-butanedioic acid 1,4 monoester-4)-beta-cyclodextrin, preparation method and application thereof
ActiveCN101928356AAchieve separationComponent separationMaterial analysis by electric/magnetic meansProcaineButanedioic acid
The invention relates to bis-[6-oxa-(2-carboxylbenzenesulfonyl-butanedioic acid 1,4 monoester-4)-beta-cyclodextrin, a preparation method and application thereof as chiral selective agent in high performance capillary electrophoresis (HPCE), namely being prepared into a chiral electrophoresis column and a mobile phase chiral additive for detachment of chiral substances. The molecular formula of the bis-[6-oxa-(2-carboxylbenzenesulfonyl-butanedioic acid 1,4 monoester-4)-beta-cyclodextrin is determined to be C64H84O49S2; beta-CD-B2 is used as the HPCE mobile phase chiral additive to separate chiral substances of phenylglycinol, anisodamine, isoprenaline and propafenone to realize baseline separation; beta-CD-B2 is used for preparing a chiral HPCE column to separate chlortrimeton, bupivacaine, procaine, atenolol, anisodamine, propafenone, lobeline and other chiral substances to realize the baseline separation. Therefore, a novel HPCE quantitative measurement method for a single enantiomerof various chiral substances can be established.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Composition of anti-HIV drugs and anti-cortisol compounds and method for decreasing the side effects of anti-HIV drugs in a human
InactiveUS20050085464A1Elevated triglycerideElevated cholesterolBiocideCarbohydrate active ingredientsProcaineSide effect
The present invention is based, in part, upon the discovery that the use of an anti-HIV drug in combination with at least one cortisol blocker such as procaine, reduces the side effects associated with anti-HIV drugs. The invention also relates to a method of treating the high cortisol catabolic effects of diseases such as AIDS in the HIV positive population and those with AIDS related complexes by the administration of a cortisol blocker. The present invention also discloses a composition comprising an anti-HIV drug and cortisol blocker. More specifically, the present invention relates to a cortisol blocking composition which comprises procaine, ascorbic acid and zinc heptahydrate.
Owner:SAMARITAN PHARMA
Disinfecting paste for dissolving wart and eliminating wart
The invention relates to cream for dissolving wart, relieving neoplasm and detoxicating, which comprises the following components by weight portion: 0 to 3.0 portions of licorice, 0 to 3.0 portions of scutellaria, 0 to 3.0 portions of phellodendron amurense, 0 to 3.0 portions of rhubarb, 0 to 1.0 portion of menthol, 2 to 60 portions of sodium hydroxide (or potassium hydroxide), 0 to 10 portions of calcium hydroxide, and 0.75 to 1.0 portion of levobupivacaine hydrochloric acid (or toad venom, bupivacaine hydrochloric acid, lidocaine, dicaine or procaine or other local anesthetics). The sodium hydroxide (or the potassium hydroxide) and the calcium hydroxide of proper concentration can quickly dissolve protein, and melt the wart, the neoplasm and various inflammatory tissues in time from 1 minute to tens of minutes; the scutellaria, the phellodendron amurense and the rhubarb resist bacteria and virus; the licorice and the scutellaria adjust immune function, and inhibit adverse tissue reaction; the toad venom has anti-inflammatory, anti-cancer and local anesthetic functions; the menthol is fresh and cool, and can deodorize and stop pain; and the levobupivacaine hydrochloric acid or other local anesthetics has local anesthetic and paregoric functions.
Owner:谭国梁
Anesthetic composition, formulation and method of use
InactiveUS20150010528A1Easy to managePromote rapid formationAmpoule syringesNervous disorderProcaineCarbocaine
An anesthetic composition for use e.g. in the administration of a local anesthetic by injection comprises a first component, which comprises hyaluronidase, and a second component which comprises an anesthetic preparation. The composition is both effective and highly shelf stable, and has as an advantage that it may be stored and administered at room temperature. In a particular embodiment, the hyaluronidase is prepared in dry powder form, as by lyophilization. The anesthetic component may be selected from a group of known anesthetics, such as lidocaine, polocaine, xylocaine, novocaine, procaine, prilocaine, bupivacaine, mepivacaine, carbocaine, etidocaine and chincocaine. The composition may be prepared in unit dosage forms, including a single dosage form, for a variety of purposes, and such unit dosage forms may be prepared in a plural chambered syringe or like dispenser, whereby the components are not mixed until administration.
Owner:WEG STUART L
Anesthetic composition, formulation and method of use
InactiveUS20090143436A1Increased shelf stabilityExtended shelf lifeBiocideNervous disorderProcaineCarbocaine
An anesthetic composition for use e.g. in the administration of a local anesthetic by injection comprises a first component, which comprises hyaluronidase, and a second component which comprises an anesthetic preparation. The composition is both effective and highly shelf stable, and has as an advantage that it may be stored and administered at room temperature. In a particular embodiment, the hyaluronidase is prepared in dry powder form, as by lyophilization. The anesthetic component may be selected from a group of known anesthetics, such as lidocaine, polocaine, xylocaine, novocaine, procaine, prilocaine, bupivacaine, mepivacaine, carbocaine, etidocaine and chincocaine. The composition may be prepared in unit dosage forms, including a single dosage form, for a variety of purposes, and such unit dosage forms may be prepared in a plural chambered syringe or like dispenser, whereby the components are not mixed until administration.
Owner:WEG STUART L
Method for simultaneously determining of concentration multi anesthesia medicament in blood plasma
InactiveCN101393196ASimple and fast operationEasy to operateComponent separationMaterial analysis by optical meansProcaineUltraviolet absorption
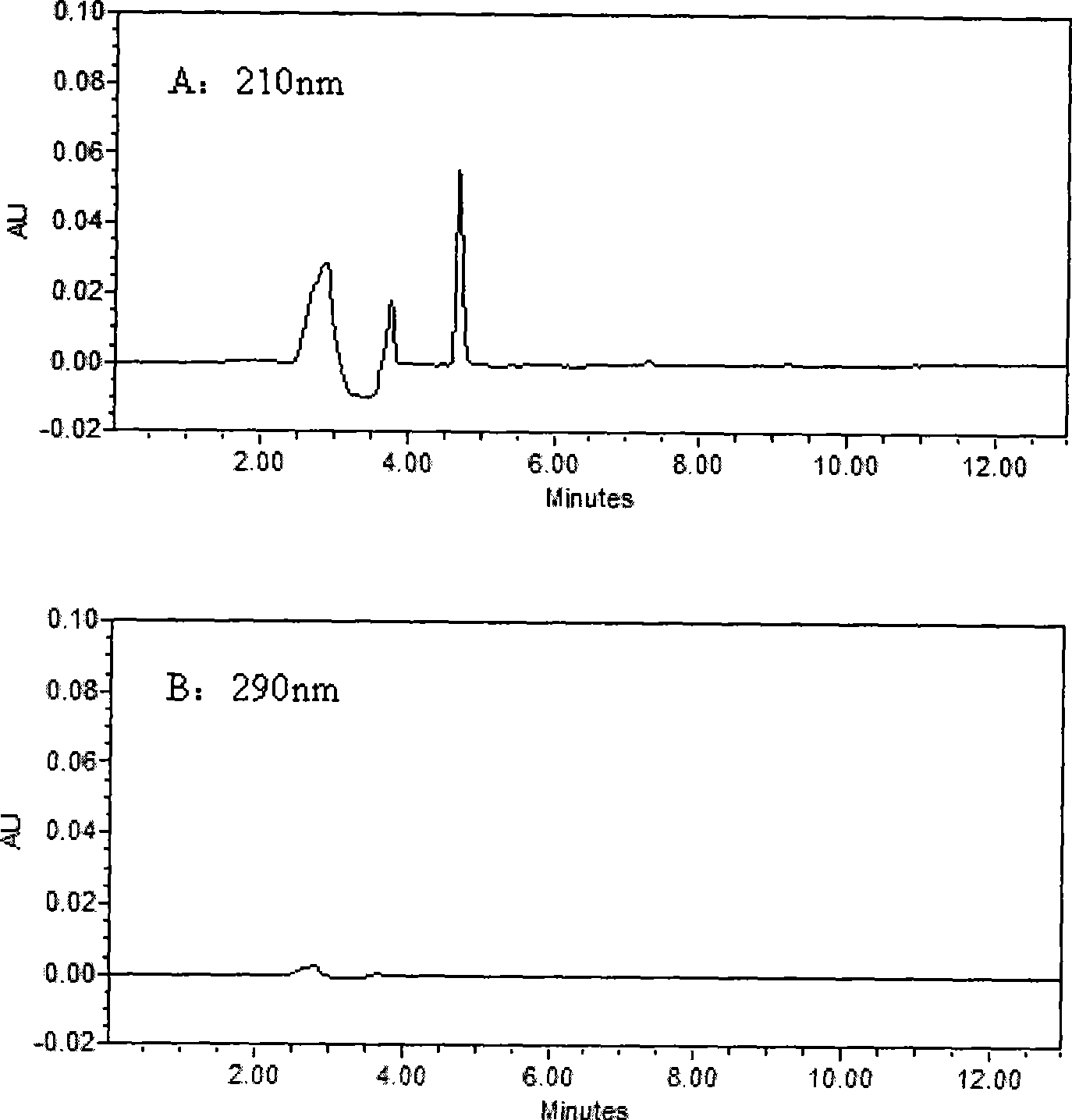
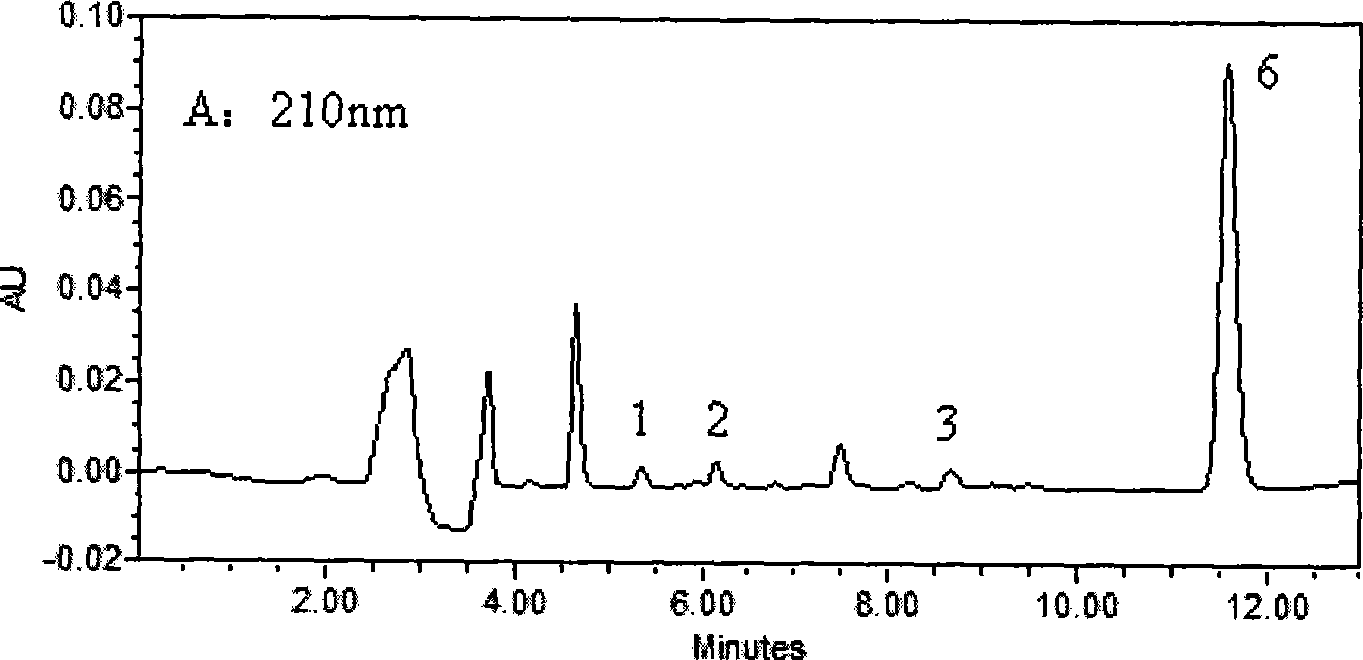
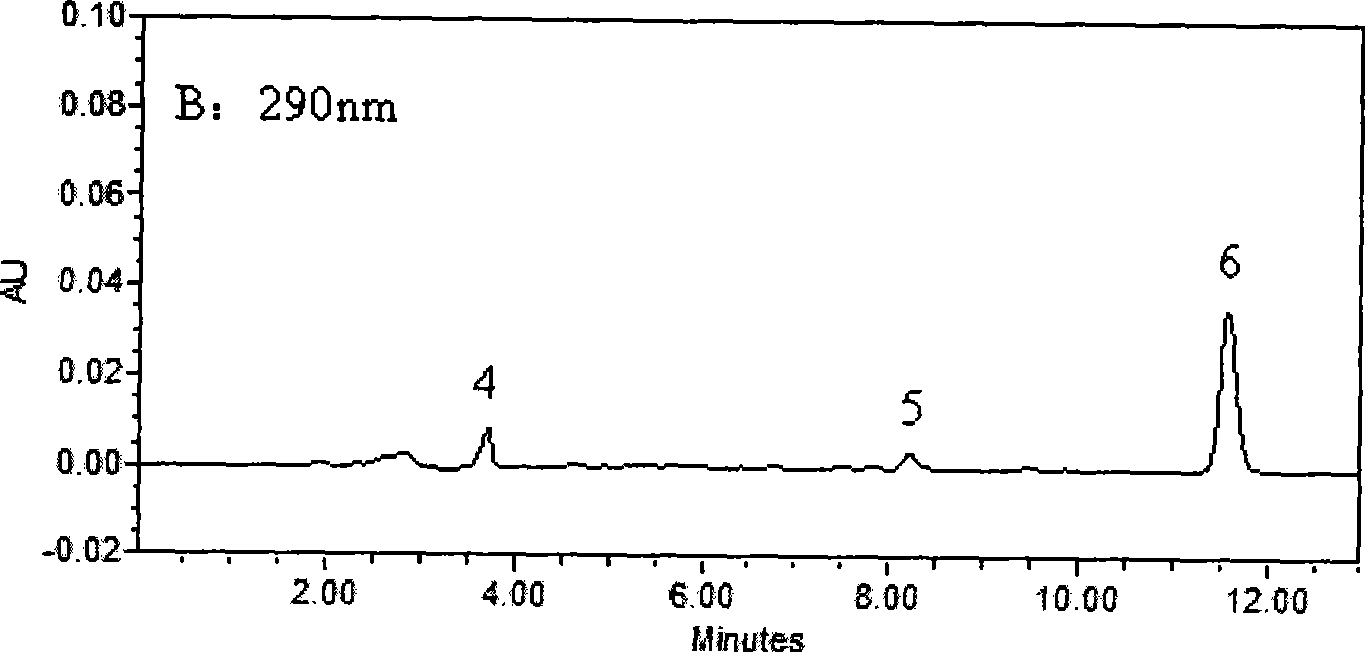
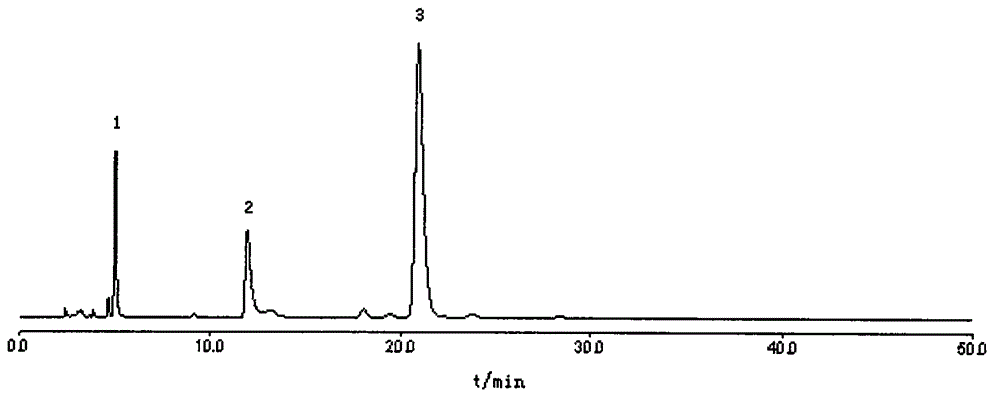
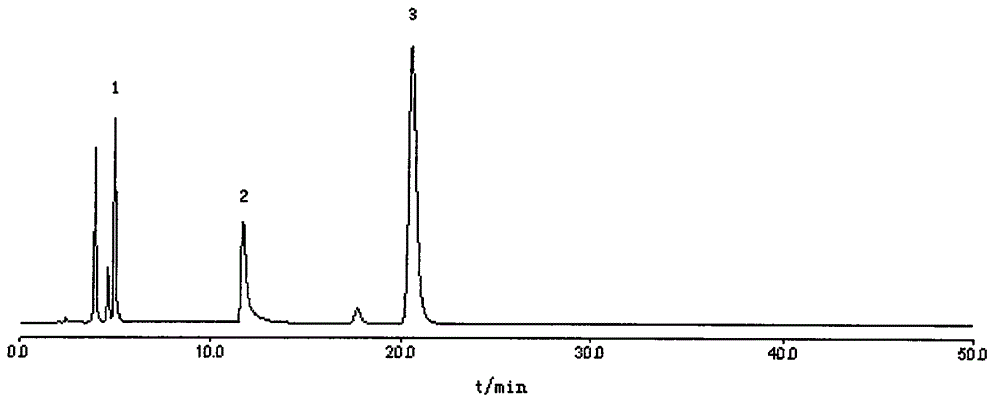
The invention belongs to the field of medical examination and relates to a method capable of synchronously determining the concentrations of a plurality of local anesthetic drugs in human plasma. The method adopts a plasma cholineesterase inhibitor to inhibit the activity of plasma cholinesterase under ice bath condition below 3 DEG C, which controls the hydrolysis of totokaine and assures the accuracy of the method; by utilizing characteristics that lidocaine, ropivacaine and bupivacaine have stronger characteristic of ultraviolet absorption at wavelength of 210nm, and procaine and the totokaine have stronger characteristic of ultraviolet absorption at wavelength of 290nm, an ultraviolet dual-wavelength method is used to detect after the separation of an acid mobile phase at a chromatographic column; and the method can ensure that the sensitivity of synchronous determination of the local anesthetic drugs is greatly improved. The method has less sampling from samples and simple, quickand sensitive pretreatment, does not need expensive equipment or reagents, has short analysis period and low cost, and is suitable for the monitoring of clinical conventional blood concentration of aplurality of drugs.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Microneedle devices and methods
A medical device, comprising: an array of microneedles, and a coating disposed on the microneedles, wherein the coating comprises: a local anesthetic selected from the group consisting of lidocaine, prilocaine, and a combination thereof; and a local anesthetic dose-extending component selected from the group consisting of tetracaine, ropivacaine, bupivacaine, procaine and a combination thereof; wherein the local anesthetic is present in an amount of at least 1 wt-% based upon total weight of solids in the coating, and wherein the local anesthetic and dose-extending component are in a non-eutectic weight ratio; a medical device, comprising an array of dissolvable microneedles, the microneedles comprising: a dissolvable matrix material; at least 1 wt-% of a local anesthetic selected from the group consisting of lidocaine, prilocaine, and a combination thereof; and a local anesthetic dose-extending component selected from the group consisting of tetracaine, ropivacaine, bupivacaine, procaine and a combination thereof; wherein the local anesthetic and dose-extending component are in a non-eutectic weight ratio, and wherein wt-% is based upon total weight of solids in all portions of the dissolvable microneedles which contain the local anesthetic; a method of extending a topically delivered local anesthetic dose in mammalian tissue using the devices; and methods of making the devices are provided.
Owner:3M INNOVATIVE PROPERTIES CO
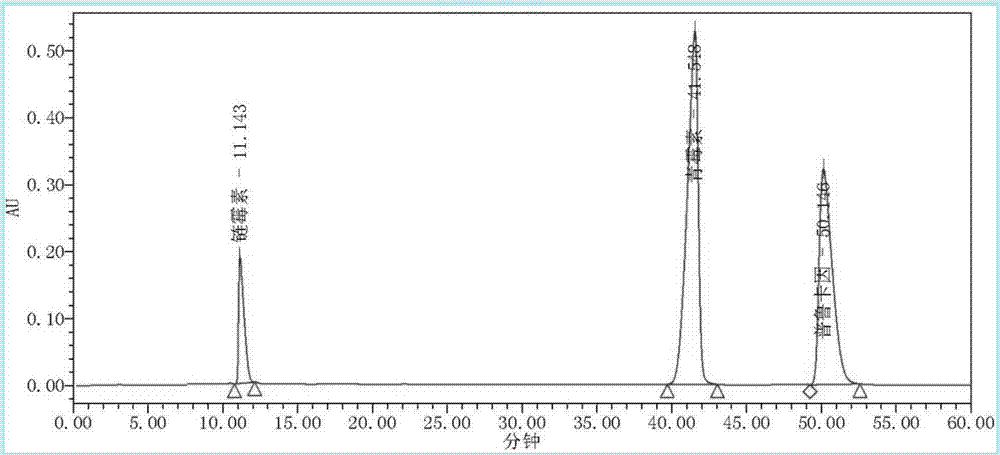
Liquid chromatography method for simultaneously determining contents of procaine, penicillin and streptomycin
InactiveCN107884498ARelief Assay ImperfectMitigation selectivityComponent separationProcaineVeterinary Drugs
The invention discloses a liquid chromatography method for simultaneously determining the contents of procaine, penicillin and streptomycin. The method includes the steps of first separately preparingstandard solutions of procaine, penicillin and streptomycin control components, then preparing a test solution containing procaine, penicillin and / or streptomycin components, using the liquid chromatograms obtained by liquid chromatography to draw the standard curves of procaine, penicillin and streptomycin, and finally testing the test solution and using the standard curves of procaine, penicillin and streptomycin to calculate the contents of procaine, penicillin and / or streptomycin. The method can accurately detect the contents of procaine, penicillin and streptomycin, has the advantages ofstrong specificity, high precision, good stability and high accuracy, is suitable for the quality control of procaine penicillin-streptomycin unilateral and compound products in the veterinary drug industry, and can effectively save the testing cost and time.
Owner:CHONGQING FANGTONG ANIMAL PHARMA
Medicinal composition for treating pneumonitis and cardiovascular diseases and its making method
InactiveCN1981762AEasy to adjustImprove motor functionSuppositories deliveryHeterocyclic compound active ingredientsProcaineClinical efficacy
A composite medicine for treating pneumonia, pulmonary fibrosis, cerebral infarction, cardiovascular and cerebrovascular ischemic diseases, ischemic eye disease, etc is prepared from anisodine (0.05-0.4 Wt portions) and procaine (5-40). Its preparing process is also disclosed.
Owner:宋琛
Sustained-release analgesic drug
The invention relates to a sustained-release analgesic drug, which is microsphere of a topical anesthesia agent. The topical anesthesia agent comprises lidocaine, tetracaine, bupivacaine, oxybuprocaine and procaine. The sustained-release microsphere provided by the invention can be prepared into a sterile microsphere injection, a powder, a spray, a tablet, a capsule, a lozenge, a soft capsule, a pill, a syrup, an external ointment, an emulsion, a bandage, a binder and a gauze. The sustained-release microsphere can be prepared by a multiple double emulsion method, a non-aqueous method, a low temperature spray extraction method or a phase coacervation method. The sustained-release analgesic drug provided by the invention can be used for pain killing of skin wounds with small area, such as cuts, abrasions and burns, and operation wounds.
Owner:伍丽娟
Medicine composition for anaesthetic pain relief after crissum operation
InactiveCN102579579BPromote absorptionCertain curative effectAntipyreticAnalgesicsProcaineCurative effect
Owner:张伟
Procaine injection
The invention discloses a procaine injection which is prepared from the following bulk pharmaceutical chemicals: procaine, xylitol, 30-70% ethanol and polyethylene glycol 400. The preparation method of the procaine injection comprises: taking procaine of a prescription amount; dissolving the procaine with ethanol; adjusting pH value; adding the xylitol; then, adding the polyethylene glycol 400 and propylene glycol; diluting to reach the whole amount; stirring; adjusting the pH value; filtering; splitting; and sterilizing by circulated steam. The injection has stable quality and high bioavailability and is especially suitable for patients with diabetes.
Owner:天津金世制药有限公司
Sterilizing and inflammation diminishing medicine for trauma in clinical laboratory and preparation method of medicine
InactiveCN104435099AExpelling wind and relieving painHeat-clearing and detoxifyingAntibacterial agentsAntimycoticsProcaineSide effect
The invention relates to a sterilizing and inflammation diminishing medicine for trauma in a clinical laboratory. The medicine comprises the following raw material drugs in parts by weight: 10-20 parts of mint, 8-16 parts of honeysuckle, 1-3 parts of propolis, 3-9 parts of wild chrysanthemums, 5-15 parts of acute turpinia leaves, 12-19 parts of centella, 3-7 parts of fossil fragments, 2-6 parts of lumbricus and 1-5 parts of aspongopus. A small quantity of procaine is added, the raw materials are combined so as to form a sponge with obvious sterilizing and inflammation diminishing effects, and the cost is low. The medicine disclosed by the invention has the efficacies of dispelling wind, relieving pain, clearing heat, removing toxicity, diminishing inflammation and resisting bacteria, and the medicine is suitable for sterilizing and diminishing inflammation for specimen extraction of trauma wound in the clinical laboratory; concerted applications of selected medicinal materials of the medicine disclosed by the invention are appropriate, and a modern advanced pharmaceutical technology is adopted to make the medicine, so that the medicine has the advantages of safe use, zero stimulus, zero toxin and zero side effects, the medicine can kill bacterial propagule, funguses, protozoon and part viruses, and the medicine is worth of wide popularization and application in the clinical laboratory.
Owner:魏国光
Method for preparing procaine
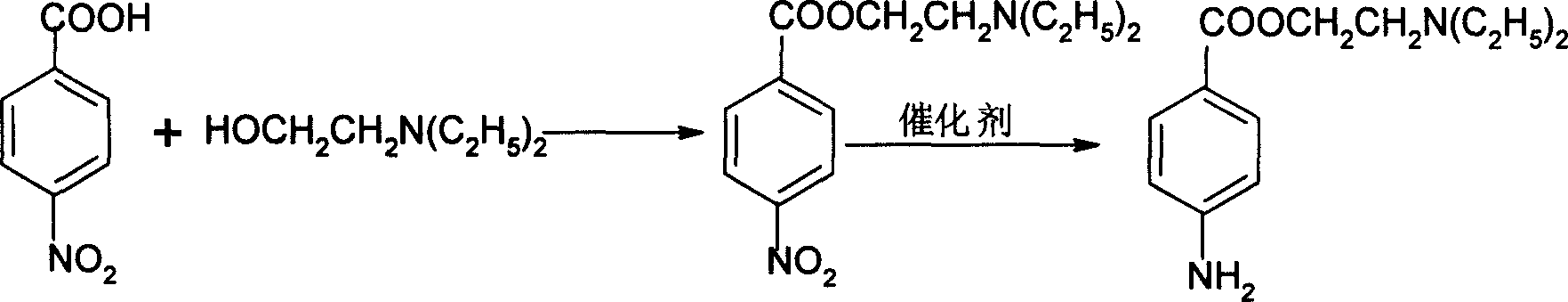
InactiveCN1562961AHigh catalytic activityLow priceOrganic compound preparationAmino-carboxyl compound preparationProcaineP-nitrobenzoic acid
This invention relates to a process for preparing procaine, by using p-nitrobenzoic acid and diethylamino ethanol as raw materials, by esterification to produce nitrocaine, which is then reduced by reductant of hydrogenin xylene solvent with catalyst of nicket compound (Leininie) in suitable temp. and pressure to produce final product-procaine. Advantages are simple process, high transforming rate, high yield, high pureness, short process, reutilization of catalyst, no three wastes.
Owner:NANJING UNIV OF TECH
HPLC measuring method for three ingredients of trinary mycin for injection
The invention relates to an HPLC measuring method for three ingredients of trinary mycin for injection. The HPLC measuring method comprises the following steps: 1), preparation of a reference solution; 2), preparation of a test solution; 3), chromatographic conditions, wherein the chromatographic column is a C18 reversed-phase chromatographic column, the mobile phase is a buffer salt solution-acetonitrile of which the volume ratio is 81:19, the buffer salt solution contains 0.21 mol / L of monopotassium phosphate and 0.16 percent of triethylamine, the pH value is 5.6, the flow velocity is 1.0 ml / min, the detection wavelength is 215 nm, and the sample size is 10 [mu]l; 4), content calculation, wherein the content calculation method adopts the external standard method, and the contents of procaine, dibenzyl ethylene diamine and penicillin in a test sample are calculated according to peak areas. According to the HPLC measuring method, the contents of the three ingredients of the trinary mycin can be measured at the same time, and the HPLC measuring method has the advantages of high repeatability, sensitivity and accuracy, and facilitates standard operation.
Owner:陈汝红
Hemorrhoid injection
InactiveCN1927219ALow cost of treatmentGood treatment effectHydroxy compound active ingredientsPharmaceutical delivery mechanismProcaineMedicine
Disclosed is an injection for treating hemorrhoid, wherein the main constituents include carbolic acid 30-35ml, glycerin 50ml, aqueous solution of saturated alum 15ml, adrenalin 6-10ml, procaine 10-14ml, water 15-30ml and baras camphor 1g.
Owner:胡学勤
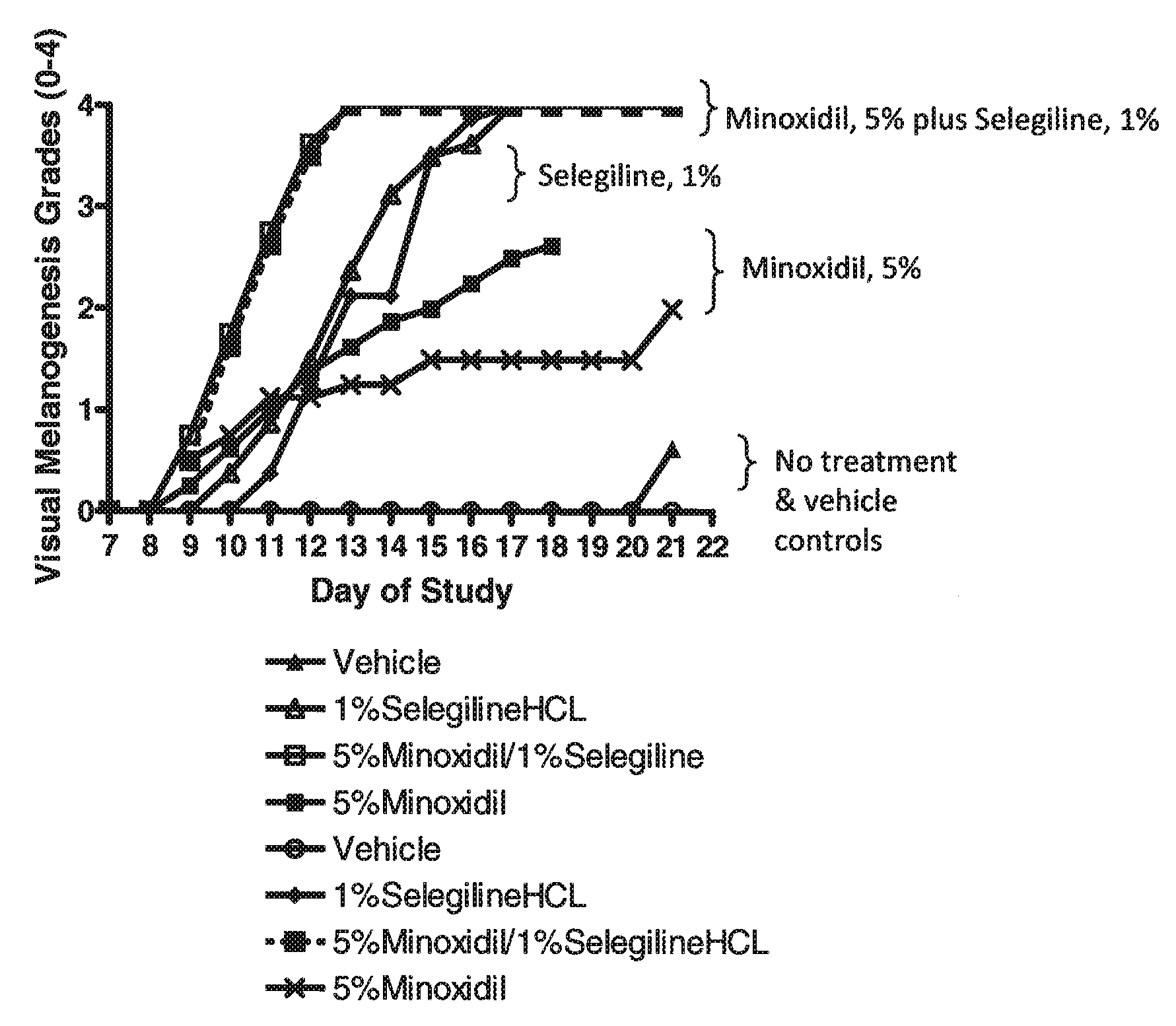
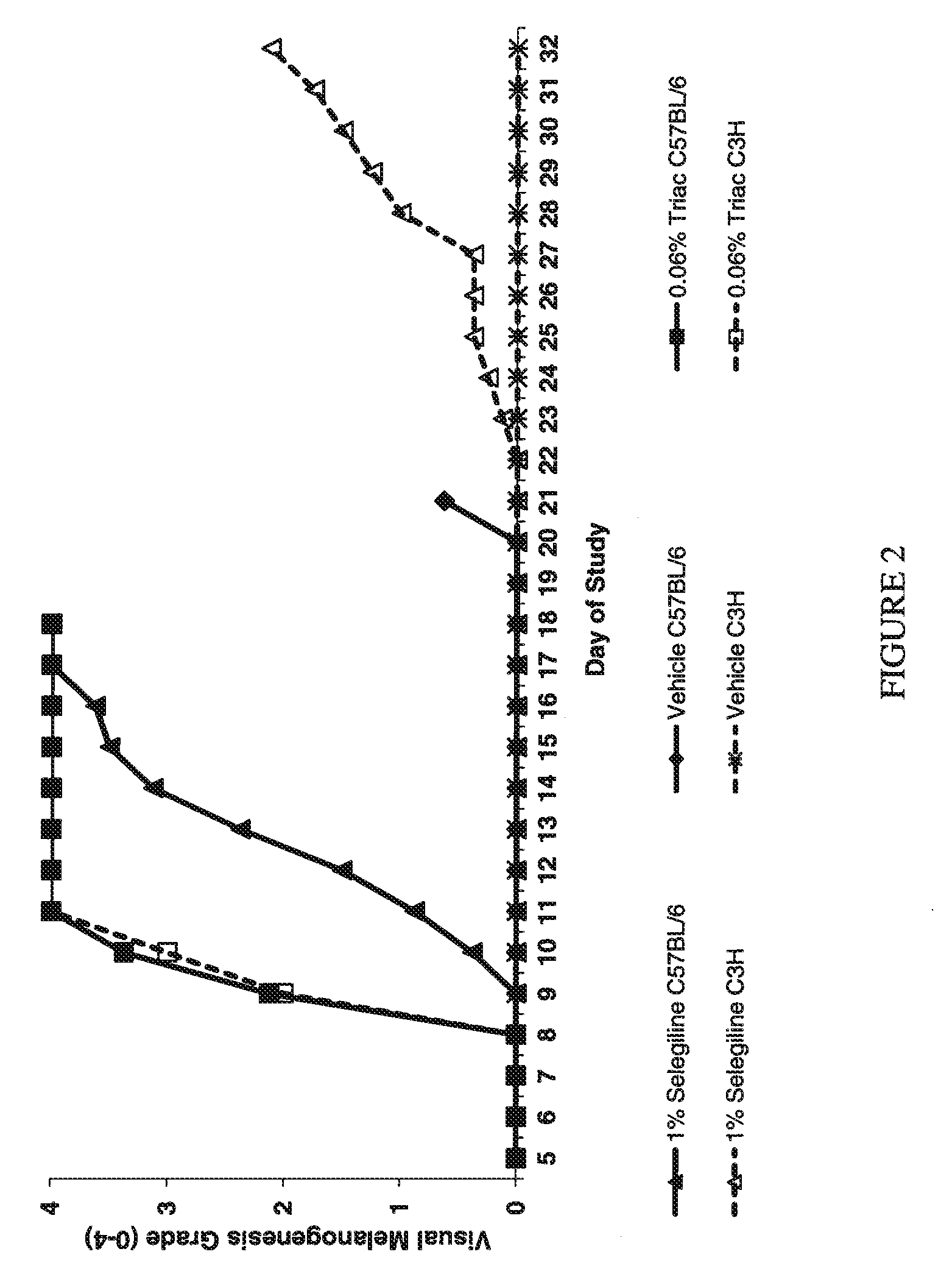
Use of monoamine oxidase inhibitors to improve epithelial biology
The invention provides a method for improving hair biology, e.g., hair growth. The method comprises administering to a subject a monoamine oxidase inhibitor and a vasodilator, a zinc salt of a carboxylic acid, a xanthine compound, pyrithione or a salt thereof, saponin, tritapene, crataegolic acid, celastrol, asiatic acid, an inhibitor of 5-alpha-reductase, 1,4-methyl-4-azasteroid, an androgen receptor antagonist, azelaic acid or a derivate thereof, cyclosporin, triiodothyronine, diazoxide, retinoic acid, a prostaglandin analog, aminexil, carnitine tartrate, apigenin, procapil, or adenosine, in an amount effective to achieve a desired effect. The invention further provides a method of reducing or delaying the appearance of an age-related skin imperfection. The method comprises administering to the subject a composition comprising an MAO inhibitor. A kit for improving hair growth also is provided.
Owner:PROCTER & GAMBLE CO
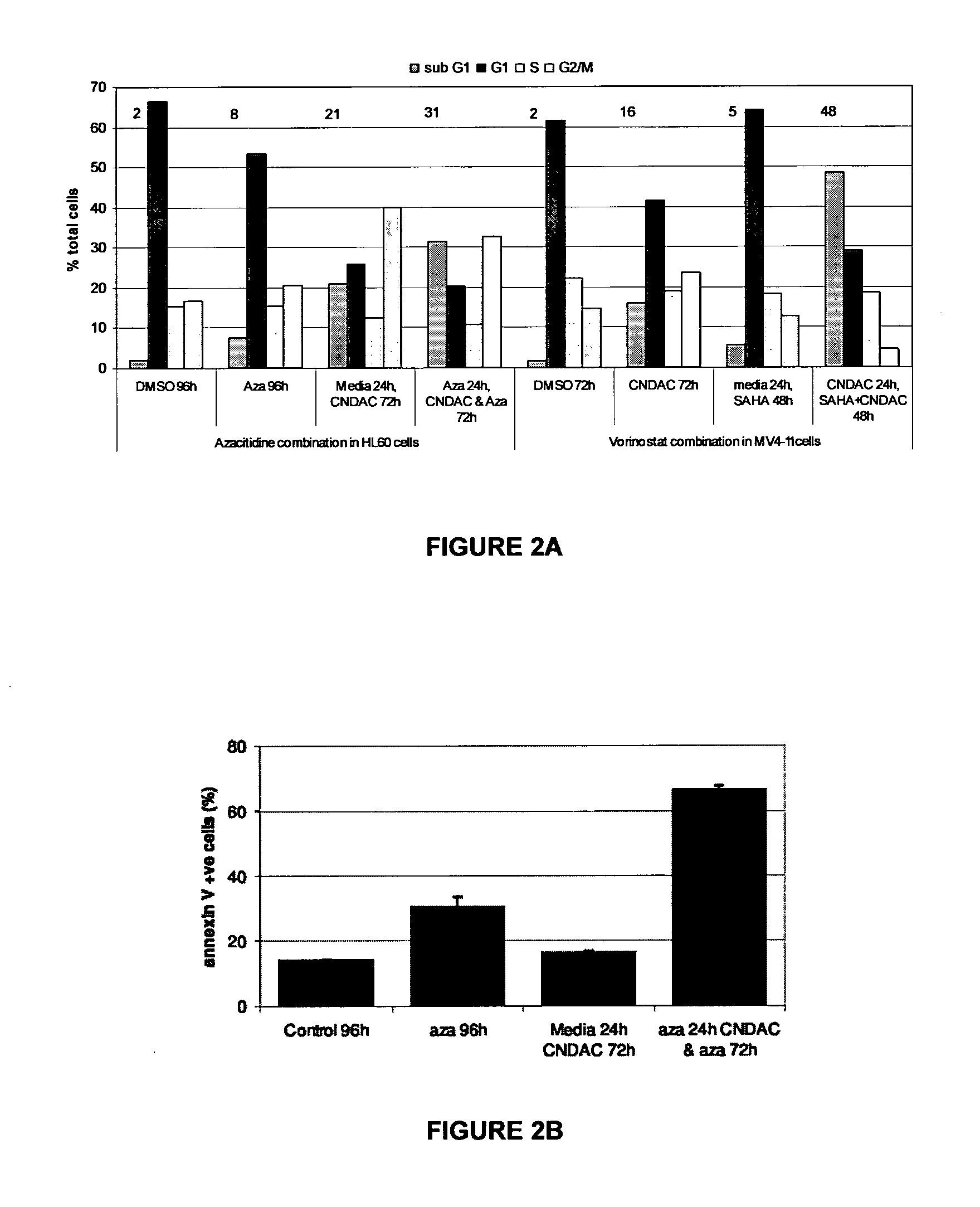
Combinations of sapacitabine or CNDAC with DNA methyltransferase inhibitors such as decitabine and procaine
InactiveUS8530445B2Good effectEase of preparation and detectabilityBiocideCarbohydrate active ingredientsProcaineSapacitabine
A first aspect of the invention relates to a combination comprising a DNA methyltransferase inhibitor and 1-(2-C-cyano-2-dioxy-β-D-arabino-pentofuranosyl)-N4-palmitoyl cytosine, or a metabolite thereof. A second aspect of the invention relates to a pharmaceutical product comprising a DNA methyltransferase inhibitor and 1-(2-C-cyano-2-dioxy-β-D-arabino-pentofuranosyl)-N4-palmitoyl cytosine, or a metabolite thereof, as a combined preparation for simultaneous, sequential or separate use in therapy. A third aspect of the invention relates to a method of treating a proliferative disorder, said method comprising simultaneously, sequentially or separately administering a DNA methyltransferase inhibitor and 1-(2-C-cyano-2-dioxy-β-D-arabino-pentofuranosyl)-N4-palmitoyl cytosine, or a metabolite thereof, to a subject.
Owner:CYCLACEL
HCV Helicase Inhibitors and Methods of Use Thereof
ActiveUS20140227225A1Inhibition of replicationTreat infectionBiocideOrganic chemistryProcaineProteinase activity
The present invention discloses thioflavine S and primuline derivatives which inhibit hepatitis C virus helicase and protease activity. Consequently, the compounds of the present invention interfere with the life cycle of the hepatitis C virus and are useful as antiviral agents. The present invention further relates to pharmaceutical compositions containing the aforementioned compounds and methods of treating an HCV infection.
Owner:UNIVERSITY OF KANSAS +1
Pharmaceutical composition and uses thereof
InactiveCN103861107ABroad antigen spectrumComprehensive antigen spectrumOrganic active ingredientsPeptide/protein ingredientsProcaineDNA Methyltransferase Inhibitor
The invention discloses a pharmaceutical composition and uses thereof. The pharmaceutical composition comprises a DNA methyltransferase inhibitor and a histone deacetylase inhibitor. The invention also discloses new uses of the pharmaceutical composition. The pharmaceutical composition is used to treat tumor cells together to obtain an exosomes tumor vaccine. The invention also discloses a method for preparing a tumor vaccine by using the pharmaceutical composition. The method comprises the following steps of using procaine and MS-275 to treat the tumor cells, and separating and purifying exosomes secreted by the tumor cells. The pharmaceutical composition disclosed by the invention improves the therapeutic effect of the exosomes tumor vaccine, and has an important clinical application value.
Owner:FIRST HOSPITAL AFFILIATED TO GENERAL HOSPITAL OF PLA
Pharmaceutical composition, patch, and preparation methods and application of pharmaceutical composition and patch
PendingCN110038130AGuaranteed thicknessGet Continuous Pain ReliefNervous disorderAntipyreticProcaineBULK ACTIVE INGREDIENT
Owner:张洁
Disinfecting paste for dissolving wart and eliminating wart
The invention relates to cream for dissolving wart, relieving neoplasm and detoxicating, which comprises the following components by weight portion: 0 to 3.0 portions of licorice, 0 to 3.0 portions of scutellaria, 0 to 3.0 portions of phellodendron amurense, 0 to 3.0 portions of rhubarb, 0 to 1.0 portion of menthol, 2 to 60 portions of sodium hydroxide (or potassium hydroxide), 0 to 10 portions of calcium hydroxide, and 0.75 to 1.0 portion of levobupivacaine hydrochloric acid (or toad venom, bupivacaine hydrochloric acid, lidocaine, dicaine or procaine or other local anesthetics). The sodium hydroxide (or the potassium hydroxide) and the calcium hydroxide of proper concentration can quickly dissolve protein, and melt the wart, the neoplasm and various inflammatory tissues in time from 1 minute to tens of minutes; the scutellaria, the phellodendron amurense and the rhubarb resist bacteria and virus; the licorice and the scutellaria adjust immune function, and inhibit adverse tissue reaction; the toad venom has anti-inflammatory, anti-cancer and local anesthetic functions; the menthol is fresh and cool, and can deodorize and stop pain; and the levobupivacaine hydrochloric acid or other local anesthetics has local anesthetic and paregoric functions.
Owner:谭国梁
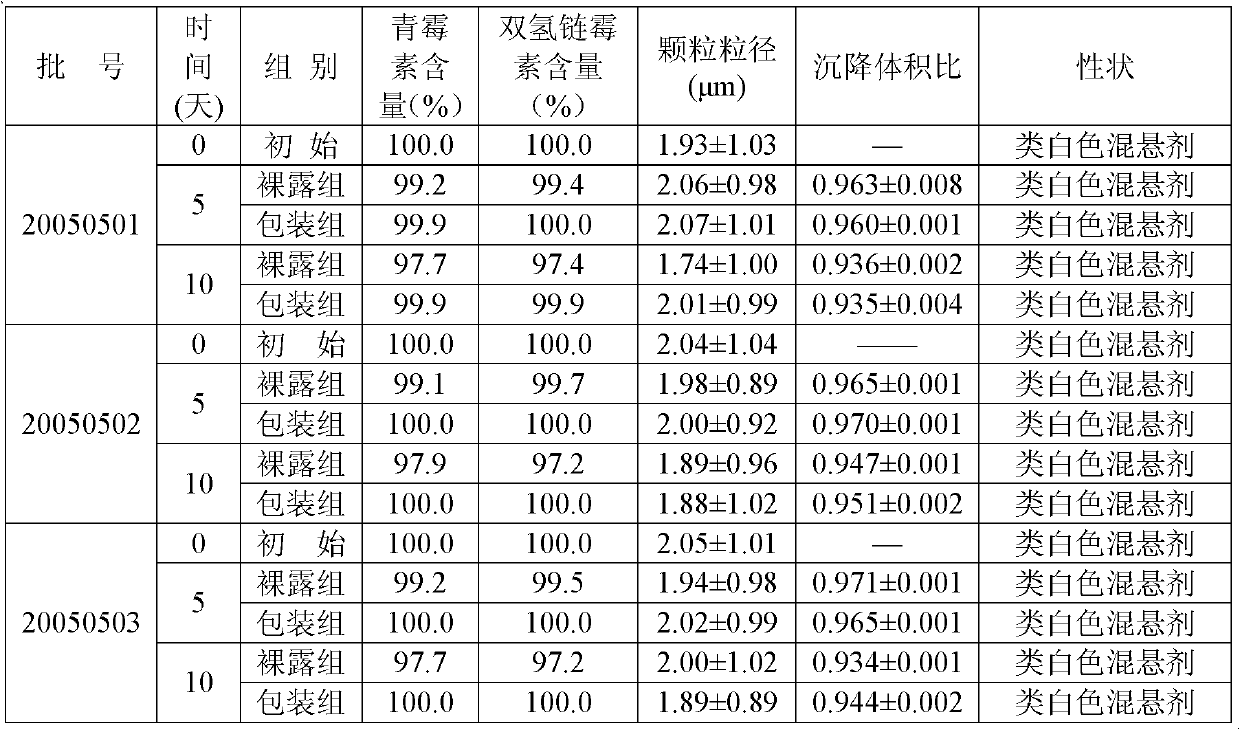
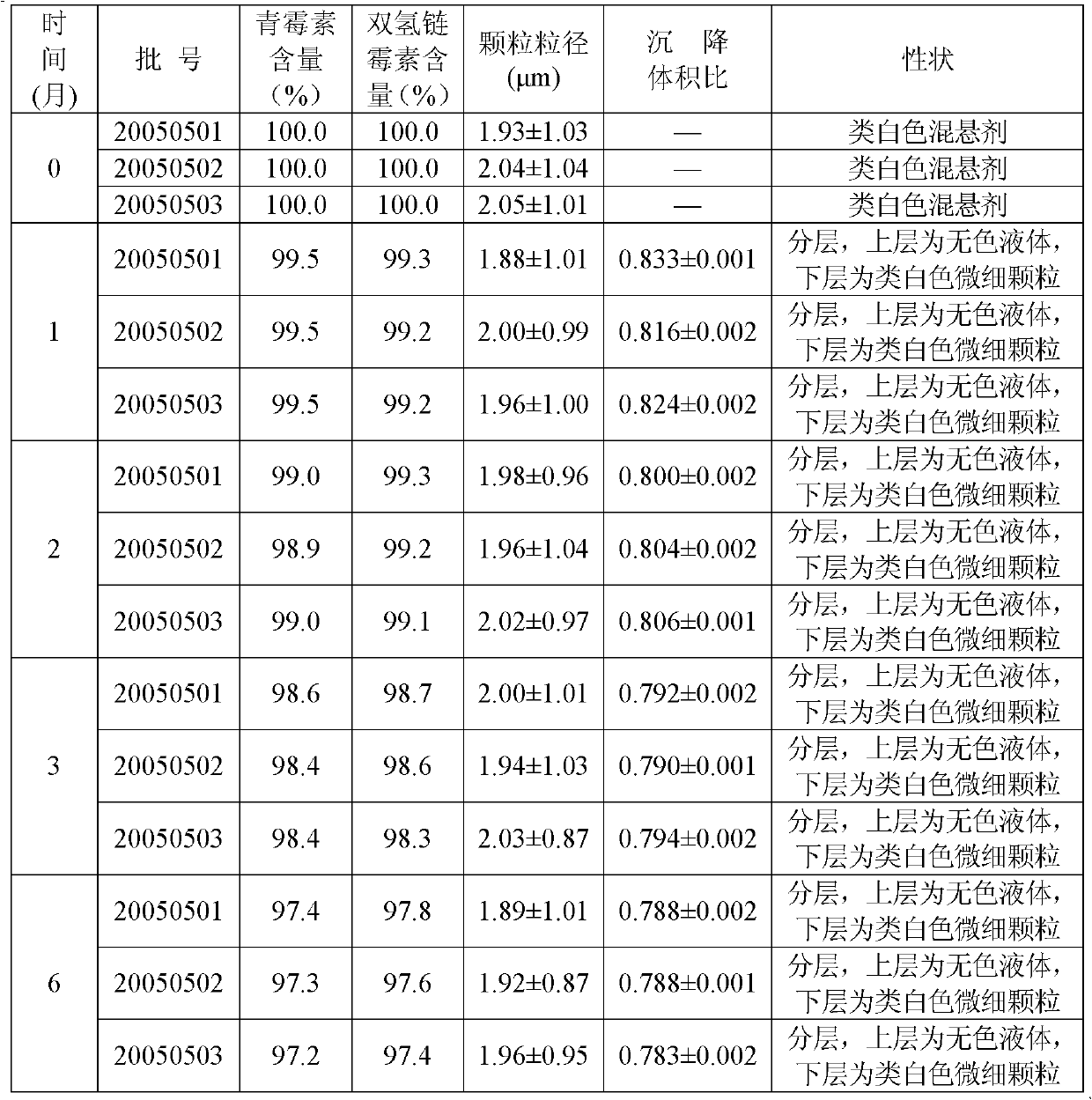
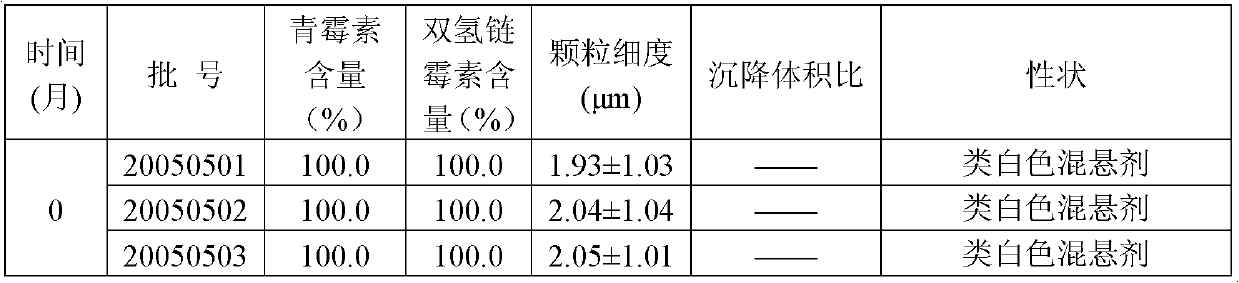
Veterinary procaine penicillin-dihydrostreptomycin sulfate suspension injection and preparation method thereof
InactiveCN103301140AImprove convenienceExpanded antimicrobial spectrumAntibacterial agentsSolution deliveryProcaineAntioxidant
The invention relates to a veterinary drug and particularly relates to a veterinary procaine penicillin-dihydrostreptomycin sulfate suspension injection and a preparation method thereof. The injection comprises 5%-30% (w / v) of procaine penicillin, 5%-30% (w / v) of dihydrostreptomycin sulfate, a proper dosage of buffer solution, 0.02%-0.2% (w / v) of a preservative, 0.5%-15% (w / v) of a dispersing agent, 0.2%-20% (w / v) of a suspending agent, 0.5%-2% (w / v) of a stabilizer and local analgesic and 0.02%-0.5% (w / v) of an antioxidant, wherein the dispersion medium is injection water. According to the invention, the procaine penicillin and the dihydrostreptomycin sulfate are creatively formed to a compound preparation, which not only improves the application convenience of the two drugs, but also expands the antibacterial spectrum of the drugs to greatly facilitate the clinical use of veterinaries, and meanwhile, specific ingredients are selected and utilized to solve the problem of preparing the suspension injection with water as medium.
Owner:CHONGQING FANGTONG ANIMAL PHARMA
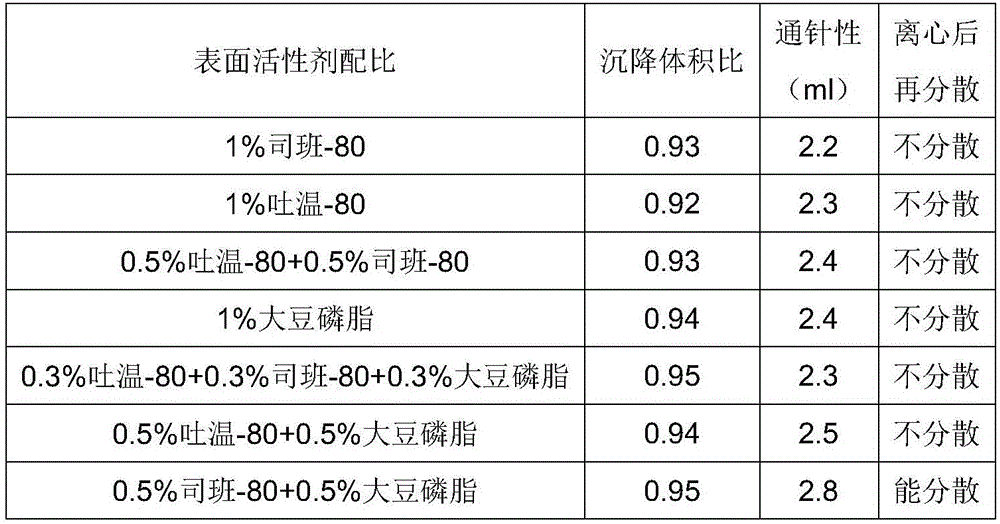
Oil suspension with procaine benzylpenicillin and method for preparing oil suspension
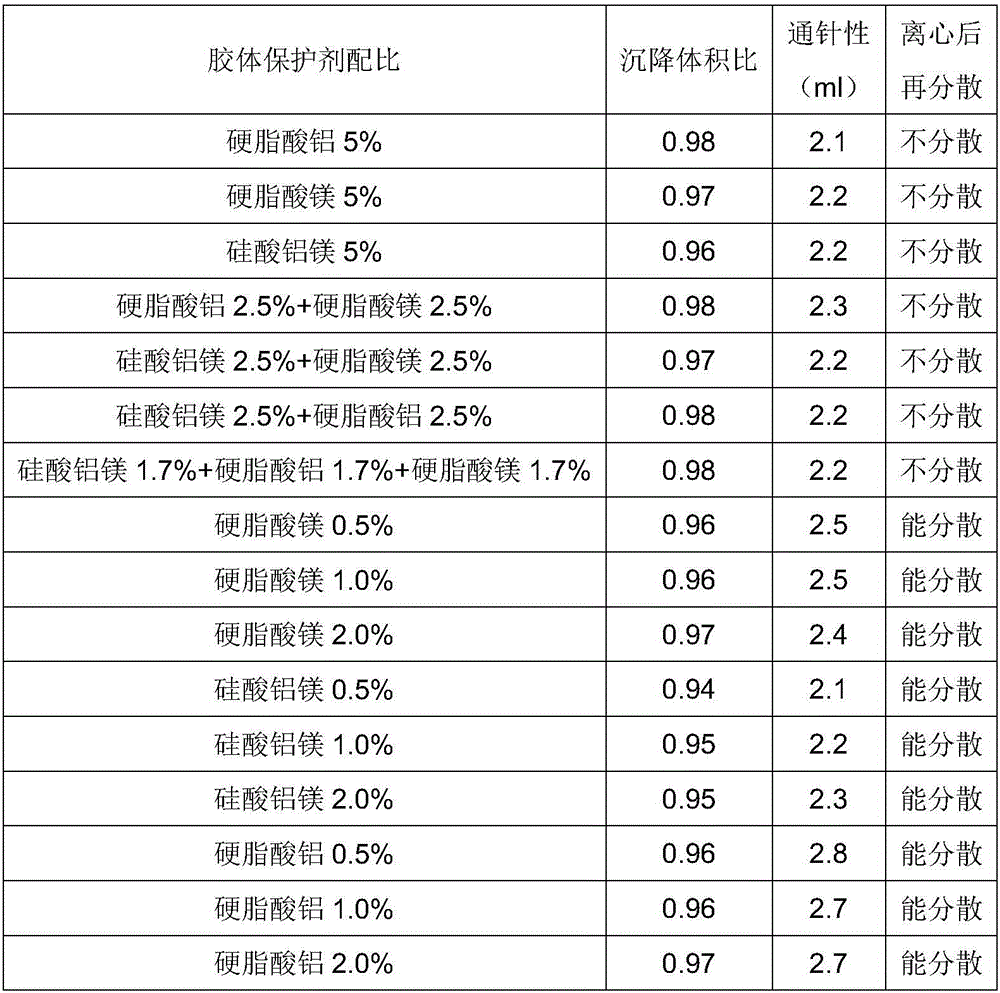
InactiveCN106420608ASedimentation volume ratio is highGood acupunctureAntibacterial agentsAntimycoticsProcaineAntioxidant
The invention provides oil suspension with procaine benzylpenicillin. Every 100 mL of oil suspension contains raw and auxiliary materials including 5-45 g of procaine benzylpenicillin, 0.5-2 g of surfactants, 0.5-2 g of colloid protective agents, 2-5 g of suspending agents, 0-0.1 g of antioxidants and the balance oil phases. The oil suspension has the advantages that the oil suspension is high in sedimentation volume ratio and excellent in syringeability and re-dispersibility; deterioration of the quality of the oil suspension is unseen after the oil suspension is stored in environments at the temperatures of 4-60 DEG C for 3 months, and accordingly the oil suspension is stable in quality and has an excellent clinical application prospect.
Owner:CHENGDU QIANKUN VETERINARY PHARMA
Method for preparing medicinal composition for perianal anaesthetic analgesia of postoperation
InactiveCN102552405APromote absorptionCertain curative effectAntibacterial agentsHydroxy compound active ingredientsProcaineHigh absorption
The invention discloses a method for preparing a medicinal composition for the perianal anaesthetic analgesia of postoperation. The medicinal composition comprises the following raw materials: procaine, eugenol, peach gum, borneol, litsea cubeba oil, thalicimine and methyl eugenol, and is characterized in that: the method for preparing the medicinal composition comprises the following steps of: adding the peach gum into isopyknic water, adding the thalicimine which is sieved with a 200-mesh sieve, and stirring continuously for 30 minutes; adding the litsea cubeba oil, eugenol and the methyl eugenol at room temperature, and continuing to stir for 15 minutes; and adding the procaine and the borneol which is sieved with the 200-mesh sieve, continuing to stir for 15 minutes and packaging intoseparated bags. Clinical experiments prove that the medicinal composition for treating the perianal anaesthetic analgesia of the postoperation has the characteristics of high absorption, definite curative effect, high safety, and is worthy of clinical application and popularization.
Owner:张伟
Mouth wash capable of preventing and treating dental ulcer
InactiveCN106924553APrevent recurrencePromote healingCosmetic preparationsHydroxy compound active ingredientsProcaineSandalwood oil
The invention discloses mouth wash capable of preventing and treating dental ulcer. The mouth wash is prepared from the following components in parts by weight: 8-12 parts of hydroxyethyl cellulose, 4-6 parts of metronidazole, 0.1-0.3 part of procaine, 0.1-0.3 part of hexadecadrol, 6-10 parts of polyving akohol, 3-5 parts of oleum menthae, 3-5 parts of succus aloes folii siccatus, 4-6 parts of borneol, 8-10 parts of honeysuckle flowers, 4-6 parts of rhizoma coptidis, 4-6 parts of radix isatidis, 4-6 parts of folium isatidis, 3-5 parts of radix zanthoxyli, 2-4 parts of sandalwood oil, 5-9 parts of bamboo salt, 0.8-1.2 parts of vitamin B12, and 80-100 parts of deionized water. For the mouth wash, the method of combining the traditional Chinese medicine and the western medicine is adopted, and the Western medicinal ingredients are utilized for rapidly killing bacteria and diminishing inflammation, relieving swelling and pain, and repairing the mucosal tissue; the traditional Chinese medicinal ingredients are utilized for clearing away heat and toxic materials, cooling blood and purging fire, and consolidating the curative effects, so that the repeated attack of dental ulcer is prevented. The mouth wash can be used by replacing medicines in daily life, the healing of ulcer is accelerated, and the relapse of dental ulcer is prevented.
Owner:QINGDAO ZHITONG SIHAI FURNITURE DESIGN RES & DEV CO LTD
HPLC-ELSD detection method of gentamicin C component content in gentamicin procaine vitamin B12 capsules
The invention belongs to the chemical detection field and especially relates to an HPLC-ELSD detection method of a gentamicin C component content in gentamicin procaine vitamin B12 capsules. The method comprises the following steps of (1) carrying out a chromatographic condition and system suitability test; (2) preparing a solution; and (3) testing and the like. Operation of the method is simple,detection efficiency is high, an error is small, and the detection method is stable; accuracy, precision, specificity, a detection limit, and durability and the like of the method accord with a methodological verification requirement; high practicality is achieved; and the gentamicin C component content in the gentamicin procaine vitamin B12 capsules can be rapidly detected so as to effectively control the quality of the gentamicin procaine vitamin B12 capsules.
Owner:广西壮族自治区食品药品检验所
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com