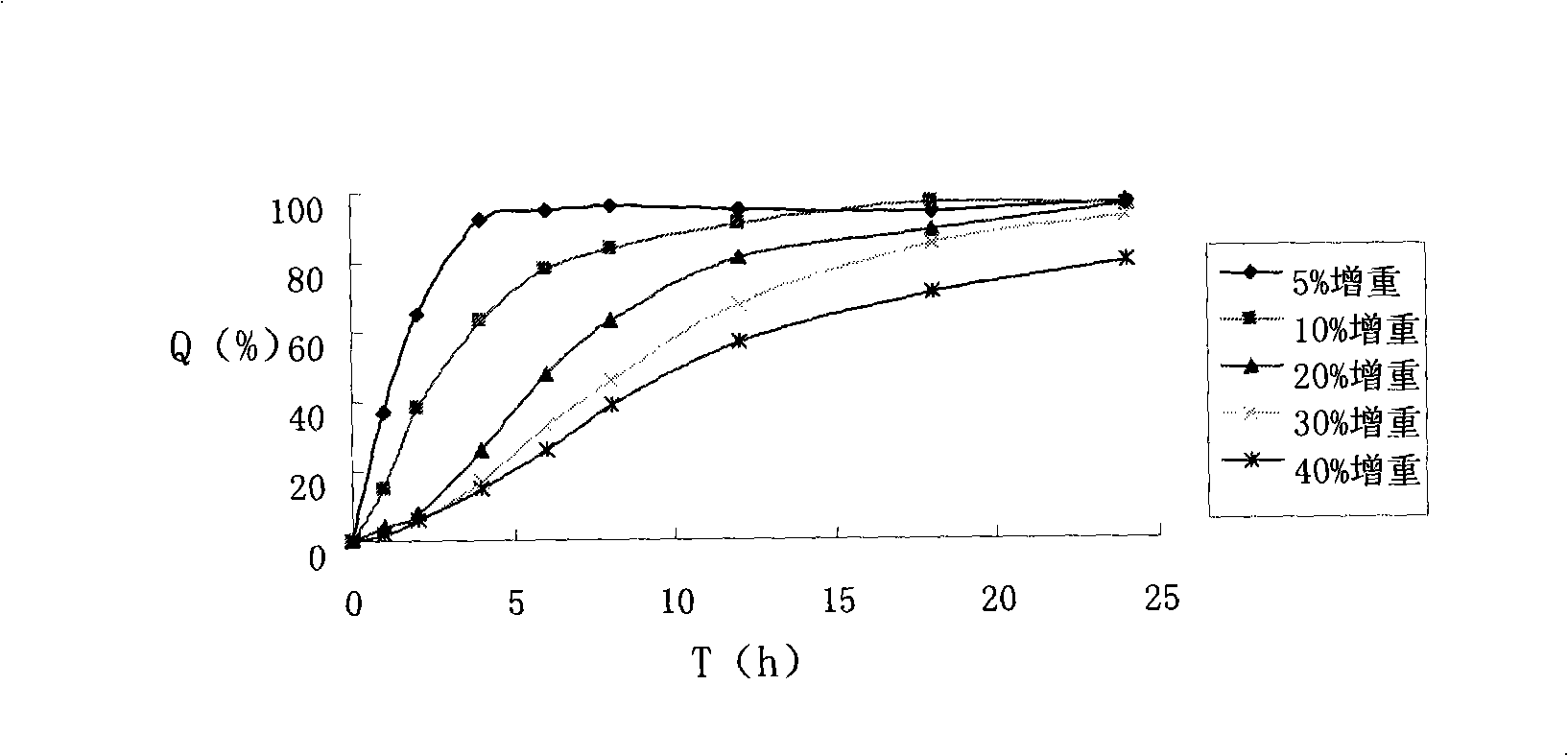
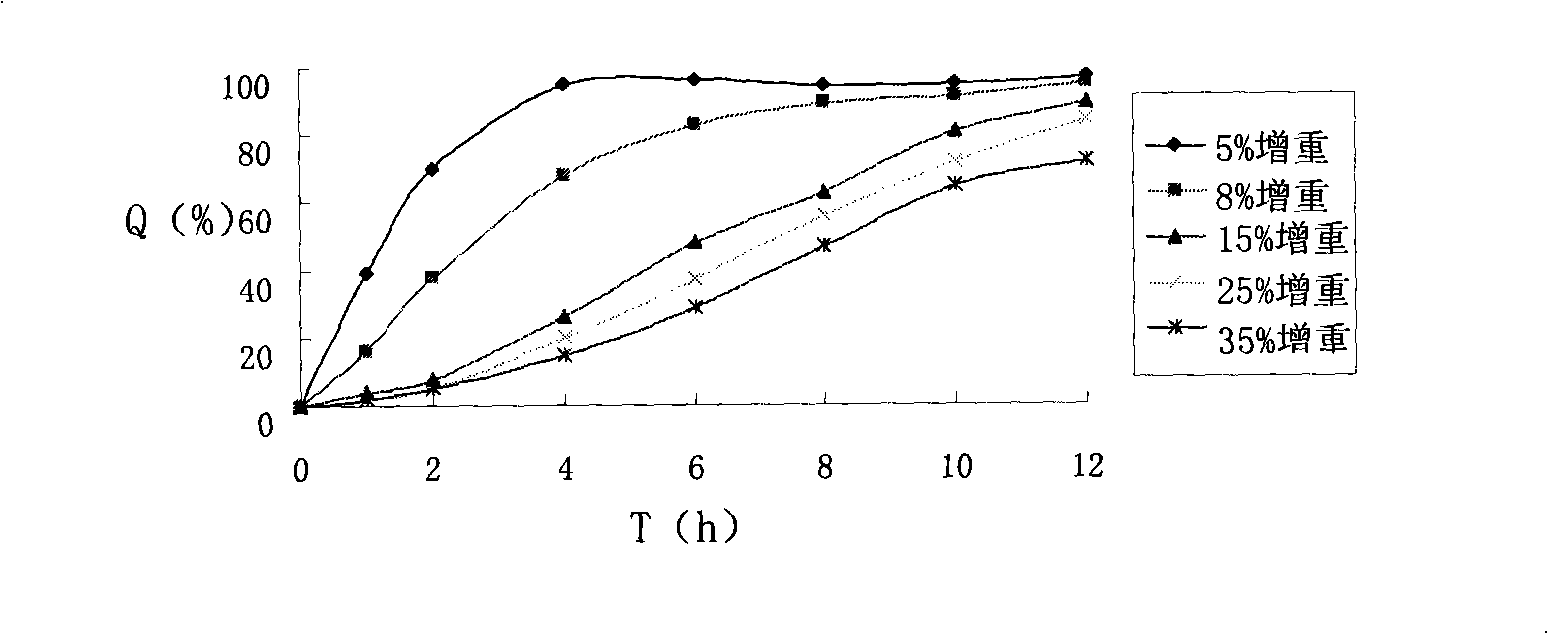
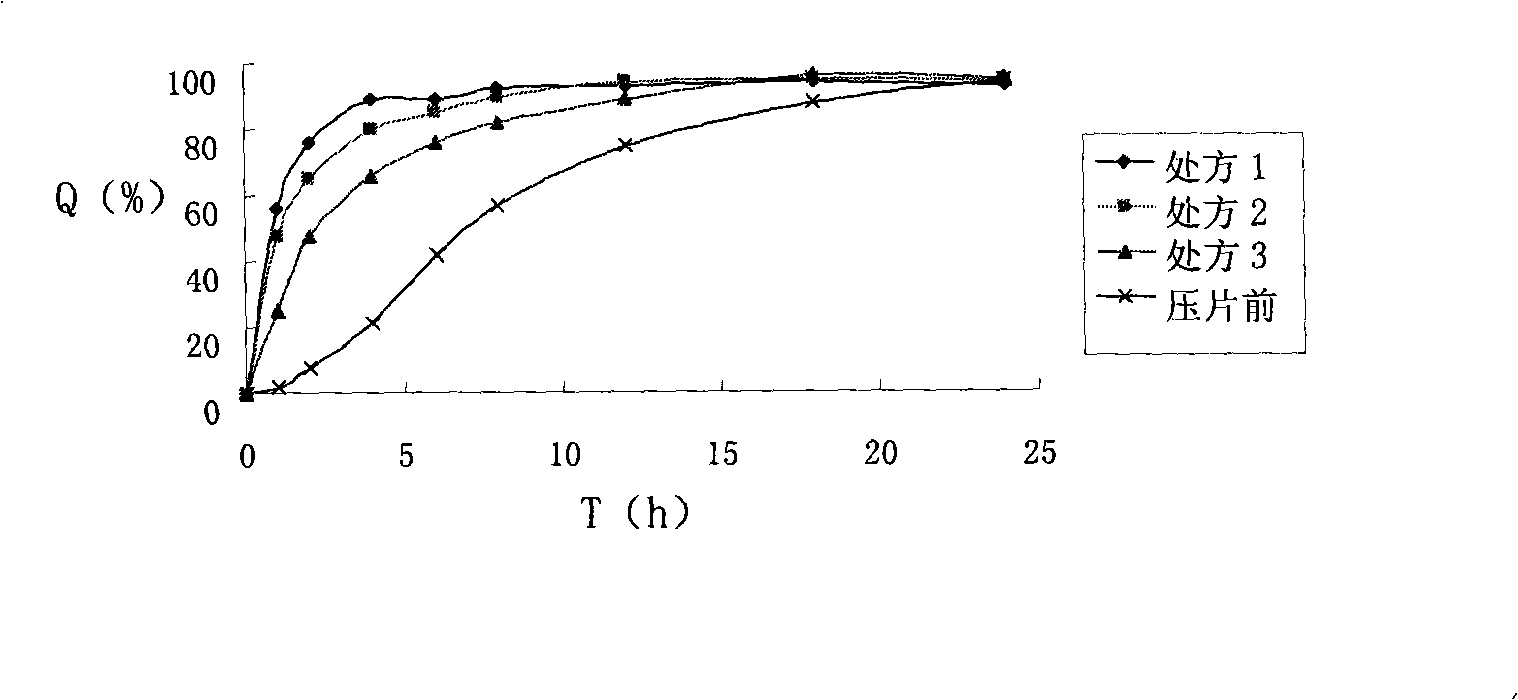
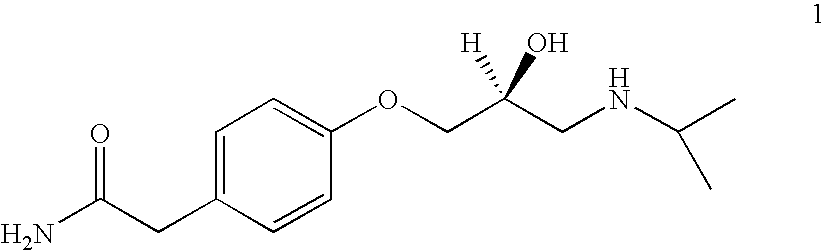
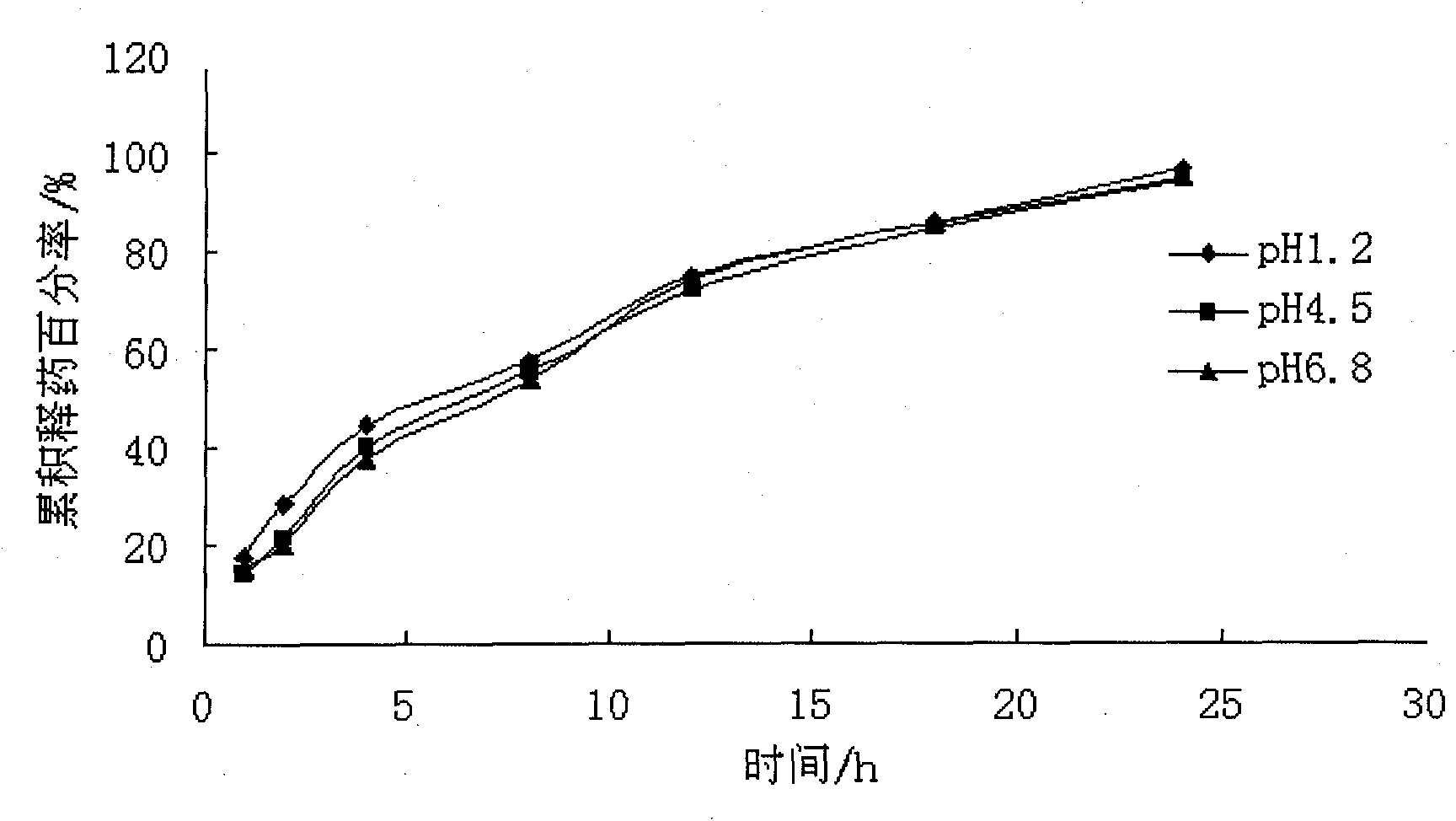
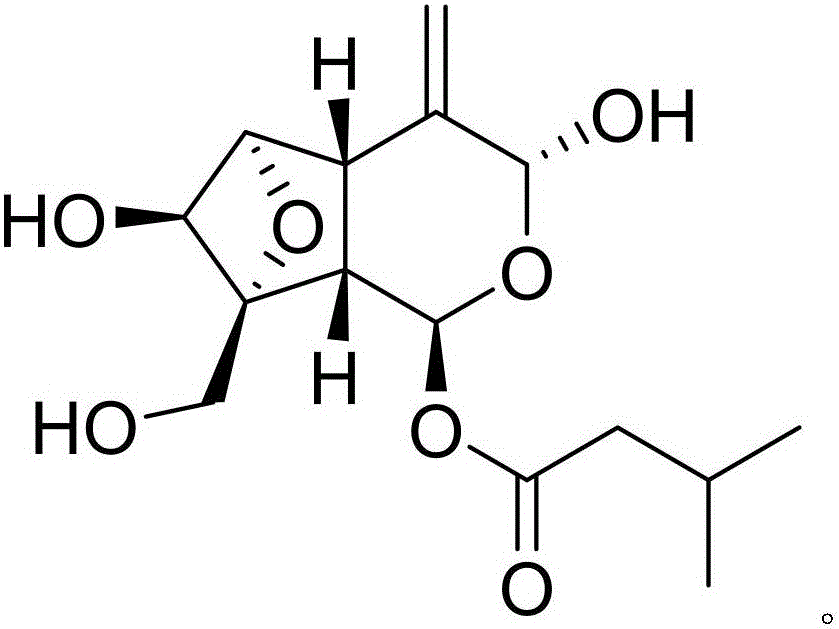
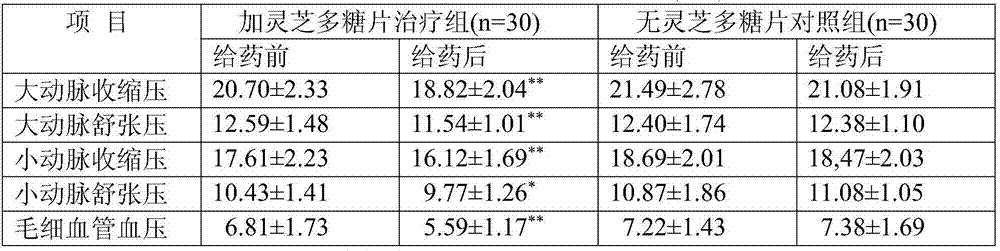
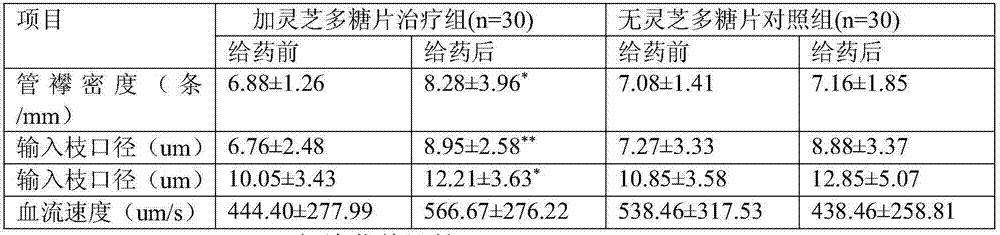
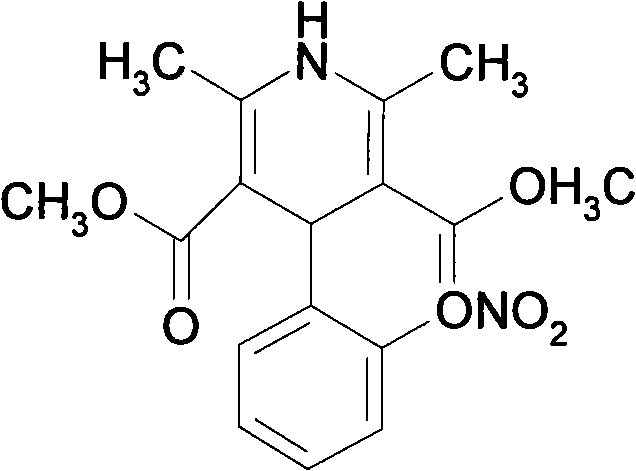
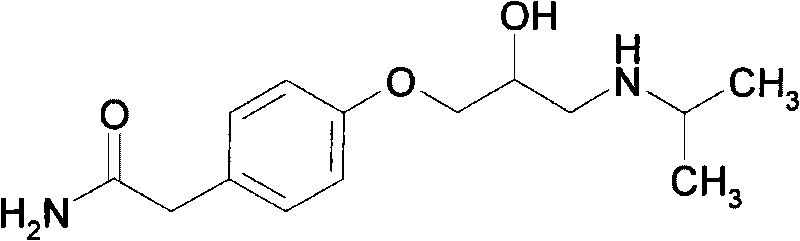
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
74 results about "Atenolol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Atenolol is used with or without other medications to treat high blood pressure (hypertension).
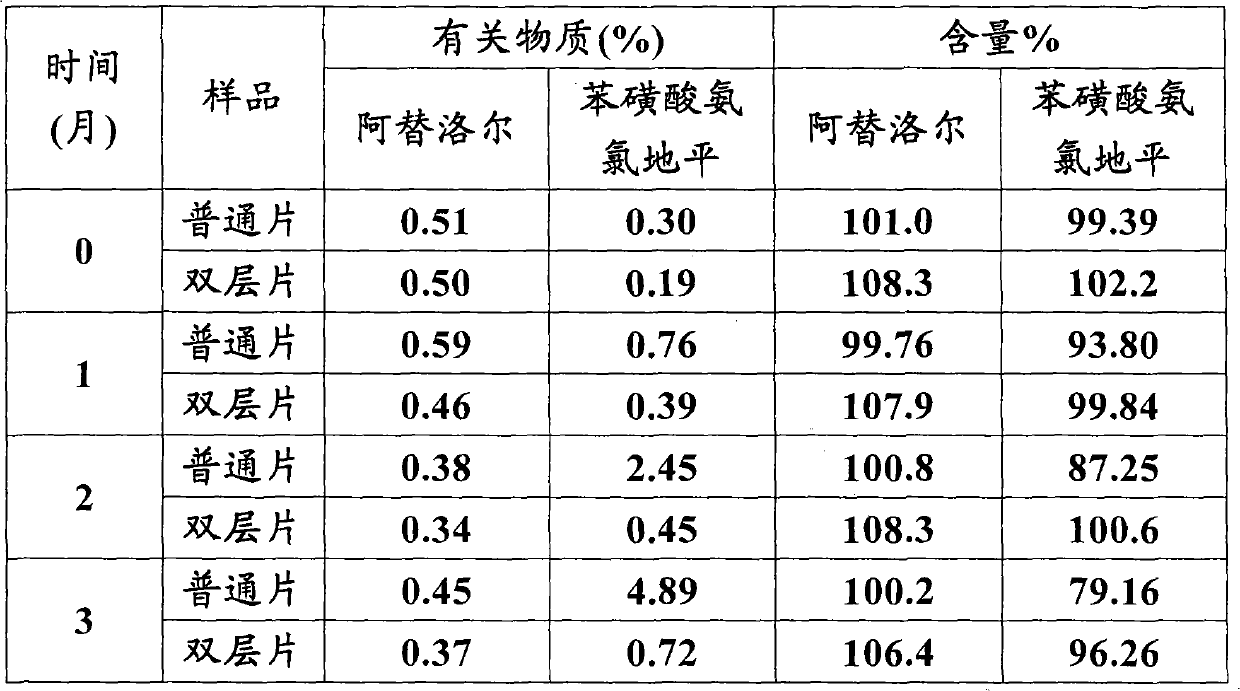
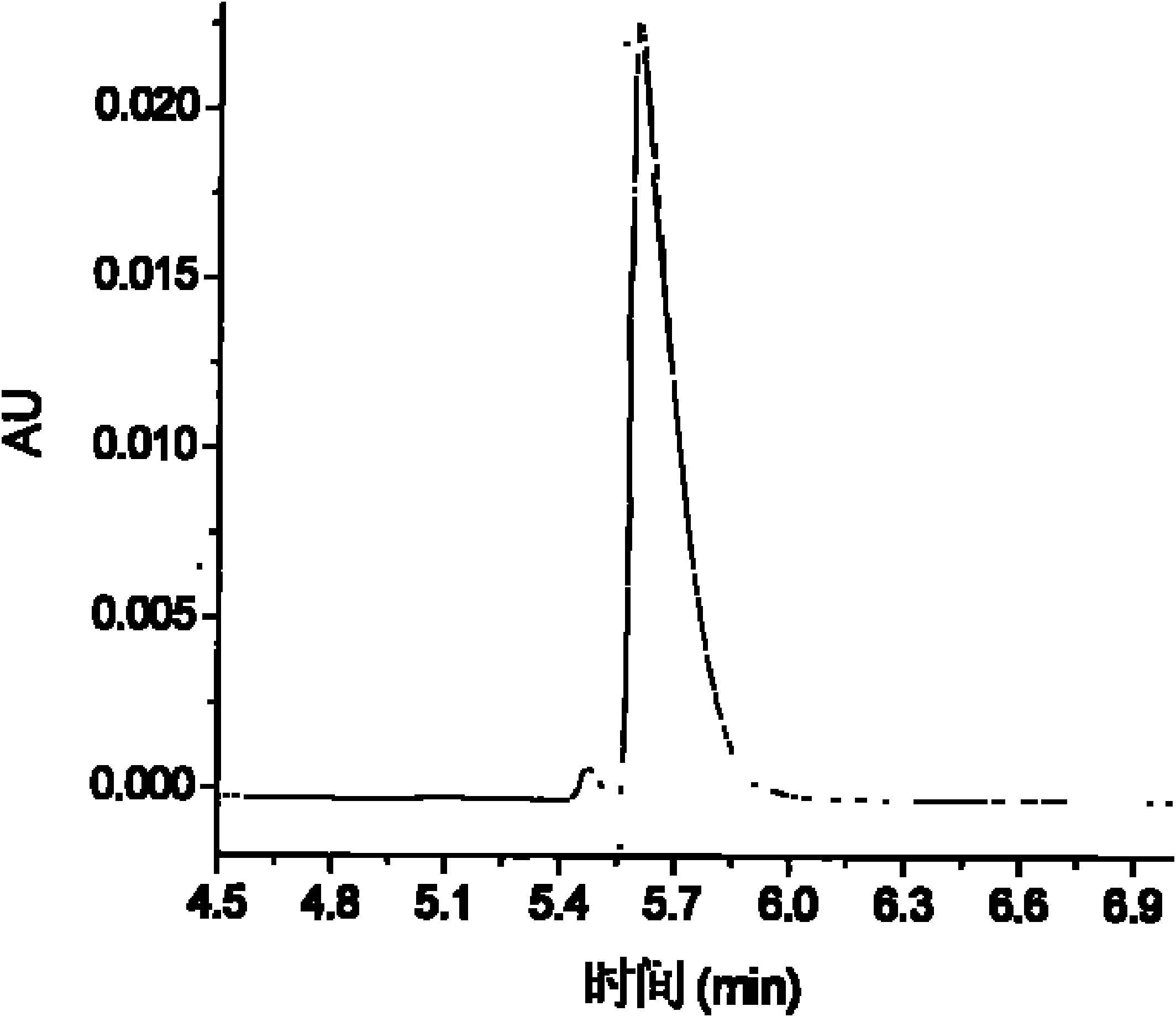
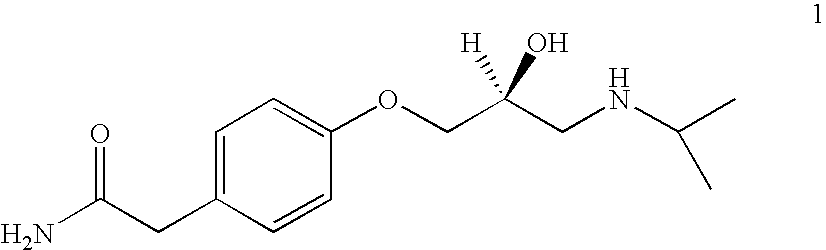
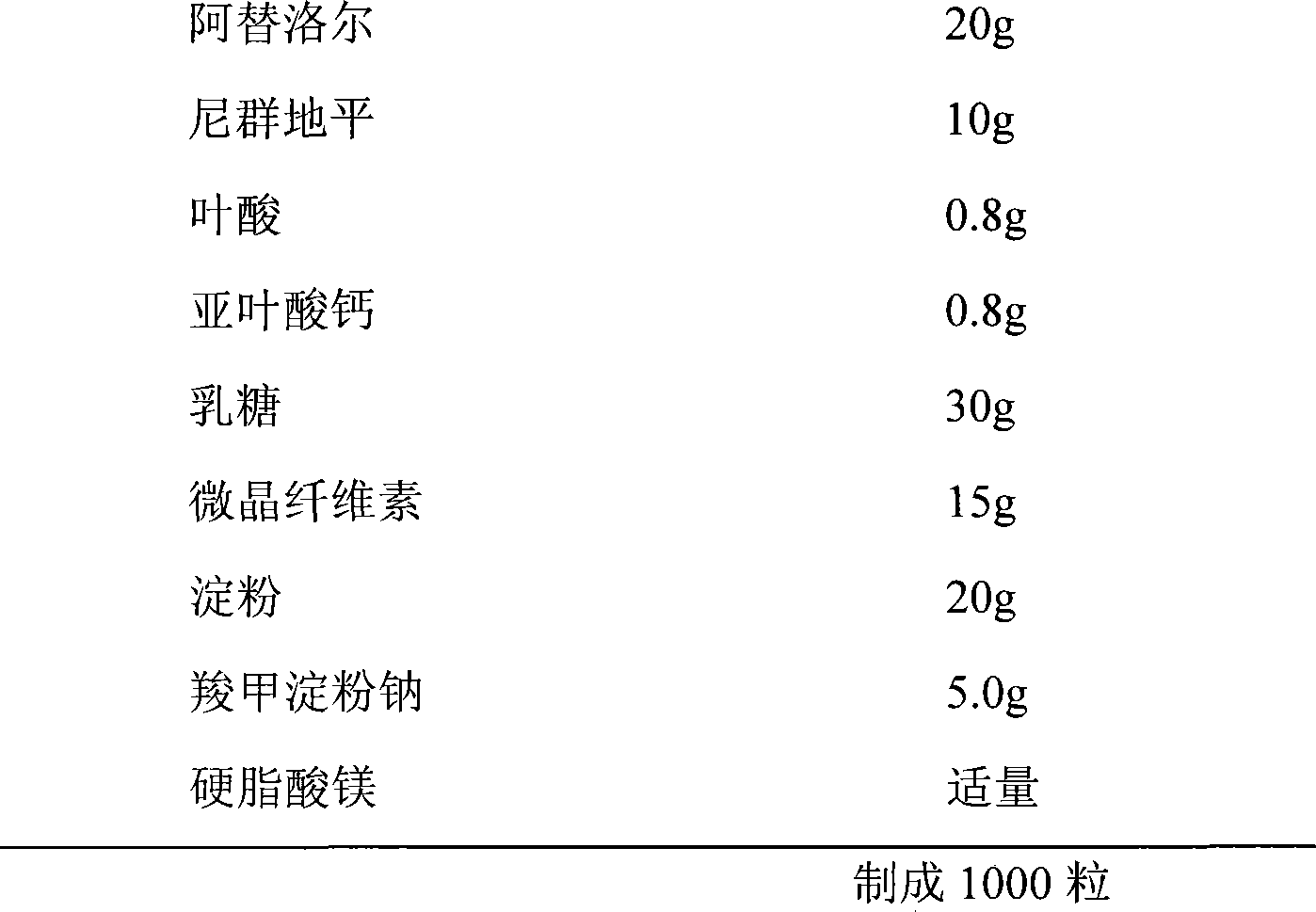
Atenolol and amlodipine bilayer tablet
ActiveCN102085201AOvercome unsatisfactory dissolutionOrganic active ingredientsPill deliveryDissolutionAmlodipine
The invention relates to an atenolol and amlodipine bilayer tablet. Particularly, the invention relates to a bilayer tablet comprising a first tablet layer and a second tablet layer, wherein the first tablet layer contains atenolol as an active constituent and a pharmaceutically acceptable excipient or accessory, and the second tablet layer contains amlodipine as an active constituent or a pharmaceutically acceptable salt thereof and a pharmaceutically acceptable excipient or accessory. The invention also provides a preparation method of the bilayer tablet. The bilayer tablet provided by the invention not only has favorable stability, but also has favorable dissolution rate.
Owner:CHINA RESOURCES DOUBLE CRANE PHARMA COMPANY
Bis-[6-oxa-(2-carboxylbenzenesulfonyl-butanedioic acid 1,4 monoester-4)-beta-cyclodextrin, preparation method and application thereof
ActiveCN101928356AAchieve separationComponent separationMaterial analysis by electric/magnetic meansProcaineButanedioic acid
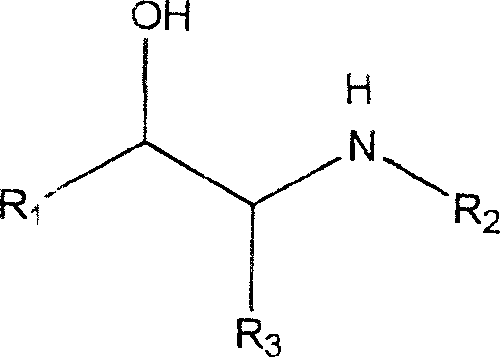
The invention relates to bis-[6-oxa-(2-carboxylbenzenesulfonyl-butanedioic acid 1,4 monoester-4)-beta-cyclodextrin, a preparation method and application thereof as chiral selective agent in high performance capillary electrophoresis (HPCE), namely being prepared into a chiral electrophoresis column and a mobile phase chiral additive for detachment of chiral substances. The molecular formula of the bis-[6-oxa-(2-carboxylbenzenesulfonyl-butanedioic acid 1,4 monoester-4)-beta-cyclodextrin is determined to be C64H84O49S2; beta-CD-B2 is used as the HPCE mobile phase chiral additive to separate chiral substances of phenylglycinol, anisodamine, isoprenaline and propafenone to realize baseline separation; beta-CD-B2 is used for preparing a chiral HPCE column to separate chlortrimeton, bupivacaine, procaine, atenolol, anisodamine, propafenone, lobeline and other chiral substances to realize the baseline separation. Therefore, a novel HPCE quantitative measurement method for a single enantiomerof various chiral substances can be established.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Compound apigenin nanoemulsion antihypertensive drug
InactiveCN102631405AEvenly distributedSystem transparencyOrganic active ingredientsEmulsion deliveryActive agentApigetrin
The invention discloses a compound apigenin nanoemulsion antihypertensive drug which comprises the following raw materials in mass percent: 1-15% of apigenin, 5-35% of surfactant, 1-20% of cosurfactant, 1-20% of oil, 0.5-10% of motherwort fruit aqueous extract, 0.5-10% of semen cassiae water extract and the balance of distilled water; and the sum of mass percents of the above raw materials is 100%. The nanoemulsion is small in emulsion droplet, uniform in distribution, low in viscosity and good in liquidity. After apigenin is prepared into a nanoemulsion, blood brain barrier transmission capacity is obviously increased, the bioavailability of a technical material is obviously improved, the half-life period of the drug can be prolonged; and the drug administration times can be reduced; with adoption of the nanoemulsion, the dissolution and infiltration capacity of atenolol can be improved and the stability of apigenin is increased; and the nanoemulsion has obvious synergistic effect compounded with the motherwort fruit and the semen cassiae; and the medicinal cure effect can be more fully played.
Owner:NORTHWEST A & F UNIV
Compound Atenolol-Nifedipine slow releasing prepn
InactiveCN1452966AEasy to take medicineAvoid missing dosesOrganic active ingredientsPharmaceutical delivery mechanismActive componentMedicine
The present invention relates to compound slow-release Atenolol-Nifedipine preparation and its medical application. The compound delayed preparation has active components Atenolol and Nifedipine anddelaying material for delayed release. The preparation includes also pharmacologically acceptable carrier.
Owner:GUANGZHOU PUIS PHARMA FACTORY
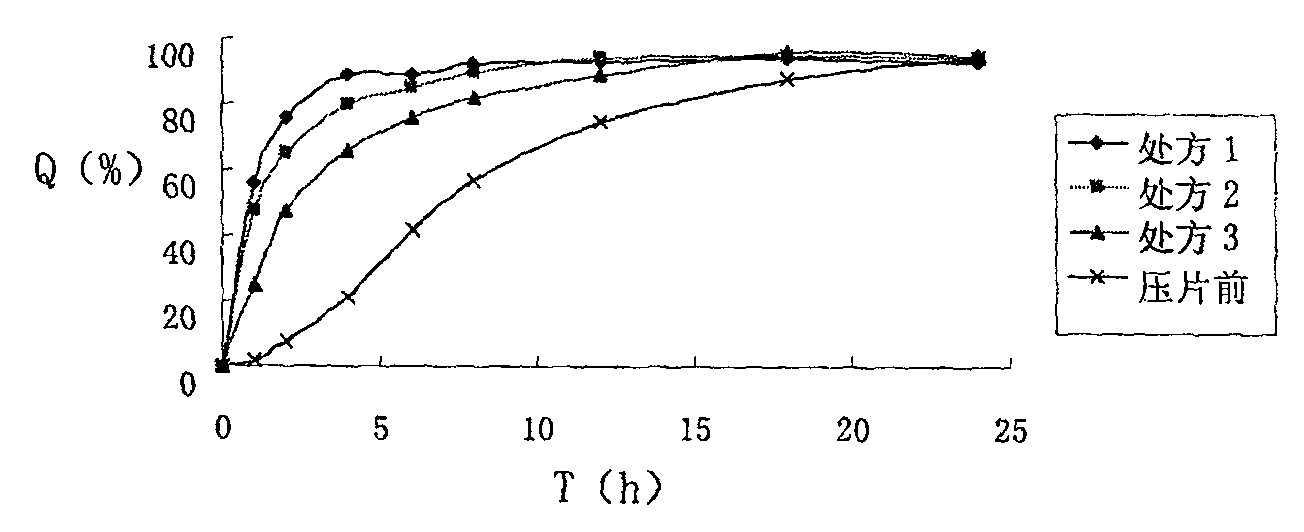
Compound sustained-release pellet tablet containing nifedipine and atenolol and preparation thereof
The invention relates to the pharmaceutical preparation field, in particular to a compound controlled release pellet tablet of nifedipine and atenolol, and is characterized in that: controlled release pellet of nifedipine, controlled release pellet of atenolol and blank pellet are pelletized to obtain the compound controlled release pellet tablet of nifedipine and atenolol, wherein the controlled release pellet of nifedipine and the controlled release pellet of atenolol are respectively obtained from drug contained core coated with acrylic resin; the weight of coating of the controlled release pellet of nifedipine is added by 10 to 30 percent while the weight of coating of the controlled release pellet of atenolol by 8 to 25 percent; the blank pellet comprises microcrystaline cellulose and one of macrogol 6000, macrogol 4000 and stearic acid or the mixture of one or two of the macrogol 6000, the macrogol 4000 and the stearic acid, wherein the blank pellet takes up 50 to 70 percent of the total weight of the compound controlled release pellet tablet of nifedipine and atenolol.
Owner:CHINA PHARM UNIV
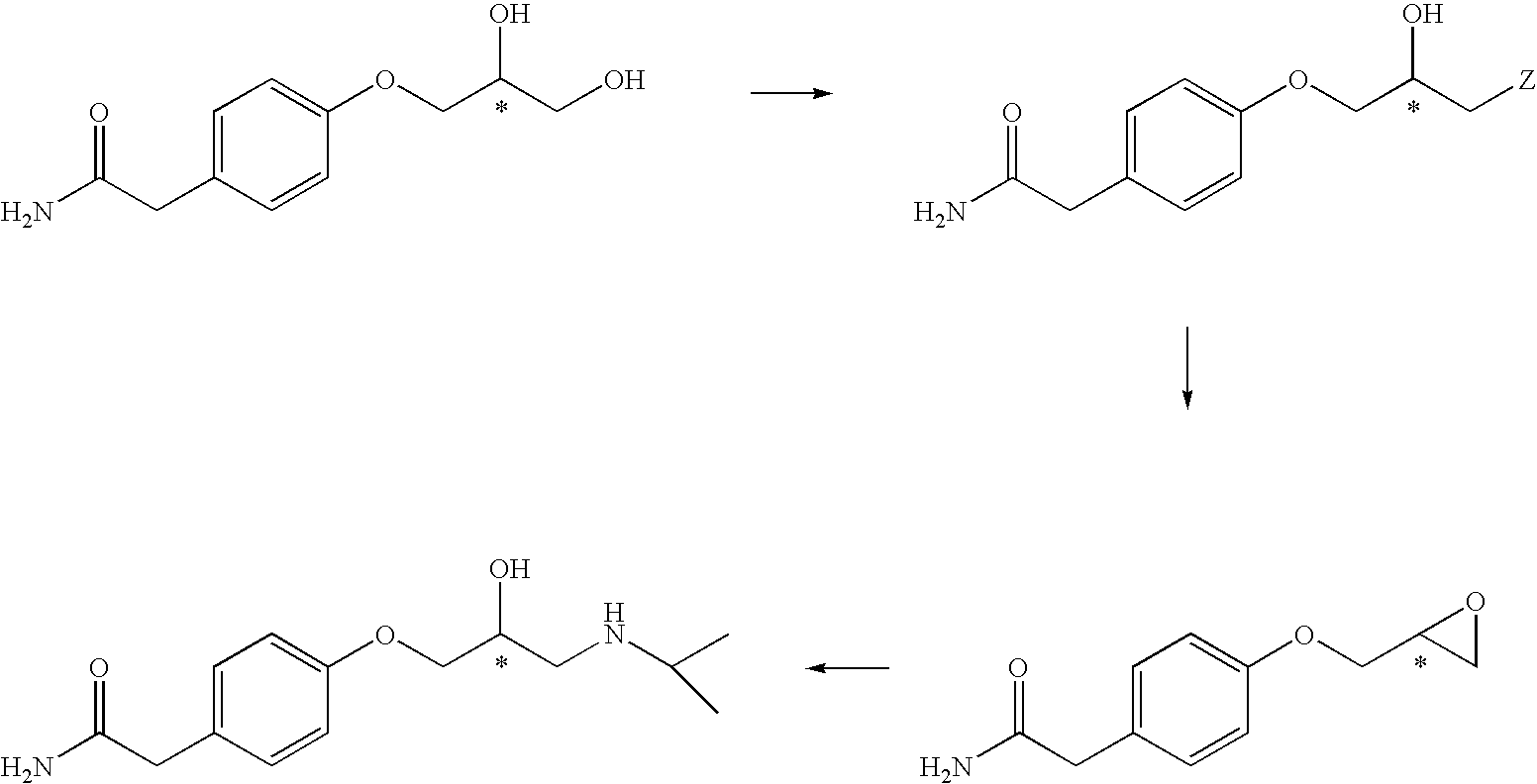
Process for producing atenolol of high optical purity
InactiveUS6982349B1High optical purityHigh yieldOrganic compound preparationCarboxylic acid amides optical isomer preparationPhenolEpichlorohydrin
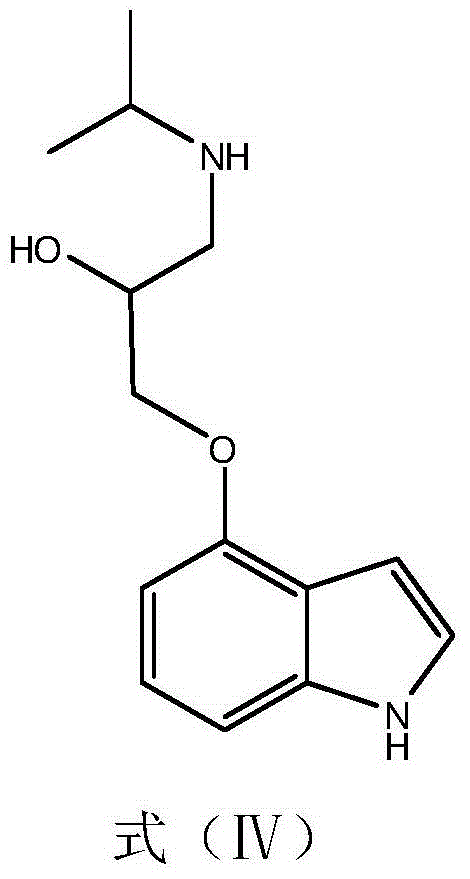
The present invention relates to an improved process for producing optically active (S)-atenolol of formula (1) in high optical purity by reacting a phenol with an epichlorohydrin.
Owner:EMCURE PHARAMACEUTICALS LTD
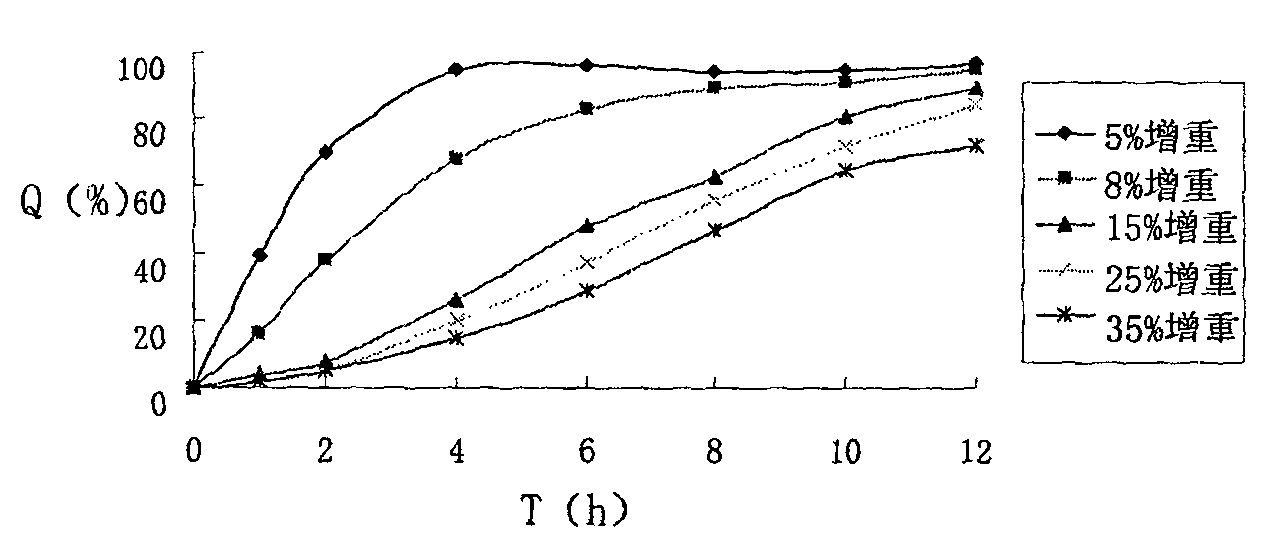
Atenolol non-pH-dependent sustained release pellets and preparation method thereof
InactiveCN101804033AStable release rateOrganic active ingredientsPharmaceutical non-active ingredientsSustained release pelletsOrganic acid
The invention relates to the field of pharmaceutical preparations, in particular to a non-pH-dependent sustained release pellet preparation containing atenolol and a preparation method thereof. The atenolol non-pH-dependent sustained release pellet preparation mainly consists of atenolol-containing pellet cores and coating materials, and is characterized in that the atenolol-containing pellet cores contain a certain proportion of solid organic acid, wherein the solid organic acid is one or more selected from fumaric acid, sorbic acid and adipic acid. The atenolol-containing pellet cores are prepared by the extrusion-spheronization method. The results show that the prepared pellets have good roundness, and the sustained release preparation can be smoothly released in different pH release media.
Owner:CHINA PHARM UNIV
Pharmaceutical composition of atenolol/amlodipine/folacin compound and uses thereof
InactiveCN101406472AImprove antihypertensive effectTake a small doseOrganic active ingredientsCardiovascular disorderDiseaseSide effect
The invention relates to a pharmaceutical composition containing atenolol, amlodipine, or folic acid compounds and application thereof. The pharmaceutical composition contains an officinal dosage of the atenolol, an officinal dosage of the amlodipine or levamlodipine, an officinal dosage of the folic acid compounds, and a pharmaceutical acceptable carrier. The dosage of the atenolol is between 5 and 50 milligrams, the dosage of the amlodipine or the levamlodipine is between 0.5 and 5.0 milligrams, and the dosage of the folic acid type compounds is between 0.2 and 1.6 milligrams. The pharmaceutical composition has the following advantages: the pharmaceutical composition enhances the hypertension curative effect through multi-target synergistic hypotensive action, and reduces the taking dosage of the amlodipine simultaneously, that is, just about one fourth of the original dosage can achieve the same hypotensive effect and reduce the side effects and medical expenses; and more importantly, the pharmaceutical composition can effectively prevent and treat or delay various complications of high blood pressure cardiovascular and cerebrovascular diseases such as cerebrovascular disorder and the like through dual targets (Hcy and blood pressure) on the basis of reducing toxic side effects. Besides, the pharmaceutical composition ensures that patients can take medicine conveniently.
Owner:史克勇
Medicinal composition of atenolol and application of medicinal composition in biological medicine
InactiveCN105837595ANovel structureInhibition of pulmonary fibrosisOrganic chemistryRespiratory disorderNatural productMedicine
The invention discloses a medicinal composition of atenolol and application of the medicinal composition in a biological medicine. The medicinal composition of the atenolol contains the atenolol and a natural product compound (I) which is separated from adenophora tetraphylla and is novel in structure; when independently used, the atenolol and the compound (I) can inhibit pulmonary fibrosis; when the atenolol and the compound (I) are used together, the effect of inhibiting pulmonary fibrosis can be further improved, so that a medicine for inhibiting pulmonary fibrosis can be developed, and compared with the prior art, the medicinal composition has outstanding practical characteristics and remarkable improvement.
Owner:徐月苗
Preparation method of atenolol injection
ActiveCN104434788AImprove solubilityQuality improvementOrganic active ingredientsPharmaceutical delivery mechanismSodium edetateSolvent
The invention discloses an atenolol injection. The atenolol injection is prepared from atenolol and pharmaceutically acceptable auxiliary materials, and the concentration of the atenolol injection is 0.45-0.55mg / mL according to the concentration of atenolol. The auxiliary materials comprise the following components: a composite solvent consisting of 1,2-propylene glycol and water, a buffer system consisting of sodium dihydrogen phosphate and disodium hydrogen phosphate, sodium chloride which is taken as an osmotic pressure regulator, and sodium edetate which is taken as a metal ion complexing agent, wherein in the composite solvent, the volume percentage of 1,2-propylene glycol accounts for 10-35%. The invention also discloses a preparation method of the atenolol injection. By adopting a mode of adding carbon twice, the purpose of adsorbing pyrogen in the injection can be achieved, and a main medicine of a solution cannot be significantly adsorbed. The atenolol injection prepared by adopting the method disclosed by the invention is good in stability and safe and effective in clinical use.
Owner:山东益康药业股份有限公司
Compound medicine preparation for treating refractory hypertension
InactiveCN107308181AAvoid careful counting of multiple drug quantitiesAvoid the hassle of dosingOrganic active ingredientsCardiovascular disorderCaptoprilSide effect
The invention discloses a compound medicine preparation for treating refractory hypertension, which is mainly prepared from the following components in parts by weight: 50 to 500 parts of ganoderan, 5 to 10 parts of nifedipine, 12.5 to 50 parts of captopril, 10 to 50 parts of hydrochlorothiazide and 12.5 to 100 parts of atenolol. According to the compound medicine preparation provided by the invention, the ganoderan is compounded with multiple antihypertensive drugs of conventional multiple-treatment to prepare a convenient-to-take compound preparation; the beneficial effects of synergetic antihypertensive effect and mutual side effect relieving / offsetting effect of the ganoderan and the four medicines are realized, the compliance of standardized medicine-taking of refractory hypertension patients is obviously improved, the therapeutic effect of obviously reducing the blood pressure level of the refractory hypertension or even realizing up-to-standard blood pressure control on part of the patients are achieved, and a novel convenient-to-take compound antihypertensive drug is provided for simplifying the drug use of the refractory hypertension patients and effectively reducing the blood pressure level.
Owner:卢广荣
A long-acting timolol transdermal preparation and its application in hemangioma
ActiveCN104274390BImproves the efficiency of drug absorptionGood treatment effectOrganic active ingredientsAerosol deliveryTherapeutic effectHemangioma
The invention relates to a timolol long-acting transdermal preparation, the preparation process of which comprises: first preparing timolol into timolol nano-medicine or timolol micropowder, and then preparing timolol nano-medicine Or timolol micropowder is prepared into timolol hydrogel transdermal preparation, ointment transdermal preparation or microneedle transdermal preparation. The present invention also relates to the application of the above-mentioned medicine in the treatment of hemangioma. Using the medicine of the invention to treat infantile hemangioma can promote the efficiency of diseased cells to absorb medicine, improve the treatment effect, reduce the dosage of medicine, and have no adverse reaction in the treatment process.
Owner:BEIJING MERSON PHARMA CO LTD
Atenolol/nitrendipine/folic-acid compound medicine combination and use thereof
InactiveCN101422459AWeaken heart rate slowing effectEasy to take medicineOrganic active ingredientsCardiovascular disorderDiseaseSide effect
The invention relates to a medicament compound of an atenolol / nitrendipine / folacin compound, and usages thereof. The invention belongs to the technical field of pharmacy. The medicament compound contains the atenolol of theriacal dosage, the nitrendipine of theriacal dosage, the folacin compound of theriacal dosage and a vector acceptable on pharmacy. The dosage of the atenolol is 5 to 50mg; the dosage of the nitrendipine is 3 to 30mg; and the dosage of the folacin compound is 0.2 to 1.6mg. The invention has the advantages that the medicament compound enhances the curative effect of pressure reduction, prolongs the lasting time of pressure reduction and removes the side effect of quickening the cardiac rate of the reflectivity of the nitrendipine in the compound by the multi-target spot cooperative pressure reduction effect; more importantly, the medicament compound can effectively prevent, cure or ease a plurality of complicating diseases of hypertension and cardiovascular and cerebrovascular diseasesin such as stroke and the like by double target spots (Hcy, blood pressure) on the foundation of reducing the side effects. Moreover, the medicament compound can lead a sufferer to be convenient to take the medicine.
Owner:史克勇
Compounds for Enhancing Hypoxia Inducible Factor Activity and Methods of Use
The present invention relates to methods for enhancing Hypoxia inducible factor-1 (HIF) activity in a cell by contacting the cell with any one of the following compounds: 3,6-bis[2-(dimethylamino)ethoxy]-9h-xanthen-9-onedihydrochloride, 2,8-bis[dimethylaminoacetyl]dibenzofurin dihydrochloride hydrate, tilorone analogue R-9536-DA, indoprofen, ciclopiroxolamine, tryptophan, ansindione, nabumetone, oxybendazole, albendazole, tropicamide, pramoxine hydrochloride, atenolol, mebendazole, carbetapentane citrate, monensin sodium, methoxyvone, hydroxyzine, phenazopyridine, clofoctol, ipraflavone, zomepirac, biochanin A, xylometazoline hydrochloride, fenbendazole, pirenzepine, triprolidine hydrochloride, daidzein, tripelennamine citrate, colchicines, aminopyridine, trimethoprim, helenine, hydroxyurea, amiodarone hydrochloride, clindamycin hydrochloride, sulfachlorpyridazine, mephenesin, semustine, clofivric acid, clofibrate, ibuprofen, hyoscyamime, nafcillin sodium, piperin, clidinium bromide, trioxsalen, hydralazine and HIF alpha protein fused to a carrier peptide.
Owner:CORNELL RES FOUNDATION INC
Atenolol frozen dry injecta and preparing method
InactiveCN101023938AImprove solubilityEasy to storePowder deliveryOrganic active ingredientsVeinFreeze-drying
The present invention discloses an atenolol freeze-dried preparation for injection. Said atenolol freeze-dried preparation has good medicine stability, can be used fro clinical intravenous injection, and can be extensively used for early treatment of arrhythmia and acute myocardiac infarction. Besides, said invention also provides its preparation method.
Owner:上海医药科技发展有限公司
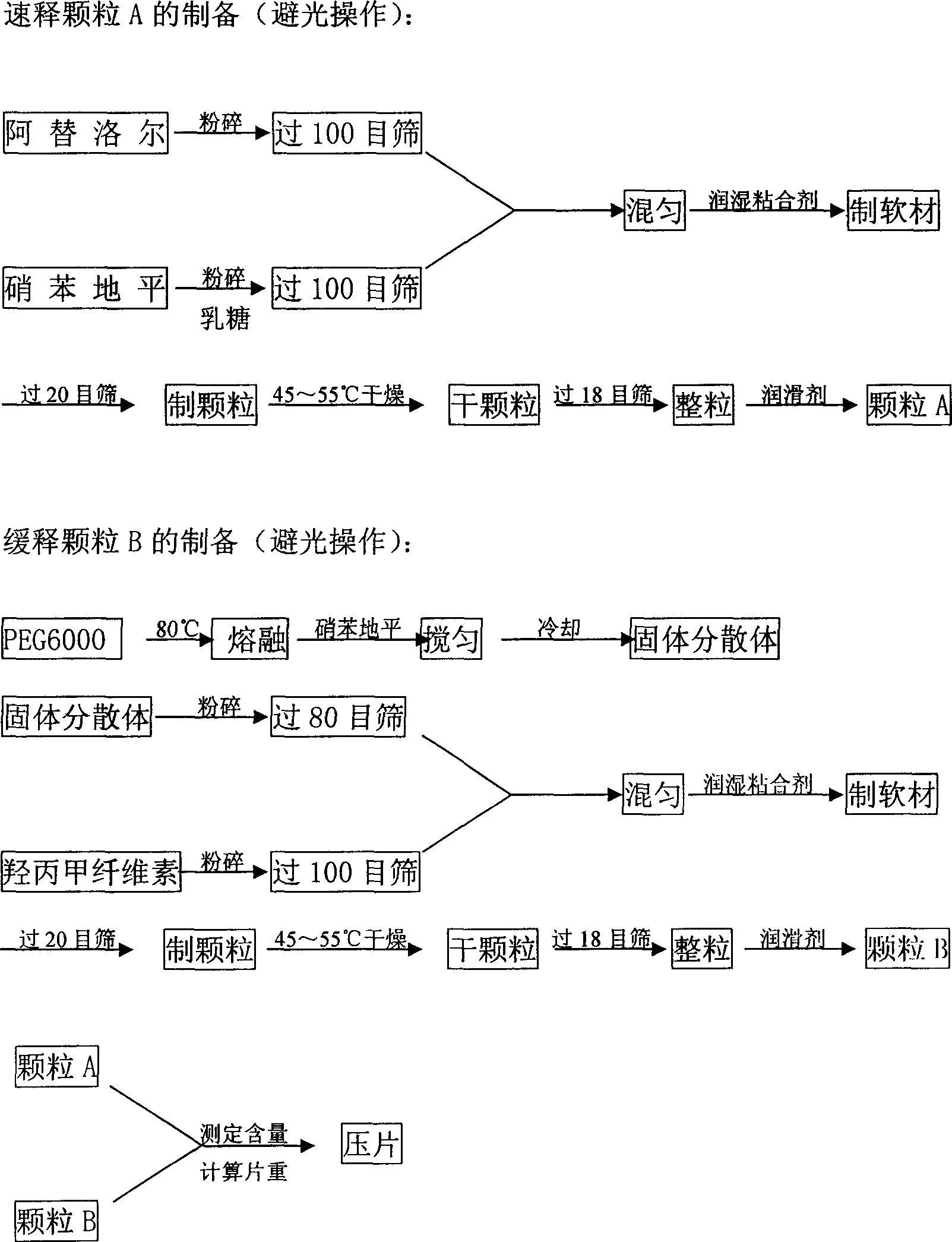
Slowly released tablet of compound atenolol, and preparation method
InactiveCN1915224AReduce adverse reactionsGood curative effectOrganic active ingredientsPill deliveryNifedipineFast release
A slow-release tablet of compound atenolol is prepared through preparing fast-release particle from atenolol and less nifedipine, preparing its slow-release layer from hydrophilic gel PEG6000, rest of nifedipine and slow-release material, and die pressing.
Owner:山东益康药业股份有限公司
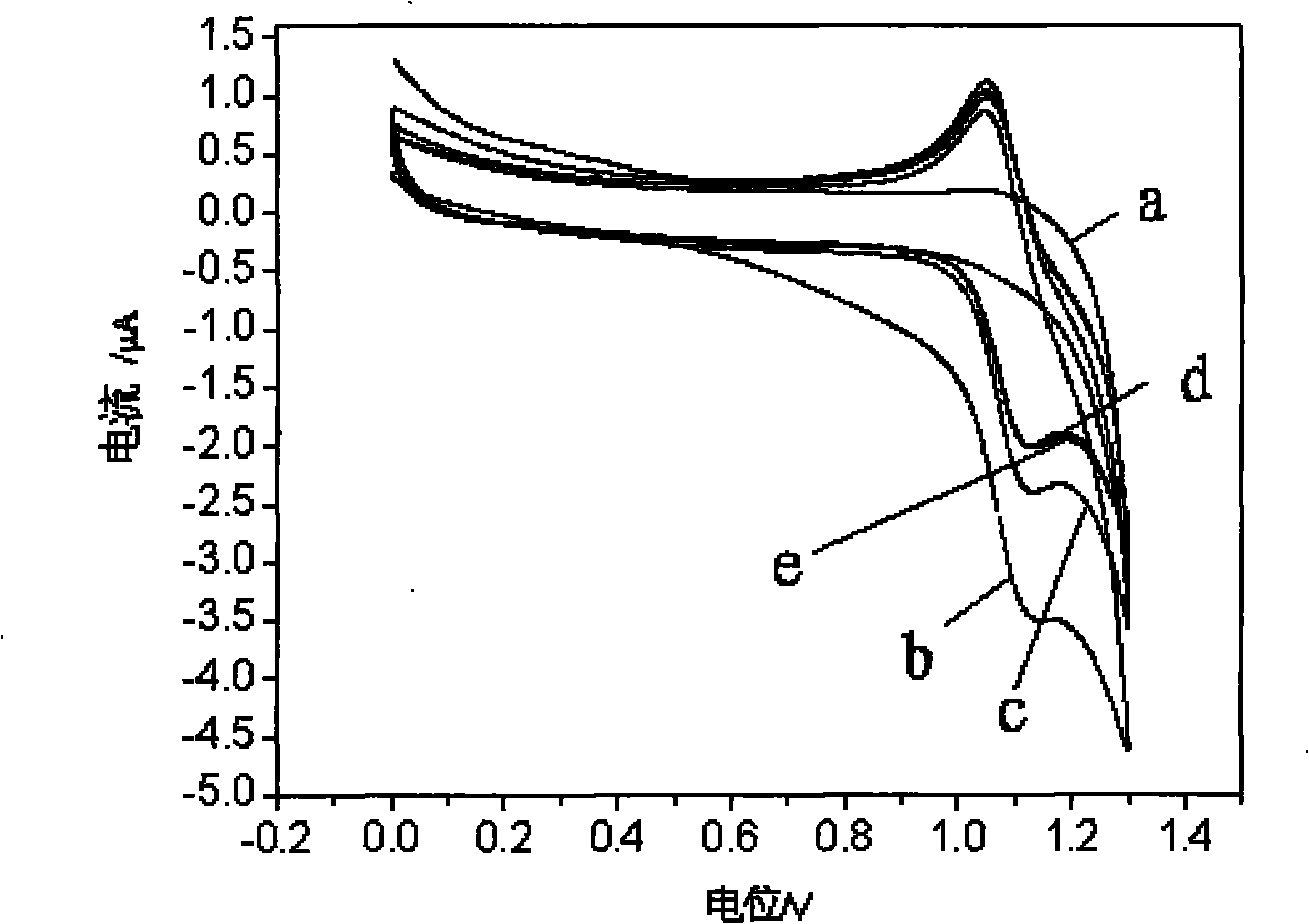
Method for simultaneously carrying out chiral separation analysis on anisodamine, atenolol and metoprolol
ActiveCN101788490ARealize simultaneous separation detectionReduce usageChemiluminescene/bioluminescenceMaterial analysis by electric/magnetic meansElectrochemistryLuminescence
The invention provides a method for simultaneously carrying out chiral separation analysis on anisodamine, atenolol and metoprolol, which comprises the following steps: putting an anisodamine sample, an atenolol sample and a metoprolol sample in a sample cell of a capillary electrophoresis electrochemistry luminescence detection hyphenated instrument and applying an injection voltage to make the samples enter a capillary of the instrument; applying a separation voltage to make the samples carry out electrophoretic separation in the capillary, wherein the capillary is filled with separation solution comprising carboxymethyl-beta-cyclodextrin and the pH value of the separation solution is between 3.0 and 6.0; and making the separated samples enter a detection cell of the instrument and applying a detection voltage to carry out detection, wherein the detection cell is filled with detection solution comprising tris (bipyridine) ruthenium and the pH value of the detection solution is between 6.0 and 9.0. Compared with the prior art, the method for carrying out chiral separation analysis, which is provided by the invention, has the advantages of less reagent use, lower detection cost, higher separation efficiency and higher detection sensitivity.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
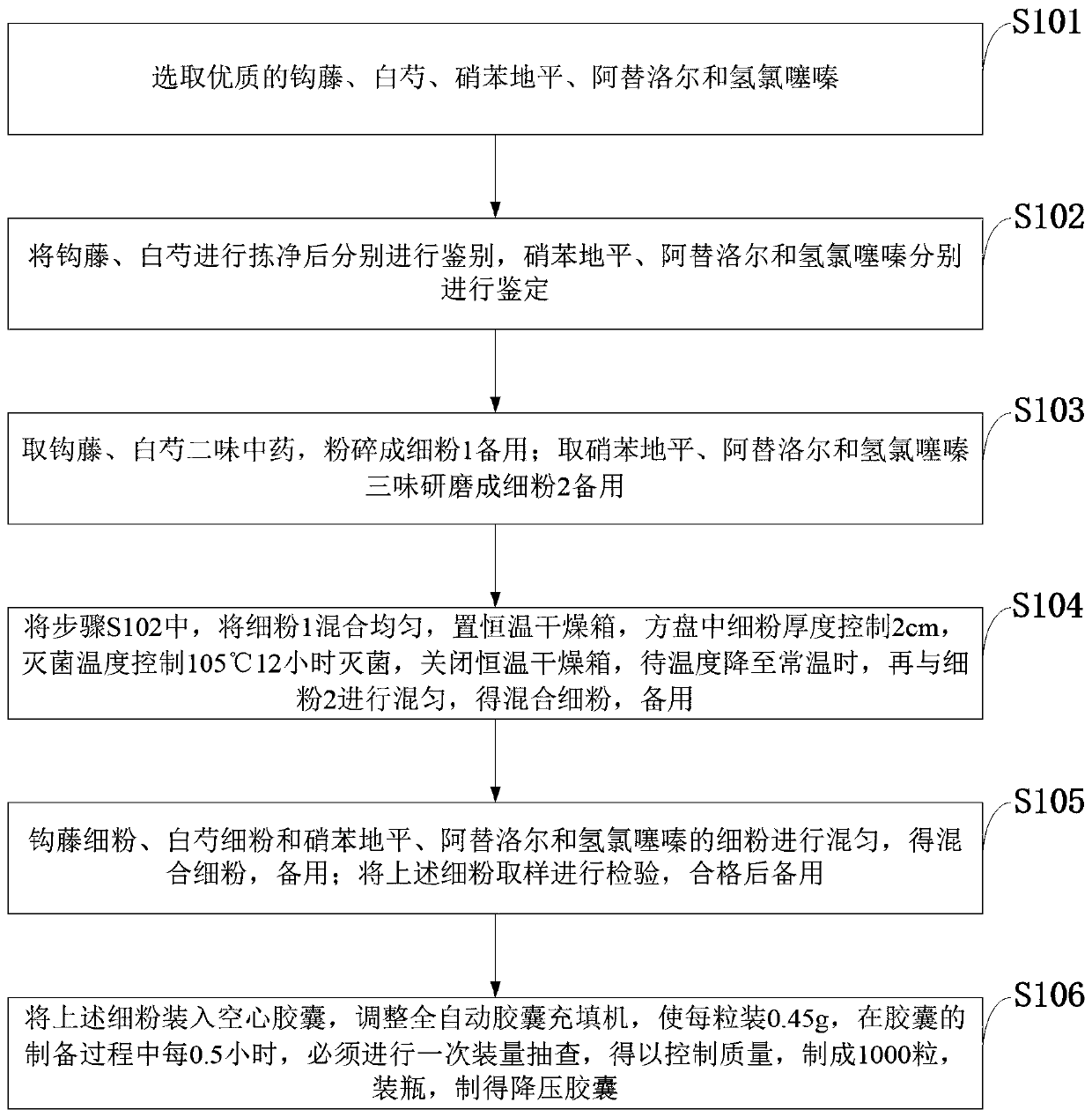
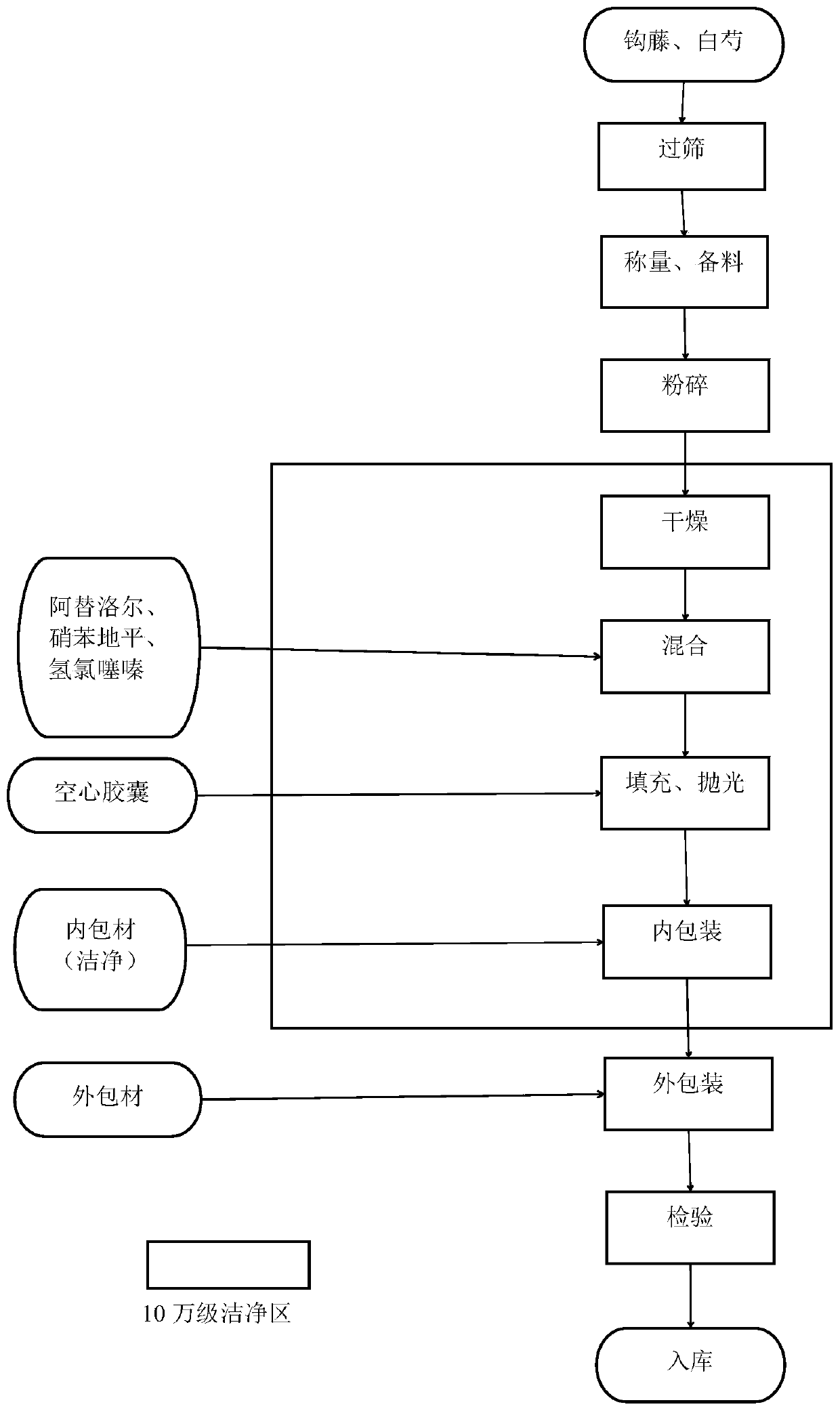
Antihypertensive capsule and preparation method
InactiveCN109771497APlay the role of increasing efficiency and reducing burdenBlood pressure level controlOrganic active ingredientsCapsule deliveryNifedipineHydrochlorothiazide
The invention belongs to the technical field of traditional Chinese medicine, and discloses an antihypertensive capsule and a preparation method. The capsule comprises 250 g of uncaria, 200 g of radixpaeoniae alba, 10.0 g of nifedipine, 12.5 g of atenolol and 6.25 g of hydrochlorothiazide. Good-quality uncaria, radix paeoniae alba, nifedipine, atenolol and hydrochlorothiazide are selected; uncaria and radix paeoniae alba are obtained and smashed into fine powder; nifedipine, atenolol and hydrochlorothiazide are ground into fine powder; sterilization is carried out, the uncaria fine powder andthe radix paeoniae alba fine powder are put into a constant-temperature drying box, the thickness of the fine powder in a square disc is controlled to be 2 cm, the sterilization temperature is controlled to be 105 DEG C, the constant-temperature drying box is shut down after 12 hours, and after the temperature is reduced to normal temperature, the fine powder is evenly mixed with the fine powder2 to obtain mixed fine powder; the uncaria fine powder, the radix paeoniae alba fine powder and the nifedipine, atenolol and hydrochlorothiazide fine powder are evenly mixed to obtain mixed fine powder for use; the mixed fine powder is loaded into empty capsules to prepare 100 capsules, the capsules are bottled, and antihypertensive capsules are obtained.
Owner:福建省南平市人民医院
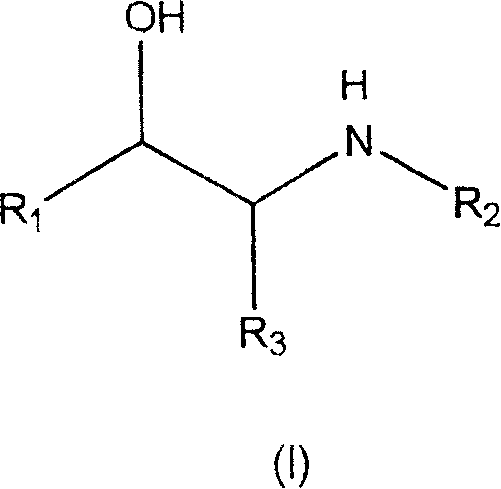
The treatment of inflammatory disorders and pain
Compounds that may be used for the treatment or prevention of a condition associated with T-cell proliferation or that is mediated by pro-inflammatory cytokines are of formula (I): wherein R1 is CHR4-OR5 or CHR4-SR5, or aryl or heteroaryl optionally substituted with one or more groups R6; R2 is alkyl or is part of a ring with R3; R3 is H, alkyl or CH2 (when forming part of a ring with R2); R4 is H or alkyl or is part of a ring with R5; R5 is aryl or heteroaryl optionally substituted with R7; each R6 is independently alkyl, CF3, OH, Oalkyl, OCOalkyl, CONH2, CN, halogen, NH2, NO2, NHCHO, NHCONH2, NHSO2alkyl, CONH2, SOMe, SO2NH2, Salkyl, CH2SO2alkyl or OCONalkyl2; R7 is R8 or (CH2)nOR8, R9, CF3, OH, OR9, OCOR9, COR9, COOR9, CONH2, CH2CONH2, CN, halogen, NH2, NO2, NHCHO, NHCONH2, NHCONHR7, NHCON(R9)2, NHCOR9, NHCOaryl, NHSO2Me, CONH2, SMe, SOMe or SO2NH2; R8 is (CH2)nOR9, (CH)nOR9, (CH2)nCOOR9 or (CH2)nCOaryl; R9 is alkyl or cycloalkyl; and n is 1 to 4; or a salt thereof, including clenbuterol and atenolol.
Owner:SOSEI R&D LTD
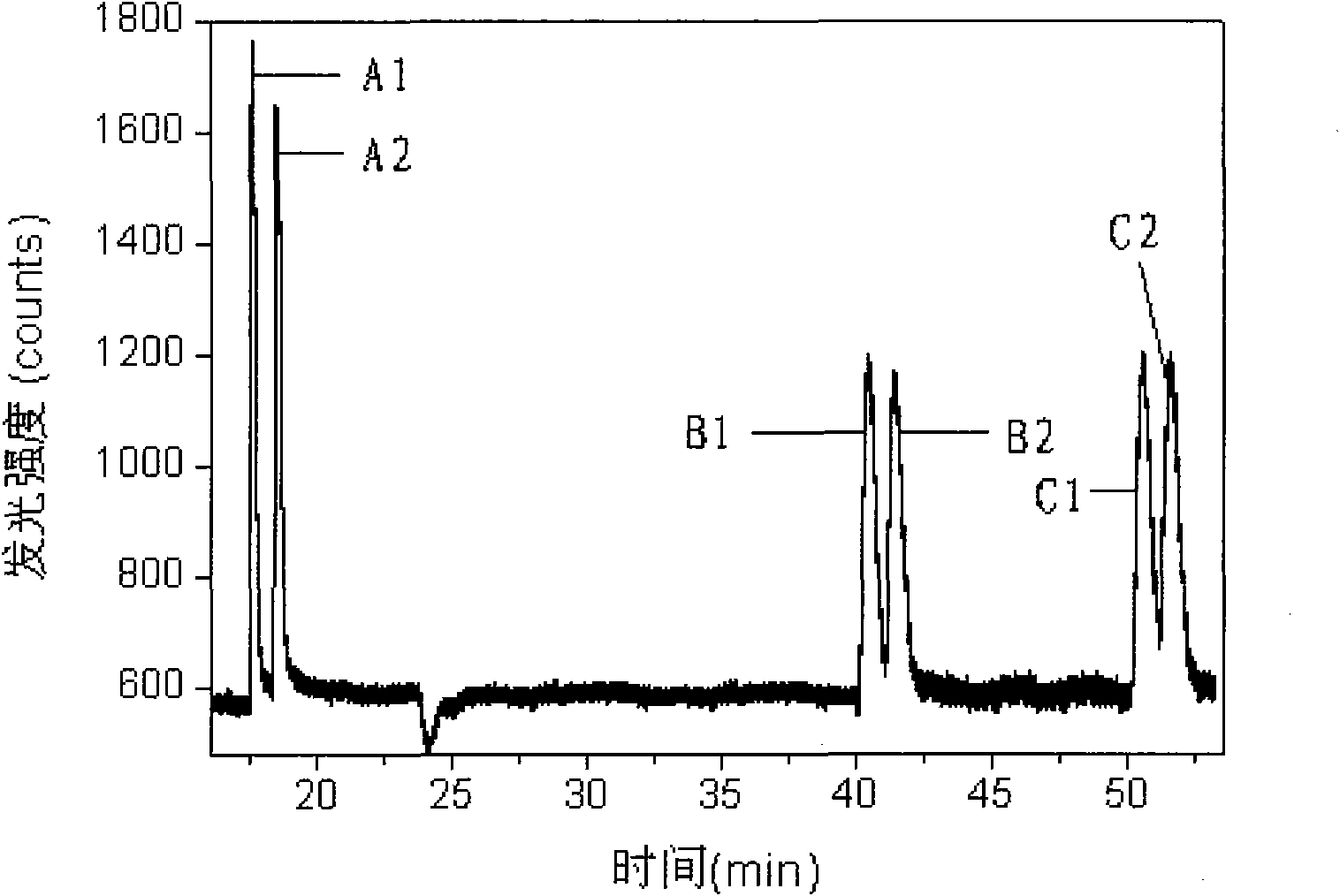
Method for capillary electrophoresis electrochemiluminescence detection of metoprolol and atenolol
InactiveCN1995983ALow costReduce instrument costChemiluminescene/bioluminescenceMaterial electrochemical variablesElectrochemiluminescenceStandard samples
The invention relates to electrocapillarity electrophoresis electrochemiluminescence inspection. It makes direct analysis for the standard sample solution and marked urine sample. It has high sensitivity and simple operation. The inspection linear scope for two standard solutions are 5*10-8-1. 0*10-5mol / L and 7. 5*10-8-1. 0*10-6mol / L, lower limit 5. 0*10-9mol / L and 7. 5*10-8mol / L. It can also be used for betaantagonist analysis with far reaching significance in betaantagonist clinical inspection.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
Atenolol sustained-release dropping pill and preparation method thereof
InactiveCN101269027ADecreased plasma renin activityLower blood pressureOrganic active ingredientsSenses disorderAdditive ingredientAngina
The invention relates to a therapeutic drug for treating arrhythmia, angina and mild and moderate hypertension as well as the preparation method thereof. The therapeutic drug aims to supplement the deficiency of the prior art and provide a sustained-release atenolol dropping pill formulation. The sustained-release atenolol dropping pill is prepared by adding stabilize Vitamin E and a hydrophobic base to the ingredients accepted in the prior art, thereby effectively overcoming the defects in the prior art and guaranteeing no occurrence of an obvious change related to the substance content for the drug during the effective storage period and has the advantages of full release, controllable release time, low frequency of drug taking, long effective hours and high bioavailability simultaneously. The sustained-release atenolol dropping pill is suitable for clinical and family use.
Owner:北京博智绿洲医药科技有限公司
Compound atenolol tablet and preparation method thereof
InactiveCN101332194AOrganic active ingredientsPharmaceutical non-active ingredientsMedicineActive component
The present invention discloses a compound atenolol tablet and a preparation method thereof, which contains active components of atenolol and chlorthalidone, auxiliary materials and a coating outside the tablet. The weight proportion of the atenolol and the chlorthalidonem is 4:1, and the auxiliary materials contain 1 to 10 parts of disintegrant, 80 to 98 parts of bulking agent, 0.1 to 5 parts of binding agent and 0.05 to 5 parts of lubricant antiplastering agent according to the total weight of the auxiliary materials. The present invention adopts appropriate auxiliary materials and well solves the relationship among disintegrating time, hardness and coating quality of the tablet. The harness of the tablet can be up to 6.5kg and the dissolubility within 60 minutes of the atenolol and chlorthalidone should be both more than 80 percent.
Owner:SHANGHAI HUA SURNAME PHARMA
Compound antihypertensive medicament
InactiveCN101756972ASuitable for long-term useGood blood pressure effectOrganic active ingredientsCardiovascular disorderNifedipineSide effect
The invention discloses a compound preparation for treating hypertension, which comprises 5-30mg of nifedipine and 12.5-75mg of atenolol. The product of the invention overcomes the defect that a side effect is markedly enhanced caused by increasing the medicament dosage for ensuring the curative effect when the nifedipine and the atenolol are clinically used at present and one medicament is singly used, and provides a new compound preparation. The nifedipine and the atenolol are combined, and the curative effect has synergistic and complementary actions; the tolerance of patients is increased, the compliance is improved, the dosage is decreased, an adverse reaction is lightened, and the compound preparation is convenient to take and has low price.
Owner:JIANGSU JIBEIER PHARMA
Novel atenolol tablets and preparation method thereof
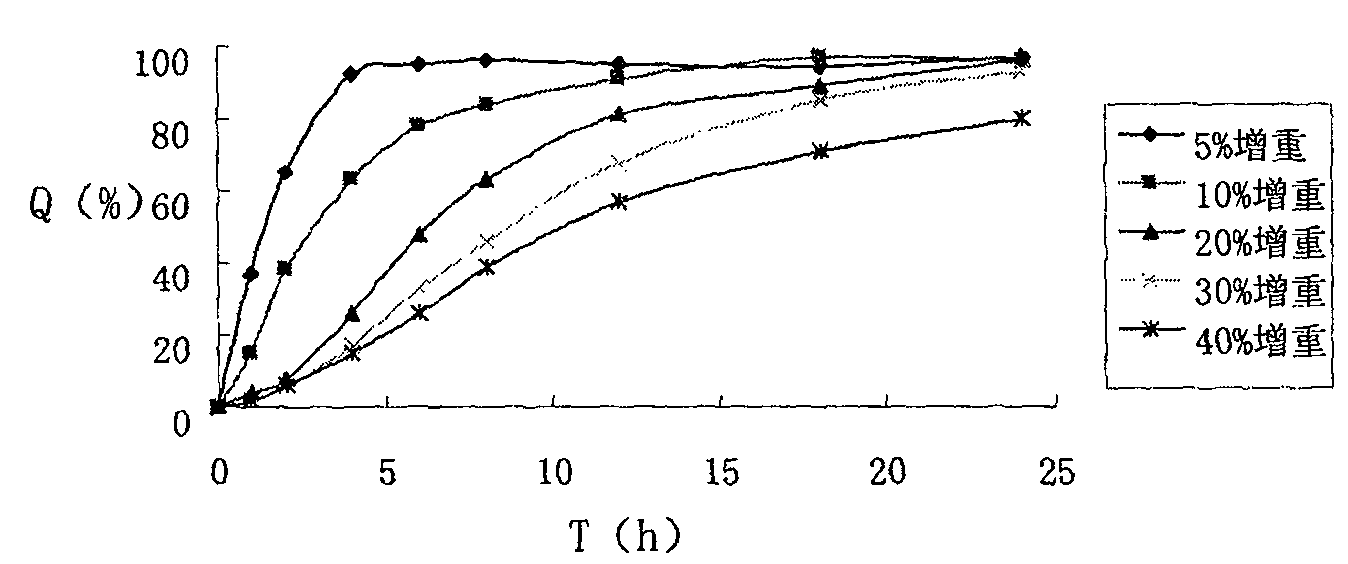
InactiveCN102512396AExtended release timeImprove bioavailabilityOrganic active ingredientsSenses disorderFoaming agentTreatment effect
The invention discloses novel atenolol tablets, which comprise the following components in percentage by weight: 10 percent of atenolol, 25 to 30 percent of hydrophilic gel material, 20 to 35 percent of framework material, 10 to 25 percent of bleaching aid, 15 to 25 percent of foaming agent and 0.1 to 3 percent of flow aid. The invention also discloses a preparation method for the novel atenolol tablets. According to the novel atenolol tablets, the defect of the atenolol in clinical application is overcome by the technical study of a preparation process for an intragastric floating retention preparation of the atenolol, so that the bioavailability of medicines is improved, the atenolol tablets have an obvious sustained-release function; and therefore, a treatment effect of the atenolol is exerted fully.
Owner:江苏黄河药业股份有限公司
Medicine composition for treating hypertension
ActiveCN101066391AStable buckLower diastolic blood pressureOrganic active ingredientsCardiovascular disorderWestern medicineGastrodia
The present invention relates to one kind of medicine composition for treating hypertension, and features that the medicine composition is prepared with medicine materials including gastrodia tuber, sealwort, fried eucommia bark, cassia obtusifolia, madder, white puncture vine, oriental water plantain, water melon seed and atenolol in certain weight proportion. Compared with singly Western medicine, the medicine composition of the present invention has the advantages of stable blood pressure lowering, effective lowering of the diastolic pressure and effect of lowering systolic pressure singly.
Owner:王忠玉
Beta-receptor blocker homogeneous enzyme immunoassay reagent and preparation and detection methods thereof
ActiveCN104535763AStrong specificityImproving immunogenicityMaterial analysisEnzyme immunoassaysImmunogenicity
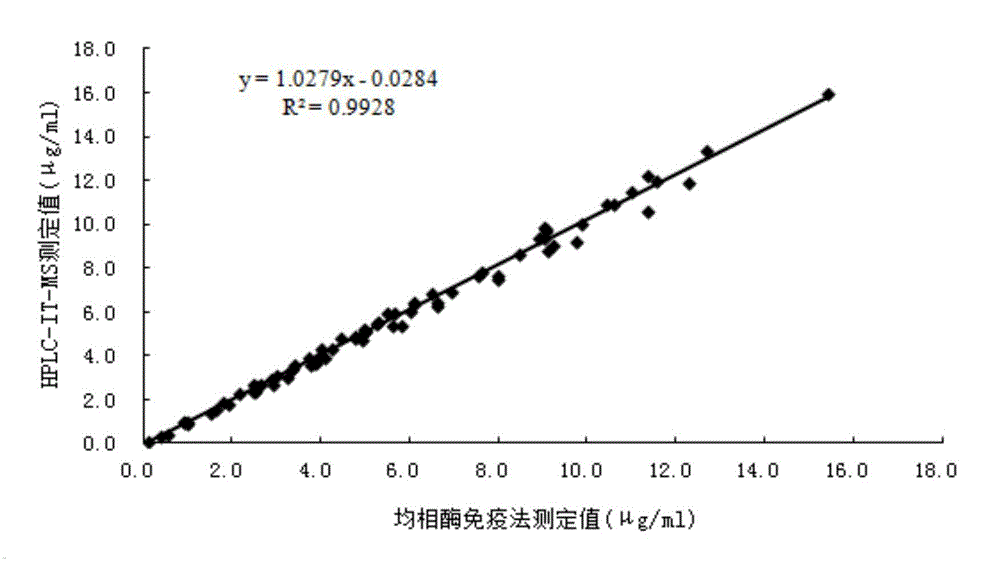
The invention relates to a beta-receptor blocker detection reagent and preparation and detection methods thereof, and specifically relates to a beta-receptor blocker homogeneous enzyme immunoassay reagent and preparation and detection methods thereof. The beta-receptor blocker homogeneous enzyme immunoassay reagent comprises an anti-beta-receptor blocker specific antibody, and an indication reagent for detecting an anti-beta-receptor blocker specific antibody-beta-receptor blocker compound; the anti-beta-receptor blocker specific antibody is obtained from a pindolol immunogen immune animal. The beta-receptor blocker detection reagent has the following beneficial effects: the pindolol immunogen is strong in specificity and high in immunogenicity, the prepared antibody is the anti-beta-receptor blocker specific antibody which can be specially bonded with various common beta-receptor blockers such as pindolol, bisoprolol, atenolol, metoprolol and propranolol, is strong in specificity and high in titer, and has no cross reaction with common 62 drugs; the homogeneous enzyme immunoassay reagent containing the anti-beta-receptor blocker specific antibody is capable of determining the content of various beta-receptor blockers in a sample conveniently, quickly and accurately; besides, a plurality of samples can be measured simultaneously on a full-automatic biochemical analyzer, and high-flux fast measurement of the beta-receptor blockers can be realized; the homogeneous enzyme immunoassay reagent is high in accuracy and strong in specificity, and are greatly improved in accuracy and detection efficiency in contrast with other methods.
Owner:AISIJIEKE GLOBAL
Compound atenolol nanoemulsion antihypertensive medicine
InactiveCN106176997AImprove solubilityReduce first pass effectOrganic active ingredientsPharmaceutical non-active ingredientsHalf-lifeDissolution
The invention discloses a compound atenolol nano-emulsion antihypertensive drug. The raw materials and the mass percentages thereof are: atenolol 1%-15%, surfactant 5%-35%, cosurfactant 1% %~20%, oil 1%~20%, Eucommia ulmoides water extract 0.5%~10%, Moutan bark water extract 0.5%~10%, the rest components are distilled water, and the sum of the mass percentages of the above raw materials is 100%. The nano-emulsion droplet has small particles, uniform distribution, low viscosity and good fluidity. After atenolol is prepared into a nanoemulsion, the ability to pass through the blood-brain barrier is significantly increased, the bioavailability of the original drug is significantly improved, the half-life of the drug is prolonged, and the number of administrations is reduced; nanoemulsion improves atenolol Atenolol's drug dissolving and penetrating ability increases the stability of atenolol; and after compounding with the water extracts of Eucommia ulmoides and Paeonia suffruticosa, it has obvious synergistic effect, and the drug efficacy is more fully exerted.
Owner:张鸿利
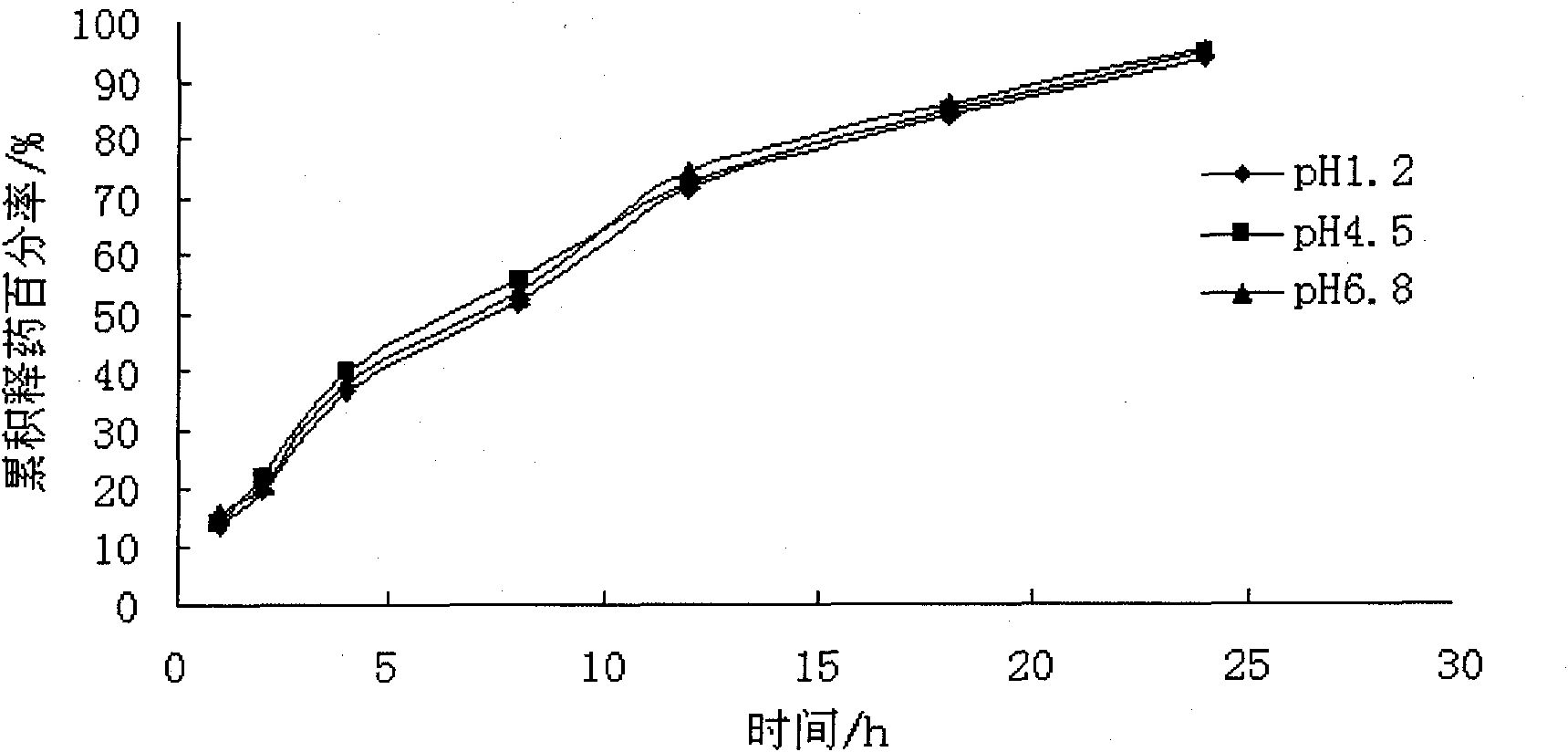
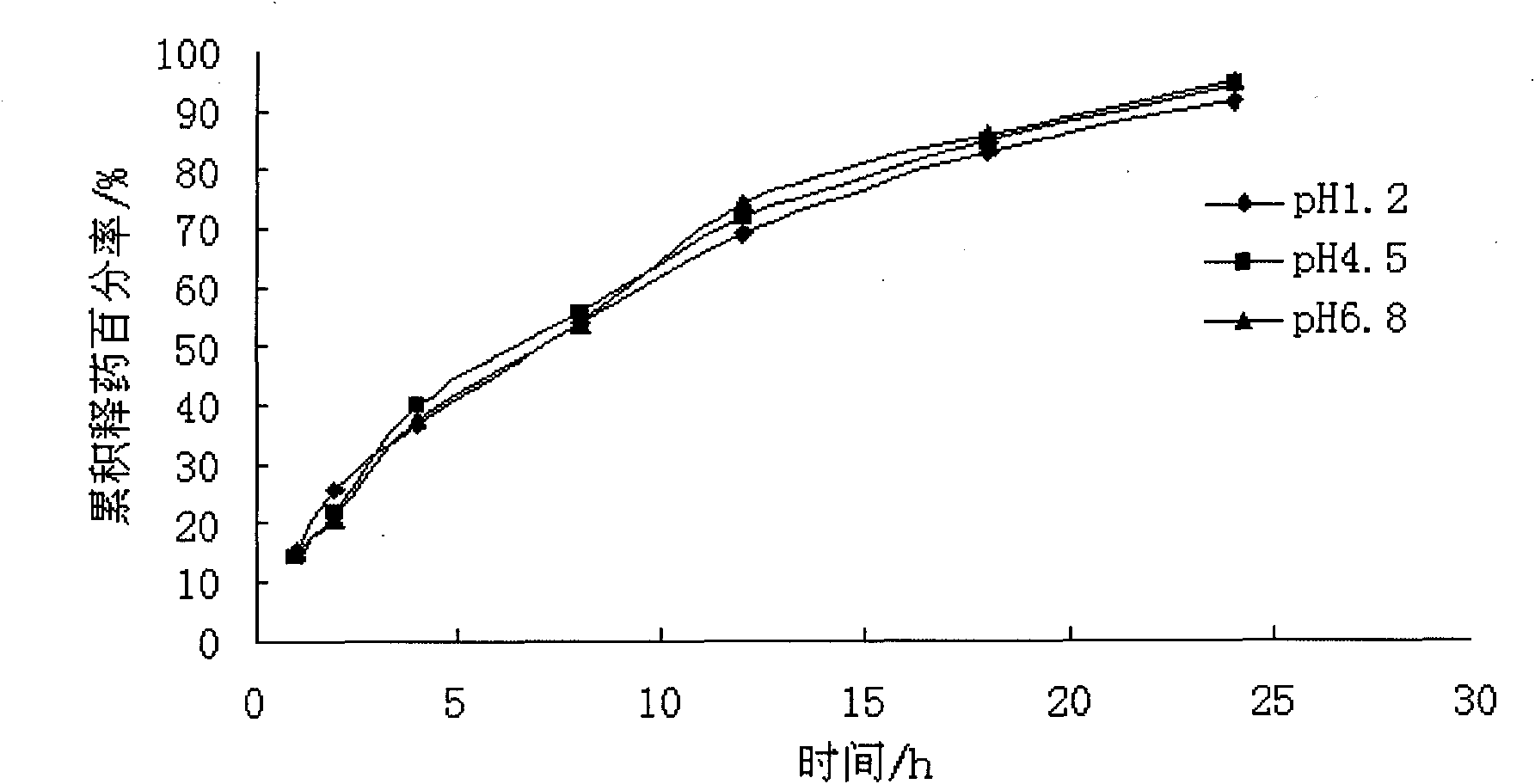
Atenolol pH independent sustained-release tablets and preparation method thereof
InactiveCN103784413APH effect is smallAvoid instabilityOrganic active ingredientsPharmaceutical delivery mechanismPolyethylene oxideEudragit RSPO
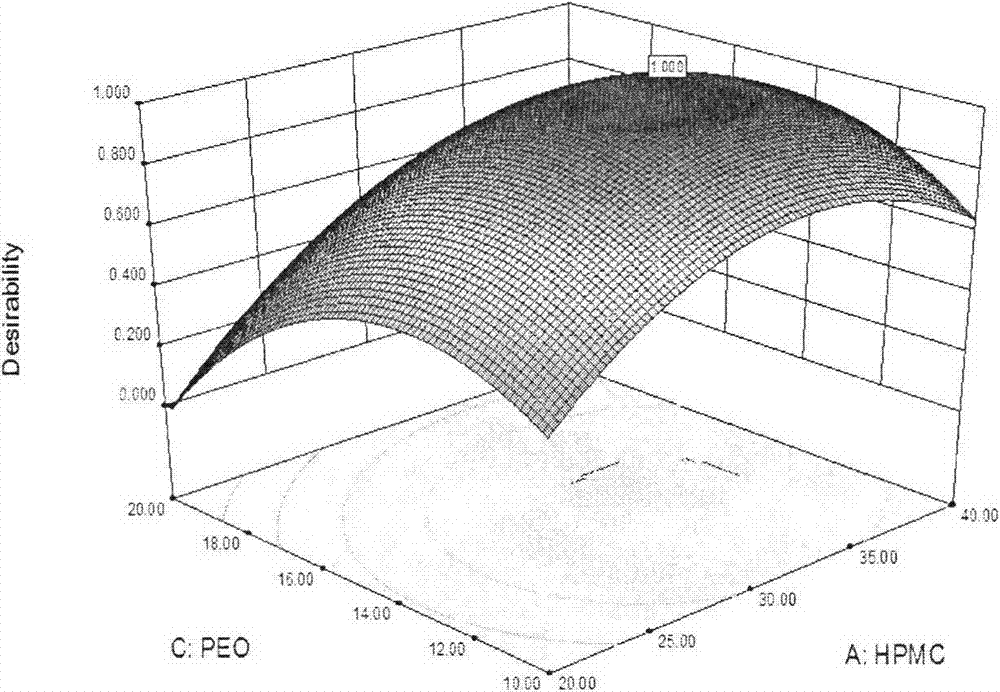
The invention relates to the pharmaceutic preparation field and in particular relates to atenolol pH independent sustained-release tablets and a preparation method thereof. The atenolol pH independent sustained-release tablets are characterized by being prepared through the steps of evenly mixing a prescription amount of atenolol, HPMC (Hydroxy propyl methyl cellulose) K15M, eudragit RSPO, high-viscosity PEO (Polyethylene Oxide) and lactose monohydrate in such a manner of progressively increasing by equal amount, next, adding a small quantity of magnesium stearate and aerosil as the lubricant, and then forming the atenolol pH independent sustained-release tablets through a powder vertical-compression process. The pH independent sustained-release tablets are prepared effectively, and the slightly alkaline drug atenolol is provided to have similar release characteristic under different simulated gastrointestinal tract pH conditions. The HPMC stable in slow release performance is taken as the slow release framework of the pH independent sustained-release tablets, the eudragit RSPO is taken as the pH regulator of the pH independent sustained-release tablets, and the high-viscosity PEO is taken as the retardant of the pH independent sustained-release tablets; the preparation process is simple, the adverse effect of the drug is remarkably reduced and the compliance of the patient is improved; and besides, the pH independent sustained-release tablets have important economic value and social significance.
Owner:CHINA PHARM UNIV
Compound sustained-release pellet tablet containing nifedipine and atenolol and preparation thereof
The invention relates to the pharmaceutical preparation field, in particular to a compound controlled release pellet tablet of nifedipine and atenolol, and is characterized in that: controlled release pellet of nifedipine, controlled release pellet of atenolol and blank pellet are pelletized to obtain the compound controlled release pellet tablet of nifedipine and atenolol, wherein the controlled release pellet of nifedipine and the controlled release pellet of atenolol are respectively obtained from drug contained core coated with acrylic resin; the weight of coating of the controlled release pellet of nifedipine is added by 10 to 30 percent while the weight of coating of the controlled release pellet of atenolol by 8 to 25 percent; the blank pellet comprises microcrystaline cellulose and one of macrogol 6000, macrogol 4000 and stearic acid or the mixture of one or two of the macrogol 6000, the macrogol 4000 and the stearic acid, wherein the blank pellet takes up 50 to 70 percent of the total weight of the compound controlled release pellet tablet of nifedipine and atenolol.
Owner:CHINA PHARM UNIV
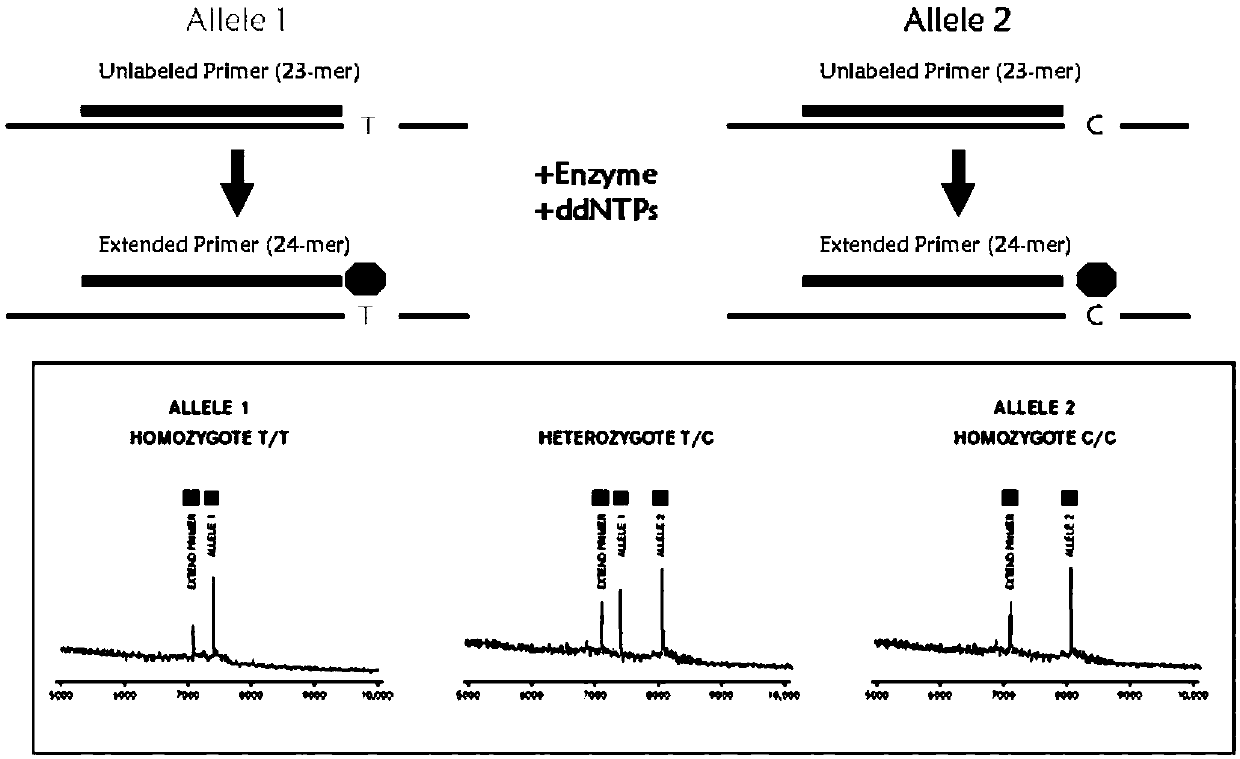
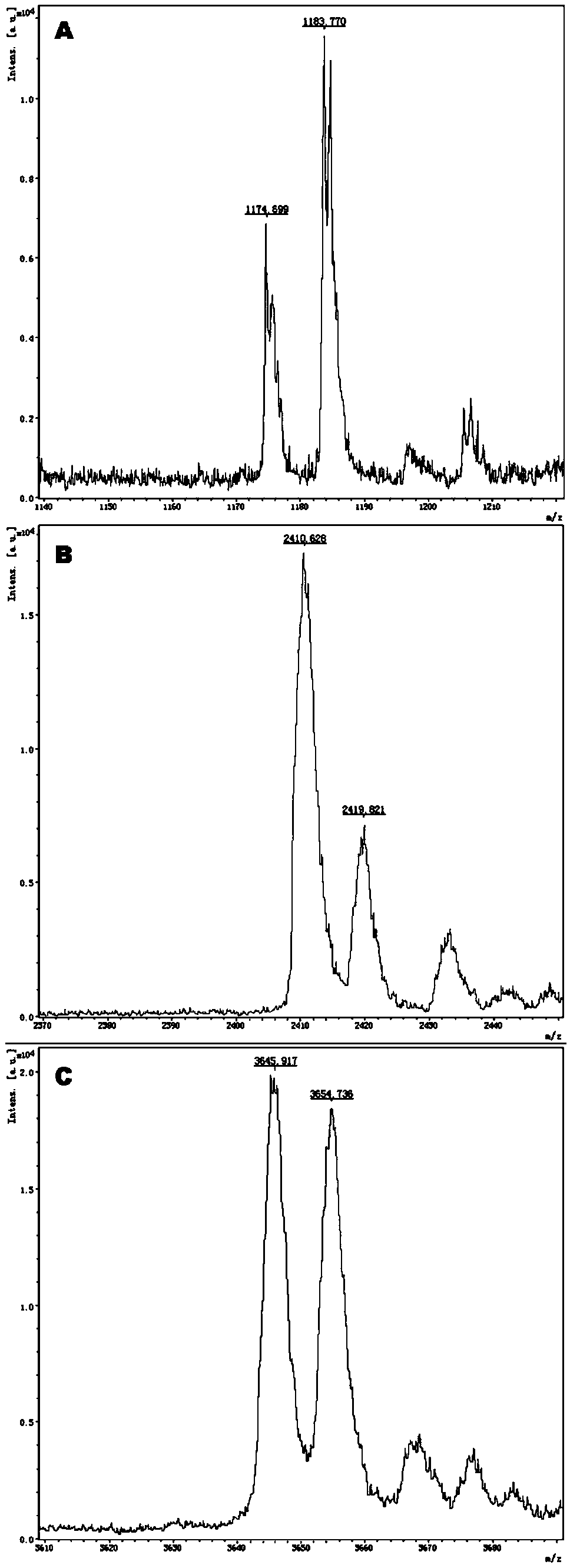
Gene detection kit for guiding medication of antihypertensive drug nitrendipine and atenolol
InactiveCN111187824AQuality Medical ServicesPromoting the process of rational drug useMicrobiological testing/measurementDNA/RNA fragmentationMultiplexGenotype
The invention provides a method and a kit for guiding medication of nitrendipine and atenolol. The method comprises the following steps: respectively designing multiple amplification primers and extension primers according to 10 to-be-detected target SNP loci; preparing a multiplex amplification primer reaction system and an extension primer reaction system; in the reaction systems, simultaneouslyand respectively carrying out amplification and single-base extension reaction on the 10 target SNP loci by using a plurality of sets of primers; and carrying out time-of-flight mass spectrometry analysis on a product after the single base extension reaction, identifying genotypes of SNP related to different drug metabolism according to products of extension primers with different molecular weights represented by a mass spectrum peak, and guiding the medication of antihypertensive drug nitrendipine and atenolol. Meanwhile, the invention provides the detection kit using the method. The methodcan simultaneously detect the 10 SNP loci related to metabolism of the antihypertensive drug nitrendipine and atenolol, and has the advantages of low cost, no need of probe synthesis, short time consumption, simple and convenient result analysis and extremely wide application field.
Owner:BIOYONG TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com