Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
83 results about "Fenbendazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fenbendazole is a broad spectrum benzimidazole anthelmintic used against gastrointestinal parasites including: giardia, roundworms, hookworms, whipworms, the tapeworm genus Taenia (but not effective against Dipylidium caninum, a common dog tapeworm), pinworms, aelurostrongylus, paragonimiasis, strongyles, and strongyloides that can be administered to sheep, cattle, horses, fish, dogs, cats, rabbits, and seals.
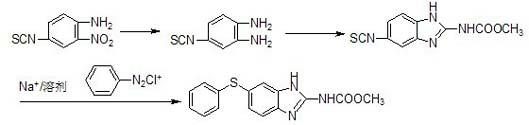
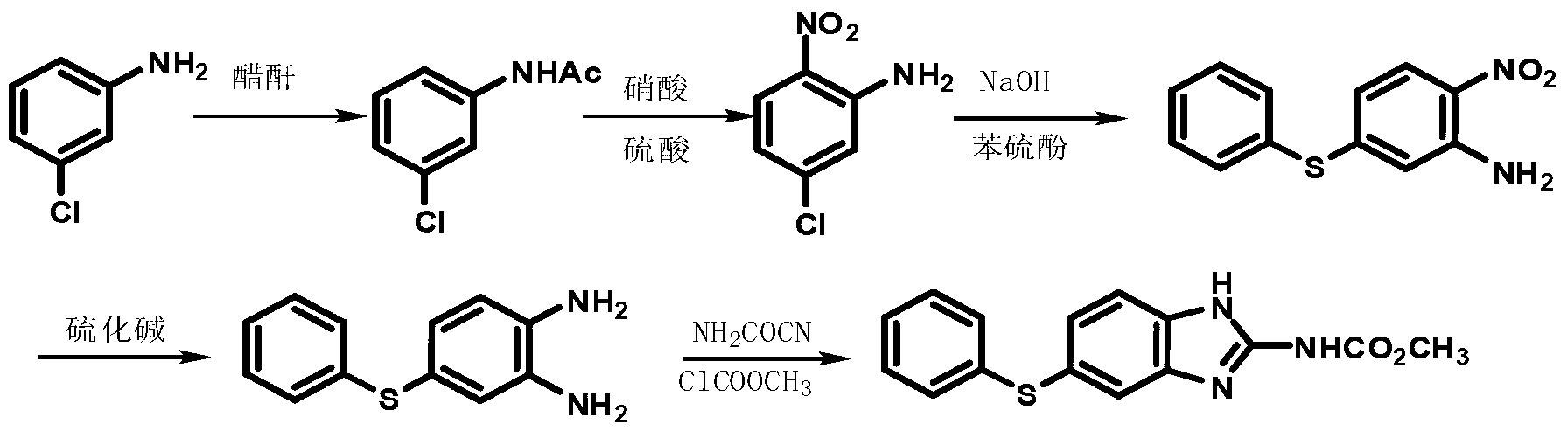
Preparation method for anthelmintic benzimidazole fenbendazole
InactiveCN102241635AOperational securitySuitable for industrial productionOrganic chemistryFenbendazoleAnthelmintic drug
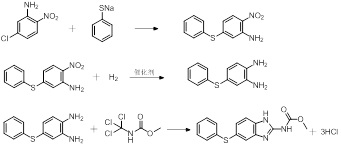
The invention discloses a preparation method for anthelmintic benzimidazole fenbendazole. The method is characterized in that: p-dichlorobenzene is used as a starting raw material which is subjected to nitration, amination, condensation, reduction and ring-closure reactions so as to obtain fenbendazole (the content of the final product being 98% to 101%). The novel preparation method has the characteristics of a simple and highly efficient process and safe operation, and is suitable for industrial production.
Owner:JIANGSU BAOZONG & BAODA PHARMACHEM
Anthelmintic formulations
The present invention provides a method for preparing a pharmaceutical formulation containing ivermectin and a method and composition that can contain ivermectin plus hexahydropyrazinoisoquinolines, tetrahydropyrimidines and benzimidazoles or febantel. Examples of hexahydropyrazinoisoquinolines, tetrahydropyrimidines and benzimidazoles include praziquantel, pyrantel pamoate and fenbendazole, respectively. A pharmaceutical formulation is provided for use in the treatment of helminthiasis of mammals, and particularly tapeworm, hookworm, roundworm, whipworm and heartworm of domestic animals and farm animals. The present invention also provides a method of treating helminthiasis in mammals, which method comprises administering to the mammal in need thereof an anthelmintically effective amount of a pharmaceutical formulation of the invention.
Owner:VIRB AC SA
Anthelmintic formulations
The present invention provides a method for preparing a pharmaceutical formulation containing ivermectin and a method and composition that can contain ivermectin plus hexahydropyrazinoisoquinolines, tetrahydropyrimidines and benzimidazoles or febantel. Examples of hexahydropyrazinoisoquinolines, tetrahydropyrimidines and benzimidazoles include praziquantel, pyrantel pamoate and fenbendazole, respectively. A pharmaceutical formulation is provided for use in the treatment of helminthiasis of mammals, and particularly tapeworm, hookworm, roundworm, whipworm and heartworm of domestic animals and farm animals. The present invention also provides a method of treating helminthiasis in mammals, which method comprises administering to the mammal in need thereof an anthelmintically effective amount of a pharmaceutical formulation of the invention.
Owner:VIRB AC SA
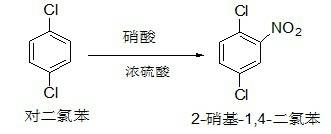
New preparation method for anthelmintic fenbendazole
InactiveCN103242237AAvoid pollutionThe new synthesis process is simple and efficientOrganic chemistryFenbendazoleM-chloroaniline
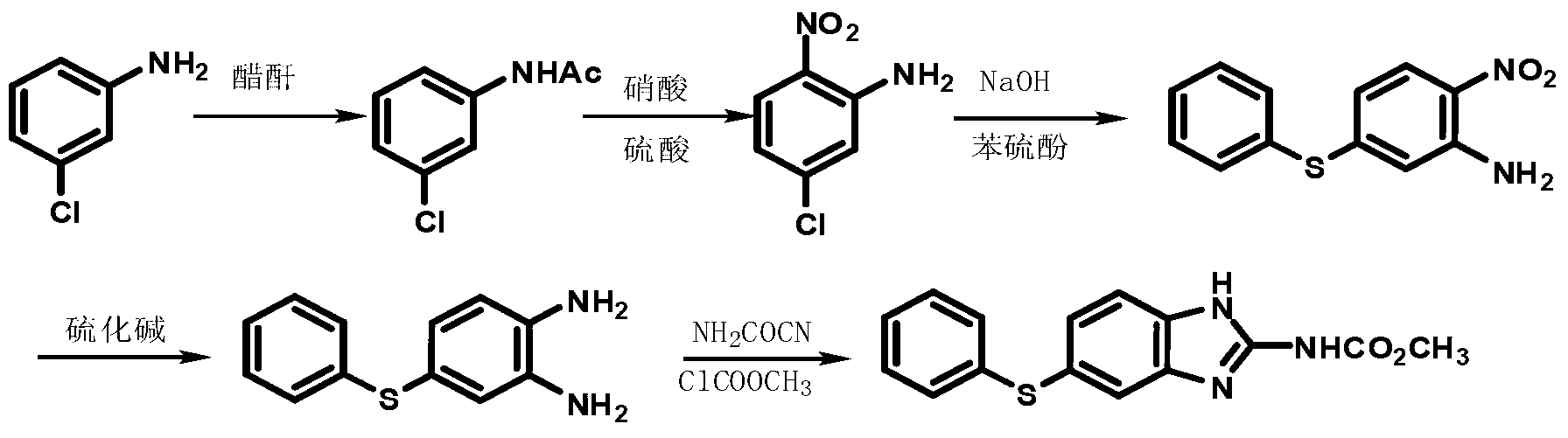
The invention discloses a new preparation method for anthelmintic fenbendazole, and provides a new synthetic route for fenbendazole. Fenbendazole is obtained by taking m-dichlorobenzene as a starting raw material through the reactions of nitration, amination, condensation, reduction and cyclization. The new preparation method is characterized in that m-chloroaniline serving as the starting raw material in the conventional industrial route is changed into m-dichlorobenzene which is low in cost; and a reduction process which uses sodium sulfide is changed into a clean and efficient reduction process. The new synthetic process is simple and efficient, low in pollution, high in quality and applicable to industrial production.
Owner:CHANGZHOU YABANG QH PHARMACHEM
Composition for preventing or treating degenerative brain diseases including compound downregulating expression of BACE1 proteins
ActiveUS20150018297A1Reduce expressionSuppress generationBiocideSugar derivativesEfavirenzRaloxifene Hydrochloride
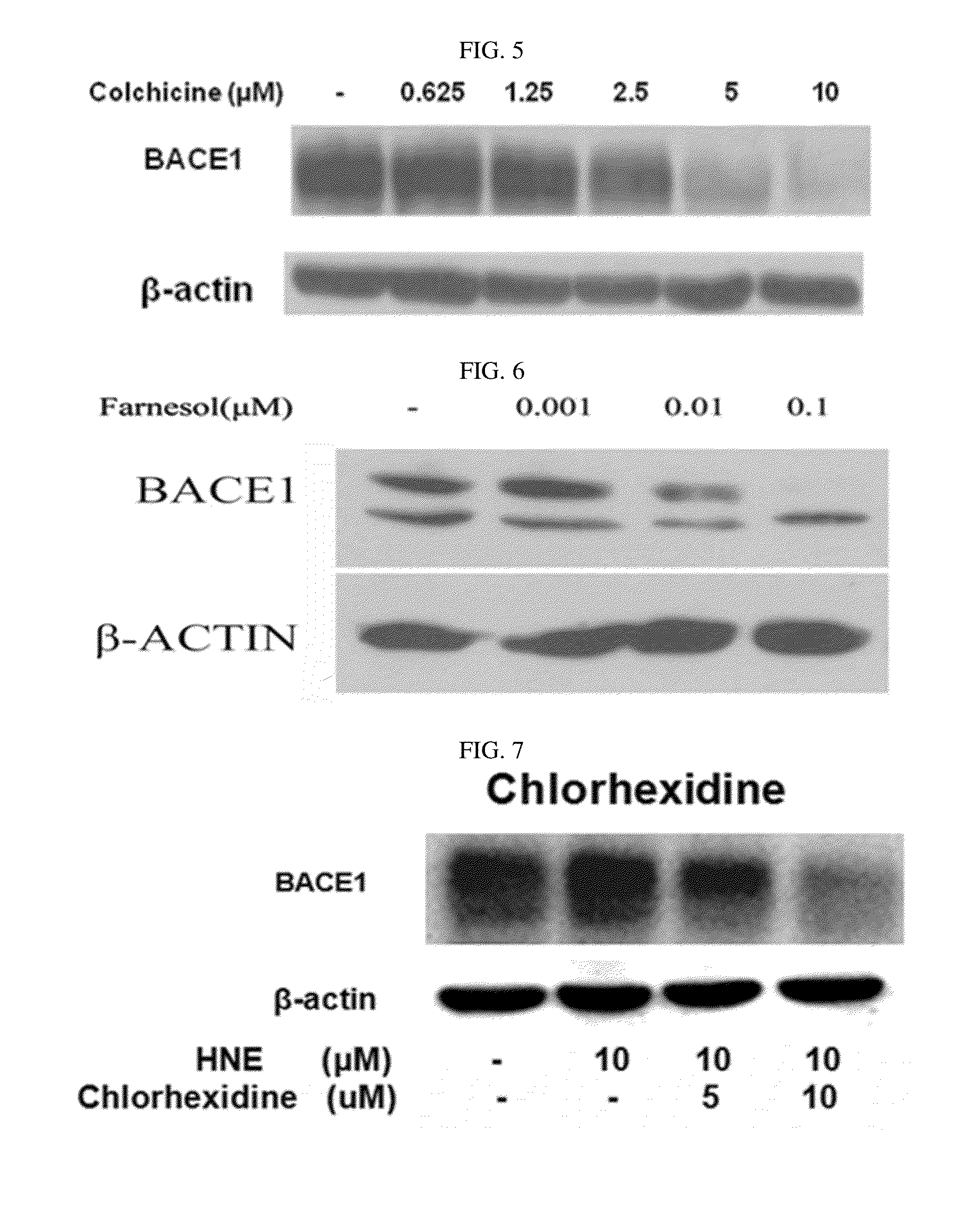
The present disclosure relates to a pharmaceutical composition, a health functional food composition, and a method for preventing or treating a brain disease or diabetes. The pharmaceutical composition, health functional food composition, and method includes at least one active ingredient that is chlorhexidine, thioguanosine, mebendazole, fenbendazole, colchicine, farnesol, trimethobenzamide hydrochloride, disulfuram, azathioprine, mebeverine hydrochloride, zaprinast, tosufloxacin hydrochloride, efavirenz, thiostrepton, probenecid, entacapone, harmine hydrochloride, flunisolide, thimerosal, hexestrol, sulfaquinoxaline sodium salt, monensin sodium salt, raloxifene hydrochloride, 2-chloropyrazine, or topotecan.
Owner:RES & BUSINESS FOUND SUNGKYUNKWAN UNIV
Broad-spectrum antiparasitic drug nano-emulsion and preparation method thereof
InactiveCN105853454AHigh thermodynamic stabilityGood storage stabilityOrganic active ingredientsPharmaceutical non-active ingredientsFenbendazolePreservative
The invention provides a broad-spectrum antiparasitic drug nanoemulsion and a preparation method thereof, which comprises the following components by weight percentage: 5-20% of fenbendazole, 0.3%-3% of ivermectin, and 0.5% of oil phase %-7%, co-surfactant 2%-12%, co-solvent 2%-5%, surfactant 10%-30%, deionized water 3-40%. The antiseptic property obtained by the invention is better, and it will not be mildewed or discolored after being placed at room temperature for three months. No additional preservatives are required, and the shelf life is long.
Owner:高艳春
Preparation method of fenbendazole
ActiveCN109467535AHigh available chlorineReduce usageOrganic chemistryChemical recyclingFenbendazoleNitration
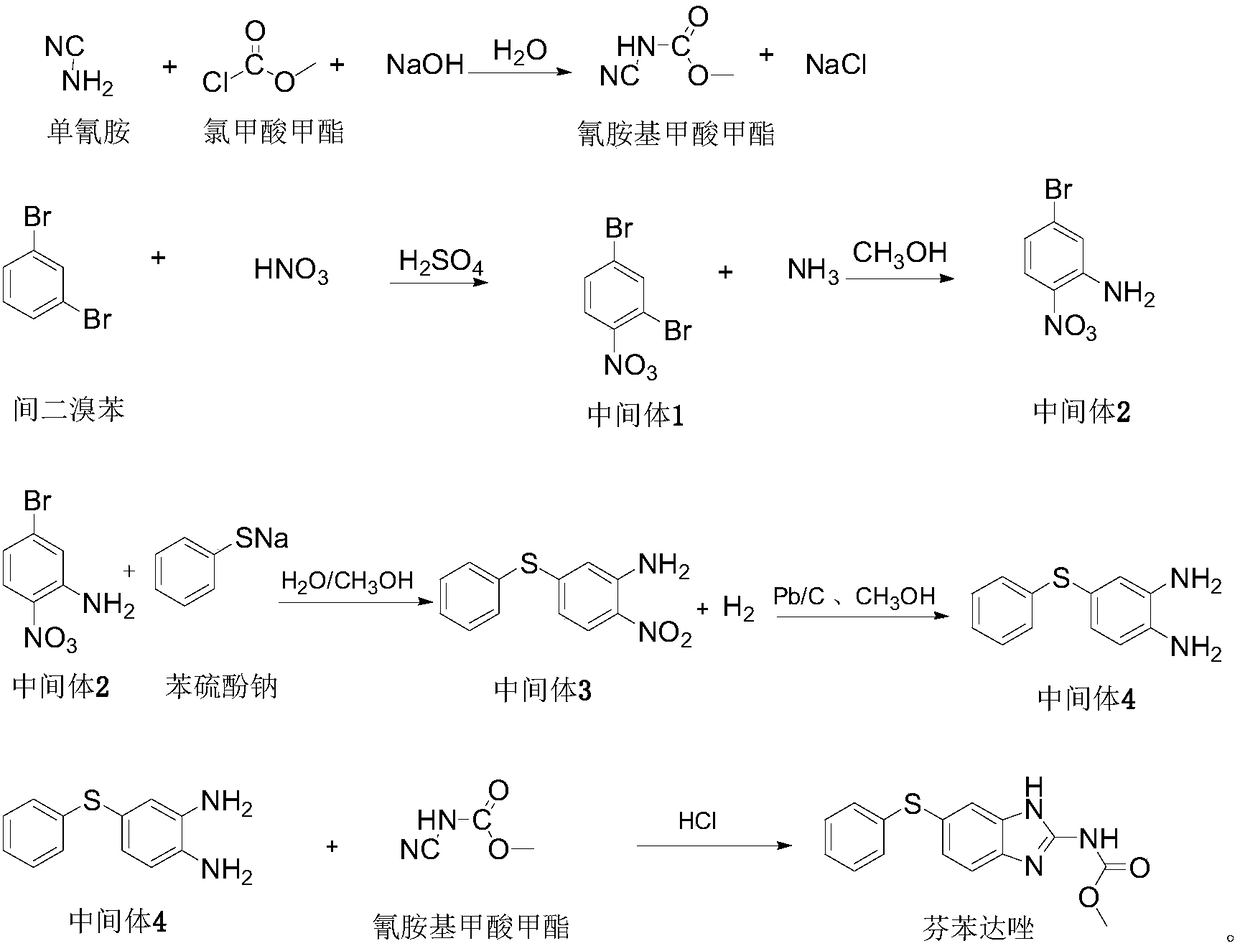
The invention discloses a preparation method of fenbendazole. The preparation method of fenbendazole is characterized by (1) taking m-dibromobenzene as a starting material, and nitrating a nitric acid / sulfuric acid system to prepare an intermediate 1(2,4-dibromonitrobenzene); (2) using the intermediate 1 as a raw material, and carrying out an ammoniation reaction with an ammonia methanol solutionto prepare an intermediate 2(5-bromo-2-nitroaniline); (3) taking the intermediate 2 and a sodium thiophenolate solution as raw materials, and carrying out condensation reaction to prepare an intermediate 3(4-phenylthio-2-nitroaniline); (4) carrying out hydrogenation reduction on the intermediate 3 through the catalysis of palladium charcoal to form intermediate 4(4-phenylthio-1,2-phenylenediamine); (5) carrying out cyclization reaction on the intermediate 4 and a methyl cyanamide aqueous solution to form the product, namely fenbendazole. The method is clean and environmentally friendly and lowin production cost, the purity of the product is more than 99.5%, and the yield is not less than 84.0%.
Owner:JIANGSU BAOZONG & BAODA PHARMACHEM
Compound fenbendazole tablet
ActiveCN105267230AThe killing effect is obviousNo side effectsOrganic active ingredientsPill deliveryReduction rateFenbendazole
The invention discloses a compound fenbendazole tablet. Specifically, each tablet is prepared from the following components by mass: 300-500mg of fenbendazole, 15-30mg of praziquantel, 0.01-0.05mg of ivermectin, 600-1000mg of a filler, 80-120mg of a disintegrating agent, 100-200mg of an adhesive, 10-20mg of a lubricant and 1-3mg of a flavoring agent. The compound fenbendazole tablet provided by the invention has significant repelling and killing effects on naturally infected worms of dogs, and single egg reduction rate reaches over 95%. The compound fenbendazole tablet can expel more species of parasites, can achieve better therapeutic effect, also is safe, has no toxic or side effect, good drug dissolution, high temperature resistance, sunlight resistance, good reproducibility, and stability.
Owner:QINGDAO AGRI UNIV
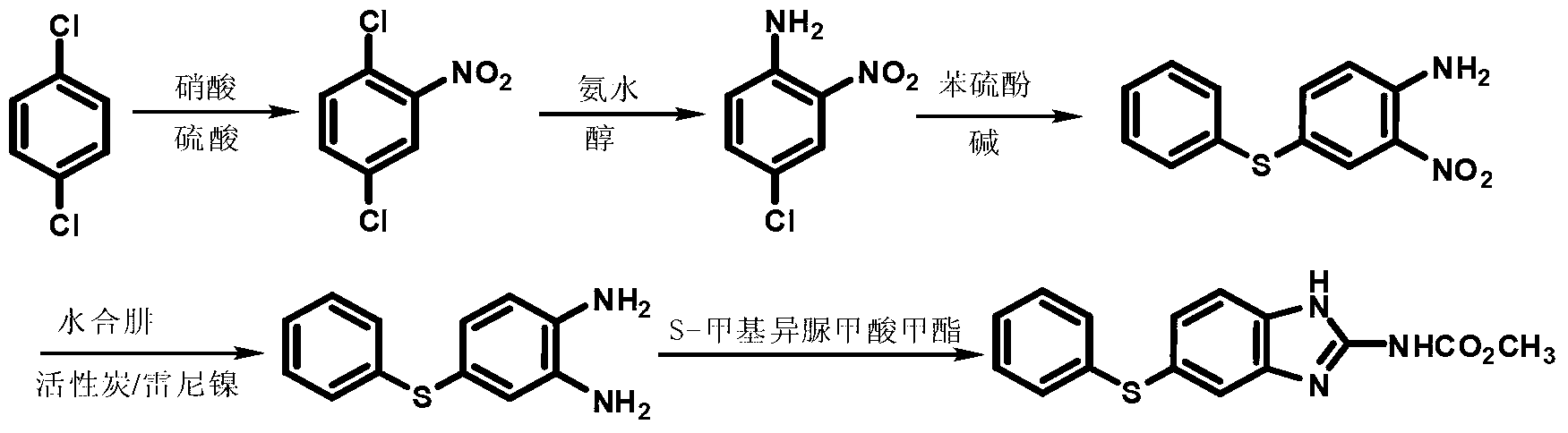
Preparation method of fenbendazole which is benzimidazole anti-helminthic drug
InactiveCN106397334AOperational securitySuitable for industrial productionOrganic chemistryFenbendazoleNitration
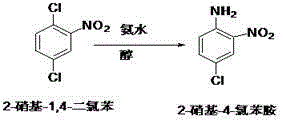
The invention discloses a preparation method of fenbendazole which is a benzimidazole anti-helminthic drug. Fenbendazole is obtained by taking p-dichlorobenzene as a raw material through nitration, amination, condensation, reduction and ring-closure, and the final product content is 98-101%. The preparation method is simple and efficient, is safe to operate, and is suitable for industrial production.
Owner:JIANGSU BAOZONG & BAODA PHARMACHEM
Fenbendazole dry emulsion and preparation method thereof
InactiveCN105456260ASettling speed is slowImprove physical stabilityPowder deliveryOrganic active ingredientsFenbendazoleSuspending Agents
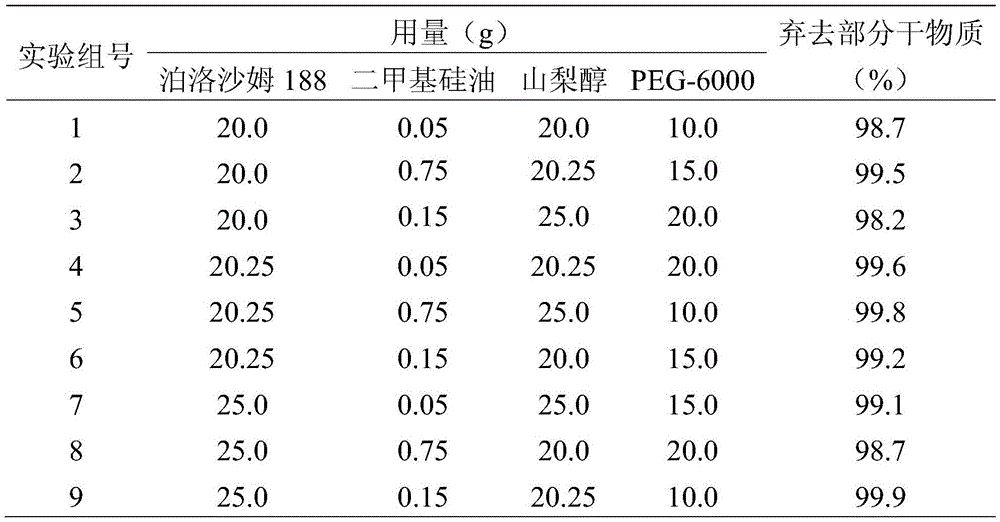
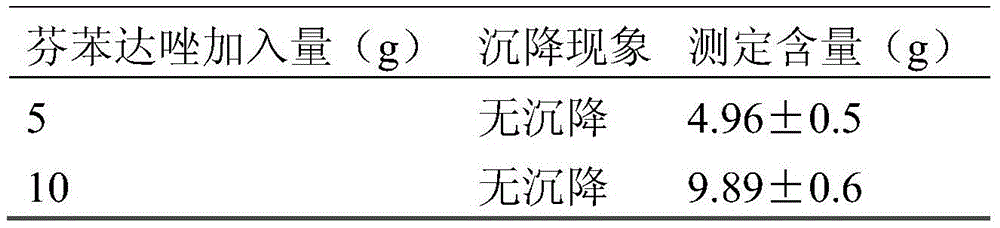
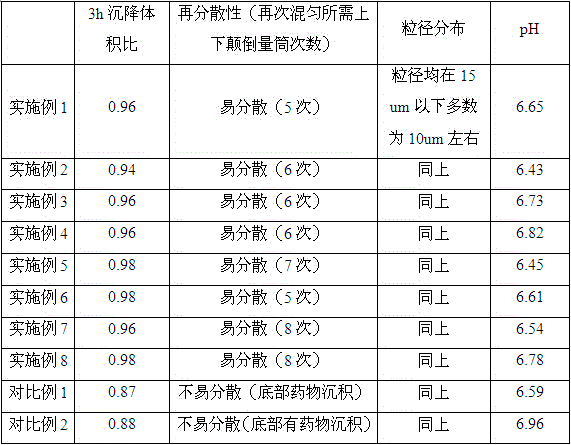
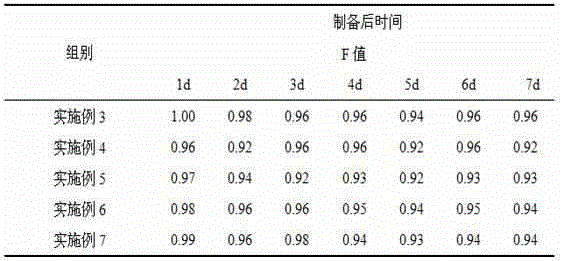
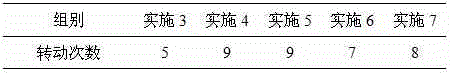
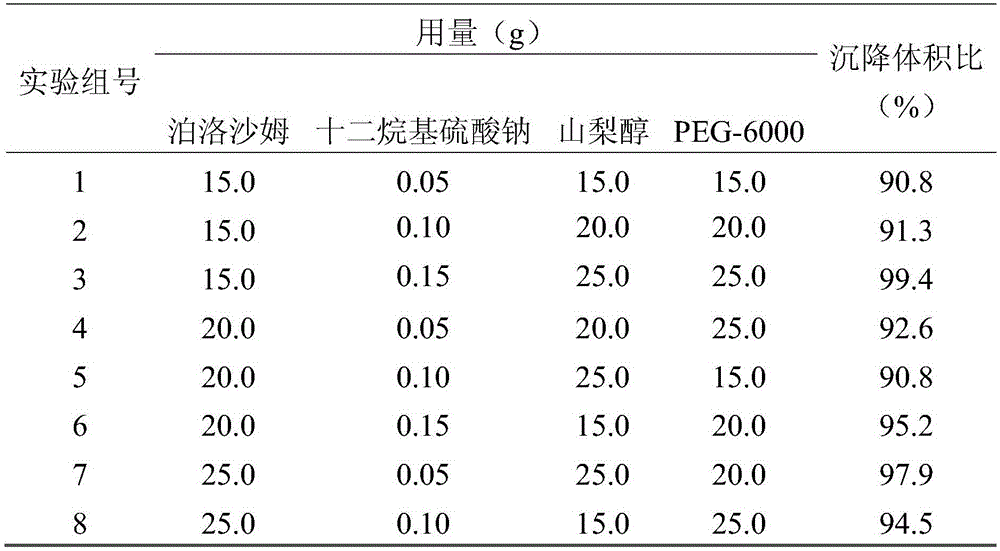
The invention discloses a fenbendazole dry emulsion. The fenbendazole dry emulsion is prepared from, by weight, 5-30 parts of fenbendazole, 20-25 parts of emulgator, 10-25 parts of suspending agent, 20-25 parts of colloid protection agent, 0.05-0.25 part of surface active agent and 0.05-1 part of oil phase. It is indicated through a quality evaluation experiment that the fenbendazole dry emulsion is low in settling velocity, good in physical stability, simple in preparation process, high in bath-to-batch quality stability and convenient to use and carry, the quality requirement of suspensions is met, and good application prospects are achieved.
Owner:CHENGDU QIANKUN VETERINARY PHARMA
Compound ivermectin injection and preparation method
InactiveCN101375856AReduce infection rateIncrease weightSulfur/selenium/tellurium active ingredientsAntiparasitic agentsFenbendazoleInfection rate
The invention discloses a compound ivermectin injection for reducing the chances of poisoning when in killing of parasites of livestock and a preparation method. The compound ivermectin injection is composed of 10-50 parts of ivermectin, 10-50 parts of fenbendazole, 50-200 parts of dimethyl sulfoxide and 30-100 parts of ethyl acetate. The preparation method is as follows: the components are weighted according to the weight; the ivermectin is added in the ethyl acetate for stirring for 10-25min till the clarification; the fenbendazole is added in the dimethyl sulfoxide for stirring for 10-25min till the clarification; the prepared solution is merged, evenly stirred and maintained for 15-20min, and propylene glycol is added till 1000 parts after the clarification of liquid medicine for the preparation. The compound preparation is effective and has high efficiency for the parasites of the livestock, and the compound preparation can be widely applied in the parasites of the livestock, reduce the infection rate of the parasites and increase the body weights of pigs, cattle, sheep and horses.
Owner:TIANJIN SHENGJI GRP CO LTD
Preparation method of compound fenbendazole tablet
ActiveCN105287613AGood effectThe killing effect is obviousOrganic active ingredientsPill deliveryFenbendazoleSide effect
The invention discloses a preparation method of a compound fenbendazole tablet. The preparation method comprises steps of preprocessing raw materials and adjuvant materials; preparing ivermectin gelatin liquid; mixing and granulating; drying and straightening granules; mixing again; tabletting; and packaging: packaging tablets passing full inspection in a brown plastic bottle. The compound fenbendazole tablet prepared by the invention has the beneficial effects that the prepared compound fenbendazole tablet is significant in effect on expelling and killing naturally infected worms in dogs, and a decreasing rate to single egg is 95% or above; the compound fenbendazole tablet is suitable for expelling many worms and is capable of achieving a treatment effect; and the compound fenbendazole tablet is better in effect, safe, free from toxic and side effects, good in drug dissolution, good in reproducibility and stable, and resists high temperature and illumination.
Owner:QINGDAO AGRI UNIV
Anthelmintic and preparation method thereof
InactiveCN103933045AEfficient killingGood effectOrganic active ingredientsPill deliveryFenbendazolePraziquantel
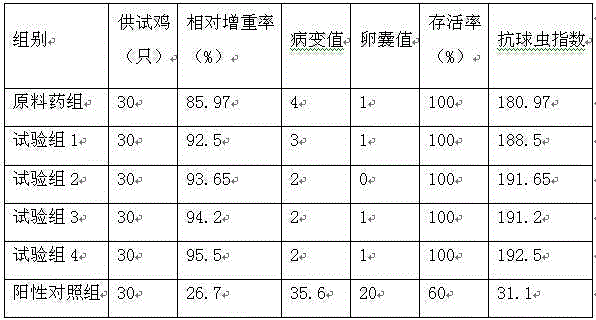
The invention discloses an anthelmintic for pets and a preparation method thereof. The anthelmintic disclosed by the invention is prepared from the following components: 83.3-87.2% of fenbendazole and 12.8-16.7% of praziquantel. The invention further provides the preparation method of the anthelmintic. The components are matched according to a specific proportion; the fenbendazole is wide in anthelmintic spectrum; the praziquantel has low toxicity to dogs, cats and the like; conversion products of the fenbendazole and the praziquantel can effectively interfere transportation of glucose by polypides in dogs and cats; the fenbendazole and the praziquantel are matched according to a specific proportion, so that the anthelmintic can take effect rapidly and is unnecessary to take on an empty stomach; in addition, the polypide-repelling degree is relatively thorough.
Owner:SHENZHEN REDRAY BIOTECHNOLOGY CORP LTD
Preparation method of fenbendazole
ActiveCN103242238AAvoid pollutionThe new synthesis process is simple and efficientOrganic chemistryM-chloroanilineFenbendazole
The invention discloses a preparation method of fenbendazole and provides a brand-new synthesis route of the fenbendazole. The fenbendazole is prepared from m-dichlorobenzene as a starting material through the steps of nitrification, condensation, amination, reduction and cyclization. The preparation method is characterized in that the starting material m-chloroaniline in the existing industrial route is changed to the cheap m-dichlorobenzene; the existing reduction technology with sodium sulfide dihydrate is changed to the clean and high-efficiency reduction technology; and the new synthesis technology is concise and simple, high in efficiency, slight in pollution, high in quality, and suitable to industrial production.
Owner:CHANGZHOU YABANG QH PHARMACHEM +2
Chinese medicinal concentrated lactating sow feed
InactiveCN103704499AEffective control of residuesPrevent red and yellowAnimal feeding stuffBiotechnologyEscherichia coli
The invention relates to a Chinese medicinal concentrated lactating sow feed, which includes feed components and Chinese medicinal components. The feed components include corn, soybean meal, fish meal, broken rice, rice skin, wheat bran, calcium hydrogen phosphate, lysine, trace elements, an enzyme preparation, choline chloride, and compound vitamin. The Chinese medicinal components comprise an antioxidant, montmorillonite, fenbendazole, Sophora flavescens, motherwort, codonopsis pilosula, astragalus, Radix Rubiae, liquorice, folium isatidis, Curcuma zedoaria, Chicken's Gizzard-membrane, and Rhizoma alismatis. According to the invention, traditional Chinese medicines are added into the feed, and mass use of antibiotics is avoided, so that the antibiotic residue in suckling pigs can be effectively controlled from the maternal source, a solid foundation for control of antibiotics in a later breeding process is laid, and support for production of harmless products and green products can be provided. The feed provided by the invention can prevent suckling pigs' red, yellow and white diarrhea diseases caused by Escherichia coli, dysentery bacillus and other pathogenic bacteria in sows, and also can promote the increase of sow's lactation yield.
Owner:GONG COUNTY JINYI ANIMAL HUSBANDRY SCI & TECH
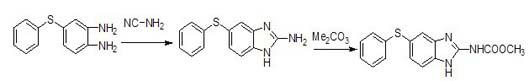
Preparation method of fenbendazole intermediate 2-nitro-4-phenylthioaniline
ActiveCN111349032AReduce manufacturing costReduce pollutionThiol preparationSulfide preparationBiotechnologyFenbendazole
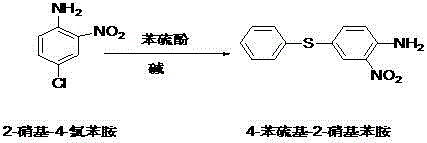
The invention relates to the technical field of veterinary drugs, and in particular, relates to a preparation method of fenbendazole intermediate 2-nitro-4-phenylthioaniline. The preparation method comprises the following steps: by taking o-nitroaniline and ammonium thiocyanate as raw materials and an organic solvent as a solvent of a reaction system, uniformly stirring and mixing, introducing chlorine, and filtering after the reaction of the raw materials is finished, so as to obtain 4-thiocyano-2-nitroaniline; taking 4-thiocyano-2-nitroaniline, adding sodium hydroxide and a reaction solvent,stirring and mixing, and filtering after the reaction is finished to obtain a sodium 4-amino-3-nitrothiophenol solution; carrying out a reaction on the sodium 4-amino-3-nitrothiophenol solution withbromobenzene under an alkaline condition, and after the reaction is finished, extracting to obtain the 2-nitro-4-phenylthioaniline. The preparation method is simple in process, the production cost isreduced, the production potential safety hazard can be reduced, the product yield is relatively high, and the environmental pollution is reduced.
Owner:SHANDONG GUOBANG PHARMA +1
Long-acting bactericide for crops
The invention relates to a long-acting bactericide for crops. The long-acting bactericide comprises a main agent and auxiliary agent, and the main agent contains fenbendazole. The animal pesticide fenbendazole is applied to the crop bactericide for the first time, and has a good effect. The long-acting bactericide disclosed in the invention has a better stability and a better rice blast control effect than the bactericide fenbendazole.
Owner:李洪军
Compounds for Enhancing Hypoxia Inducible Factor Activity and Methods of Use
The present invention relates to methods for enhancing Hypoxia inducible factor-1 (HIF) activity in a cell by contacting the cell with any one of the following compounds: 3,6-bis[2-(dimethylamino)ethoxy]-9h-xanthen-9-onedihydrochloride, 2,8-bis[dimethylaminoacetyl]dibenzofurin dihydrochloride hydrate, tilorone analogue R-9536-DA, indoprofen, ciclopiroxolamine, tryptophan, ansindione, nabumetone, oxybendazole, albendazole, tropicamide, pramoxine hydrochloride, atenolol, mebendazole, carbetapentane citrate, monensin sodium, methoxyvone, hydroxyzine, phenazopyridine, clofoctol, ipraflavone, zomepirac, biochanin A, xylometazoline hydrochloride, fenbendazole, pirenzepine, triprolidine hydrochloride, daidzein, tripelennamine citrate, colchicines, aminopyridine, trimethoprim, helenine, hydroxyurea, amiodarone hydrochloride, clindamycin hydrochloride, sulfachlorpyridazine, mephenesin, semustine, clofivric acid, clofibrate, ibuprofen, hyoscyamime, nafcillin sodium, piperin, clidinium bromide, trioxsalen, hydralazine and HIF alpha protein fused to a carrier peptide.
Owner:CORNELL RES FOUNDATION INC
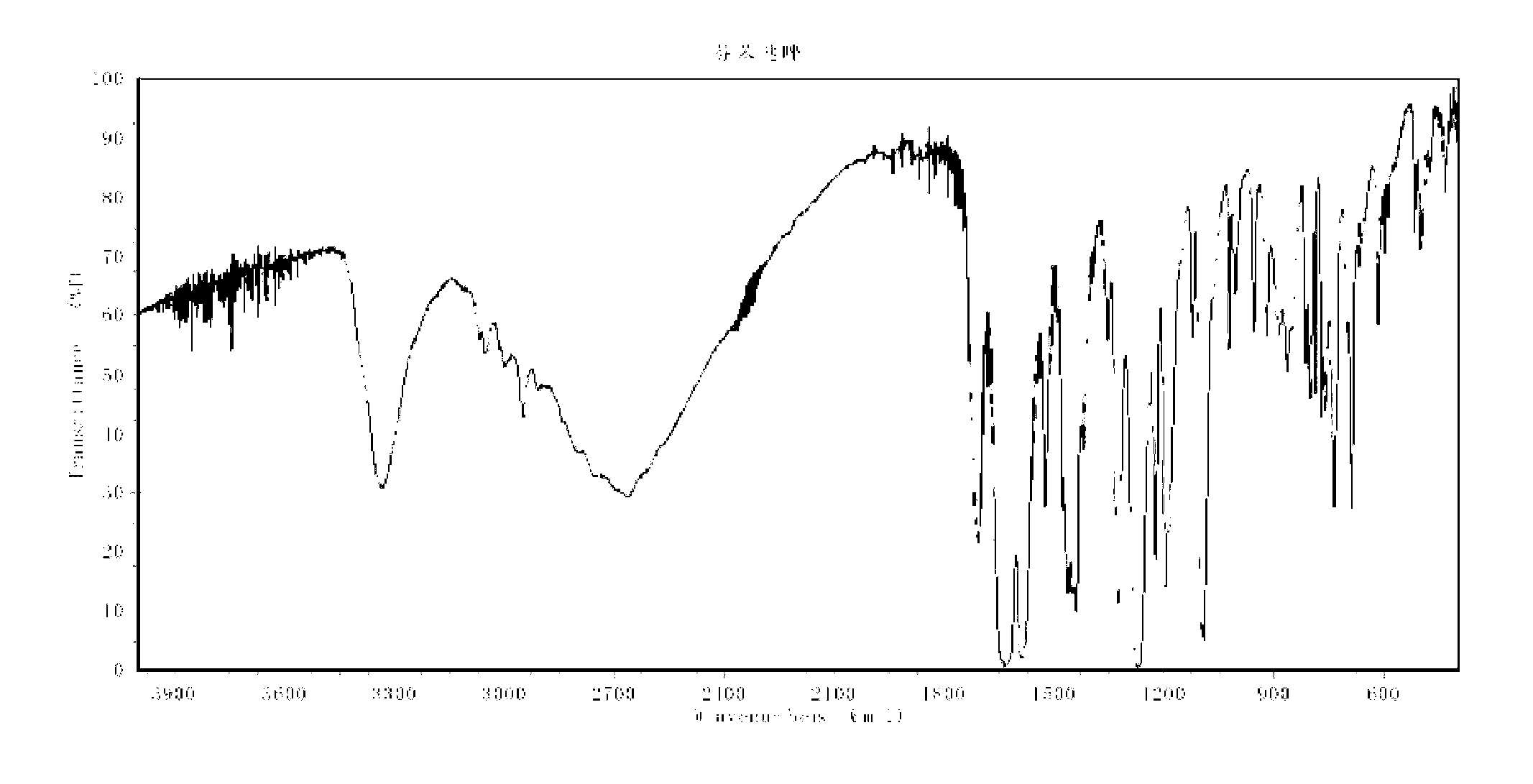
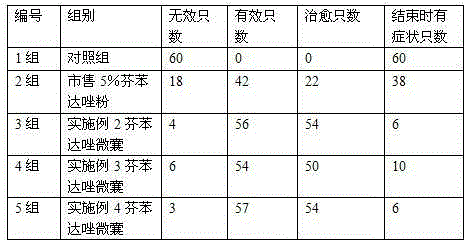
Fenbendazole micro-capsules and preparation method thereof
ActiveCN105343032AExtended half-lifeImprove stabilityOrganic active ingredientsAntiparasitic agentsFenbendazoleEmulsion
Disclosed are fenbendazole micro-capsules and a preparation method thereof. The preparation method includes, firstly, preparing fenbendazole emulsion and gelatin solution, adding the gelatin solution into the fenbendazole emulsion while stirring continuously until forming capsules, cooling by ice bath, adding in formaldehyde solution and sodium hydroxide solution to regulate pH value, standing the mixture until the mixture is in a layering state, removing supernatant, and then washing and drying to obtain finished products. The preparation method of fenbendazole micro-capsules is simple, is low in cost, easy to operate, low in loss and high in total yield; fenbendazole micro-capsules produced by the method are white or light-yellow or yellow spherical particles with the diameter ranging from 200 micrometers to 850 micrometers, are semi-transparent in microscope, are good in flowability and accordingly are easy to mix with food.
Owner:ZHENGZHOU HOUYI PHARMA
Traditional Chinese medicine type nonpregnant sow concentrated feed
InactiveCN103719589APrevention of Syndromic Reproductive DisordersPromote estrusAnimal feeding stuffDiseaseFenbendazole
The invention relates to a traditional Chinese medicine type nonpregnant sow concentrated feed which comprises feed components and traditional Chinese medicine components, wherein the feed components comprises corn, soybean meal, fish meal, broken rice, silverskin, wheat bran, calcium hydrophosphate, lysine, microelement, an enzymic preparation, choline chloride and compound vitamin; the traditional Chinese medicine components comprises an anti-oxidant, salt, montmorillonite, fenbendazole, radix sophorae flavescentis, cyrtomium fortunei, polygonum multiflorum, astragalus membranaceus, raspberry, semen allii tuberose, rosalaevigata mickx, semen cuscutae, epimedium, morinda officinalis, cynomorium songaricum, codonopsis pilosula, bighead atractylodes rhizome, poria, radix glycyrrhizae and Chinese angelica. According to the invention, the traditional Chinese medicines are added into the feed, too much used antibiotics are avoided, antibiotic residue in the body of porkers is effectively controlled from parent source, firm basis is established on antibiotic control during later stage breeding process, and support is provided for the production of public hazard product and green product. Obstetric diseases such as hysteritis of the sow and the comprehensive breeding difficulty disease of the sow can be prevented, empathema and ovulation of the sow can also be prevented, and the conception rate of the sow is improved.
Owner:GONG COUNTY JINYI ANIMAL HUSBANDRY SCI & TECH
Invermectin and fenbendazole suspension injection and preparing method thereof
ActiveCN105708850APromote absorptionImprove bioavailabilityOrganic active ingredientsSolution deliveryFenbendazoleOrganic solvent
The invention discloses an invermectin and fenbendazole suspension injection and a preparing method thereof. Each 100 ml of the invermectin and fenbendazole suspension injection is prepared from, by mass, 2.5-5 g of fenbendazole, 0.2-0.4 g of invermectin, 0.2-1 g of a wetting agent, 0.8-1.8 g of a suspending agent, 0.1-0.4 g of a preservative, 1.0-2.0 g of a deflocculant and 0.002-0.008 g of a pH regulator. The preparing method of the suspension injection comprises the following steps that firstly, the wetting agent is added with water to be dissolved; secondly, the suspending agent, the preservative, the deflocculant and the pH regulator are added with water to be dissolved respectively; thirdly, invermectin and fenbendazole are added in the solution obtained in the first step and evenly mixed; fourthly, the solution obtained in the second step is added into the solution obtained in the third step under a shearing condition, and shearing is carried out. The preparing method of the invermectin and fenbendazole suspension injection is easy to implement, and a small dose of organic solvents are used; in addition, the insect resistant spectrum is enlarged through joint use of invermectin and fenbendazole, and the cost and toxicity of pesticide are greatly reduced.
Owner:SOUTH CHINA AGRI UNIV
Preparation method of fenbendazole microspheres
InactiveCN104473880AAids in cross-linking and curingHigh drug loadingOrganic active ingredientsOrganic non-active ingredientsFenbendazoleMicrosphere
The invention relates to a preparation method of fenbendazole microspheres, which comprises the following steps: (1) water phase preparation: taking gelatin and water, preparing a gelatin solution, heating to 45-55 DEG C, and adding fenbendazole until the gelatin concentration is 0.02-0.4 g / ml and the fenbendazole concentration is 0.01-0.5 g / ml; (2) oil phase preparation: taking liquid paraffin and 1.0-2.0 wt% Span 80, and mixing according to the volume ratio of (4-6):1; (3) adding the water phase in the step (1) into the oil phase in the step (2) according to the volume ratio of 1:(5-7), stirring, cooling to 5 DEG C below, and adding glutaric dialdehyde to perform crosslinking curing, wherein the volume ratio of the glutaric dialdehyde to the water phase in the step (3) is 1:(1-10); and (4) washing with isopropanol, washing with petroleum ether, and carrying out vacuum drying. The preparation method is simple to operate, has the advantages of cheap and accessible reagents, high simpleness and safety, environmental protection and lower preparation cost, and can easily implement popularization and application.
Owner:ZHENGZHOU HOUYI PHARMA
Fenbendazole nano suspension and preparation method thereof
InactiveCN105982854AIncrease Saturation SolubilityImprove dissolution rateOrganic active ingredientsSolution deliveryFenbendazoleSide effect
The invention discloses a fenbendazole nano suspension which is composed of, by mass, 2-8% of fenbendazole, 0.5-2% of a suspending agent, 0.05-0.15% of a wetting agent, 0.5-2% of a flocculating agent, 0.05-0.15% of an antibacterial agent and the balanced being water for injection. Compared with the prior art, the fenbendazole is processed into the nano suspension, so that saturated solubility, dissolving rate and mucous membrane adhesion are improved, a problem of low bioavailability of the medicine is overcome and effectiveness, safety and stability of the medicine are improved. The preparation method and formula in the invention are simple. The fenbendazole nano suspension has uniform particle size distribution, is easy to apply and is suitable for industrial production. The nano suspension has excellent stability, improves solubility and oral-taking bioavailability of fenbendazole, is reduced in toxic and side effects and has excellent practical value and application prospect.
Owner:HENAN HUITONG TIANXIA BIO ENG
Synthetic method of fenbendazole
A synthetic method of fenbendazole belongs to the technical field of insect repellents, and specifically comprises the following steps: carrying out condensation reaction on 5-chloro-2-nitroaniline and a sodium thiophenol aqueous solution in a mixed solution of n-propyl alcohol and water to obtain 5-thiophenyl-2-nitroaniline; carrying out reduction reaction on the 5-thiophenyl-2-nitroaniline in a high-pressure kettle under the catalysis of raney nickel to generate 4-thiophenyl o-phenylenediamine; and mixing the 4-thiophenyl o-phenylenediamine and N-(trichloromethyl) methyl carbamate for a cyclization reaction to obtain fenbendazole; the method has the advantages that conditions are mild, operation is easy and convenient, ammonium chloride can be prevented from being generated in the cyclization process, and the three-waste cost is low; the generation of amine salt is avoided, and the treatment cost of three wastes is greatly reduced; and the yield of the fenbendazole can reach 84.27% to 89.99%, and the purity of the fenbendazole can reach 96.39% to 99.71%.
Owner:SHANDONG GUOBANG PHARMA +1
Fenbendazole dry suspension and preparation method thereof
InactiveCN105456199ASettling speed is slowImprove physical stabilityPowder deliveryOrganic active ingredientsFenbendazoleSuspending Agents
The invention discloses a fenbendazole dry suspension and a preparation method thereof. The fenbendazole dry suspension is prepared from, by weight, 50-80 parts of fenbendazole, 15-25 parts of emulgator, 10-25 parts of suspending agent, 10-25 parts of colloid protective agent and 0.05-0.25 part of surface active agent. It is indicated through a quality evaluation experiment that the fenbendazole dry suspension is low in settling velocity, good in physical stability, simple in preparation process, high in bath-to-batch quality stability and convenient to use and carry, the quality requirement of suspensions is met, and good application prospects are achieved.
Owner:CHENGDU QIANKUN VETERINARY PHARMA
Preparation method of high-dissolution-rate fenbendazole medicinal preparation
ActiveCN104523684AEasy to useReduce pollutionOrganic active ingredientsPharmaceutical non-active ingredientsFenbendazoleAnthelmintic drug
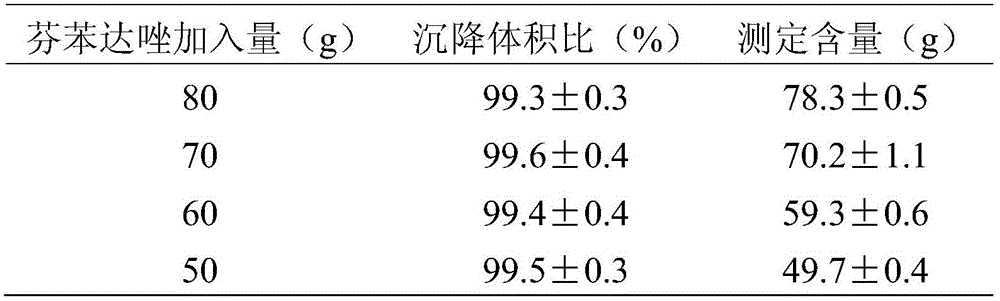
The invention relates to a technology for preparing high-dissolution-rate antiparasitic drug fenbendazole and belongs to the field of veterinary preparation. A preparation method of the high-dissolution-rate antiparasitic drug fenbendazole comprises the following steps: adding a fenbendazole crude drug in food grade solvent, heating to dissolving fenbendazole, and preparing fenbendazole solution; adding a food grade emulsifying agent in the fenbendazole solution, and stirring, so that the food grade emulsifying agent is uniformly dispersed, and fenbendazole emulsion is obtained; slowly adding the fenbendazole emulsion into a food grade adsorbent, mixing, stirring continuously, and thus the high-dissolution-rate fenbendazole solid pharmaceutical preparation can be prepared. The high-dissolution-rate antiparasitic drug fenbendazole has the advantages that dissolution efficiency of insoluble fenbendazole in a gastrointestinal tract is improved to the utmost extent, and dissolution rate is increased to more than 70% from the original less than 1%.
Owner:ZHEJIANG WANFANG BIO TECH CO LTD
Method for significant reduction of solvent dosage in oxfendazole production process
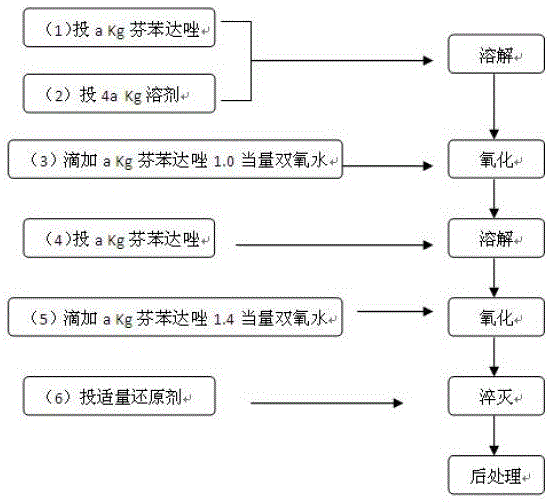
The invention discloses a method for significant reduction of solvent dosage in an oxfendazole production process. In the oxfendazole production process adopting fenbendazole as the starting material and using hydrogen peroxide as the oxidant, because of the poor solubility of fenbendazole, large solvent dosage and no recoverability, the process is high in cost and is environmentally unfriendly. According to the characteristics of material crystal transformation, through ingenious design of the feeding sequence, feeding mode, feeding amount and feeding time, the solvent dosage can be greatly saved. The process is simple to operate, also is economical and environmentally friendly, and is suitable for industrial production.
Owner:JIANGSU BAOZONG & BAODA PHARMACHEM
Fenbendazole effervescence particle for livestock and poultry and preparation method thereof
InactiveCN102846578AGood curative effectImprove bioavailabilityOrganic active ingredientsPharmaceutical delivery mechanismFenbendazoleDisease
The invention relates to a fenbendazole effervescence particle for livestock and poultry and a preparation method thereof. The fenbendazole effervescence particle comprises the following raw materials and accessories expressed in weight parts: 30 to 80 parts of fenbendazole, 100 to 140 parts of citric acid, 120 to 170 parts of sodium bicarbonate, 20 to 60 parts of polyethylene glycol 6000, 190 to 260 parts of dextrin and 310 to 390 parts of cane sugar. The preparation method comprises the following steps: respective crushing of the raw materials and the accessories; mixing; granulation; drying; size finishing; and subpackage. The effervescence particle provided by the invention is developed and prepared by using modern detection means and modern pharmaceutical equipment according to GMP standard for veterinary drugs and has the advantages of good curative effects, safety and reliability; the accessories and an effervescent disintegrating agent are added into the raw materials so as to prepare an effervescent granule, and livestock and poultry with diseases take the effervescent granule through oral administration, and good taste, stable quality and improved bioavailability of drugs are realized.
Owner:GREAT BIOLOGY PHARMA TIANJIN
Air-brake hose material
The invention discloses an air-brake hose material which comprises the following raw materials in parts by weight: 93-100 parts of ethylene-propylene-diene monomer rubber 4770R, 7-10 parts of polydicyclopentadiene, 4-5 parts of polyvinylidene fluoride, 2-3 parts of polyvinyl alcohol, 1-2 parts of fenbendazole, 4-6 parts of poly(butyl acrylate), 2-3 parts of monopotassium phosphate, 0.2-0.4 part of trimethylhexamethylenediamine, 2-4 parts of calcium aluminate, 4-7 parts of carbon fiber, 20-24 parts of carbon black, 1-1.6 parts of 2,4-dichlorobenzoyl peroxide, 0.8-1 part of an accelerating agent CSB, 0.4-1 part of an accelerating agent CPB and 16-27 parts of a composite filler. An air-brake hose provided by the invention is good in positioning performance, high in overall strength and good in aging resistance and corrosion resistance.
Owner:WUHU JIACHENG ELECTRONICS TECH
Wettable fenbendazole ivermectin powder
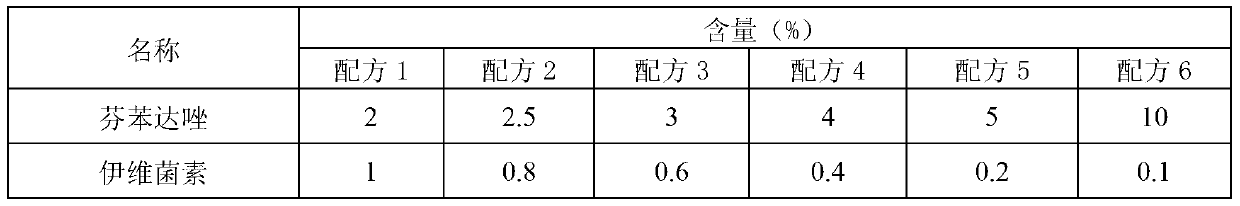
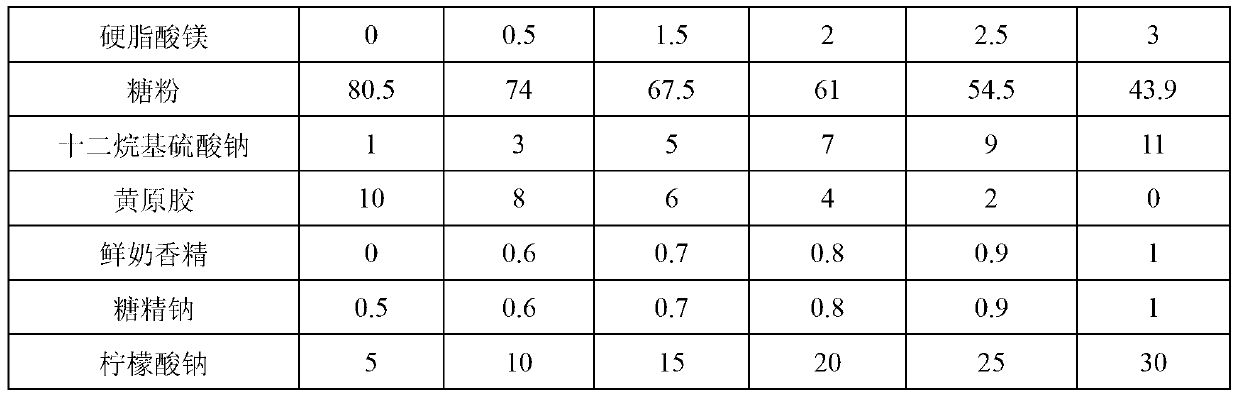
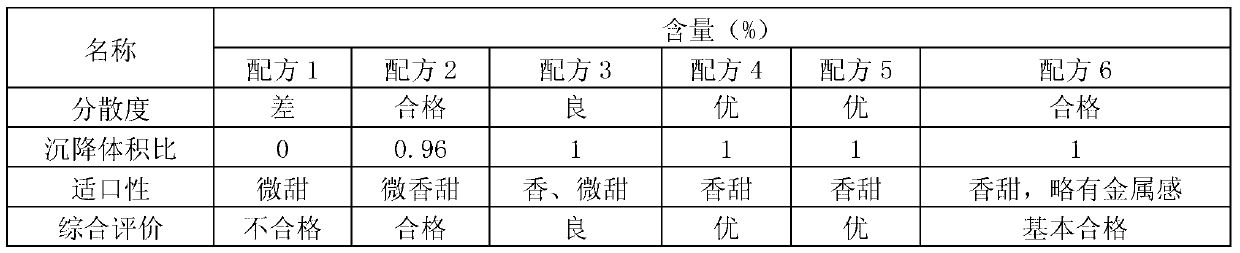
InactiveCN103340884AGood dispersionEvenly distributedPowder deliveryOrganic active ingredientsDispersityIcing sugar
The invention provides wettable fenbendazole ivermectin powder which is prepared from the following raw materials and auxiliary materials in parts by weight: 2.5-10 parts of fenbendazole, 0.1-0.8 part of ivermectin, 0.5-3 parts of magnesium stearate, 43.9-74 parts of powdered sugar, 3-11 parts of sodium dodecyl sulfate, 0-8 parts of xanthan gum, 0.6-1 part of fresh milk essence, 0.6-1 part of saccharin sodium and 10-30 parts of sodium citrate. The invention also provides a preparation method and application of the wettable fenbendazole ivermectin powder. The wettable fenbendazole ivermectin powder significantly improves the dispersity of the ivermectin and fenbendazole in water to ensure that the ivermectin and the fenbendazole can be uniformly distributed in the water for a long time to facilitate clinical water drinking and administration; and wettable fenbendazole ivermectin powder has a food calling function, namely that the fenbendazole ivermectin powder has a food calling function when used on faunas with sensitive tastes, such as pigs and the like. The wettable fenbendazole ivermectin powder is simple in preparation process, low in production cost and suitable for industrial large-scale production.
Owner:CHENGDU QIANKUN VETERINARY PHARMA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com