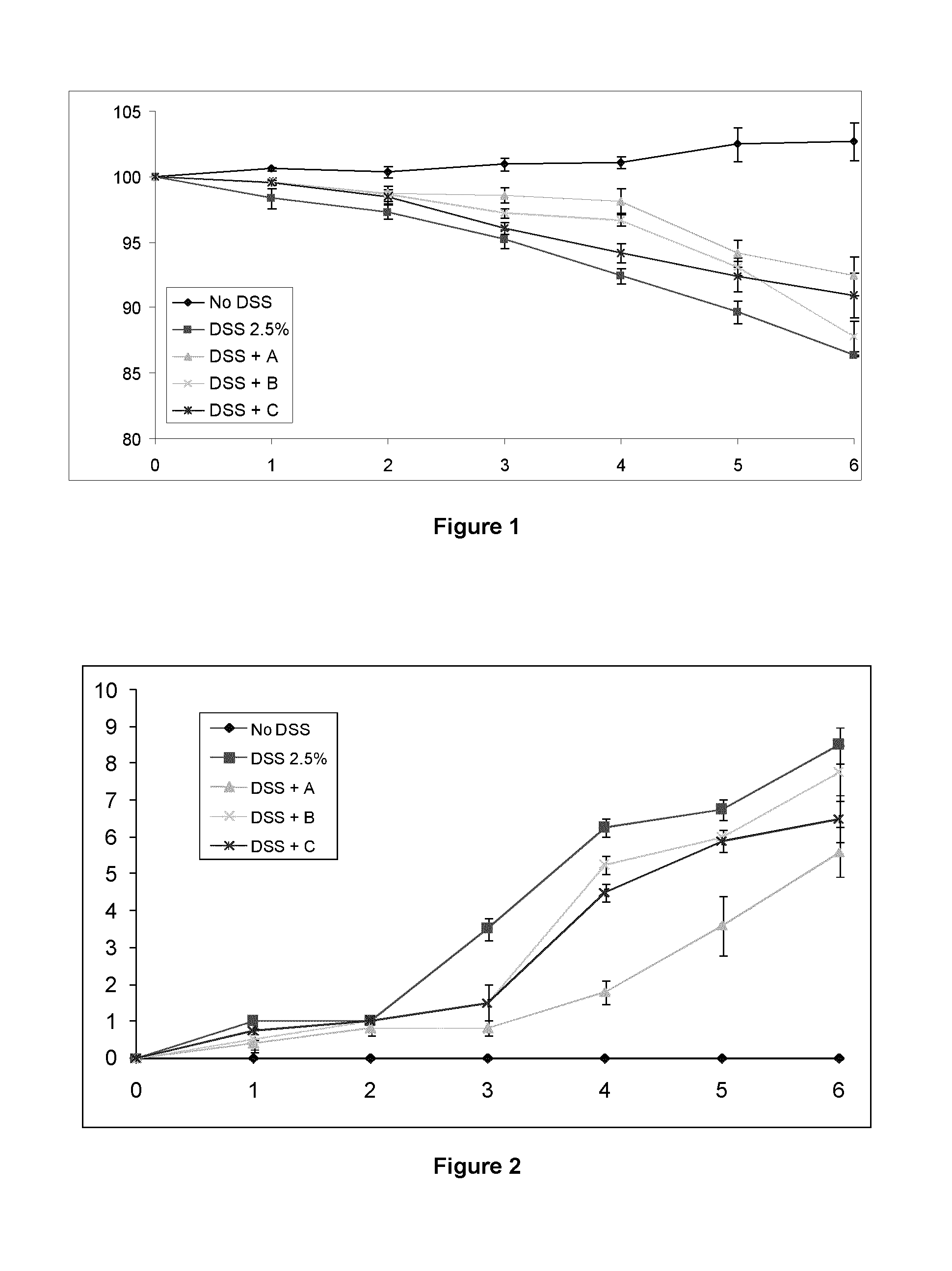
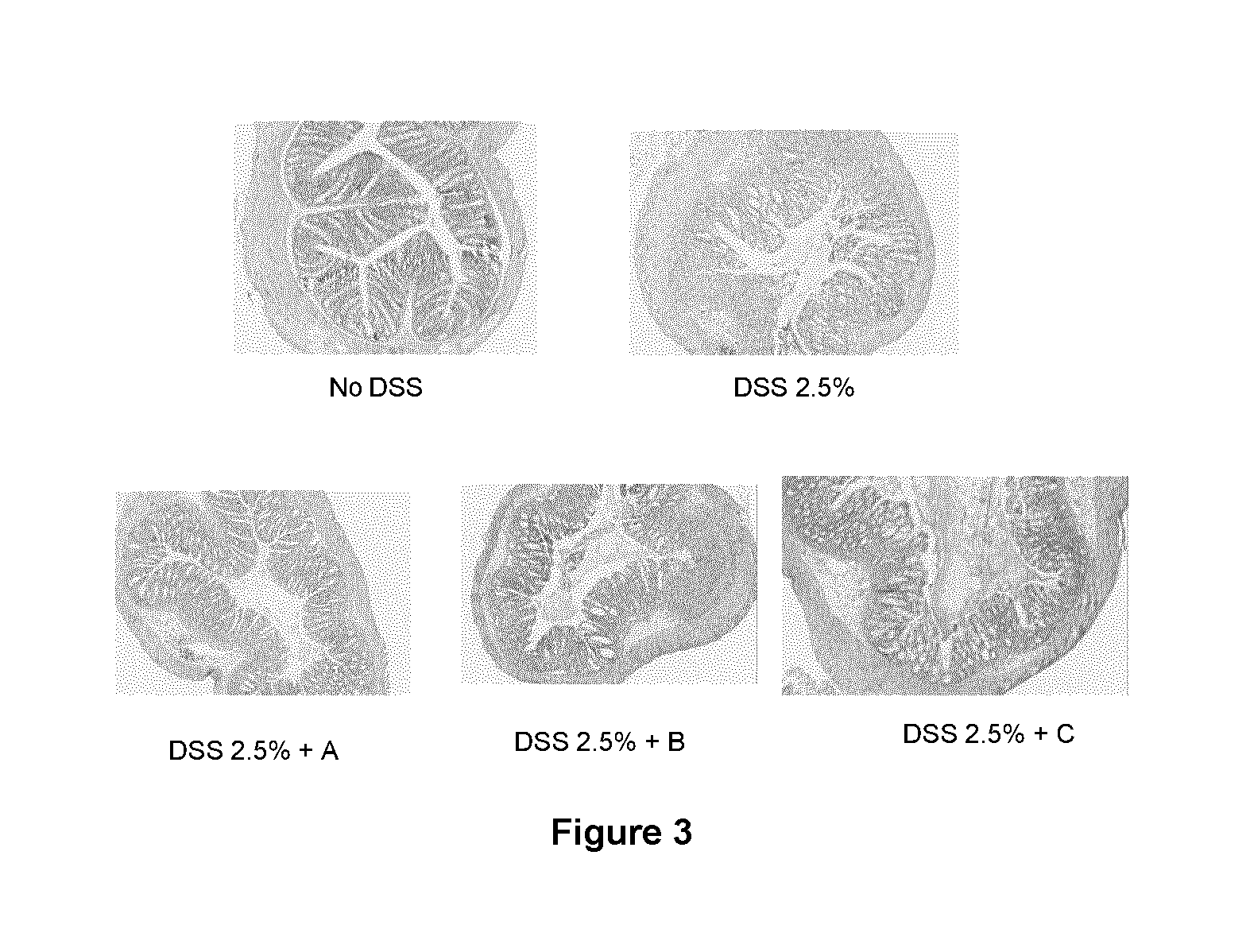
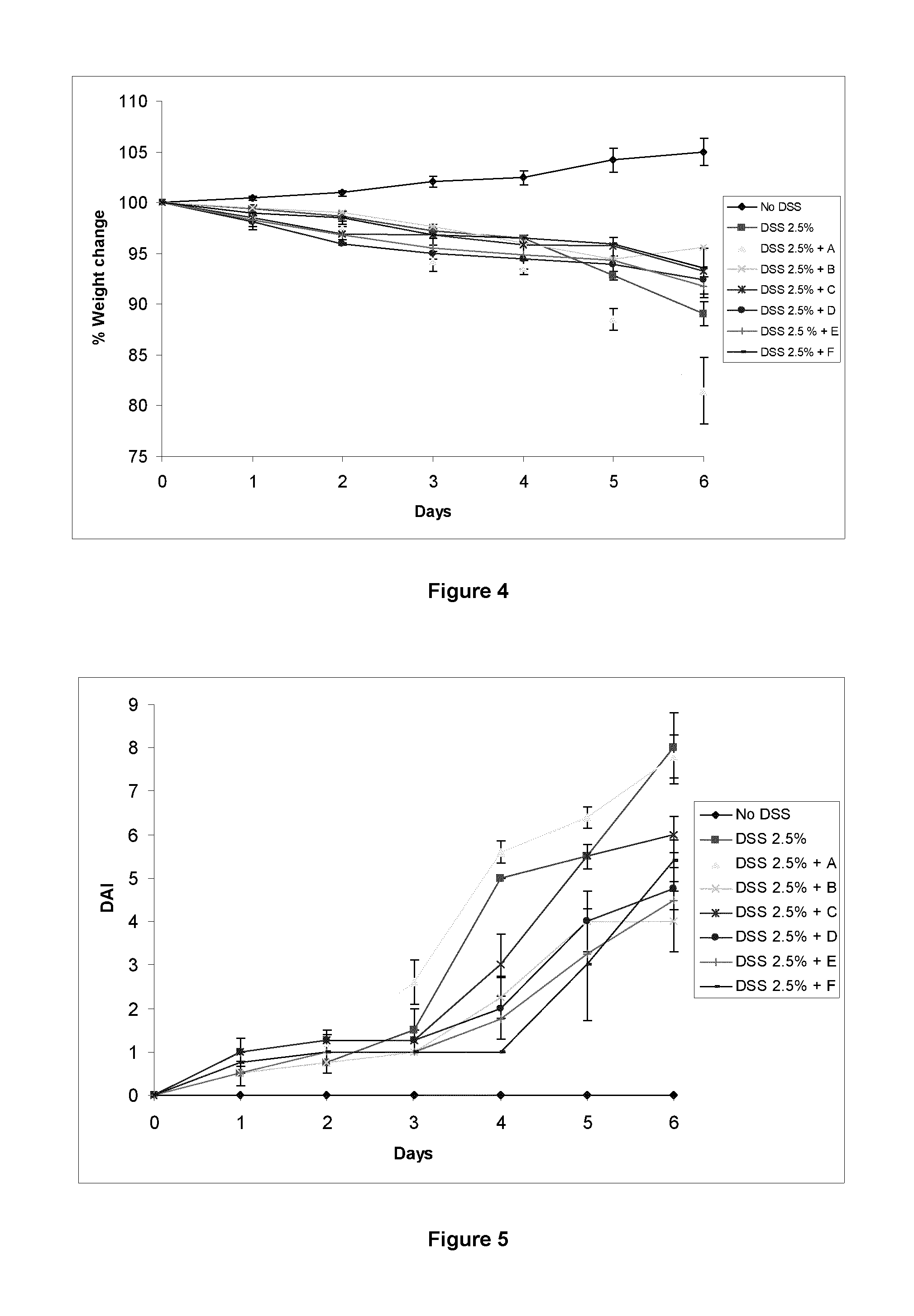
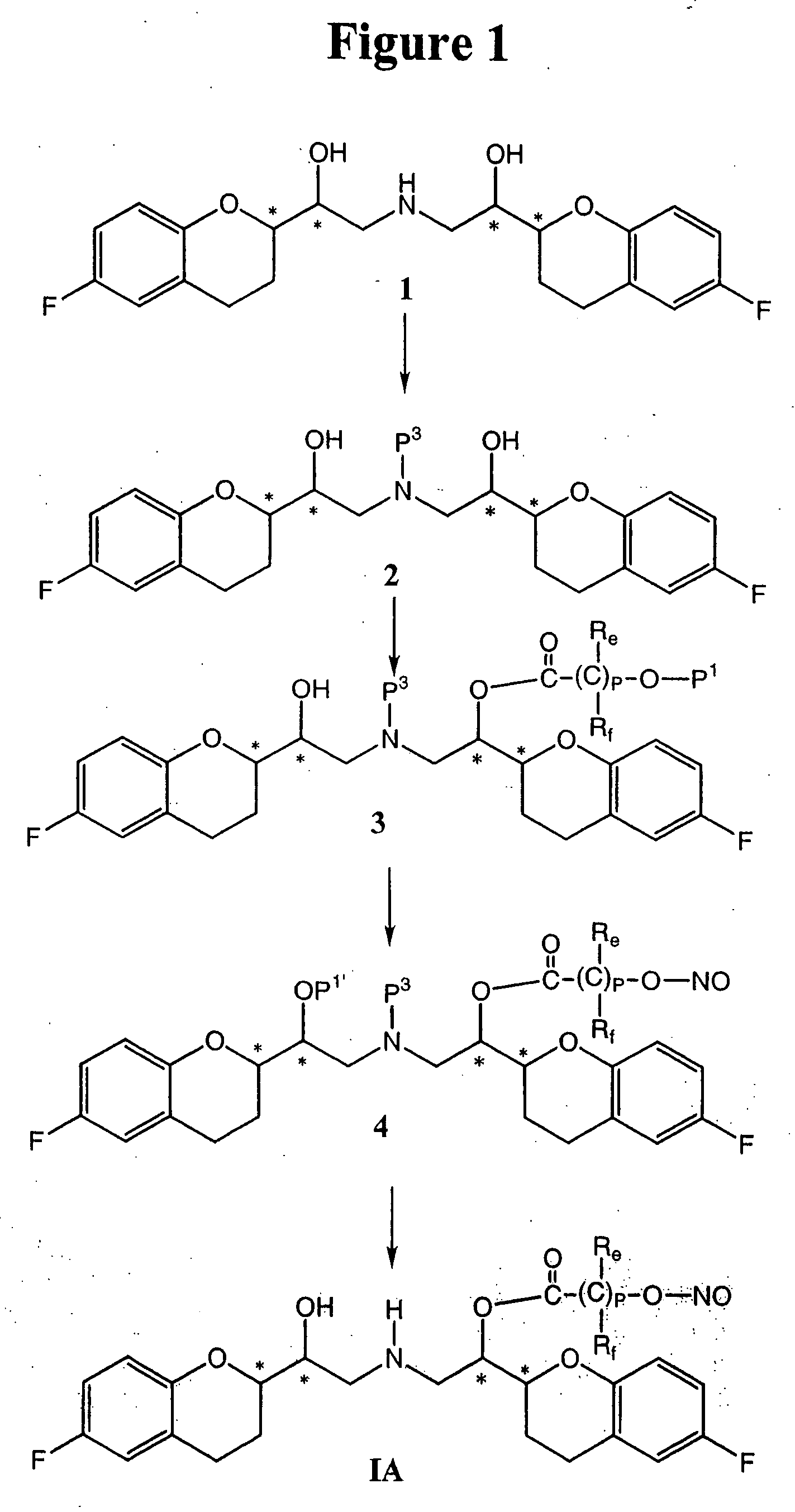
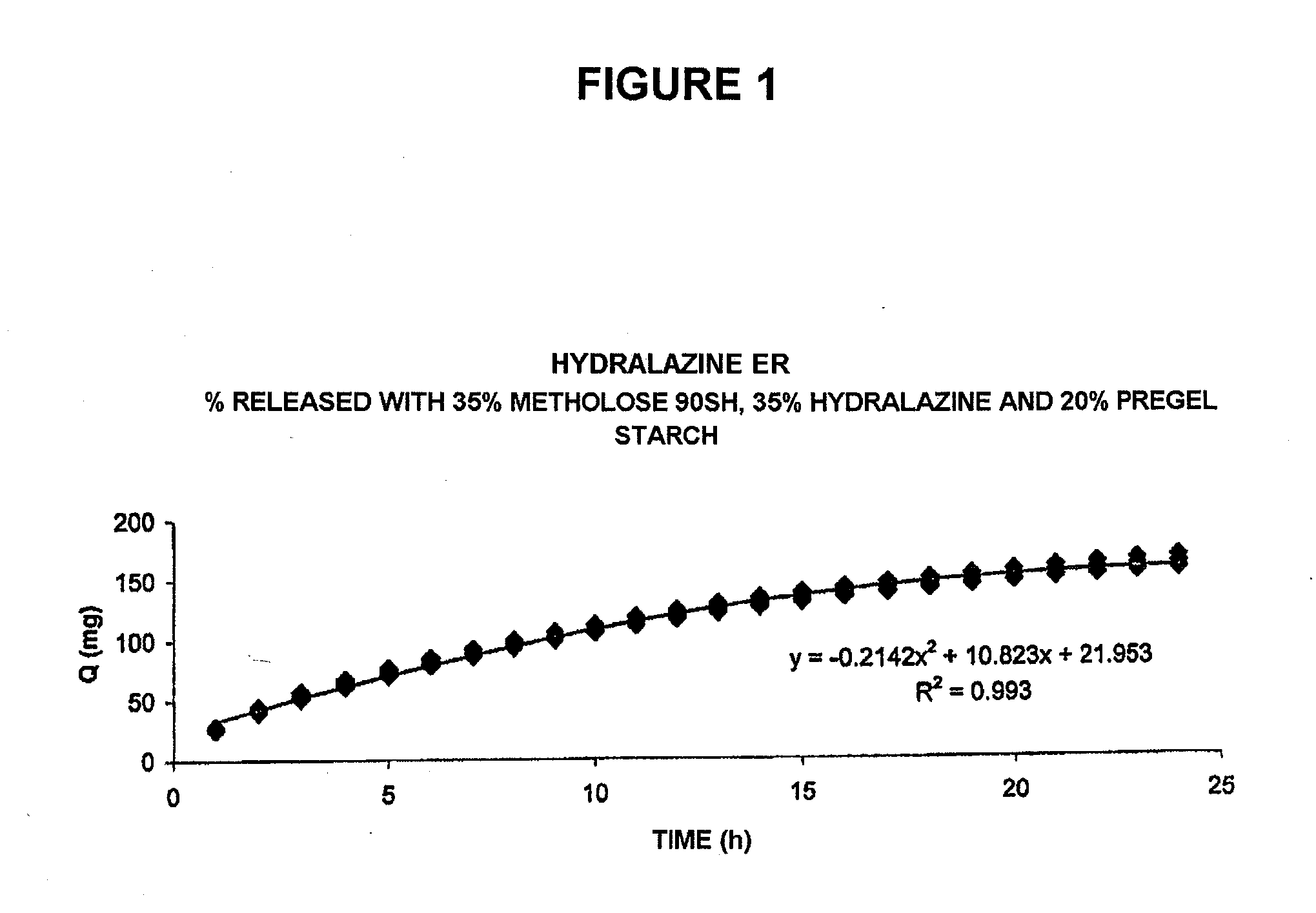
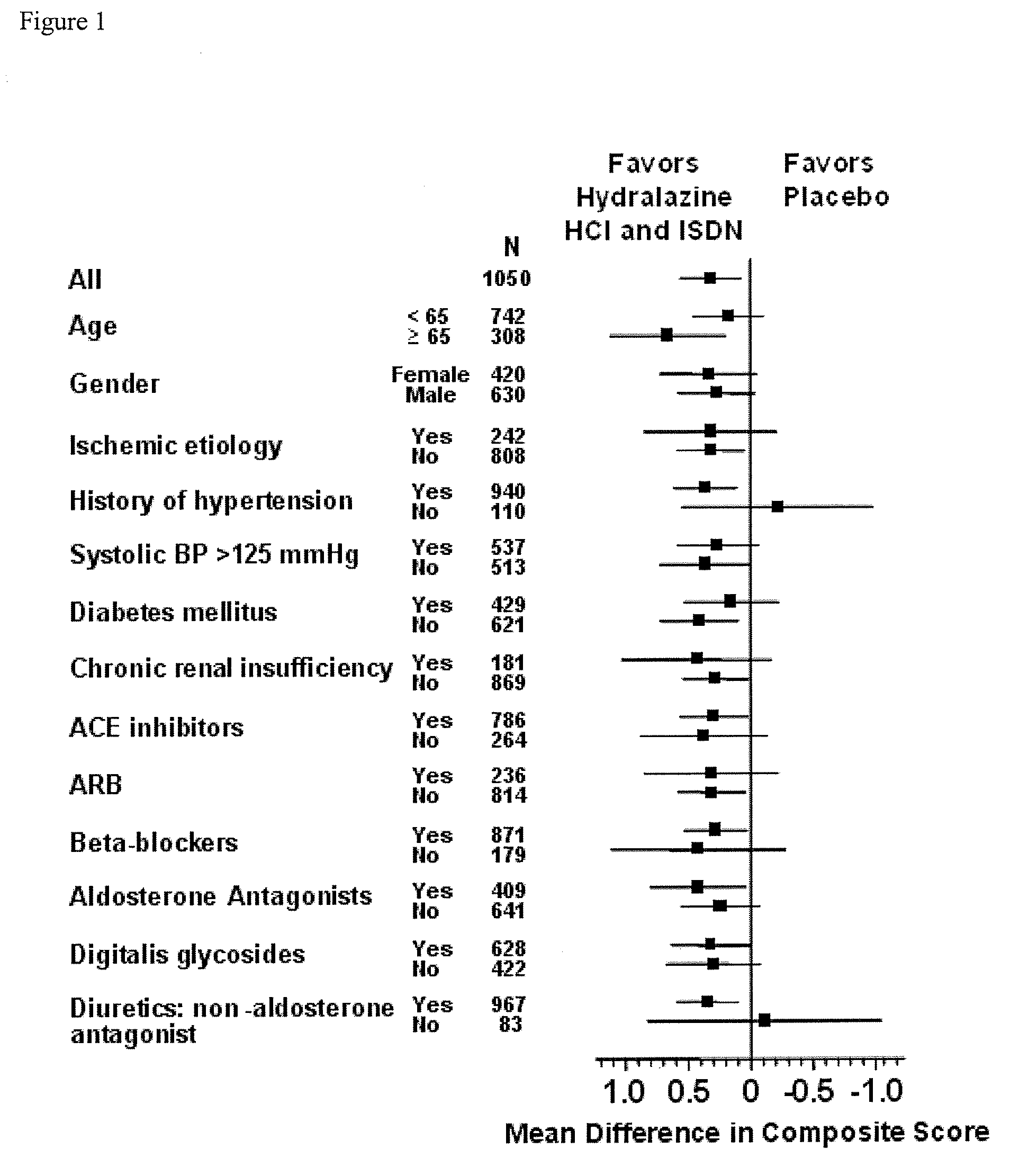
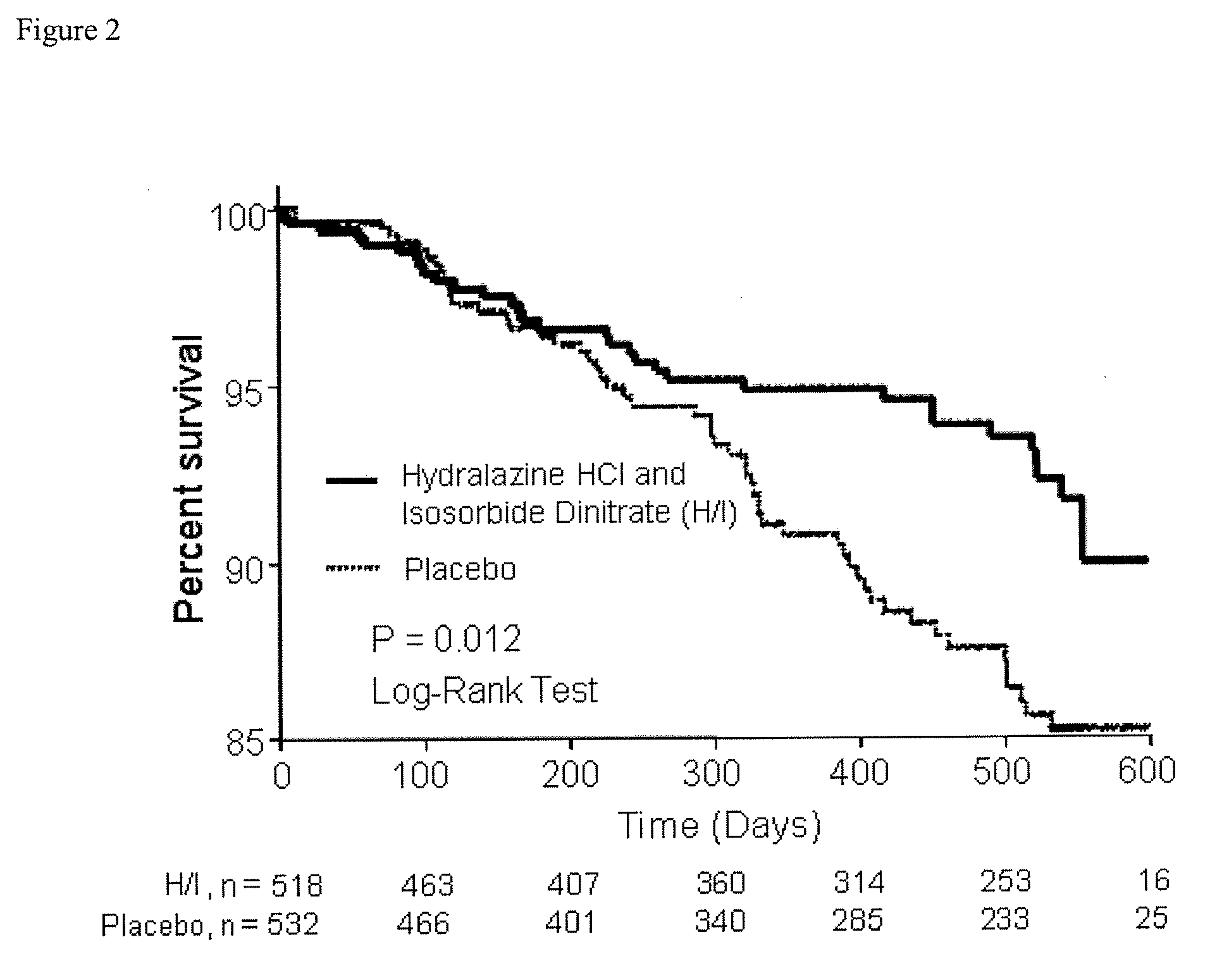
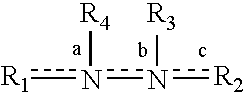Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35 results about "Hydralazine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hydralazine is used with or without other medications to treat high blood pressure.
Immunomodulatory compositions
InactiveUS20130243873A1Promote dissolutionFacilitated releaseOrganic active ingredientsNervous disorderHydralazineDopamine hydroxylase inhibitor
Immunomodulator formulations for use in the treatment of disease of the GI tract. The formulations comprise a hydroxylase inhibitor and / or an immunosuppressant. Exemplary formulations comprise hydralazine as a hydroxylase inhibitor and / or cyclosporin A as an immunosuppressant.
Owner:SIGMOID PHARM LIMITED
Methods for treating blood disorders with nitric oxide donor compounds
The invention describes methods for treating blood disorders or for treating the symptoms and / or complications associated with blood disorders by administering a therapeutically effective amount of at least one nitric oxide donor compound and optionally at least one antioxidant, or a pharmaceutically acceptable salt thereof, and / or at least one therapeutic agent. The antioxidant is preferably a hydralazine compound or a pharmaceutically acceptable salt thereof. The nitric oxide donor compound is preferably N-hydroxy-L-arginine and / or isosorbide dinitrate and / or isosorbide mononitrate. The blood disorder is preferably sickle cell anemia. The complication resulting from a blood disorder is preferably pulmonary hypertension.
Owner:NITROMED
Nitrosated and nitrosylated nebivolol and its metabolites, compositions and methods of use
InactiveUS7138430B2Prevention of platelet aggregation and platelet adhesionAntibacterial agentsBiocideMetaboliteAntioxidant
Owner:NICOX SA
Compositions and methods related to heart failure
InactiveUS20060014828A1Reduce in quantityShorten the construction periodBiocideNervous disorderDiseaseType B Natriuretic Peptide
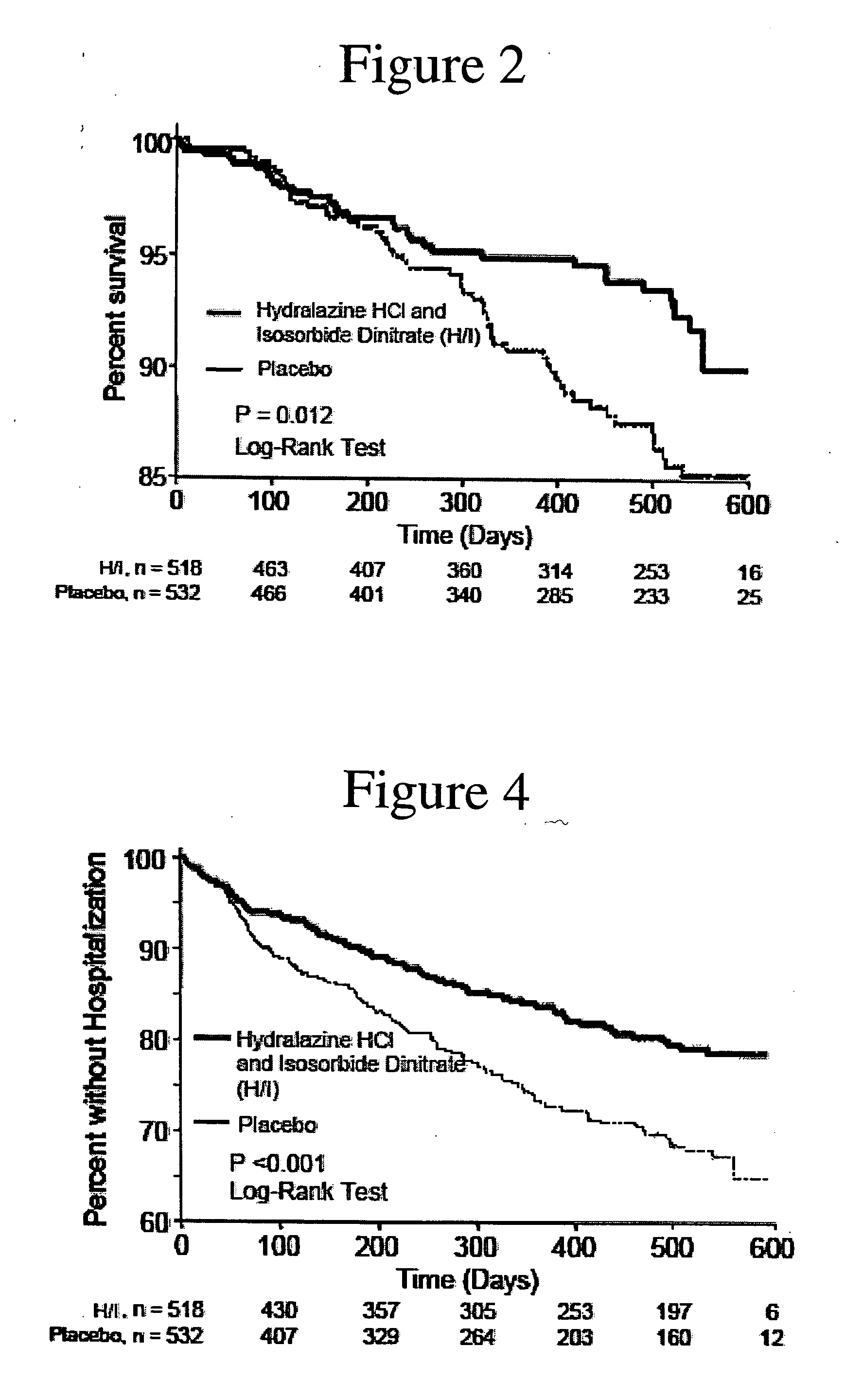
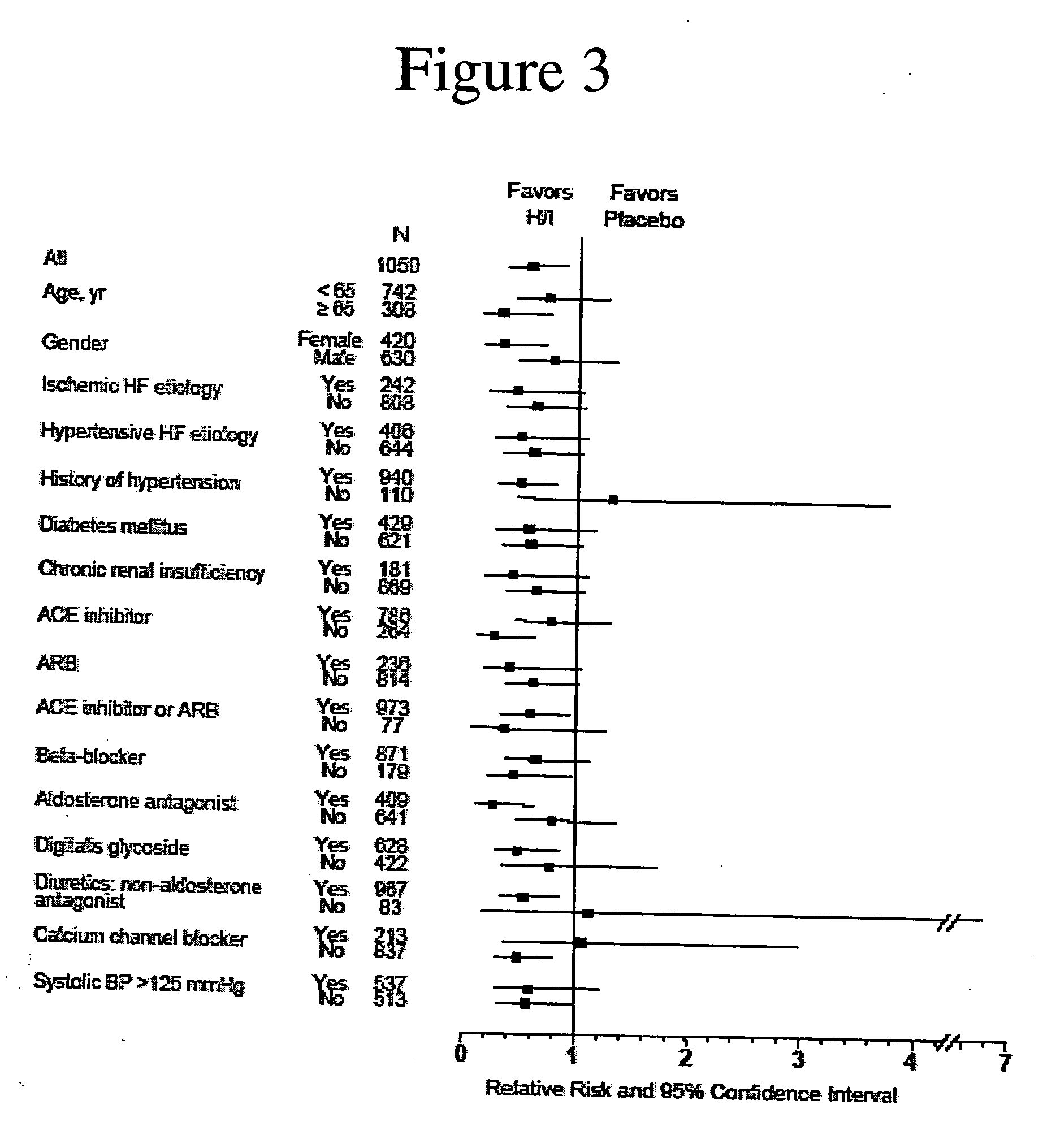
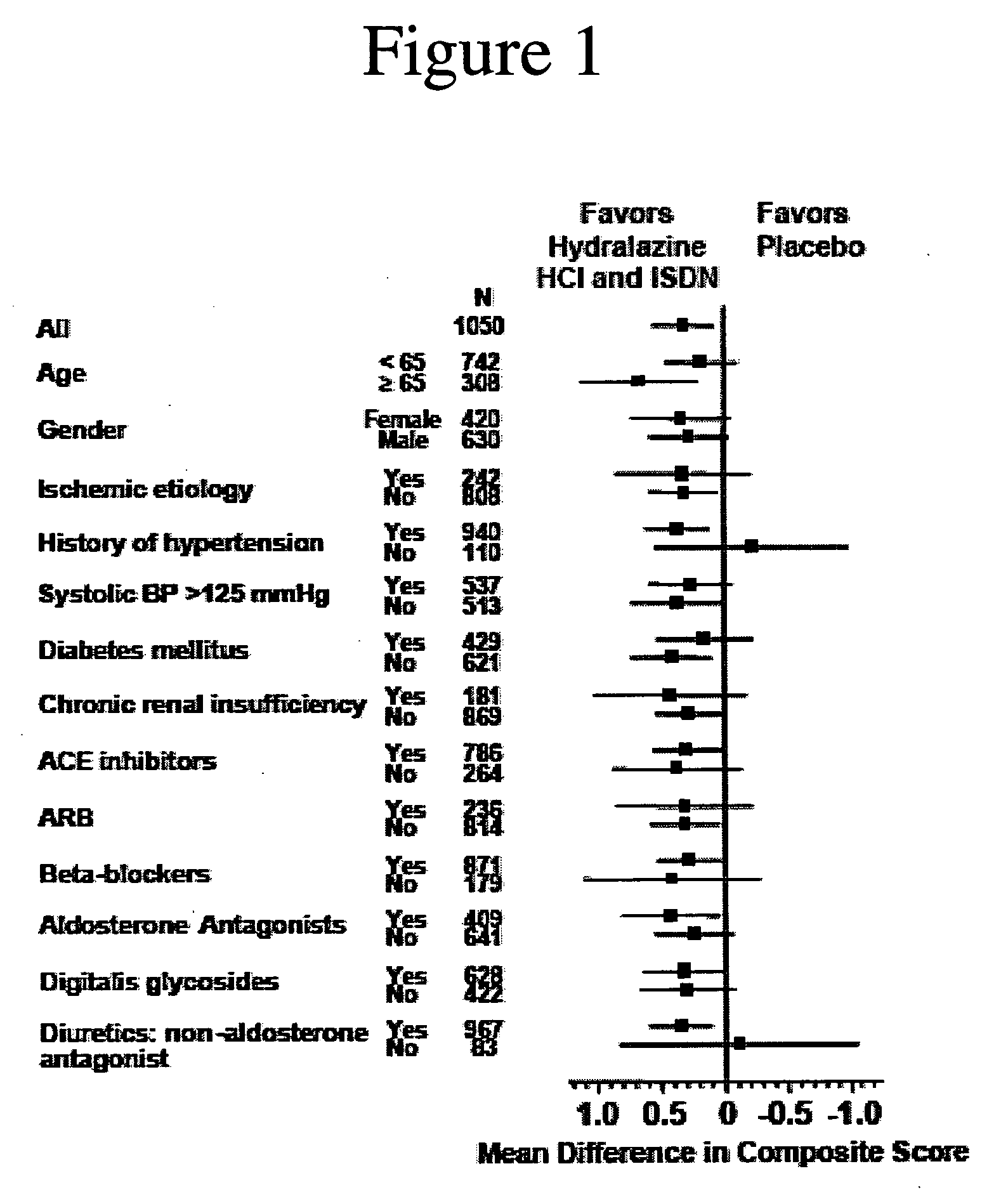
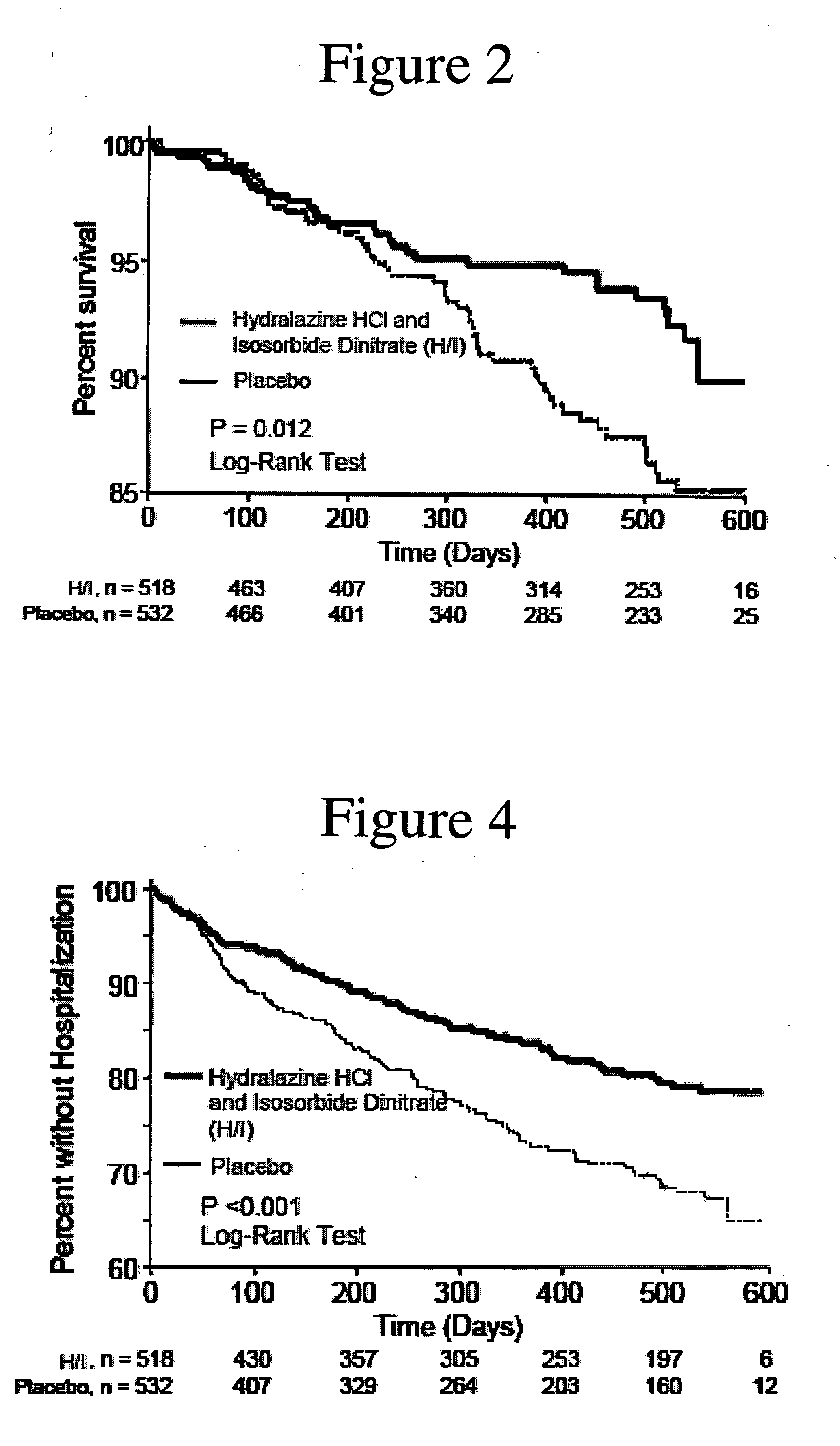
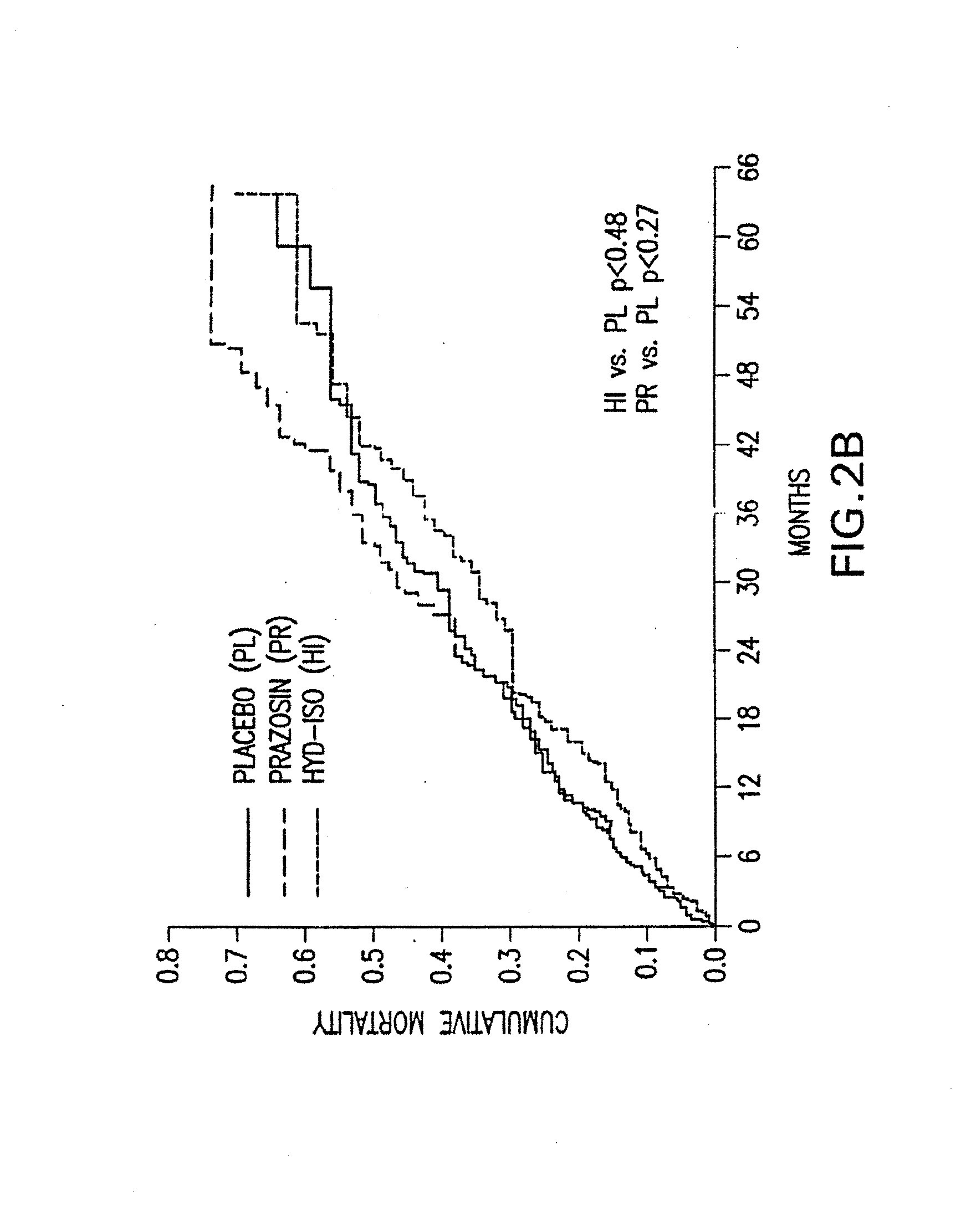
The invention provides methods for (a) prolonging time to hospitalization for heart failure; (b) prolonging time to first hospitalization for heart failure; (c) reducing the total number of days a patient with heart failure spends in the hospital for heart failure for a single hospital stay (i.e., reducing the duration of a single hospital stay for a patient with heart failure); (d) reducing the total number of days a patient spends in the hospital for heart failure for multiple hospital stays (i.e., two or more hospital stays); (e) reducing the number of hospital admissions for heart failure; (f) reducing mortality and reducing hospitalizations for heart failure (e.g., the total number of days in the hospital and / or the number of hospital visits); (g) increasing the left ventricular ejection fraction in a heart failure patient; (h) treating a sexual dysfunction (e.g., erectile dysfunction and female sexual dysfunction) (j) treating a headache in a heart failure patient by administering a non-steroidal antiinflammatory compound (i.e., NSAIDs); (k) treating a heart failure patient who has a history of hypertension (but who is not currently diagnosed with hypertension); (l) improving the quality of life in a heart failure patient based on the Minnesota Living with heart failure questionnaire; (m) decreasing the levels of B-type natriuretic peptide; (n) treating hypertension in a heart failure patient; (o) lowering blood pressure in a heart failure patient; (p) treating labile hypertension; (q) treating idiopathic hypertension; (r) increasing patient compliance with medication dosing in a heart failure patient; (s) treating hypertension in a patient with a dilated heart; (t) treating ischemic disease and / or coronary artery disease; and (u) reducing cardiomegaly in a patient in need thereof comprising administering to the patient a therapeutically effective amount of (i) a hydralazine compound or pharmaceutically acceptable salt thereof, (ii) isosorbide dinitrate and / or isosorbide mononitrate, and (iii) optionally at least one compound selected from the group consisting of angiotensin converting enzyme inhibitors, β-adrenergic antagonists, angiotensin II antagonists, aldosterone antagonists, cardiac glucosides (digitalis), and diuretic compounds.
Owner:NITROMED
Methods for reducing hospitalizations related to heart failure
InactiveUS20060014829A1Reduce in quantityShorten the construction periodBiocideNervous disorderDigitalisMortality rate
The invention provides methods for (a) prolonging time to hospitalization for heart failure; (b) prolonging time to first hospitalization for heart failure; (c) reducing the total number of days a patient with heart failure spends in the hospital for heart failure for a single hospital stay (i.e., reducing the duration of a single hospital stay for a patient with heart failure); (d) reducing the total number of days a patient spends in the hospital for heart failure for multiple hospital stays; (e) reducing the number of hospital admissions for heart failure; and (f) reducing mortality and reducing hospitalizations for heart failure (e.g., the total number of days in the hospital and / or the number of hospital visits) in a patient in need thereof comprising administering to the patient a therapeutically effective amount of (i) a hydralazine compound or pharmaceutically acceptable salt thereof, (ii) isosorbide dinitrate and / or isosorbide mononitrate, and (iii) optionally at least one compound selected from the group consisting of angiotensin converting enzyme inhibitors, β-adrenergic antagonists, angiotensin II antagonists, aldosterone antagonists, cardiac glucosides (digitalis), and diuretic compounds.
Owner:NITROMED
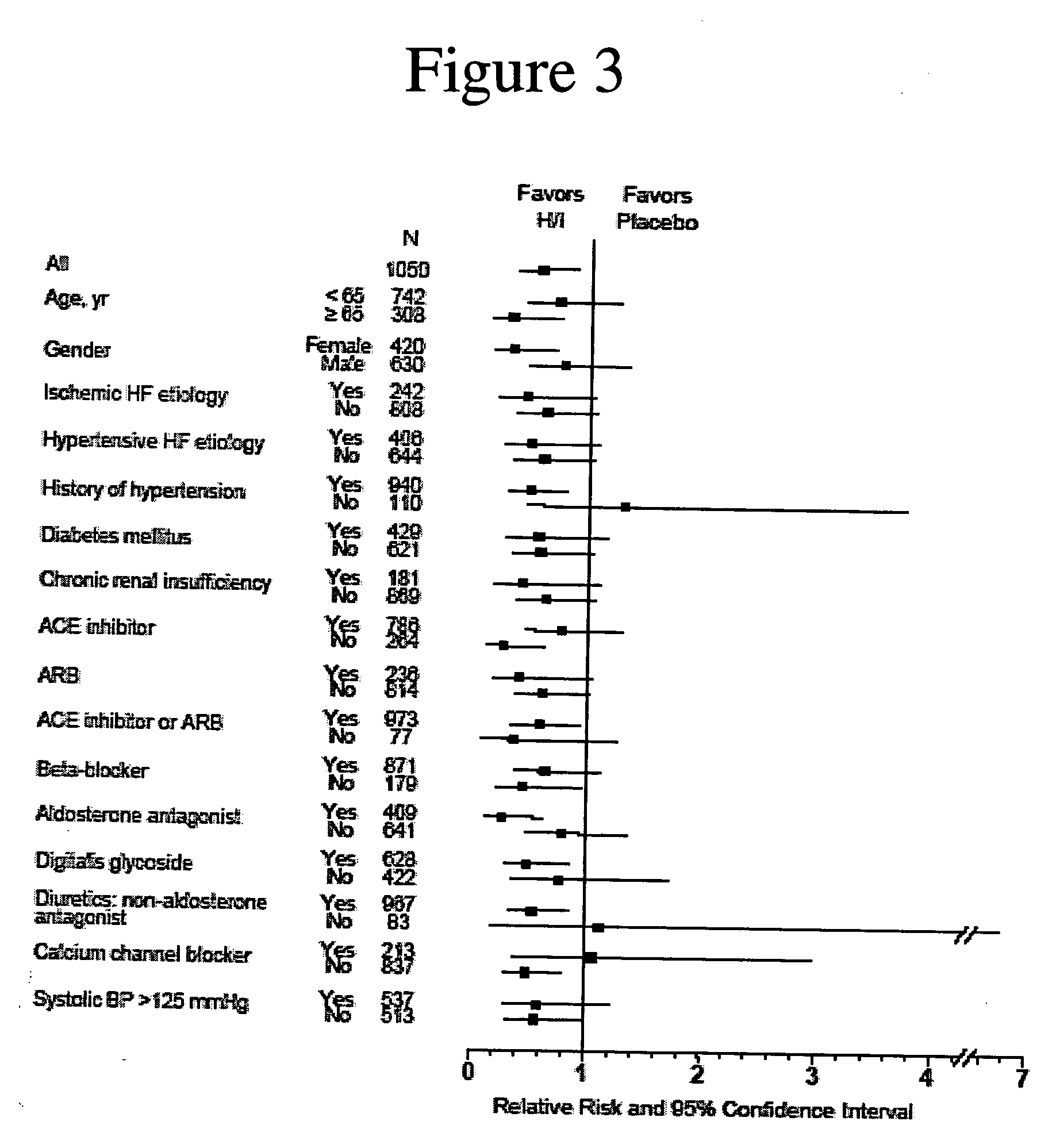
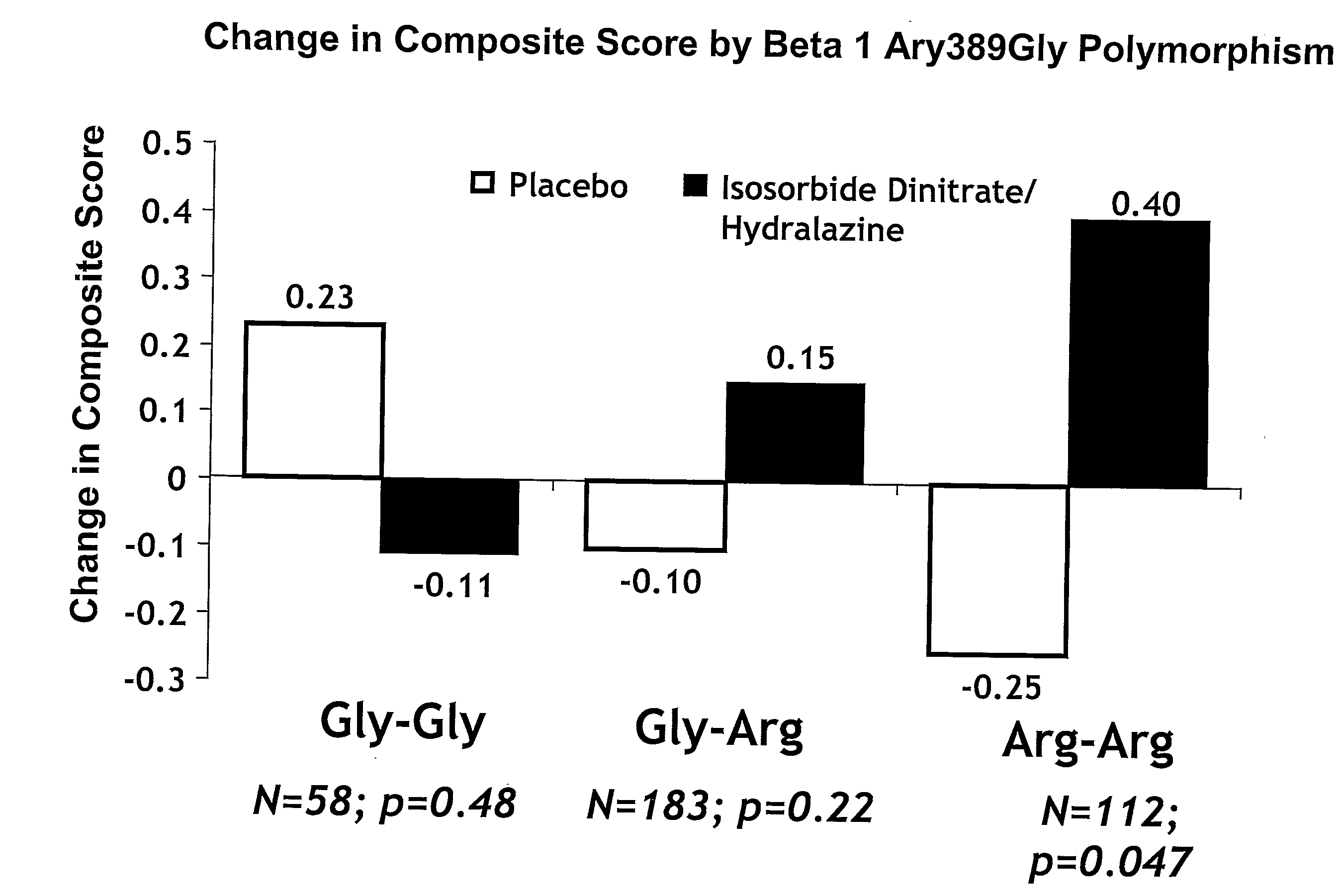
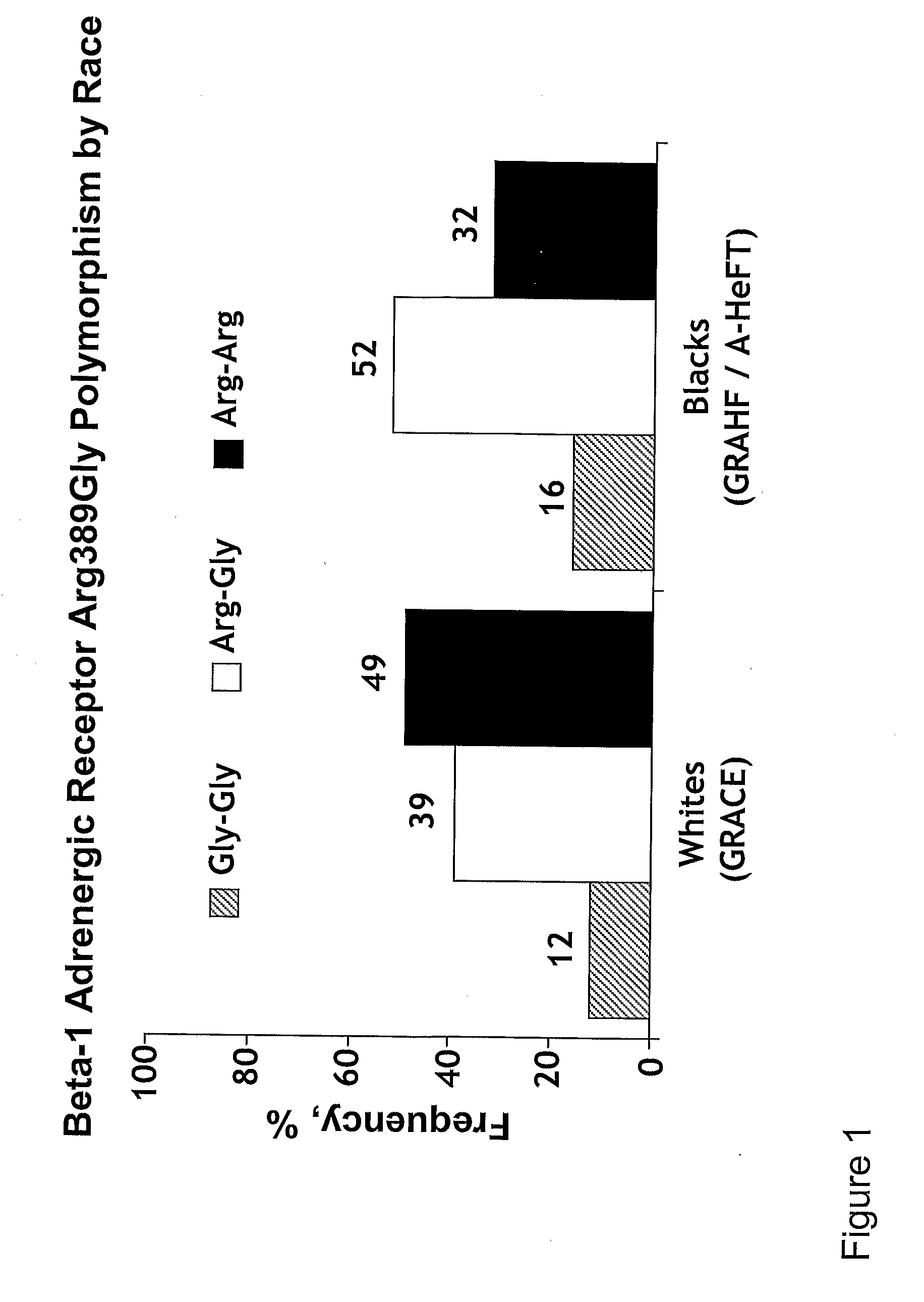
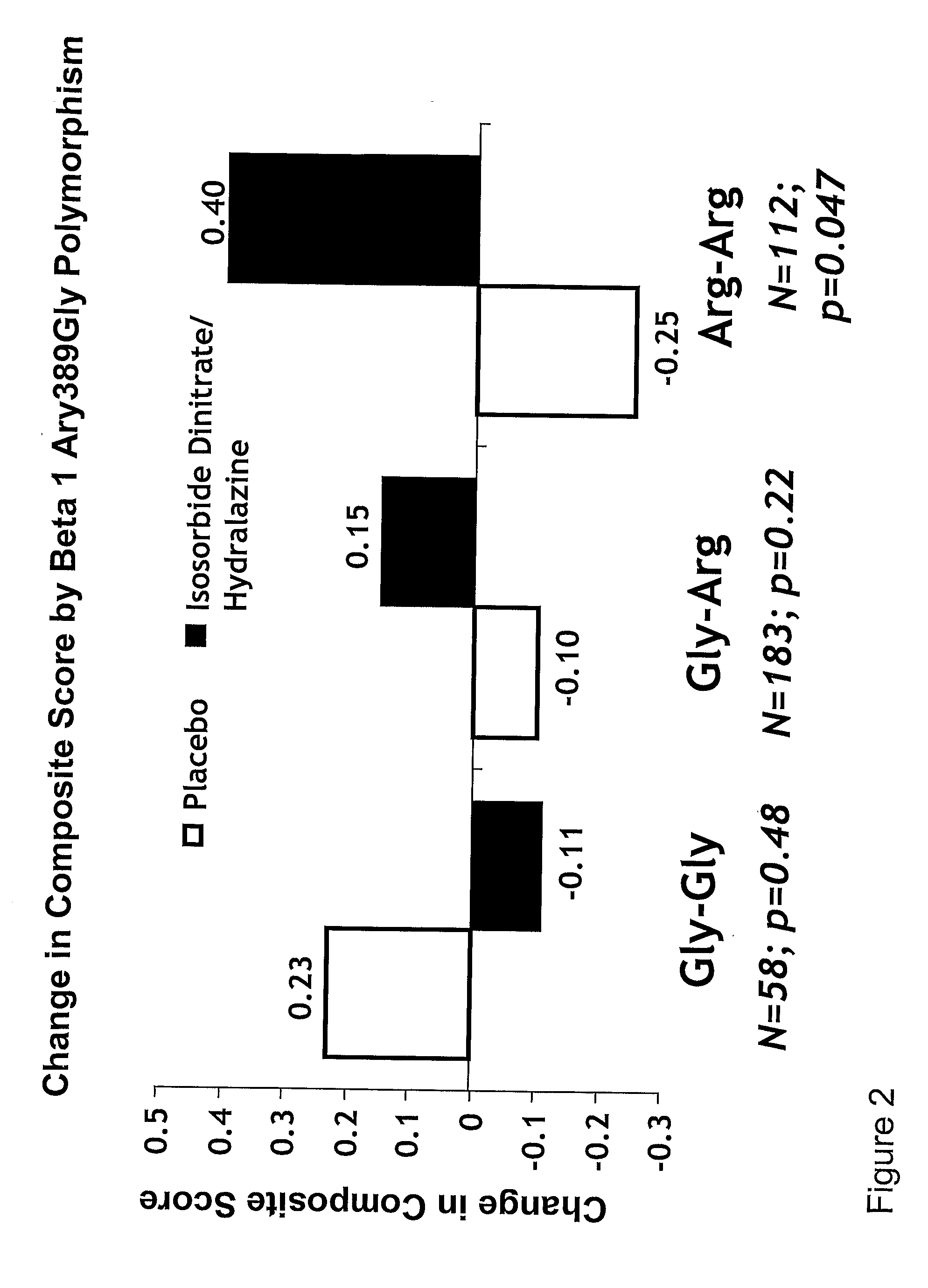
Genetic risk assessment in heart failure: impact of genetic variation of beta 1 adrenergic receptor gly389arg polymorphism
InactiveUS20090192128A1Reduce mortalityIncreased oxygen consumptionBiocideMicrobiological testing/measurementAntioxidantLeft ventricular size
The invention provides methods for (a) reducing mortality associated with heart failure; (b) improving oxygen consumption; (c) treating heart failure; (d) treating hypertension; (e) improving the quality of life in a heart failure patient; (f) inhibiting left ventricular remodeling; (g) reducing hospitalizations related to heart failure; (h) improving exercise tolerance; (j) increasing left ventricular ejection fraction; (k) decreasing levels of B-type natriuretic protein; (l) treating renovascular diseases; (m) treating end-stage renal diseases; (n) reducing cardiomegaly; (o) treating diseases resulting from oxidative stress; (p) treating endothelial dysfunctions; (q) treating diseases caused by endothelial dysfunctions; or (r) treating cardiovascular diseases; in a patient in need thereof, wherein the patient has a Arg389Arg polymorphism and / or a Gly389Gly polymorphism in the beta 1 adrenergic receptor gene, comprising administering to the patient (i) at least one antioxidant compound or a pharmaceutically acceptable salt thereof; (ii) at least one nitric oxide enhancing compound; and (iii) optionally the best current therapy for the treatment of cardiovascular diseases. In one embodiment the antioxidant is a hydralazine compound or a pharmaceutically acceptable salt thereof and the nitric oxide enhancing compound is isosorbide dinitrate and / or isosorbide mononitrate.
Owner:NITROMED +1
Tumour vaccine and preparation method thereof
InactiveCN103816535AEfficient killingBroad spectrum of tumor antigensTumor/cancer cellsAntibody medical ingredientsMedicineCurative effect
The invention discloses a tumour vaccine and a preparation method thereof. According to the invention, hydralazine and SAHA are adopted to jointly treat tumor cells so as to obtain an exosmoes tumour vaccine. The invention further discloses a preparation method of the tumour vaccine, which comprises the steps that the hydralazine cooperates with the SAHA to treat the tomour cells, and separate and purify exosomes excreted by the tomour cells. The tumour vaccine disclosed by the invention improves the curative effect of the exosomes tumour vaccine, and has important clinic application value.
Owner:FIRST HOSPITAL AFFILIATED TO GENERAL HOSPITAL OF PLA
Nebivolol and its metabolites in combination with nitric oxide donors, compositions and methods of use
InactiveUS20060009513A1Prevention of platelet aggregation and platelet adhesionAntibacterial agentsBiocideMetaboliteAntioxidant
The invention describes novel compositions comprising nebivolol and / or at least one metabolite of nebivolol and at least one nitric oxide donor, and, optionally, at least one antioxidant or a pharmaceutically acceptable salt thereof, and / or at least one compound used to treat cardiovascular diseases or a pharmaceutically acceptable salt thereof, and / or at least one nitrosated compound used to treat cardiovascular diseases. The compounds and compositions of the invention can also be bound to a matrix. The nitric oxide donor is a compound that donates, transfers or releases nitric oxide, elevates endogenous levels of endothelium-derived relaxing factor, stimulates endogenous synthesis of nitric oxide or is a substrate for nitric oxide synthase and may preferably be isosorbide dinitrate and / or isosorbide mononitrate. The antioxidant may preferably be a hydralazine compound or a pharmaceutically acceptable salt thereof. The invention also provides methods for treating and / or preventing vascular diseases characterized by nitric oxide insufficiency; and for treating and / or preventing Raynaud's syndrome; and for treating and / or preventing cardiovascular diseases or disorders.
Owner:NICOX SA
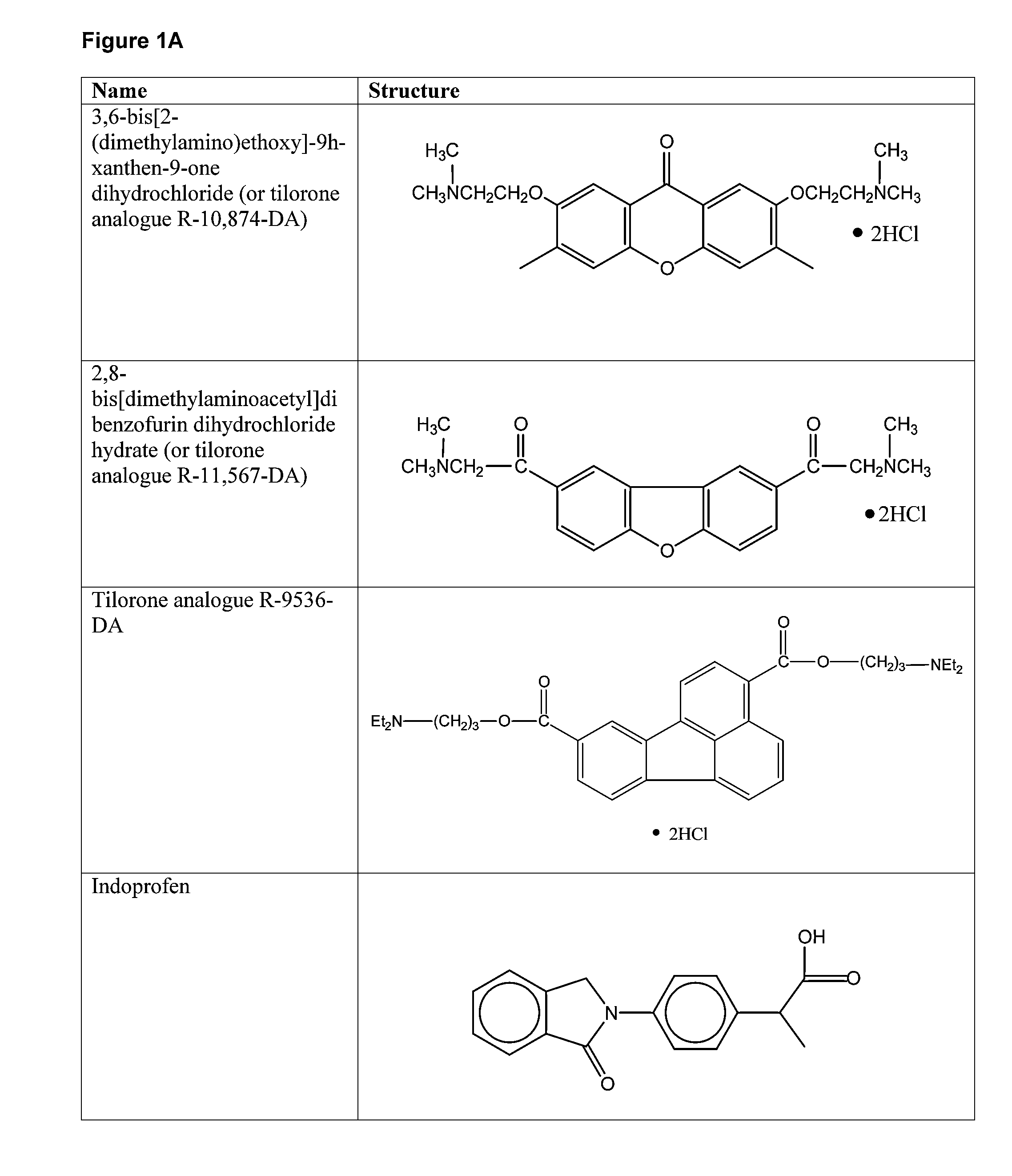
Compounds for Enhancing Hypoxia Inducible Factor Activity and Methods of Use
The present invention relates to methods for enhancing Hypoxia inducible factor-1 (HIF) activity in a cell by contacting the cell with any one of the following compounds: 3,6-bis[2-(dimethylamino)ethoxy]-9h-xanthen-9-onedihydrochloride, 2,8-bis[dimethylaminoacetyl]dibenzofurin dihydrochloride hydrate, tilorone analogue R-9536-DA, indoprofen, ciclopiroxolamine, tryptophan, ansindione, nabumetone, oxybendazole, albendazole, tropicamide, pramoxine hydrochloride, atenolol, mebendazole, carbetapentane citrate, monensin sodium, methoxyvone, hydroxyzine, phenazopyridine, clofoctol, ipraflavone, zomepirac, biochanin A, xylometazoline hydrochloride, fenbendazole, pirenzepine, triprolidine hydrochloride, daidzein, tripelennamine citrate, colchicines, aminopyridine, trimethoprim, helenine, hydroxyurea, amiodarone hydrochloride, clindamycin hydrochloride, sulfachlorpyridazine, mephenesin, semustine, clofivric acid, clofibrate, ibuprofen, hyoscyamime, nafcillin sodium, piperin, clidinium bromide, trioxsalen, hydralazine and HIF alpha protein fused to a carrier peptide.
Owner:CORNELL RES FOUNDATION INC
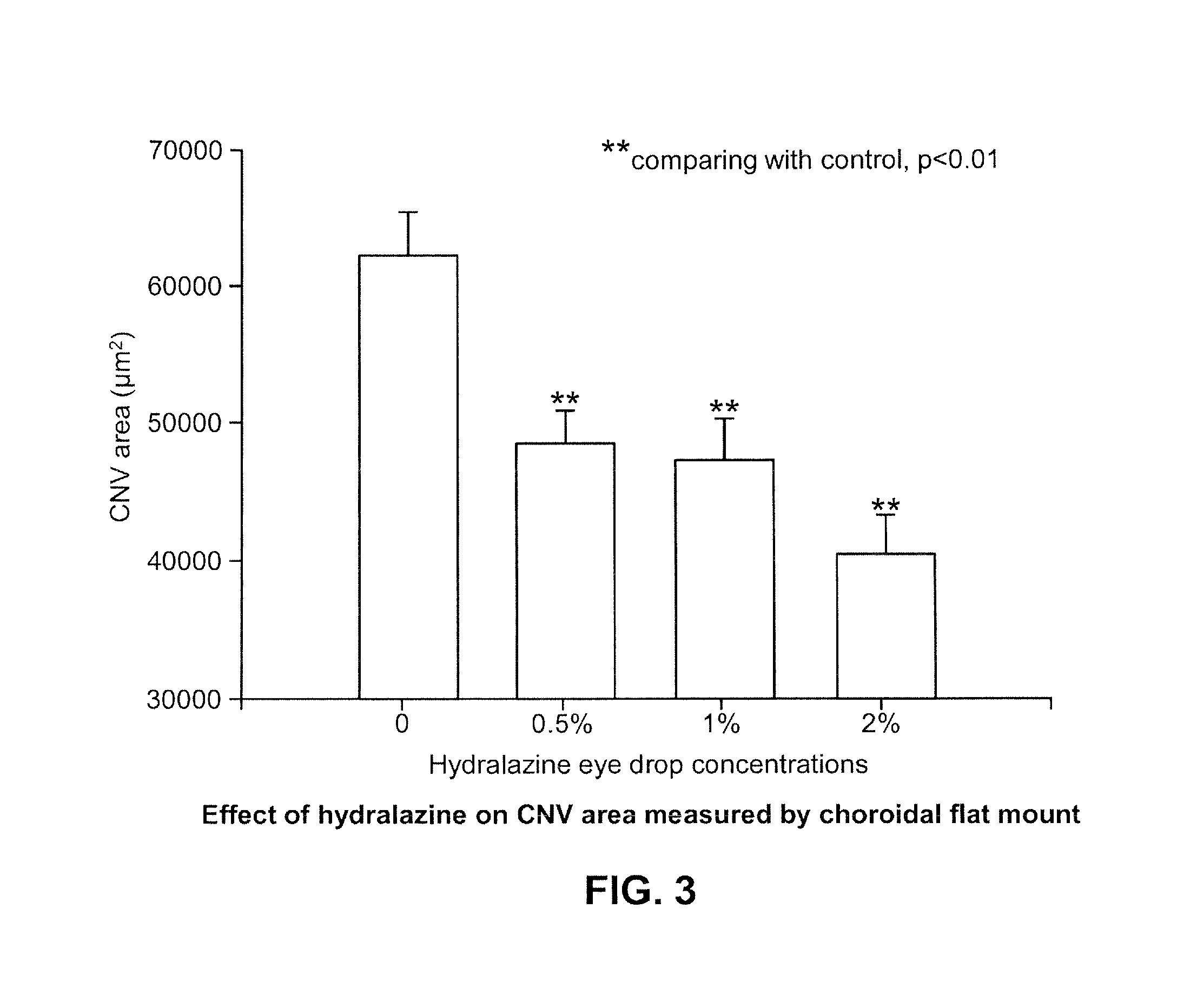
Therapeutic formulation and methods of treatment
The present disclosure relates pharmaceutical formulations comprising hydralazine in the treatment of eye diseases and conditions with the formulations. The present disclosure also related to methods of preparing the pharmaceutical formulations.
Owner:MACUCLEAR
Trimetaphan camsilate and linseed oil nanoemulsion antihypertensive drug
InactiveCN102697856AEvenly distributedSystem transparencyOrganic active ingredientsEmulsion deliveryHalf-lifeAdditive ingredient
The invention discloses an oil-in-water type trimetaphan camsilate and linseed oil nanoemulsion antihypertensive drug which comprises the following raw materials in percentage by weight: 1%-15% of trimetaphan camsilate, 15%-35% of surfactant, 0-20% of cosurfactant, 1%-25% of linseed oil and the balance of distilled water, wherein the sum of the mass percent of the raw materials is 100%. The nanoemulsion has the advantages of small emulsion drop granule, uniform distribution, small viscosity and good flowability. In the nanoemulsion dosage form, the hydralazine which is a water-soluble medicine and the fat soluble linseed oil are organically combined, so the dissolving and infiltration capacities of the linseed oil are improved, and the stability and the pesticide effect of the trimetaphan camsilate are improved; and after the trimetaphan camsilate and the linseed oil are prepared into the nano dosage form, the advantages of the trimetaphan camsilate and the linseed oil are combined, so the antihypertensive effect is obviously increased, the half-life period of the drug is prolonged, and the drug administration times is reduced.
Owner:NORTHWEST A & F UNIV
Nitrosated And Nitrosylated Cardiovascular Compounds, Compositions And Methods Of Use
InactiveUS20070238740A1Improve propertiesBiocideSenses disorderRenovascular diseaseAngiotensin ii antagonist
The invention describes novel nitrosated and / or nitrosylated cardiovascular compounds or pharmaceutically acceptable salts thereof, and novel compositions comprising at least one nitrosated and / or nitrosylated cardiovascular compound, and, optionally, at least one nitric oxide donor and / or at least one therapeutic agent. The invention also provides novel compositions and kits comprising at least one cardiovascular compound of the invention, that is optionally nitrosated and / or nitrosylated, and, optionally, at least one nitric oxide donor compound and / or at least one therapeutic agent. The invention also provides methods for (a) treating cardiovascular diseases; (b) treating renovascular diseases; (c) treating diabetes; (d) treating diseases resulting from oxidative stress; (e) treating endothelial dysfunctions; (f) treating diseases caused by endothelial dysfunctions; (g) treating cirrhosis; (h) treating pre-eclampsia; (j) treating osteoporosis; and (k) treating nephropathy. The nitrosated and / or nitrosylated cardiovascular compounds are preferably nitrosated and / or nitrosylated aldosterone antagonists, nitrosated and / or nitrosylated angiotensin II antagonists, nitrosated and / or nitrosylated calcium channel blockers, nitrosated and / or nitrosylated endothelin antagonists, nitrosated and / or nitrosylated hydralazine compounds, nitrosated and / or nitrosylated neutral endopeptidase inhibitors and nitrosated and / or nitrosylated renin inhibitors.
Owner:NICOX SA
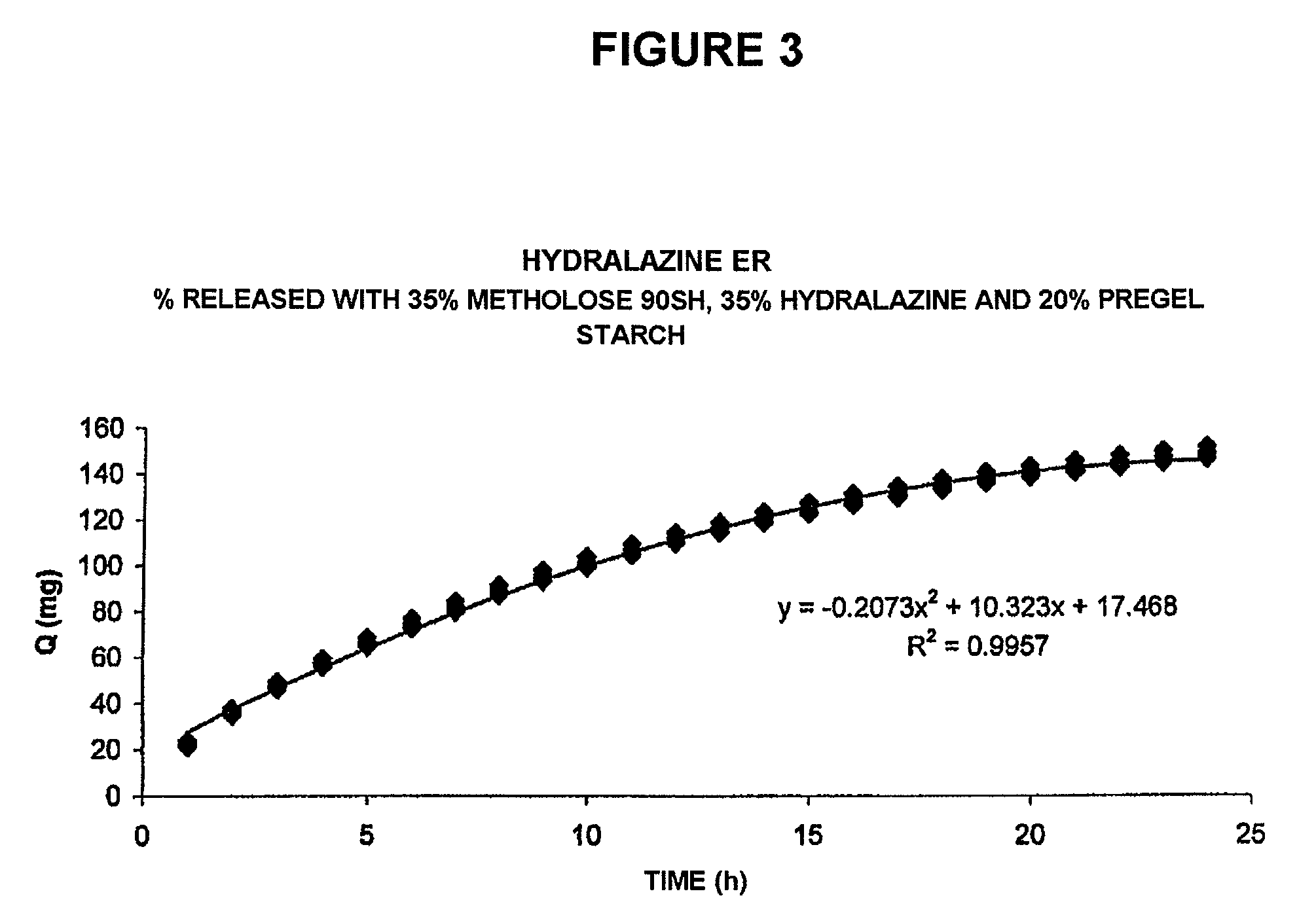
Pharmaceutical composition for the sustained release of hydralazine and use thereof as a support for cancer treatment
The invention relates to a substained release form of hydralazine for use in cancer therapy. The substained release form of hydralazine can be used to obtain a constant concentration of the active principle in the blood, thereby enabling the demethylating effect of the hydralazine without producing the hypotensive action thereof, such that the inventive composition can be used in cancer therapy.
Owner:PSICOFARMA S A DE
Compounds and methods for treatment of solid tumors
The present invention relates to pharmaceutical compositions containing targetable bioconjugates of hydralazine, a direct vasodilating agent previously shown to decrease tumor blood flow, oxygenation and interstitial fluid pressure in solid tumors. These bioconjugates are hydralazine prodrugs that contain hydralazine conjugated to biocompatible carrier molecules which specifically bind to sites that are expressed on a diverse variety of tumor cell types. These hydralazine prodrugs are preferably conjugated through an acid-labile hydrazone link that is designed to be stable in plasma and release hydralazine through acid-catalyzed hydrolysis in the acidic environment of the target tumor. Because these prodrugs are stable at physiological pH and in plasma, they are devoid of systemic vasoactive activity; however, they are acid-labile conjugates that can be hydrolyzed upon reaching the more acid environment of the tumor where the vasoactive activity of hydralazine is restored. These prodrugs selectively bind to tumor-specific receptors on tumor cells, and are degraded in the acidic tumor cell environment or the acidic lysosomal compartments after being internalized into the cell.
Owner:B& G PARTNERS
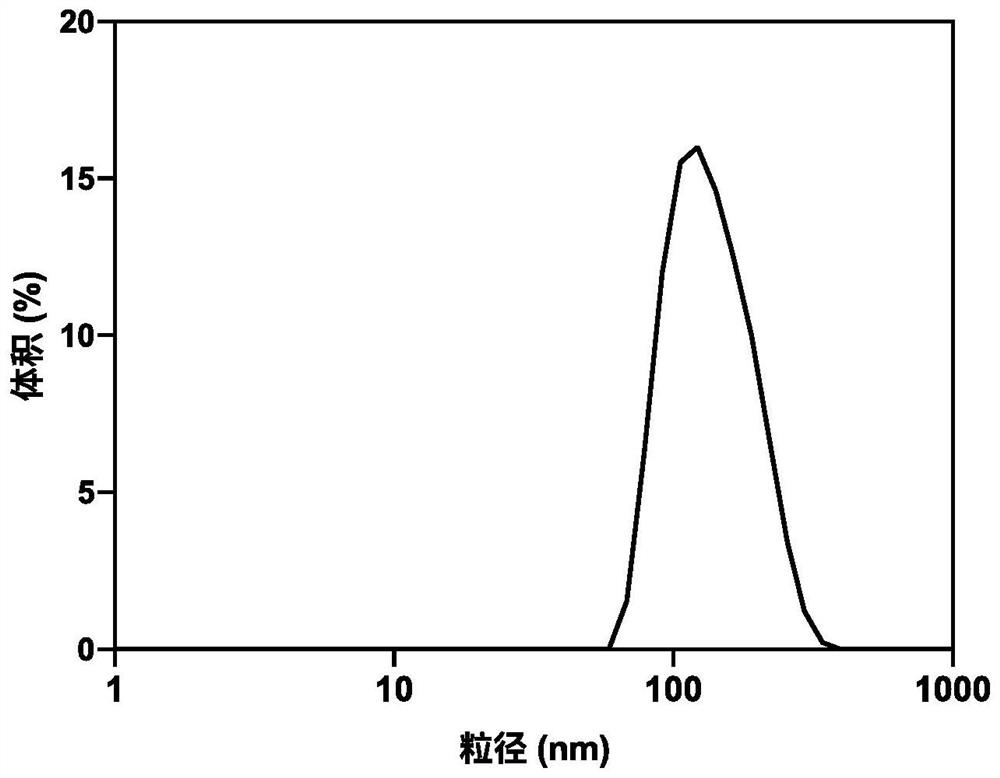
Nanometer medicine for improving tumor microenvironment based on hydralazine as well as preparation and application of nanometer medicine
ActiveCN114099705AImprove delivery efficiency to tumor tissueEnhance tumor selectivityPowder deliveryDipeptide ingredientsPolyethylene glycolBoronic acid
The invention discloses a nano-drug for improving a tumor microenvironment based on hydralazine as well as preparation and application of the nano-drug, and belongs to the technical field of medicines. The nano-drug is a micelle type nano-particle formed by self-assembling an amphiphilic polymer and a hydrophobic chemotherapeutic drug containing a boric acid group in water; a hydrophilic segment of the amphiphilic polymer is polyethylene glycol, and a hydrophobic segment of the amphiphilic polymer is a polymer formed by connecting a pH response type hydralazine bond with hydralazine. The nano-drug can efficiently release small-molecule hydralazine in an acidic tumor environment, and hydralazine improves the efficiency of conveying an entrapped chemotherapeutic drug to tumor tissues by expanding tumor blood vessels. In addition, the nano-drug can efficiently load chemotherapeutic drugs, and is intensively released in tumor acidic tissues, so that the treatment effect of the chemotherapeutic drugs is enhanced, the toxicity to normal tissues is low, and the tumor selectivity of the nano-drug is remarkably enhanced.
Owner:ZHEJIANG UNIV HANGZHOU GLOBAL SCI & TECH INNOVATION CENT
Immunomodulatory compositions
InactiveUS9821024B2Promote dissolutionFacilitated releaseOrganic active ingredientsNervous disorderDiseaseHydralazine
Immunomodulator formulations for use in the treatment of disease of the GI tract. The formulations comprise a hydroxylase inhibitor and / or an immunosuppressant. Exemplary formulations comprise hydralazine as a hydroxylase inhibitor and / or cyclosporin A as an immunosuppressant.
Owner:SIGMOID PHARM LIMITED
Therapeutic formulation and methods of treatment
The present disclosure relates pharmaceutical formulations comprising hydralazine in the treatment of eye diseases and conditions with the formulations. The present disclosure also related to methods of preparing the pharmaceutical formulations.
Owner:MACUCLEAR
Pharmaceutical Composition For The Sustained Release Of Hydralazine And Use Thereof As A Support For Cancer Treatment
The invention relates to a sustained release form of hydralazine for use in cancer therapy. Said sustained release form of hydralazine can be used to obtain a constant concentration of the active principle in the blood, thereby enabling the demethylating effect of the hydralazine without producing the hypotensive action thereof, such that the inventive composition can be used in cancer therapy.
Owner:PSICOFARMA S A DE
Genetic risk assessment in heart failure: impact of the genetic variation of g-protein beta 3 subunit polymorphism
InactiveUS20090306027A1Reduce mortalityIncreased oxygen consumptionBiocideAnimal repellantsAntioxidantNitric oxide
The invention provides methods for treating various indications and diseases in a patient in need thereof, wherein the patient has a C825T polymorphism in the G protein beta3 subunit (GNB3), comprising administering to the patient (i) at least one antioxidant compound or a pharmaceutically acceptable salt thereof; (ii) at least one nitric oxide enhancing compound; and (iii) optionally the best current therapy for the treatment of cardiovascular diseases. In one embodiment the antioxidant is a hydralazine compound or a pharmaceutically acceptable salt thereof and the nitric oxide enhancing compound is isosorbide dinitrate and / or isosorbide mononitrate.
Owner:NITROMED +1
Compound ramipril nano-emulsion for antihypertension
InactiveCN102423483AEvenly distributedSystem transparencyOrganic active ingredientsDipeptide ingredientsAdjuvantActive agent
The invention discloses an oil-in-water type compound ramipril nano-emulsion, which is prepared from 1%-25% of ramipril, 0.1%-25% of hydralazine, 0.1%-25% of lisinopril, 10%-55% of surfactant, 5%-30% of oil and the balance distilled water, wherein the sum of the mass percent of the ingredients is 100%. The nano-emulsion is small in emulsion droplet granule, even in distribution, low in viscosity, and good in liquidity and has antihypertensive capacity, the dissolubility of the ramipril is enhanced, the bioavailability and the drug stability of the ramipril are improved, the metabolic time of the nano-emulsion in vivo is delayed, and the problem of hypertension can be synthetically solved because the hydralazine and the lisinopril are synthesized. With the adoption of the compound ramipril nano-emulsion, the dosage of the adjuvants of the antihypertension is reduced, the toxicity of the antihypertension to body is lowered, and the production cost is lowered; and furthermore, the preparation method of the compound ramipril nano-emulsion is simple and is low in energy consumption.
Owner:NORTHWEST A & F UNIV
Methods for reducing hospitalizations related to heart failure
InactiveUS20090118293A1Reduce in quantityShorten the construction periodBiocideNervous disorderDigitalisMortality rate
The invention provides methods for (a) prolonging time to hospitalization for heart failure; (b) prolonging time to first hospitalization for heart failure; (c) reducing the total number of days a patient with heart failure spends in the hospital for heart failure for a single hospital stay (i.e., reducing the duration of a single hospital stay for a patient with heart failure); (d) reducing the total number of days a patient spends in the hospital for heart failure for multiple hospital stays; (e) reducing the number of hospital admissions for heart failure; and (f) reducing mortality and reducing hospitalizations for heart failure (e.g., the total number of days in the hospital and / or the number of hospital visits) in a patient in need thereof comprising administering to the patient a therapeutically effective amount of (i) a hydralazine compound or pharmaceutically acceptable salt thereof, (ii) isosorbide dinitrate and / or isosorbide mononitrate, and (iii) optionally at least one compound selected from the group consisting of angiotensin converting enzyme inhibitors, β-adrenergic antagonists, angiotensin II antagonists, aldosterone antagonists, cardiac glucosides (digitalis), and diuretic compounds.
Owner:NITROMED
Pharmaceutical composition for treating hypertension and preparation method thereof
ActiveCN103800367BComponent stabilityEasy to makePill deliveryAluminium/calcium/magnesium active ingredientsVitamin b6Potassium
A pharmaceutical composition for treating hypertension and a preparation method thereof. A compound reserpine tablet is provided, the tablet includes reserpine, hydrochlorothiazide, vitamin B6, vitamin B1, calcium pantothenate, magnesium trisilicate, potassium chloride, dihydralazine sulfate, promethazine hydrochloride And a pharmaceutically acceptable carrier, wherein the vitamin B1 exists in the form of coated granules.
Owner:SHANGHAI SINE PHARMA LAB
Kits of hydralazine compounds and isosorbide dinitrate and/or isosorbide mononitrate
InactiveUS20080226752A1Improving oxygen consumptionImprove exercise toleranceBiocideInorganic active ingredientsVascular diseaseDigitalis
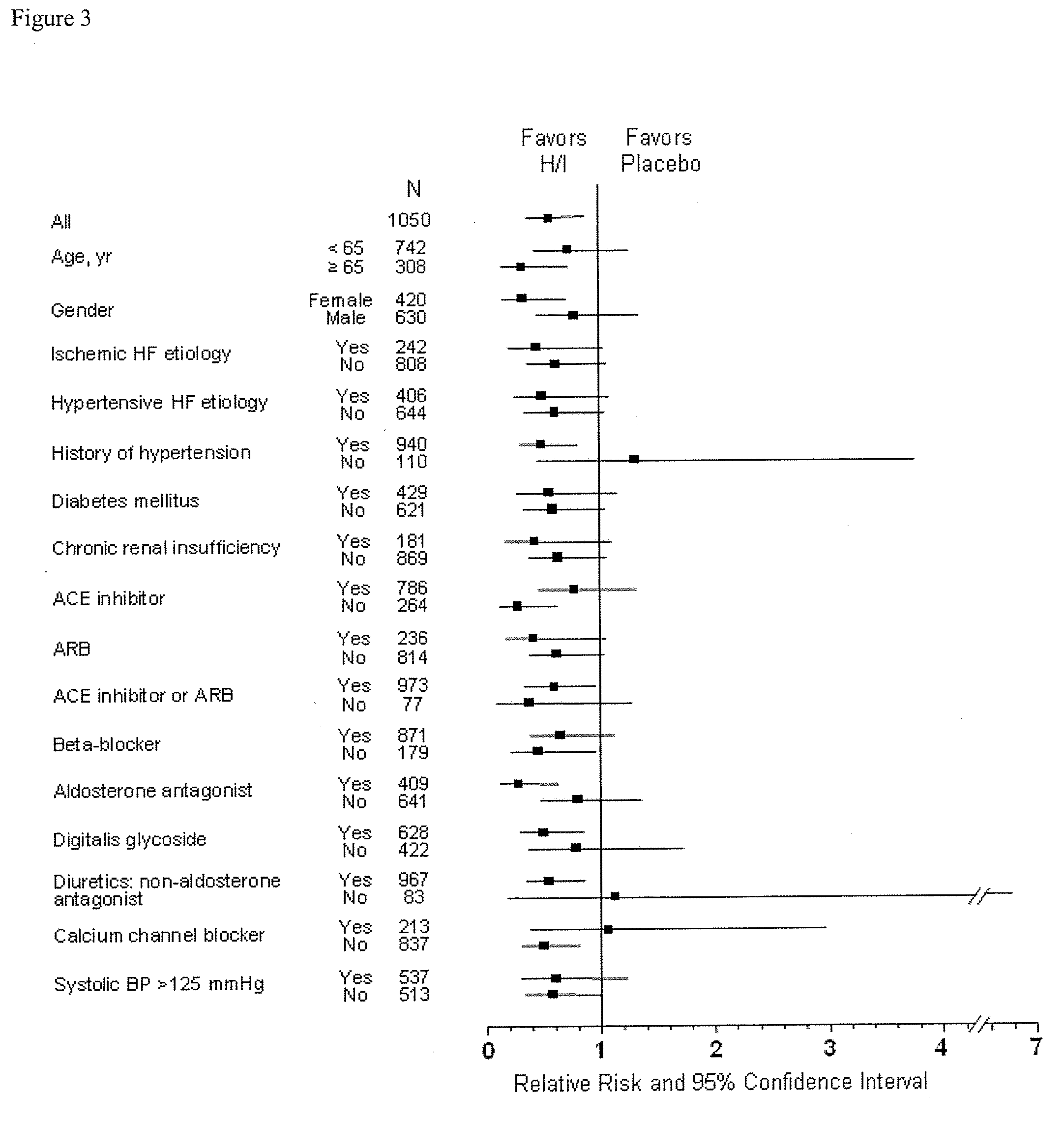
The present invention provides methods of treating and preventing mortality associated with heart failure in an African American patient with hypertension and improving oxygen consumption, quality of life and exercise tolerance by administering a therapeutically effective amount of at least one hydralazine compound and at least one of isosorbide dinitrate and isosorbide mononitrate, and, optionally, one or more compounds, such as, for example, a digitalis, a diuretic compound, or a compound used to treat cardiovascular diseases. In the present invention, the hydralazine compound is preferably hydralazine or a pharmaceutically acceptable salt thereof. Preferred methods of the invention comprise administering hydralazine or a pharmaceutically acceptable salt thereof and isosorbide dinitrate.
Owner:NITROMED
Methods of treating vascular diseases characterized by nitric oxide insufficiency
The invention provides methods of treating and / or preventing vascular diseases characterized by nitric oxide insufficiency by administering a therapeutically effective amount of at least one nitrosated angiotensin-converting enzyme inhibitor, nitrosated beta-adrenergic blocker, nitrosated cholesterol reducer, nitrosated calcium channel blocker, nitrosated endothelin antagonist, nitrosated angiotensin II receptor antagonist, nitrosated renin inhibitor, and optionally at least one compound used to treat cardiovascular diseases and / or at least one antioxidant, or a pharmaceutically acceptable salt thereof, and / or at least one compound that donates, transfers or releases nitric oxide, elevates endogenous levels of endothelium-derived relaxing factor, stimulates endogenous synthesis of nitric oxide or is a substrate for nitric oxide synthase. The antioxidant may preferably be a hydralazine compound or a pharmaceutically acceptable salt thereof. The compound that donates, transfers or releases nitric oxide, elevates endogenous levels of endothelium-derived relaxing factor, stimulates endogenous synthesis of nitric oxide or is a substrate for nitric oxide synthase may preferably be isosorbide dinitrate and / or isosorbide mononitrate. The vascular diseases characterized by nitric oxide insufficiency include a cardiovascular disease and a disease resulting from oxidative stress.
Owner:NITROMED
Use of Transcriptome Modifying Agents and Chemotherapy or Radiotherapy Against Cancer
The use of transcriptome-modifying agents is disclosed in order to prevent malignant cells from undergoing the necessary genetic changes in order to combat cell insult and survive chemotherapy or radiotherapy. The combination of transcriptome-modifying agents comprises agents that inhibit the DNA methylation machinery plus a substance that inhibits histone deacetylation. A treatment kit is disclosed which includes an effective dose of hydralazine and valproic acid or a salt of same in the case of magnesium valproate, which is intended for use with radiotherapy or chemotherapy in the treatment of patients cancer.
Owner:UNIV NAT AUTONOMA DE MEXICO
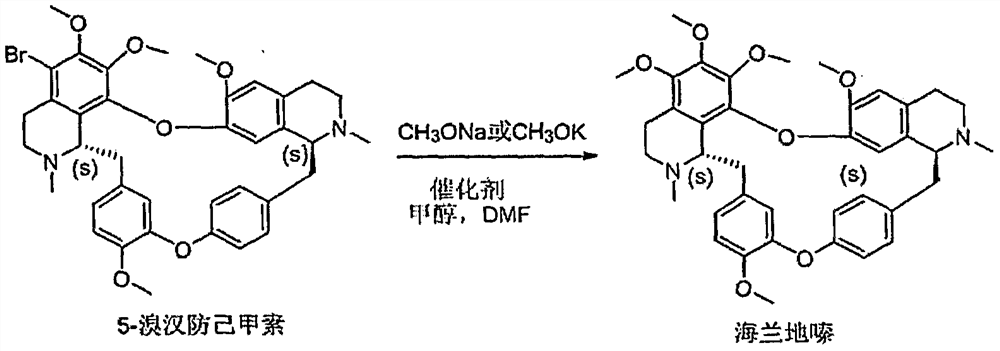
Preparation method of Helanadizine
ActiveCN109280057BEasy to purifyHigh reaction yieldOrganic chemistryBiochemical engineeringCombinatorial chemistry
The invention relates to a preparation method of Helanadizine. The invention discloses a method for preparing helanadizine from 5-bromotetrandrine. The invention also discloses a method for preparing helanadizine by using tetrandrine as a raw material through bromination and methoxylation. The preparation method disclosed in the invention uses tetrandrine as a raw material, has high reaction yield, easy purification of intermediates and products, and can be used for the industrial preparation of helandizine.
Owner:YU JIE BIOTECH SHANGHAI CO LTD
Transdermal patch composition for treating vascular diseases characterized by nitric oxide insufficiency
The present invention provides novel transdermal patch comprising a therapeutic amount of a hydralazine compound and at least one of isosorbide dinitrate and isosorbide mononitrate in therapeutically effective dosage of each of the aforementioned compounds.
Owner:NITROMED
Ophthalmic preparation
The invention relates to an ophthalmic preparation composition to provide a stable hydralazine topical preparation for the treatment of ocular diseases and illness conditions. The composition contains0.02%-2% by weight of a hydralazine pharmaceutically active drug, wherein ethylene dinitrilotetra-acetic acid radical ions used for chelating metal ions are basically not contained. The invention also relates to a method of preparing the above ophthalmic composition.
Owner:SYNCORE BIOTECH
Hydralazine nanoemulsion antihypertensive drug
InactiveCN106109410AReduce first pass effectGood treatment effectOrganic active ingredientsPharmaceutical non-active ingredientsHalf-lifeMedicine
The invention discloses an oil-in-water hydralazine nanoemulsion antihypertensive drug. The raw materials and the mass percentages of each raw material are: 1%-18% of hydralazine, 25%-45% of surfactant, and 25%-45% of surface active agent. The active agent is 0-20%, the remaining components are distilled water, and the sum of the mass percentages of the above-mentioned raw materials is 100%. The nano-emulsion droplet has small particles, uniform distribution, low viscosity and good fluidity. The nanoemulsion-type water-soluble drug hydralazine increases the stability and efficacy of hydralazine, significantly increases the antihypertensive effect, prolongs the half-life of the drug, and reduces the number of administrations.
Owner:张鸿利
Compounds For Enhancing Hypoxia Inducible Factor Activity And Methods Of Use
The present invention relates to methods for enhancing Hypoxia inducible factor-1 (HIF) activity in a cell by contacting the cell with any one of the following compounds: 3,6-bis[2-(dimethylamino)ethoxy]-9h-xanthen-9-onedihydrochloride, 2,8-bis[dimethylaminoacetyl]dibenzofurin dihydrochloride hydrate, tilorone analogue R-9536-DA, indoprofen, ciclopiroxolamine, tryptophan, ansindione, nabumetone, oxybendazole, albendazole, tropicamide, pramoxine hydrochloride, atenolol, mebendazole, carbetapentane citrate, monensin sodium, methoxyvone, hydroxyzine, phenazopyridine, clofoctol, ipraflavone, zomepirac, biochanin A, xylometazoline hydrochloride, fenbendazole, pirenzepine, triprolidine hydrochloride, daidzein, tripelennamine citrate, colchicines, aminopyridine, trimethoprim, helenine, hydroxyurea, amiodarone hydrochloride, clindamycin hydrochloride, sulfachlorpyridazine, mephenesin, semustine, clofivric acid, clofibrate, ibuprofen, hyoscyamime, nafcillin sodium, piperin, clidinium bromide, trioxsalen, hydralazine and HIF alpha protein fused to a carrier peptide.
Owner:CORNELL RES FOUNDATION INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com