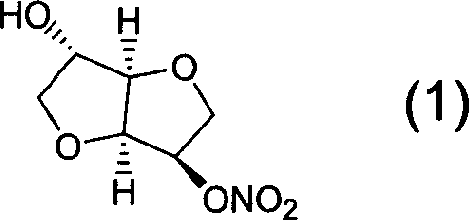
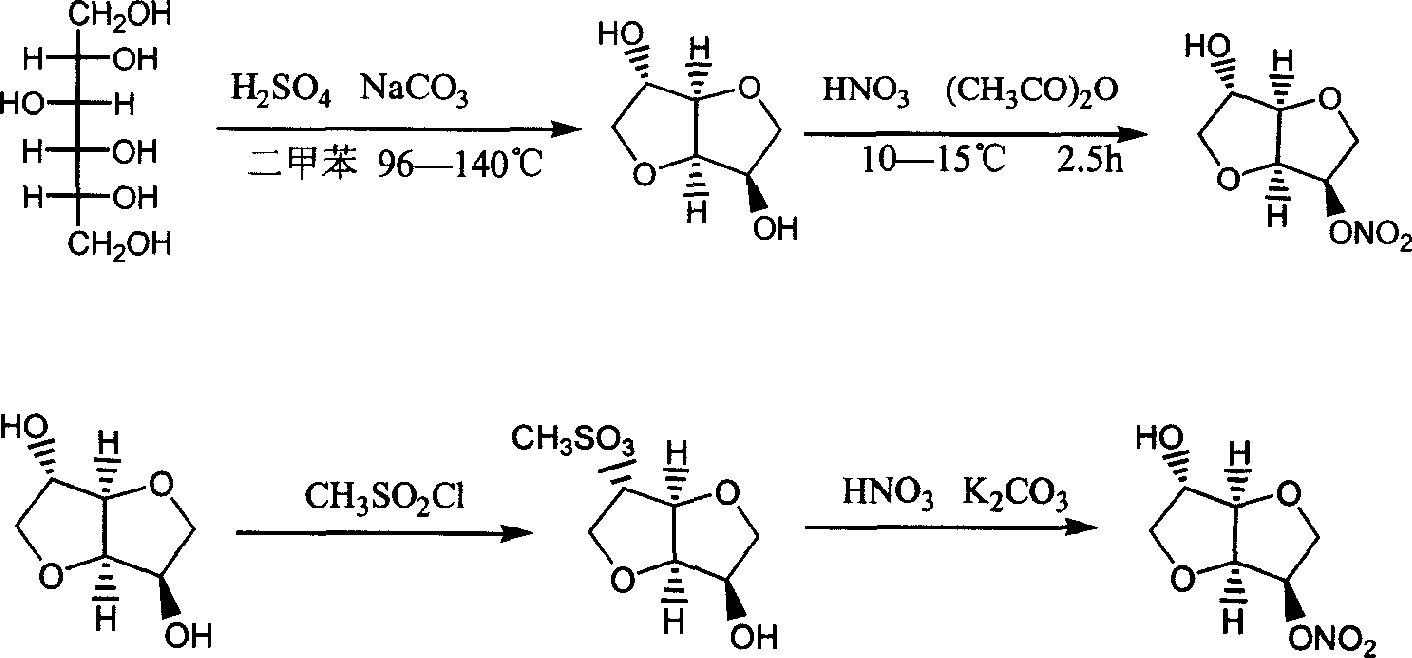
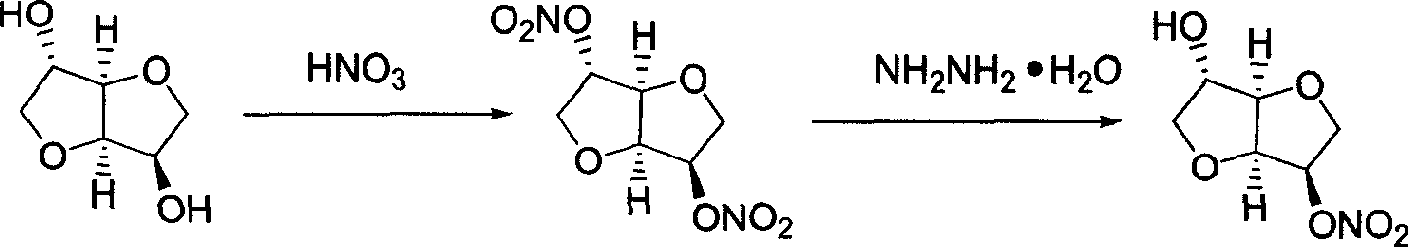
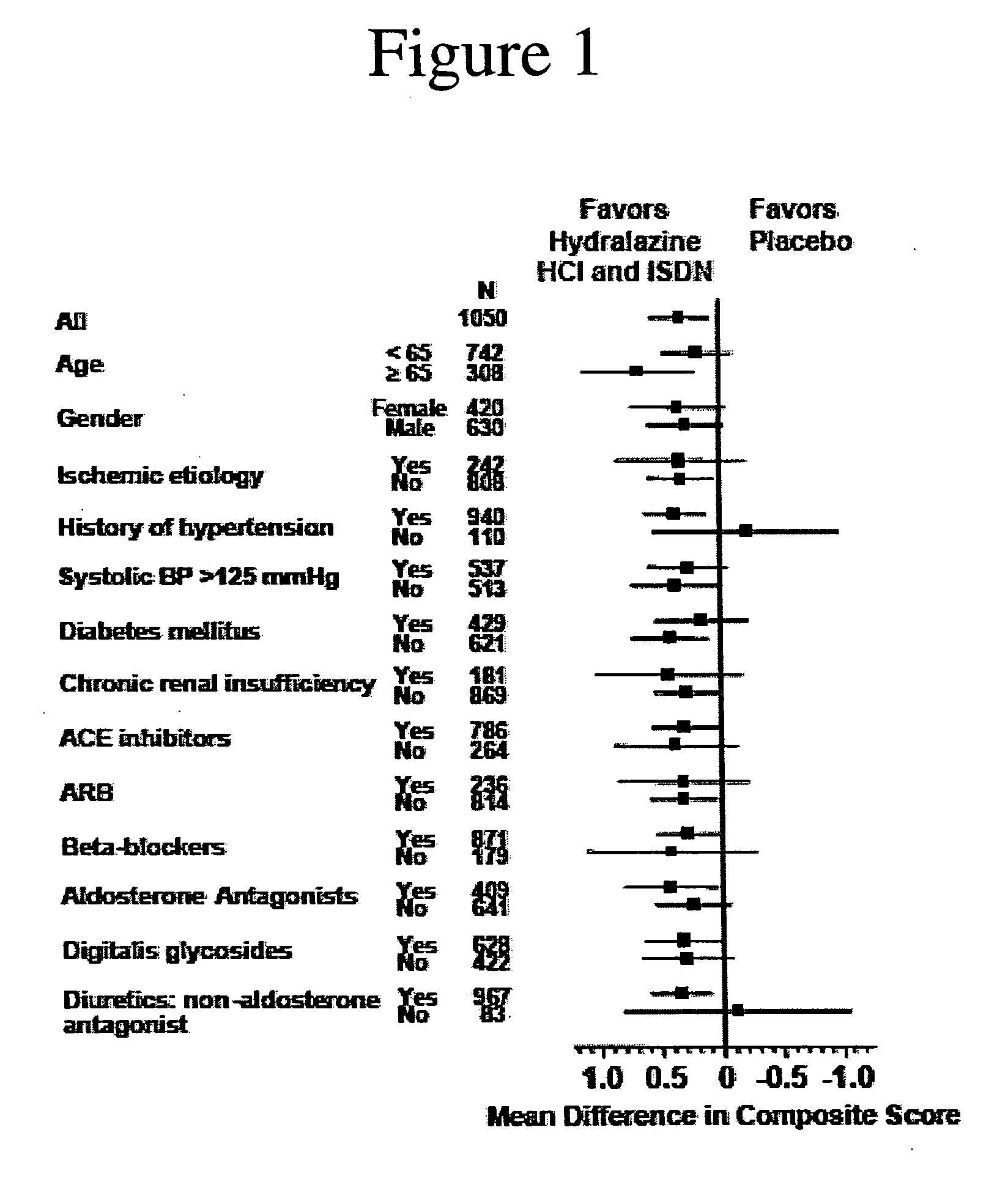
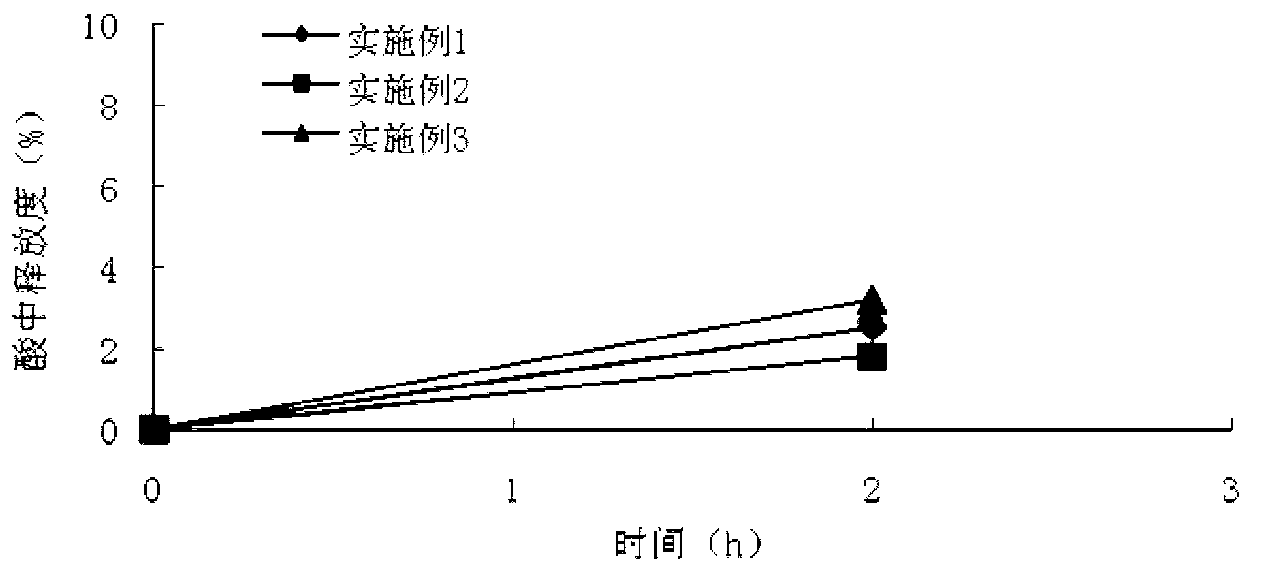
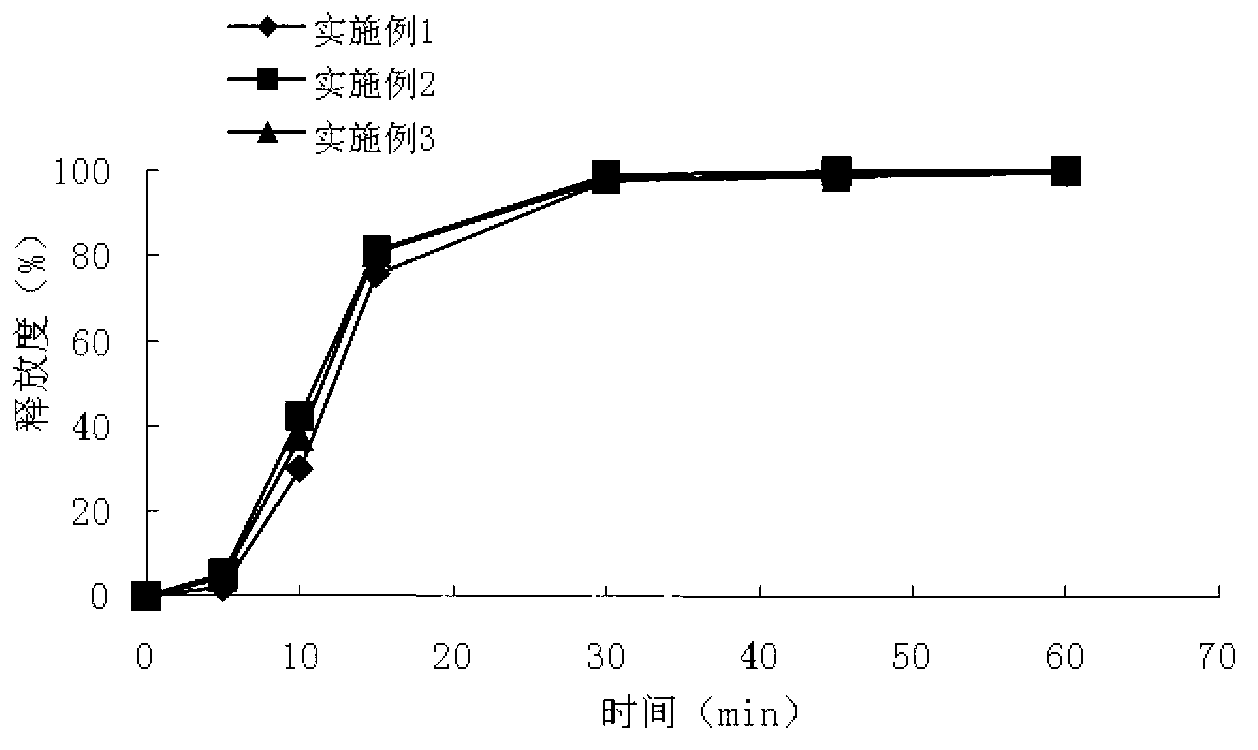
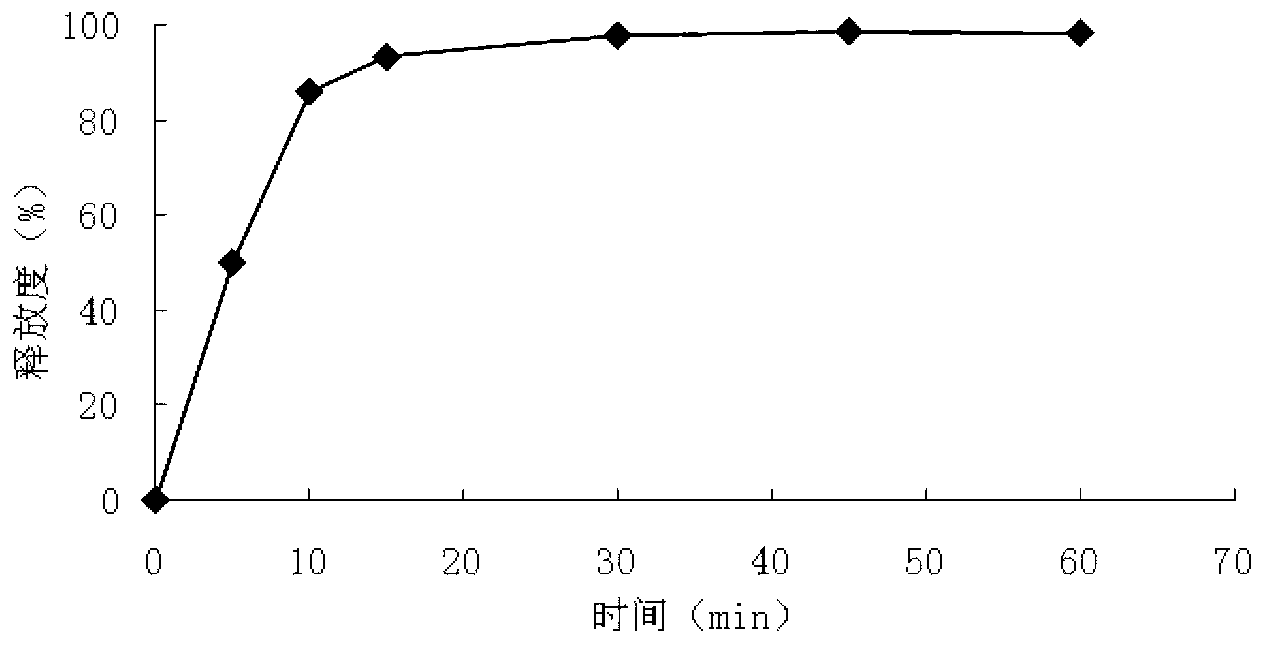
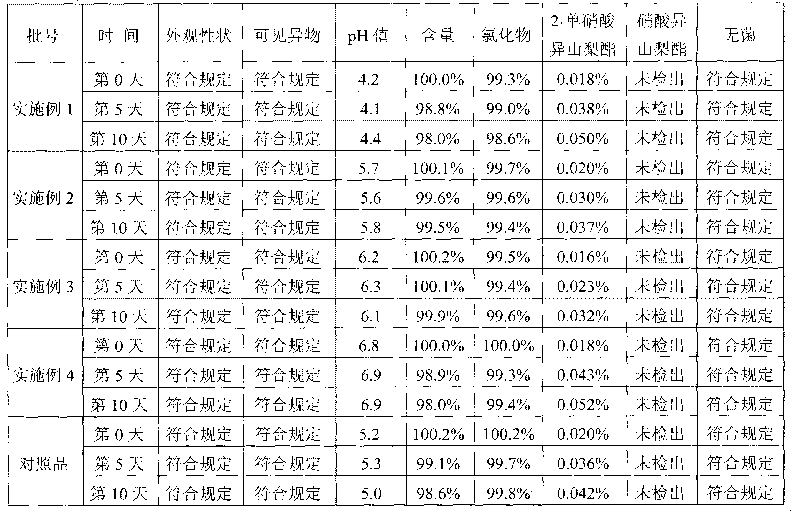
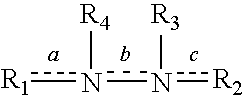Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
118 results about "Isosorbide dinitrate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Isosorbide dinitrate is used to prevent chest pain (angina) in patients with a certain heart condition (coronary artery disease).
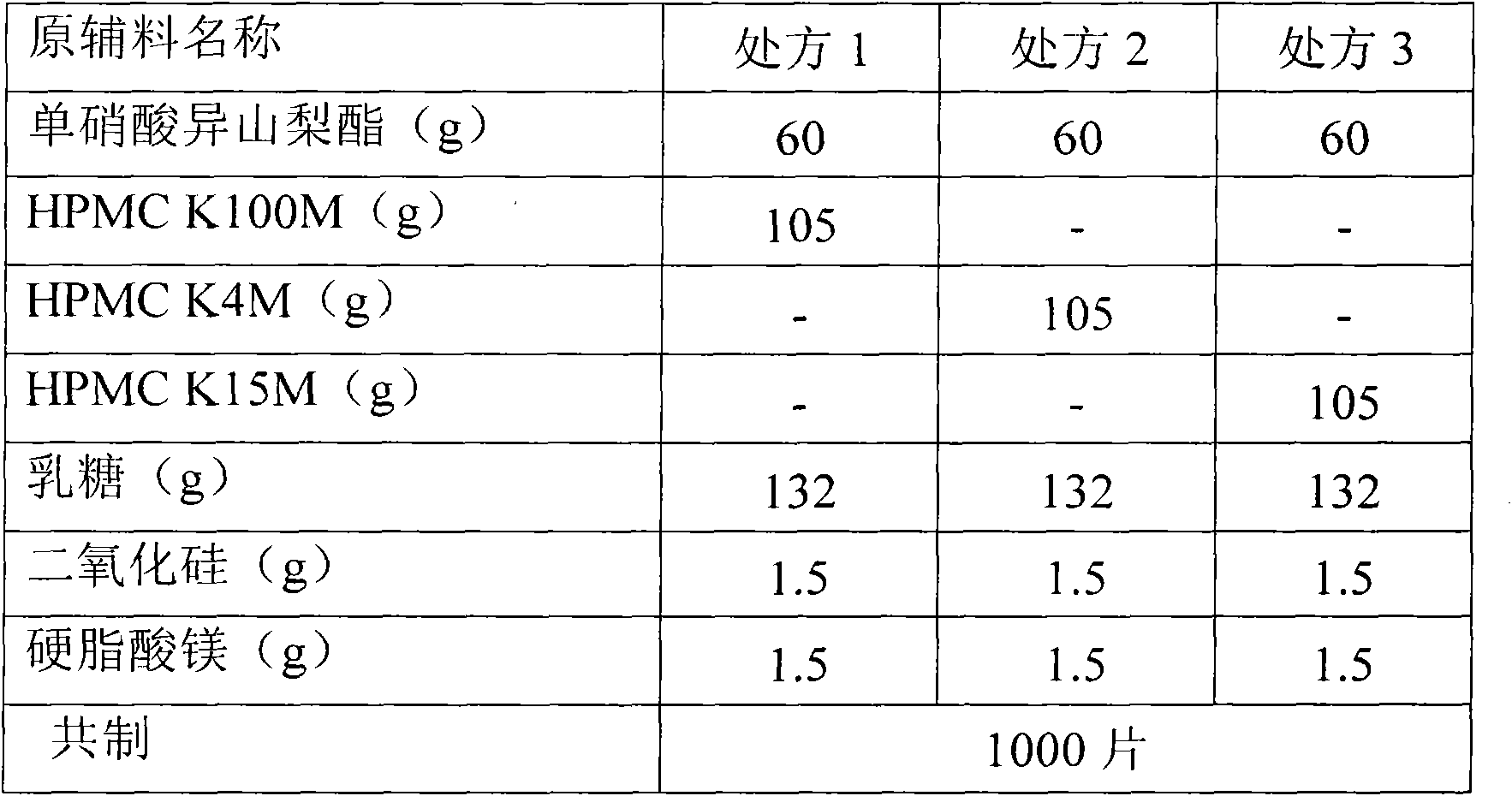
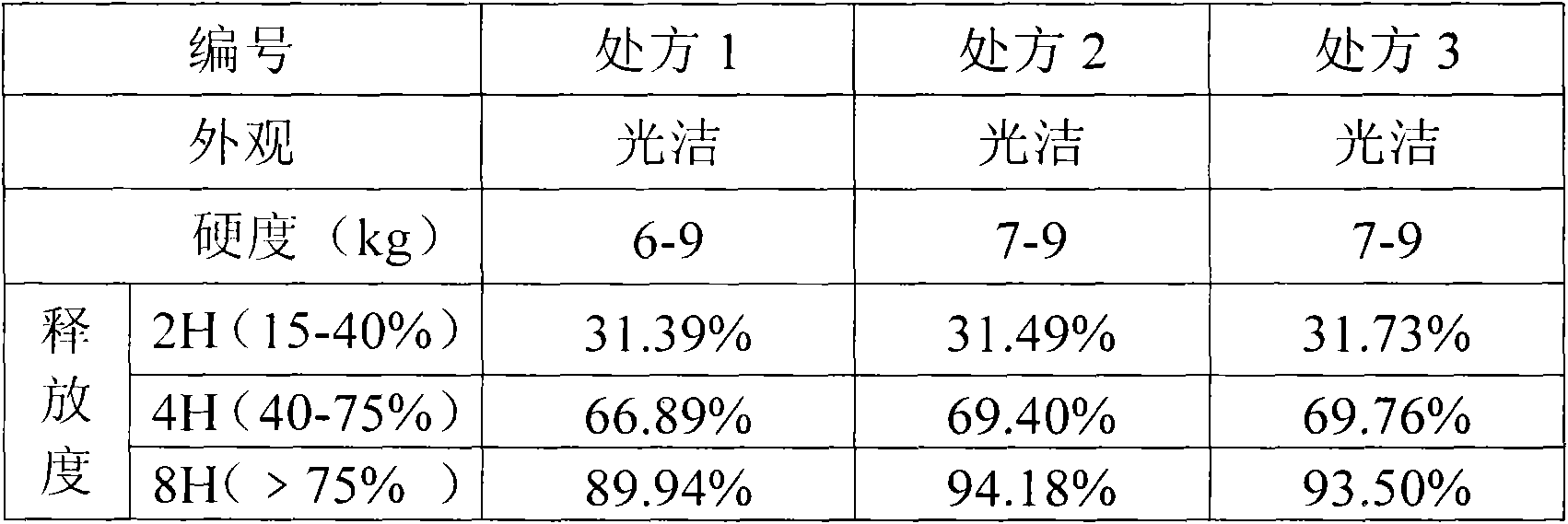
Single nitrate isosorbide delayed-release tablets
InactiveCN1679536AEasy to takeHelp flushingPharmaceutical delivery mechanismCardiovascular disorderCoronary artery diseaseCoronary heart disease
A slow-releasing tablet of isosorbide mononitrate for treating coronary heart disease and angina pectoris is prepared proportionally from isosorbide mononitrate, slow releasing agent, adhesive, filler and lubricant through proportional mixing, granulating, and tabletting.
Owner:LUNAN PHARMA GROUP CORPORATION
Methods for treating blood disorders with nitric oxide donor compounds
The invention describes methods for treating blood disorders or for treating the symptoms and / or complications associated with blood disorders by administering a therapeutically effective amount of at least one nitric oxide donor compound and optionally at least one antioxidant, or a pharmaceutically acceptable salt thereof, and / or at least one therapeutic agent. The antioxidant is preferably a hydralazine compound or a pharmaceutically acceptable salt thereof. The nitric oxide donor compound is preferably N-hydroxy-L-arginine and / or isosorbide dinitrate and / or isosorbide mononitrate. The blood disorder is preferably sickle cell anemia. The complication resulting from a blood disorder is preferably pulmonary hypertension.
Owner:NITROMED
Compositions and Methods Using Apocynin Compounds and Nitric Oxide Donors
The invention describes novel compositions comprising at least one apocynin compound or a pharmaceutically acceptable salt thereof, and at least one nitric oxide donor, and, optionally, at least one therapeutic agent. The invention also provides novel kits comprising at least one apocynin compound or a pharmaceutically acceptable salt thereof, and at least one nitric oxide donor compound, and, optionally, at least one therapeutic agent. The invention also provides methods for (a) treating cardiovascular diseases; (b) treating renovascular diseases; (c) treating diabetes; (d) treating diseases resulting from oxidative stress; (e) treating endothelial dysfunctions; (f) treating diseases caused by endothelial dysfunctions; (g) treating gastrointestinal disorders; (h) treating inflammatory disorders; and (j) treating respiratory disorders. The apocynin compound may preferably be apocynin. The nitric oxide donor compound may preferably be isosorbide dinitrate and / or isosorbide mononitrate.
Owner:ARBOR PHARMA LLC
Nitrosated and nitrosylated nebivolol and its metabolites, compositions and methods of use
InactiveUS7138430B2Prevention of platelet aggregation and platelet adhesionAntibacterial agentsBiocideMetaboliteAntioxidant
Owner:NICOX SA
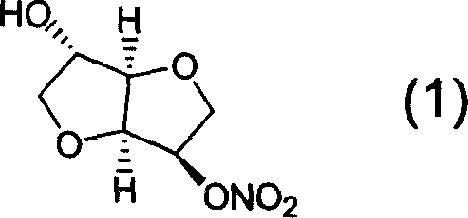
Derivatives of isosorbide mononitrate and its use as vasodilating agents with reduced tolerance
InactiveUS6858632B2Potent vasodilating effectSmall and null tolerance effectBiocideOrganic chemistryArylTolerability
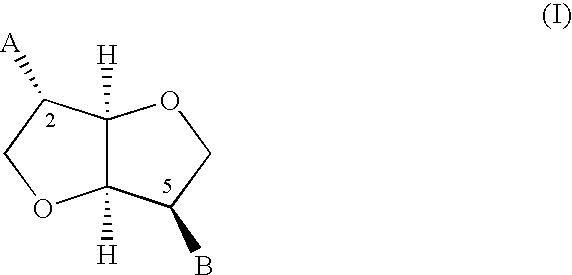
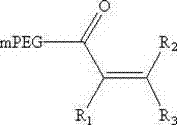
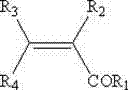
Novel derivatives of isosorbide mononitrate and its pharmaceutically acceptable salts, which have vasodilating activity with a reduced effect of tolerance, of the general formula (I) in which A and B independently represent any of the groups—ONO2 and —Z—CO—R, wherein Z is an oxygen atom or sulphur atom and R is an alkyl C1-C4 group, an aryl group or an aralkyl group, eventually substituted, or the group in which R1 is hydrogen, or an alkyl C1-C4 group, an aryl group or an aralkyl group, eventually substituted, with the proviso that one of A or B is always —ONO2, but never both of them at the same time, when Z is an sulphur atom R is an alkyl C1-C4 group, an aryl group or an aralkyl group, eventually substituted, and when Z is an oxygen atom R is the group
Owner:LACER SA
Diesel cycle fuel compositions containing dianhydrohexitols and related products
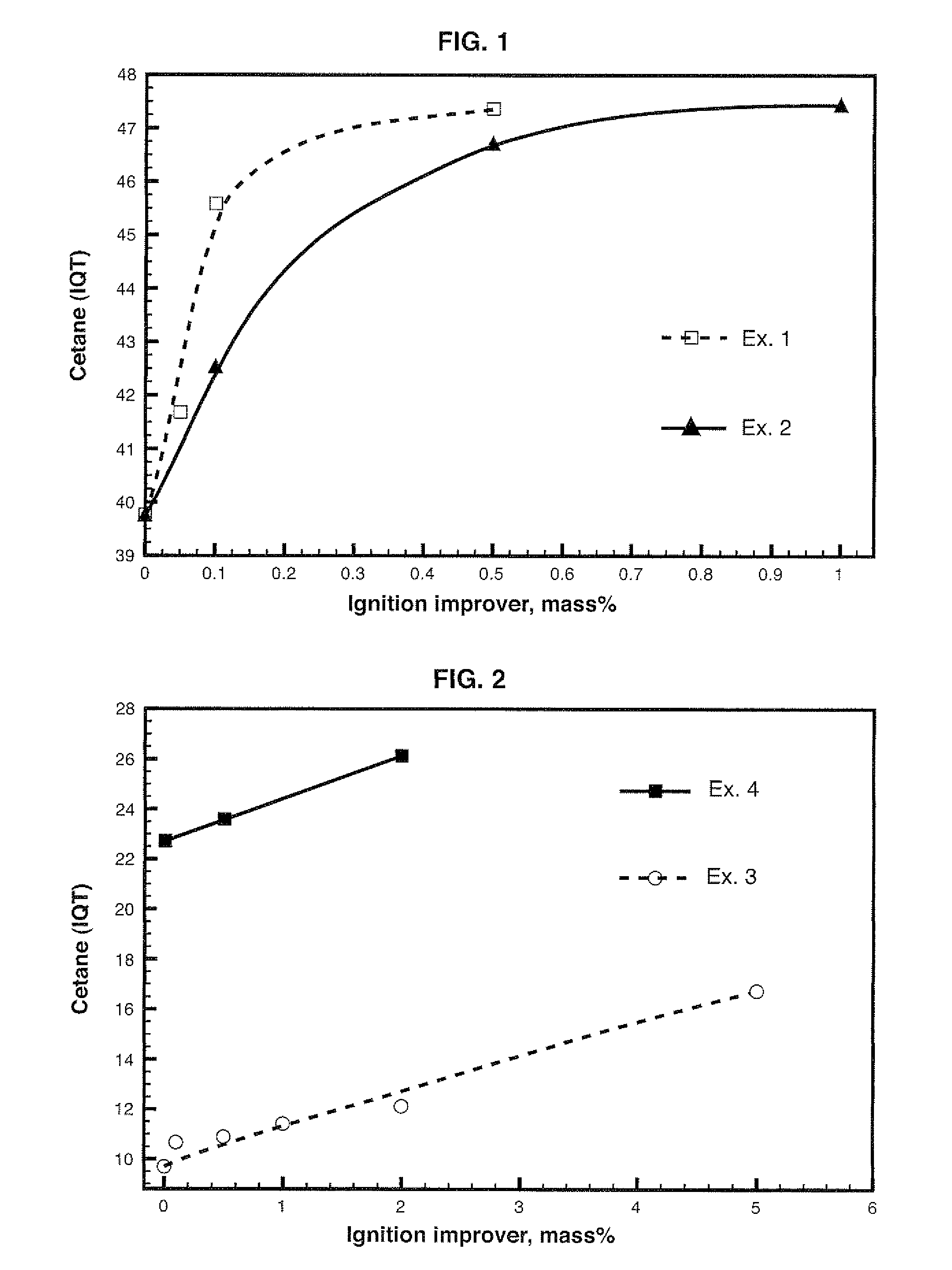
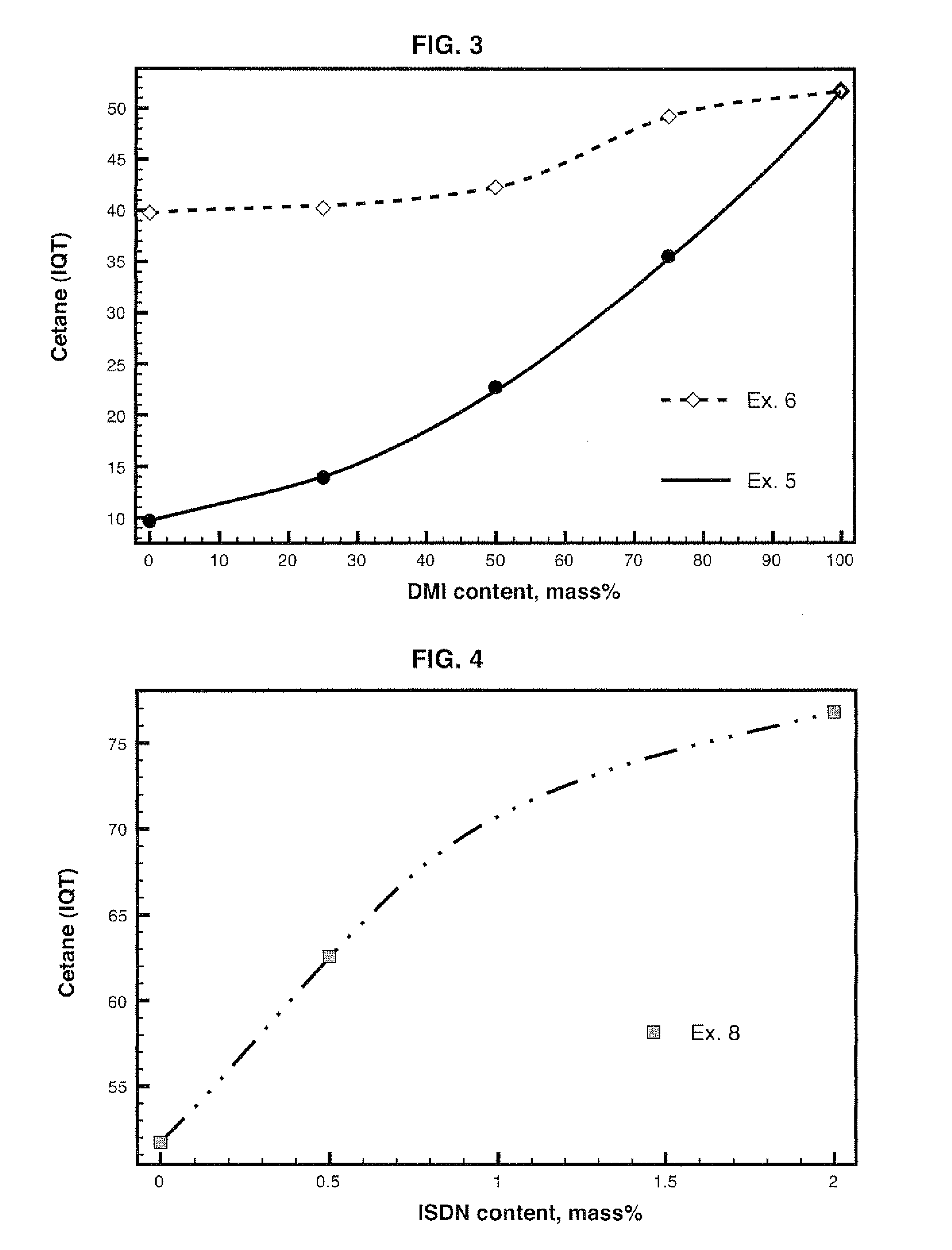
Diesel cycle fuel compositions are described containing at least one dianhydrohexitol compound according to the general formula 2 and / or itsderived hydrocarbyl ethers or nitric ethers compounds, where the R′ and R″ substituents are both H or one or both of R′ and R″ is alkyl, cycloalkyl or phenyl, or one or both are —NO2. A preferred fuel composition is that containing dimethyl isosorbide (DMI) added or not of isosorbide dinitrate (ISDN) as ignition improver. The dianhydrohexitols compounds form compositions with at least one of the components selected among petroleum-derived diesel fuel, biodiesel, ethanol and water. The mixture of DMI and ISDN has excellent cetane number (IQT). Still, the oxygenated nature of the dianhydrohexitols and related compounds of the fuel compositions inhibits soot formation upon burning of the said Diesel cycle fuel compositions.
Owner:PETROLEO BRASILEIRO SA (PETROBRAS)
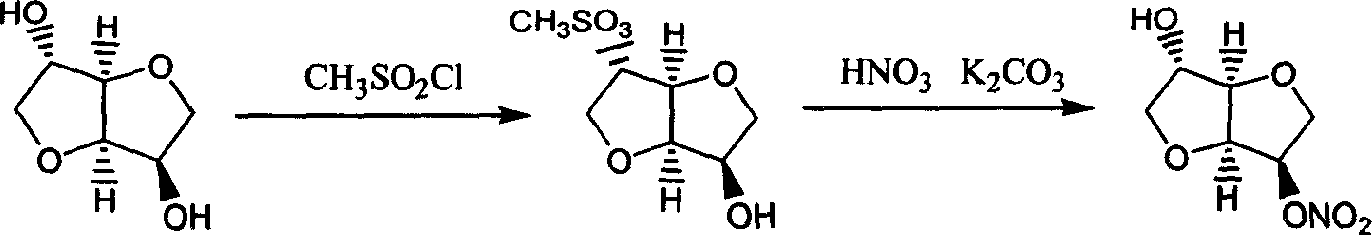
Preparation process of isosorbide mononitrate
InactiveCN1618798AHarsh reaction conditionsLow yieldOrganic chemistryCardiovascular disorderAnginaAcetic oxide
A process for preparing the isosorbide mononitrate from sorbitol includes dewatering sorbitol by p-methylphenyl sulfonic acid to obtain anhydrosorbitol, protecting by acetic oxide under existance of N,N-dimethylaminopyridine, nitrating by nitric acid / acetic oxide / acetic acid system, and removing protection by potassium carbonate-methanol system. It can be used to treat angina pectoris.
Owner:LUNAN PHARMA GROUP CORPORATION
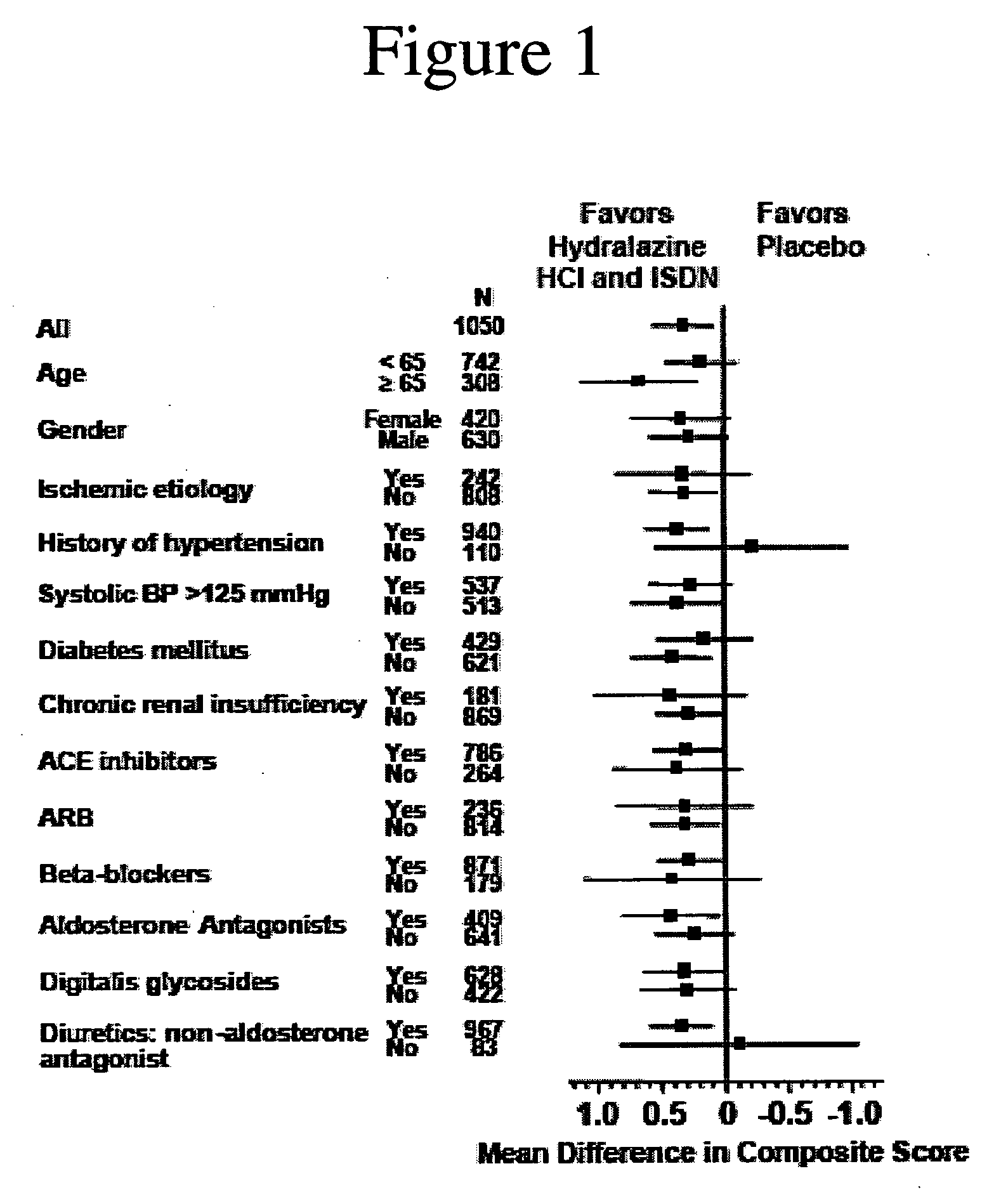
Compositions and methods related to heart failure
InactiveUS20060014828A1Reduce in quantityShorten the construction periodBiocideNervous disorderDiseaseType B Natriuretic Peptide
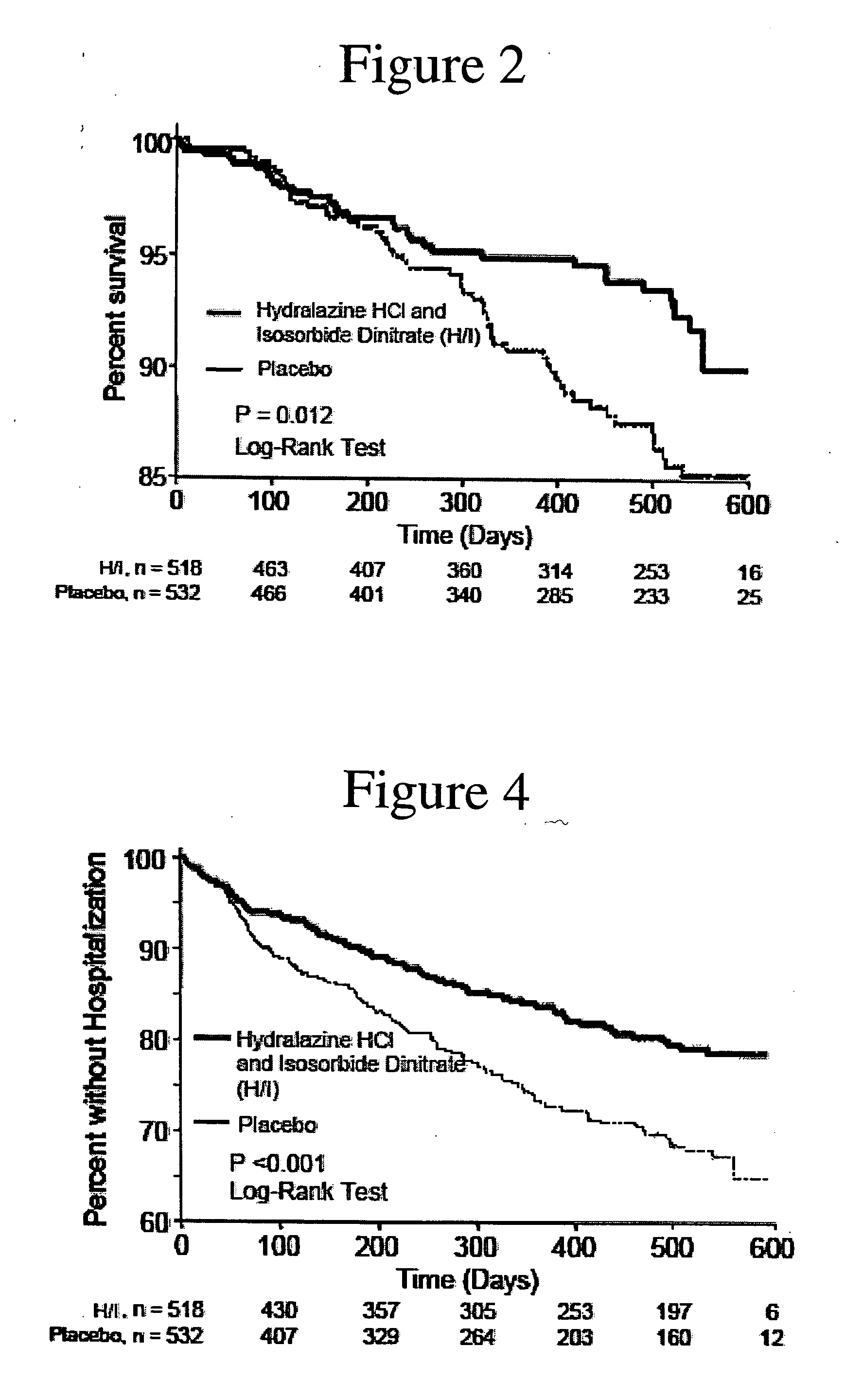
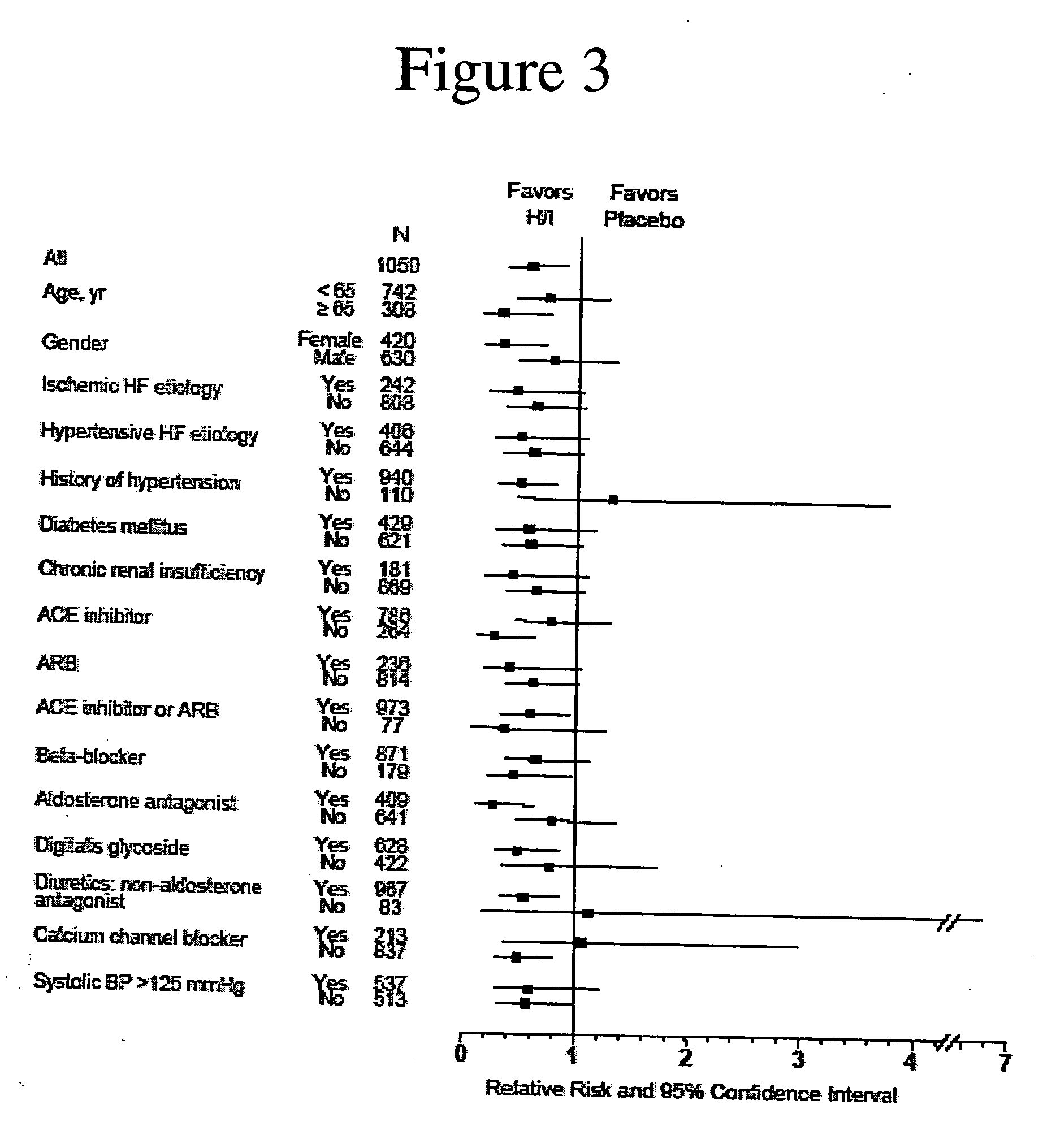
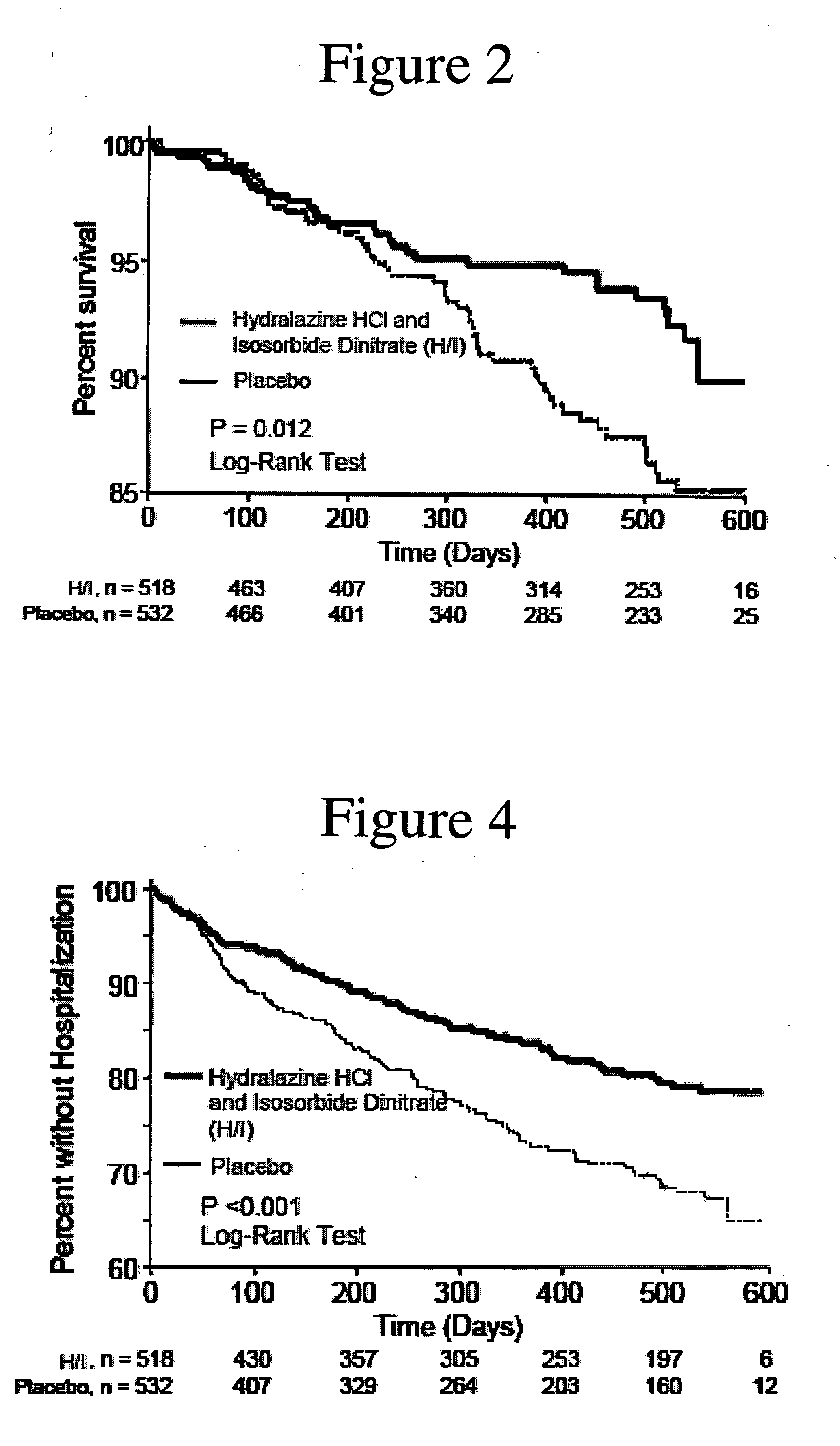
The invention provides methods for (a) prolonging time to hospitalization for heart failure; (b) prolonging time to first hospitalization for heart failure; (c) reducing the total number of days a patient with heart failure spends in the hospital for heart failure for a single hospital stay (i.e., reducing the duration of a single hospital stay for a patient with heart failure); (d) reducing the total number of days a patient spends in the hospital for heart failure for multiple hospital stays (i.e., two or more hospital stays); (e) reducing the number of hospital admissions for heart failure; (f) reducing mortality and reducing hospitalizations for heart failure (e.g., the total number of days in the hospital and / or the number of hospital visits); (g) increasing the left ventricular ejection fraction in a heart failure patient; (h) treating a sexual dysfunction (e.g., erectile dysfunction and female sexual dysfunction) (j) treating a headache in a heart failure patient by administering a non-steroidal antiinflammatory compound (i.e., NSAIDs); (k) treating a heart failure patient who has a history of hypertension (but who is not currently diagnosed with hypertension); (l) improving the quality of life in a heart failure patient based on the Minnesota Living with heart failure questionnaire; (m) decreasing the levels of B-type natriuretic peptide; (n) treating hypertension in a heart failure patient; (o) lowering blood pressure in a heart failure patient; (p) treating labile hypertension; (q) treating idiopathic hypertension; (r) increasing patient compliance with medication dosing in a heart failure patient; (s) treating hypertension in a patient with a dilated heart; (t) treating ischemic disease and / or coronary artery disease; and (u) reducing cardiomegaly in a patient in need thereof comprising administering to the patient a therapeutically effective amount of (i) a hydralazine compound or pharmaceutically acceptable salt thereof, (ii) isosorbide dinitrate and / or isosorbide mononitrate, and (iii) optionally at least one compound selected from the group consisting of angiotensin converting enzyme inhibitors, β-adrenergic antagonists, angiotensin II antagonists, aldosterone antagonists, cardiac glucosides (digitalis), and diuretic compounds.
Owner:NITROMED
Prepn process of isosorbide mononitrate
ActiveCN1609108ALow boiling pointReduce the temperatureOrganic chemistryCardiovascular disorderRutheniumIsosorbide mononitrate
The preparation process of isosorbide mononitrate as angina pectoris resisting medicine includes the following steps: directly nitrating sorbitol with fuming nitric acid and concentrated sulfuric acid in 0-0.5 time to obtain 2, 5-dinitro isosorbate; and the subsequent catalytic hydrogenation with ruthenium complex as catalyst and selective reduction of 2-nitro group. The present invention has simple process and high yield.
Owner:SHANDONG NEWTIME PHARMA
Single nitrate isosorbide sodium chloride injection
InactiveCN1679537AFast and reliableGood curative effectPharmaceutical delivery mechanismHeterocyclic compound active ingredientsCoronary artery diseaseAngina
A liquid sodium chloride injection of isosorbide mononitrate for treating coronary heart disease and angina pectoris is prepared from isosorbide mononitrate and sodium chloride. It can quickly take its high curative effect.
Owner:LUNAN PHARMA GROUP CORPORATION
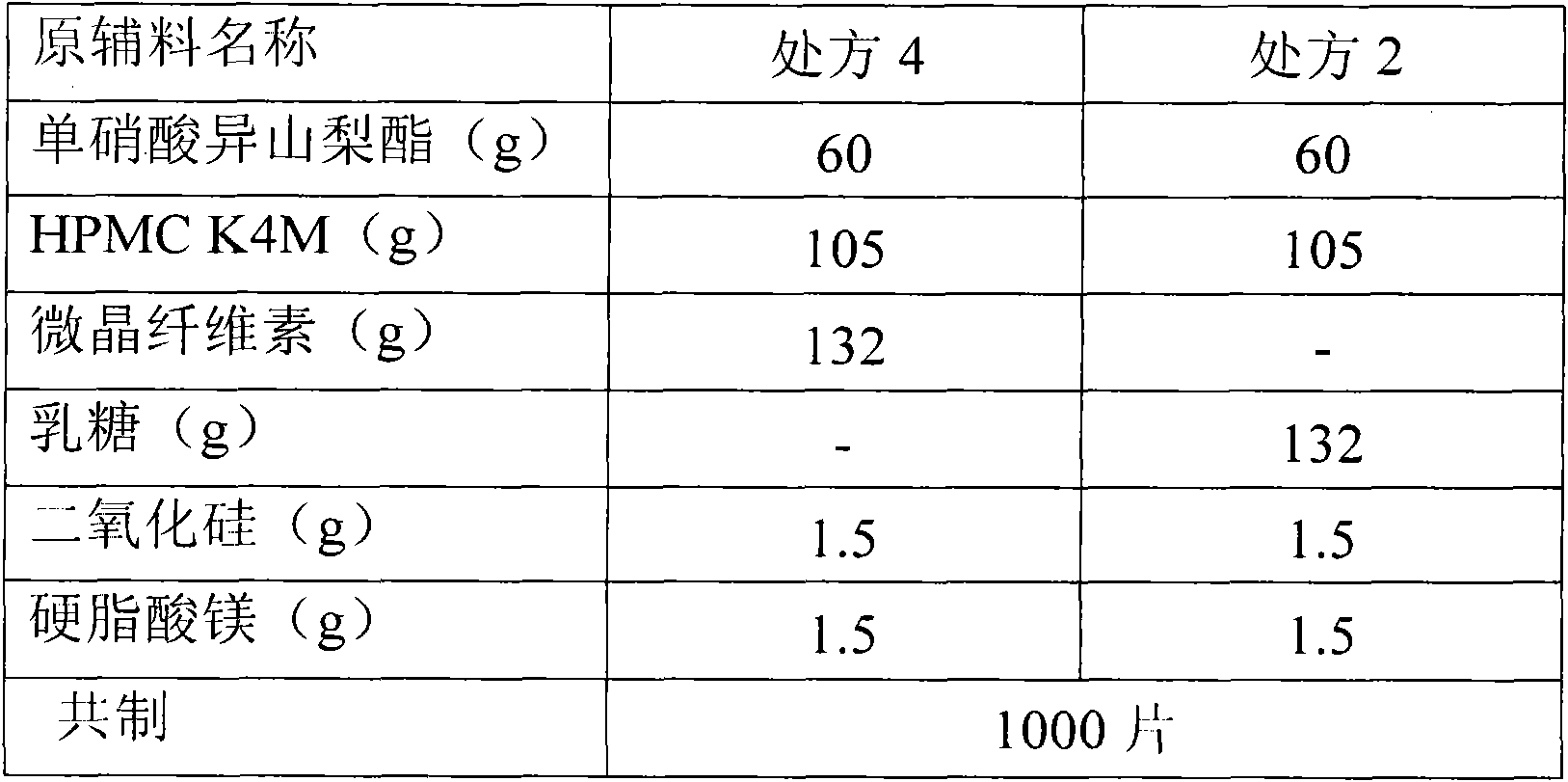
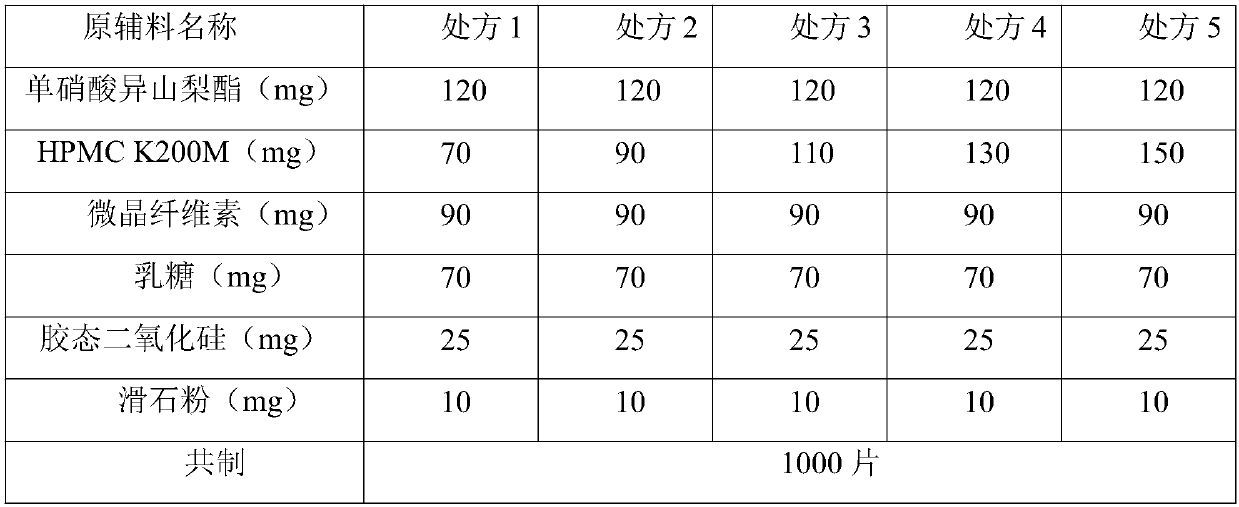
Isosorbide mononitrate sustained release tablet and preparation method thereof
ActiveCN102688212APharmaceutical non-active ingredientsDrageesSustained Release TabletIsosorbide mononitrate
The invention belongs to the field of pharmaceutic preparation, and particularly relates to an isosorbide mononitrate sustained release tablet and a preparation method thereof. The tablet prescription of the sustained release tablet in the application comprises the following components: isosorbide mononitrate, hydroxypropyl methylcellulose K4M, microcrystalline cellulose, silicon dioxide and sodium dodecyl sulfate. The isosorbide mononitrate sustained release tablet prepared by the components has better stability.
Owner:北京均大检测科技有限公司
Methods for reducing hospitalizations related to heart failure
InactiveUS20060014829A1Reduce in quantityShorten the construction periodBiocideNervous disorderDigitalisMortality rate
The invention provides methods for (a) prolonging time to hospitalization for heart failure; (b) prolonging time to first hospitalization for heart failure; (c) reducing the total number of days a patient with heart failure spends in the hospital for heart failure for a single hospital stay (i.e., reducing the duration of a single hospital stay for a patient with heart failure); (d) reducing the total number of days a patient spends in the hospital for heart failure for multiple hospital stays; (e) reducing the number of hospital admissions for heart failure; and (f) reducing mortality and reducing hospitalizations for heart failure (e.g., the total number of days in the hospital and / or the number of hospital visits) in a patient in need thereof comprising administering to the patient a therapeutically effective amount of (i) a hydralazine compound or pharmaceutically acceptable salt thereof, (ii) isosorbide dinitrate and / or isosorbide mononitrate, and (iii) optionally at least one compound selected from the group consisting of angiotensin converting enzyme inhibitors, β-adrenergic antagonists, angiotensin II antagonists, aldosterone antagonists, cardiac glucosides (digitalis), and diuretic compounds.
Owner:NITROMED
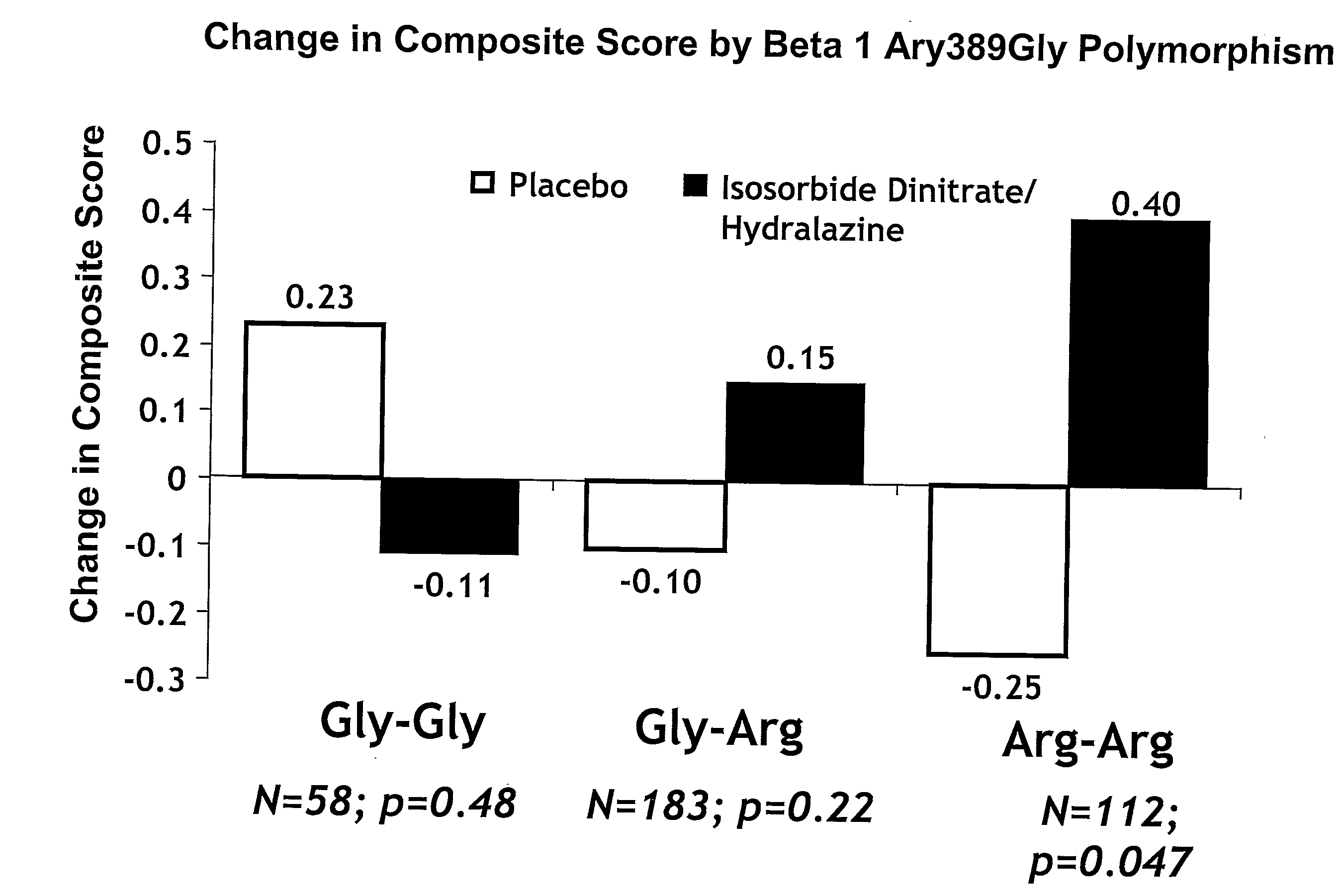
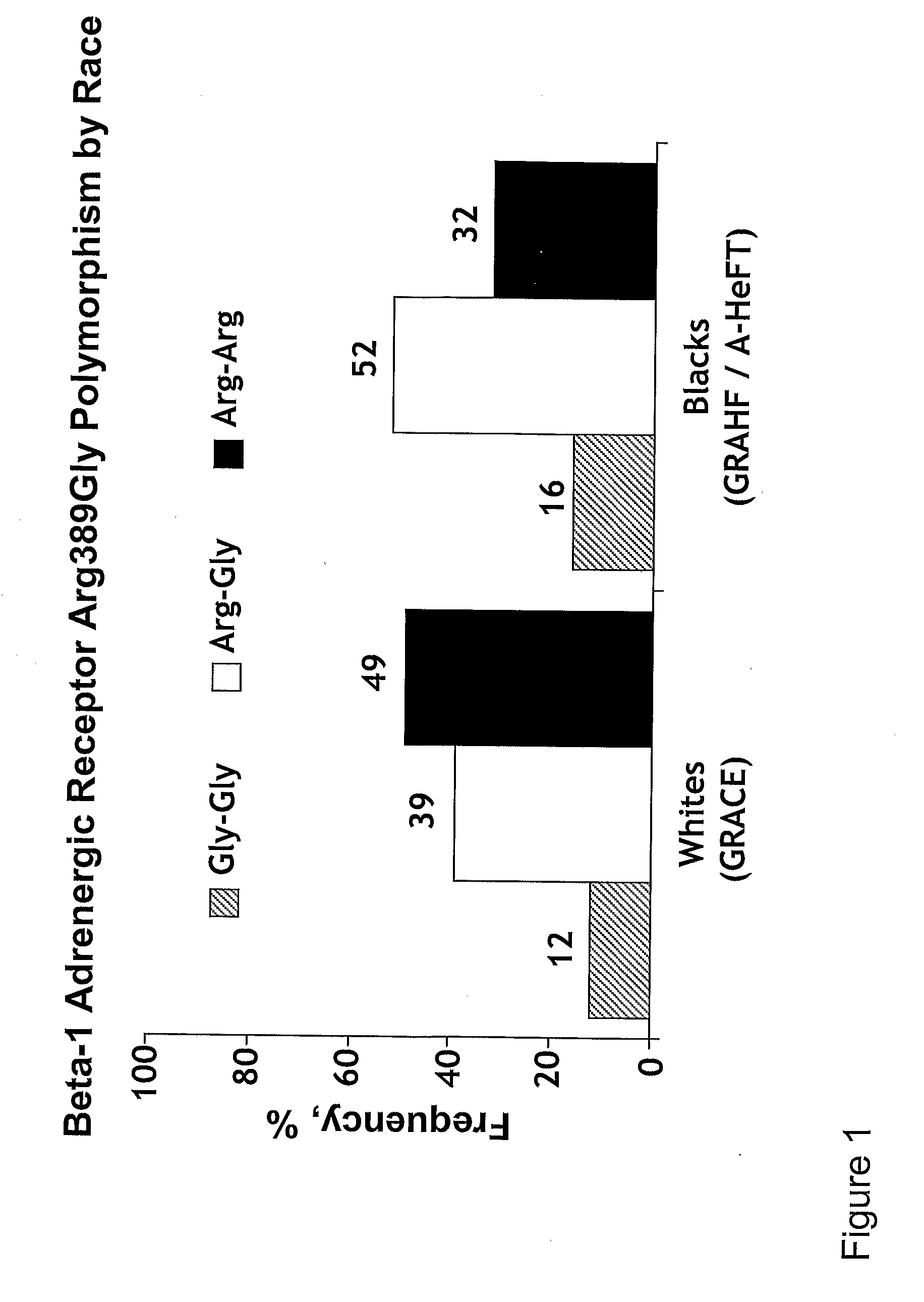
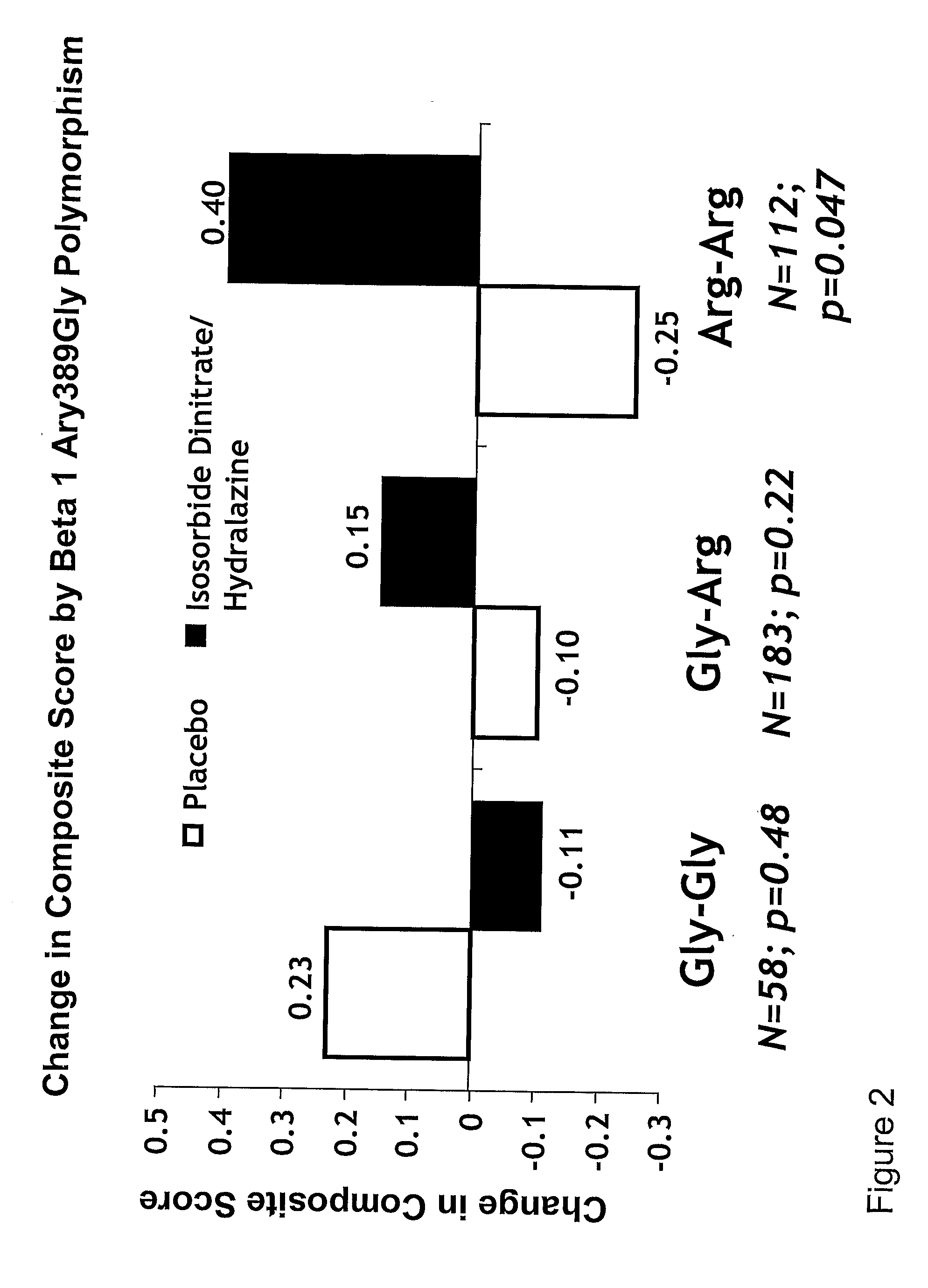
Genetic risk assessment in heart failure: impact of genetic variation of beta 1 adrenergic receptor gly389arg polymorphism
InactiveUS20090192128A1Reduce mortalityIncreased oxygen consumptionBiocideMicrobiological testing/measurementAntioxidantLeft ventricular size
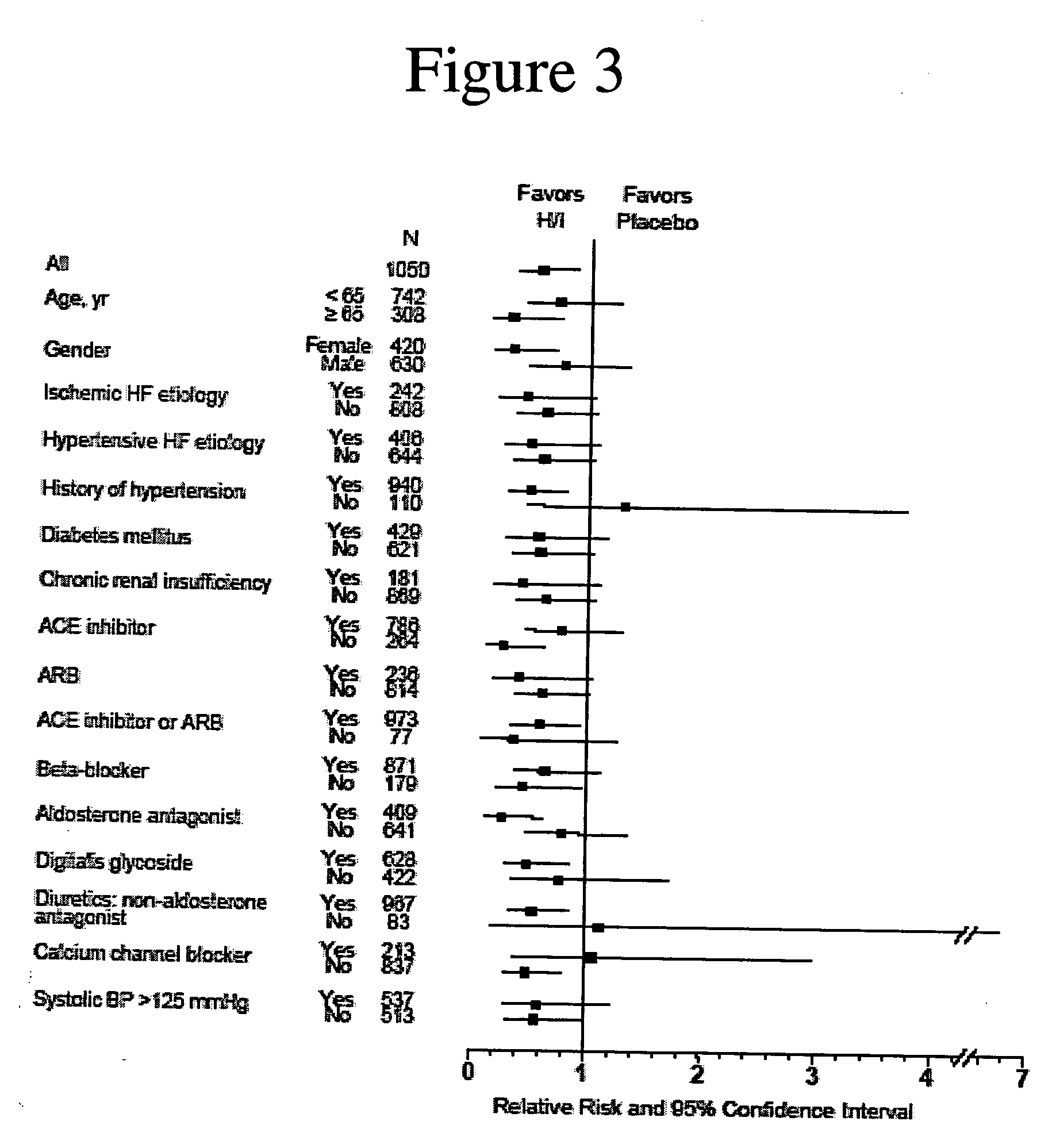
The invention provides methods for (a) reducing mortality associated with heart failure; (b) improving oxygen consumption; (c) treating heart failure; (d) treating hypertension; (e) improving the quality of life in a heart failure patient; (f) inhibiting left ventricular remodeling; (g) reducing hospitalizations related to heart failure; (h) improving exercise tolerance; (j) increasing left ventricular ejection fraction; (k) decreasing levels of B-type natriuretic protein; (l) treating renovascular diseases; (m) treating end-stage renal diseases; (n) reducing cardiomegaly; (o) treating diseases resulting from oxidative stress; (p) treating endothelial dysfunctions; (q) treating diseases caused by endothelial dysfunctions; or (r) treating cardiovascular diseases; in a patient in need thereof, wherein the patient has a Arg389Arg polymorphism and / or a Gly389Gly polymorphism in the beta 1 adrenergic receptor gene, comprising administering to the patient (i) at least one antioxidant compound or a pharmaceutically acceptable salt thereof; (ii) at least one nitric oxide enhancing compound; and (iii) optionally the best current therapy for the treatment of cardiovascular diseases. In one embodiment the antioxidant is a hydralazine compound or a pharmaceutically acceptable salt thereof and the nitric oxide enhancing compound is isosorbide dinitrate and / or isosorbide mononitrate.
Owner:NITROMED +1
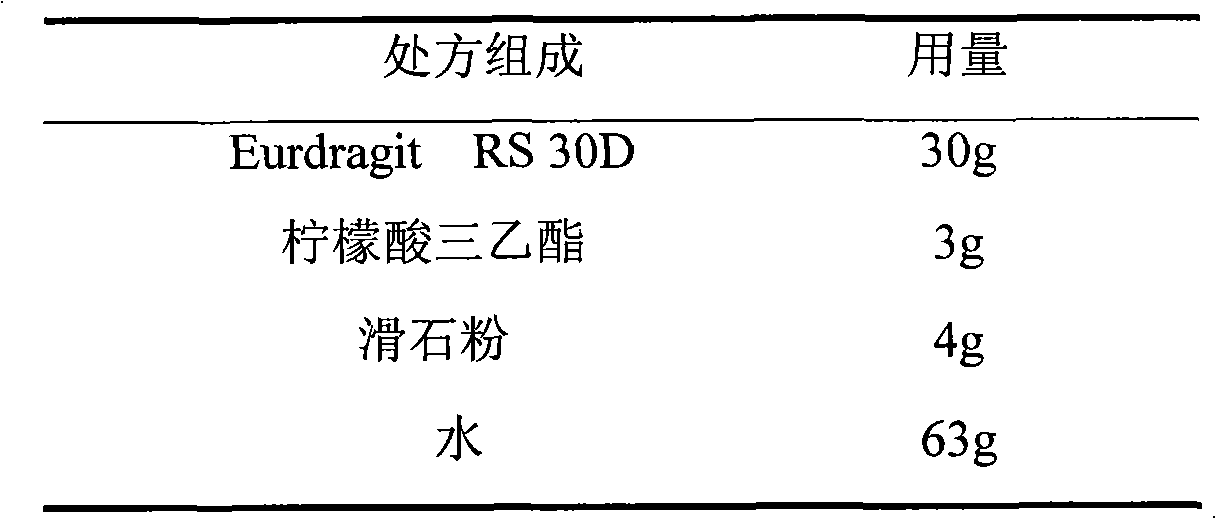
Isosorbide mononitrate sustained-release pellet, and isosorbide mononitrate quick-release and sustained-release pellet capsule adopting it
ActiveCN103211768AAccelerated agingReduce permeabilityPharmaceutical non-active ingredientsGranular deliverySustained release pelletsMedicine
The invention relates to an isosorbide mononitrate sustained-release pellet, and an isosorbide mononitrate quick-release and sustained-release pellet capsule adopting it. The sustained-release film of the sustained-release pellet adopts Eurdragit RS 30D as a film forming material, the core of the sustained-release pellet contains high-expansibility sodium carboxymethyl starch and a pharmaceutically-acceptable excipient commonly used for sustained-release pellets, and optimally, the excipient is microcrystalline cellulose, wherein the weight percentage of sodium carboxymethyl starch in the core of the sustained-release pellet is 5-20%. The sustained-release film of the sustained-release pellet includes the Eurdragit RS 30D, a plasticizer triethyl citrate and an anti-adherent talcum powder, the optimal ratio of the Eurdragit RS 30D to triethyl citrate to the talcum powder is 30:3:4, and the optimal coating weight gain is 19-38%. The core will obviously expand after absorbing water because of the containment of sodium carboxymethyl starch highly expanding after contacting with water, so the sustained-release film is stretched, the thickness of the film is thinned, the apertures of water-pervious micro-pores are increased, the permeability is good, and the permeability decrease caused by film ageing is compensated, thereby the middle and later stage release speed is basically constant, the last stage residue is small, and a stable release performance is always maintained prior to the expiration date.
Owner:北京天衡药物研究院有限公司
Compound isosorbide mononitrate aspirin sustained-release capsule preparation and preparation method
ActiveCN103285017AReduce adverse reactionsAvoid adverse reactionsPharmaceutical delivery mechanismHeterocyclic compound active ingredientsSustained release pelletsSustained Release Capsule
The invention discloses a compound isosorbide mononitrate aspirin sustained-release capsule preparation. The compound isosorbide mononitrate aspirin sustained-release capsule preparation is characterized by comprising an isosorbide mononitrate sustained-release capsule preparation and an aspirin enteric-coated preparation, wherein the isosorbide mononitrate sustained-release capsule preparation contains 40-80 parts by weight of isosorbide mononitrate and comprises an immediate-release pellet with 30 percent of isosorbide mononitrate and a sustained-release pellet with 70 percent of isosorbide mononitrate, and the aspirin enteric-coated preparation contains 50-90 parts by weight of aspirin. The invention also provides a preparation method of the compound capsule preparation. With the adoption of the compound isosorbide mononitrate aspirin sustained-release capsule preparation, the curative effects are better improved, the adverse reaction due to stimulation from the aspirin to a gastric mucosa is better reduced, and meanwhile, the isosorbide mononitrate can also satisfy the requirement of stable release in a stomach.
Owner:吉林天衡药业有限公司
Isosorbide mononitrate sodium chloride injection
ActiveCN101708157AEnsure safetyEnsure effectivenessInorganic non-active ingredientsPharmaceutical delivery mechanismSodium Chloride InjectionIsosorbide mononitrate
The invention relates to isosorbide mononitrate sodium chloride injection which belongs to the field of medical preparation. The isosorbide mononitrate sodium chloride injection is prepared from 10-40mg of isosorbide mononitrate, 450-1800mg of sodium chloride, an amount of pH regulator which regulates the pH to 4.0-7.0 and water for injection, wherein the volume of the water for injection is fixed to 100ml. The isosorbide mononitrate sodium chloride injection has high stability and simple preparation process.
Owner:LUNAN PHARMA GROUP CORPORATION
Isosorbide mononitrate sustained release tablet and preparation method thereof
ActiveCN110403911AImprove liquidityPharmaceutical non-active ingredientsPill deliverySustained Release TabletLactose
The invention belongs to the field of pharmaceutical preparations, and particularly discloses a large-sized isosorbide mononitrate sustained release tablet and a preparation method thereof. Accordingto the prescription, the sustained release tablet is prepared from the components: isosorbide mononitrate:lactose (6:4), hydroxypropyl methylcellulose K200M, microcrystalline cellulose, mannitol, hydrogenated castor oil, silica and magnesium stearate. According to the large-sized isosorbide mononitrate sustained release tablet and the preparation method thereof, the microcrystalline cellulose, themannitol and the hydrogenated castor oil of a certain proportion are mixed to greatly improve the fluidity of mixed powder, and requirements of producing pressed tablets are met.
Owner:LUNAN PHARMA GROUP CORPORATION
Nebivolol and its metabolites in combination with nitric oxide donors, compositions and methods of use
InactiveUS20060009513A1Prevention of platelet aggregation and platelet adhesionAntibacterial agentsBiocideMetaboliteAntioxidant
The invention describes novel compositions comprising nebivolol and / or at least one metabolite of nebivolol and at least one nitric oxide donor, and, optionally, at least one antioxidant or a pharmaceutically acceptable salt thereof, and / or at least one compound used to treat cardiovascular diseases or a pharmaceutically acceptable salt thereof, and / or at least one nitrosated compound used to treat cardiovascular diseases. The compounds and compositions of the invention can also be bound to a matrix. The nitric oxide donor is a compound that donates, transfers or releases nitric oxide, elevates endogenous levels of endothelium-derived relaxing factor, stimulates endogenous synthesis of nitric oxide or is a substrate for nitric oxide synthase and may preferably be isosorbide dinitrate and / or isosorbide mononitrate. The antioxidant may preferably be a hydralazine compound or a pharmaceutically acceptable salt thereof. The invention also provides methods for treating and / or preventing vascular diseases characterized by nitric oxide insufficiency; and for treating and / or preventing Raynaud's syndrome; and for treating and / or preventing cardiovascular diseases or disorders.
Owner:NICOX SA
Polymethyl polyglycol methacrylate containing sorbitol ester mononitrate structure as well as preparation method and use method thereof
InactiveCN102344521AWith analgesic functionExcellent anticoagulant propertiesPharmaceutical containersMedical packagingReaction temperatureNitrogen gas
The invention discloses a polymethyl polyglycol methacrylate containing an isosorbide sorbitol ester structure as well as a preparation method and a use method thereof. The preparation method comprises the following steps of: mixing 2-acrylic acid-5-isosorbide dinitrate, methyl acrylate compounds and methylpropenoic polyglycol acid ester compounds; polymerizing free groups under the protection of nitrogen gas; reacting at the temperature of 20-90DEG C for 1-20 hours; repeatedly precipitating and dissolving generated polymerisate by petroleum ether and tetrahydrofuran; and finally, vacuum drying. The obtained polymer has a certain analgesic function and a better anticoagulation effect.
Owner:TIANJIN PLASTICS RES INST CO LTD
Isosorbide dinitrate oral administration impulse pellet preparations
ActiveCN101269059AGood reproducibilityImprove consistencyGranular deliveryOil/fats/waxes non-active ingredientsDiseaseAdjuvant
The invention discloses an oral pulse pellet pharmaceutical preparation of isosorbide esters, which consists of an immediate-release pellet core, an alkaline layer and a retardation layer containing isosorbide esters of 10 to 50 mg, wherein, the retardation layer contains polyacrylic resin 3, the weight increment of which is 80 to 200 percent of the immediate-release pellet core; the alkaline layer is a medicinal water-soluble alkaline adjuvant, the weight of which is 10 to 30 percent of the immediate-release pellet core. The pharmaceutical preparation has the characteristics that the drug does not release immediately after being taken orally, releases immediately from the pellet after 3 to 4 hours time lagging and the plasma concentration presents a pulse peak value. The pharmaceutical preparation can achieve the goal of preventing angina pectoris and other diseases from being triggered due to the rise of blood pressure and heart rate within a few hours after a patient awakens and wakes up in the early morning.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH +1
Isosorbide dinitrate sodium chloride injection
ActiveCN104027329AImprove solubilityImprove stabilityPharmaceutical delivery mechanismPharmaceutical non-active ingredientsSolubilitySodium Chloride Injection
The invention relates to an isosorbide dinitrate sodium chloride injection which comprises the following components in effective dose: 1200-1300mg of isosorbide dinitrate, 9000-15000mg of sodium chloride, 650-700mg of a cosolvent and the balance of water for injection, wherein a pH adjustor adjusts the pH to 5.0-5.5, and the water for injection is added until the volume of the injection is 2000ml. The isosorbide dinitrate sodium chloride injection provided by the invention has good water solubility and stability.
Owner:JIANGXI DONGFU PHARMA CO LTD
Isosorbide mononitrate sustained release tablet and preparation method thereof
ActiveCN110420192AGood sustained release effectImprove liquidityPill deliveryOil/fats/waxes non-active ingredientsSustained Release TabletWestern medicine
The invention belongs to the technical field of Western medicine preparations, and particularly provides an isosorbide mononitrate sustained release tablet and a preparation method thereof. Accordingto the prescription, the sustained release tablet comprises isosorbide mononitrate, lactose, sustained release materials, microcrystalline cellulose, a glidant and a lubricant. An isosorbide mononitrate mixed powder prepared by a special preparation process according to the formula has good fluidity and is suitable for direct tablet compression, and the prepared sustained release tablet is good instability and better in sustained release effect.
Owner:LUNAN PHARMA GROUP CORPORATION
Method for preparing isosorbide mononitrate sustained-release capsules
ActiveCN106138012ASolve the crystallization problemAvoid easy cakingGranular deliveryMicrocapsulesAdjuvantSustained Release Capsule
The invention belongs to the field of medicine industry and particularly relates to a method for preparing isosorbide mononitrate sustained-release capsules. The method for preparing the isosorbide mononitrate sustained-release capsules, provided by the invention, comprises the steps: (1) preparing isosorbide mononitrate quick-release micropellets; (2) preparing isosorbide mononitrate sustained-release micropellets; (3) carrying out mixing: mixing the isosorbide mononitrate quick-release micropellets with the isosorbide mononitrate sustained-release micropellets, and carrying out encapsulation, thereby obtaining the product. Shown by experimental determination, the product prepared by the technical scheme provided by the invention is good in stability, the problem, i.e., crystallization of isosorbide mononitrate is solved, and the problems of the existing processes that principal drugs and adjuvants are prone to caking and heated softening, the removal of residual solvent is not facilitated, and the micropellets are adhered are also avoided. Shown by in-vitro dissolution and in-vivo biological equivalent experimental investigations, the isosorbide mononitrate sustained-release capsules provided by the invention have bioequivalence compared with original triturates.
Owner:广东隆信制药有限公司
Methods for treating respiratory disorders
The invention provides methods for treating respiratory disorders in a patient in need thereof comprising administering an effective amount of (i) at least one hydralazine compound or a pharmaceutically acceptable salt thereof, (ii) isosorbide dinitrate and / or isosorbide mononitrate, and (iii) optionally at least one therapeutic agent. The hydralazine compound may be hydralazine hydrochloride. The respiratory disorders may be chronic obstructive pulmonary disease, pulmonary hypertension, emphysema, asthma, cystic fibrosis and bronchitis.
Owner:NITROMED
Vitamin E and trace element selenium aqueous solution and preparation method thereof
InactiveCN102726615AImprove stabilityNot easily oxidizedAnimal feeding stuffAnti stressSelenium deficiency
The invention provides a vitamin E and trace element selenium aqueous solution and a preparation method thereof, and relates to a feed additive. The vitamin E and trace element selenium aqueous solution capable of preventing and treating chicken vitamin E and selenium deficiency diseases, enhancing chicken productivity and anti-stress capacity, and enhancing chicken immunity, and the preparation method of the aqueous solution, are provided. The raw materials of the vitamin E and trace element selenium aqueous solution in percentage by mass are as follows: 10%-12% of vitamin E oil, 20%-30% of polyoxyethylene isosorbide dinitrate mono-oleate, 8%-12% of n-butyl alcohol, 0.01%-0.02% of sodium selenite, and 46%-62% of purified water. The vitamin E oil, the polyoxyethylene isosorbide dinitrate mono-oleate and the n-butyl alcohol are added into a stirrer for stirring, then the sodium selenite and the purified water are added for continuously stirring, and finally the product is obtained.
Owner:XIAMEN HUIYING ANIMAL TECH
Emulsion-type paraffin remover, and preparation method and application thereof
The invention provides an emulsion-type paraffin remover, and a preparation method and application thereof. The paraffin remover is prepared from the following components in percentage by volume: 55-60% of oily solvent, 0.5-3% of surfactant and the balance of water, wherein the surfactant is sodium dodecyl benzene sulfonate and / or isosorbide dinitrate laurate. The paraffin remover is simple in formula, on the one hand, the surfactant adopted by the paraffin remover can make a system to form stable emulsion at the normal temperature, and the stable time is long; and on the other hand, when theparaffin remover is added into a formation and the temperature rises, the system can rapidly break the emulsion, and thus an organic solvent can exert a paraffin dissolving effect with the greater efficacy. The surfactant adopted by the paraffin remover can further form a layer of water film on the surface of an oil pipe, so that the effects of decreasing the depositing quantity of oil well paraffin and prolonging the paraffin removing period are achieved.
Owner:中国石油工程建设有限公司华北分公司
Pharmaceutical composition containing isosorbide mononitrate for treating high blood pressure
InactiveCN1634022AGood synergistic antihypertensive effectGood curative effectCardiovascular disorderHeterocyclic compound active ingredientsClinical efficacyPulse pressure
The expansion of pulse pressure (PP) is the individual dangerous cardiovascular (CV) factor, by integrating Isosorbide Mononitrate with other hypertension resisting medicaments, good clinic curative effect is obtained, and synergy effect is achieved in terms of reduction of contraction pressure, wherein the combination of calcium ion antagonist, rennin-angiotensins-aldosterone system antagon, blood vessel expanding agent, epinephrine acceptor retarding agent can make the most ideal effect. The invention realizes the functions of reducing contraction pressure, mitigating unwanted reaction caused by hypertension medicament, and effectively increasing survival rate of hypertension patients.
Owner:LUNAN PHARMA GROUP CORPORATION
New application of composition containing isosorbide mononitrate and ivabradine
ActiveCN106176761AGood treatment effectImprove diastolic function of the heartCardiovascular disorderHeterocyclic compound active ingredientsSide effectIsosorbide mononitrate
The invention belongs to the field of medicine and particularly relates to an application of a pharmaceutical composition taking isosorbide mononitrate and ivabradine as active pharmaceutical ingredients in preparation of medicine for treating diastolic heart failure. By conformation through a lot of pharmacology experiment, the isosorbide mononitrate and the ivabradine can obviously improving heart diastolic function of patients with diastolic heart failure, and remarkable synergistic effect is achieved in the aspect of treating the diastolic heart failure. The pharmaceutical composition is obvious in treating effect and little in side effects in treating the diastolic heart failure, and is quite good in medical application prospect.
Owner:LUNAN PHARMA GROUP CORPORATION
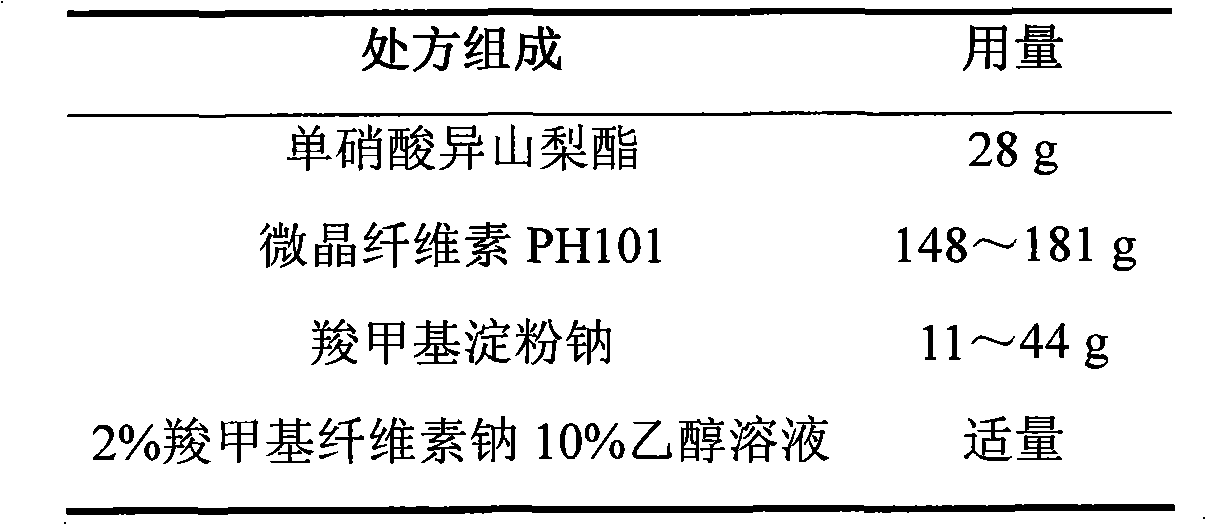
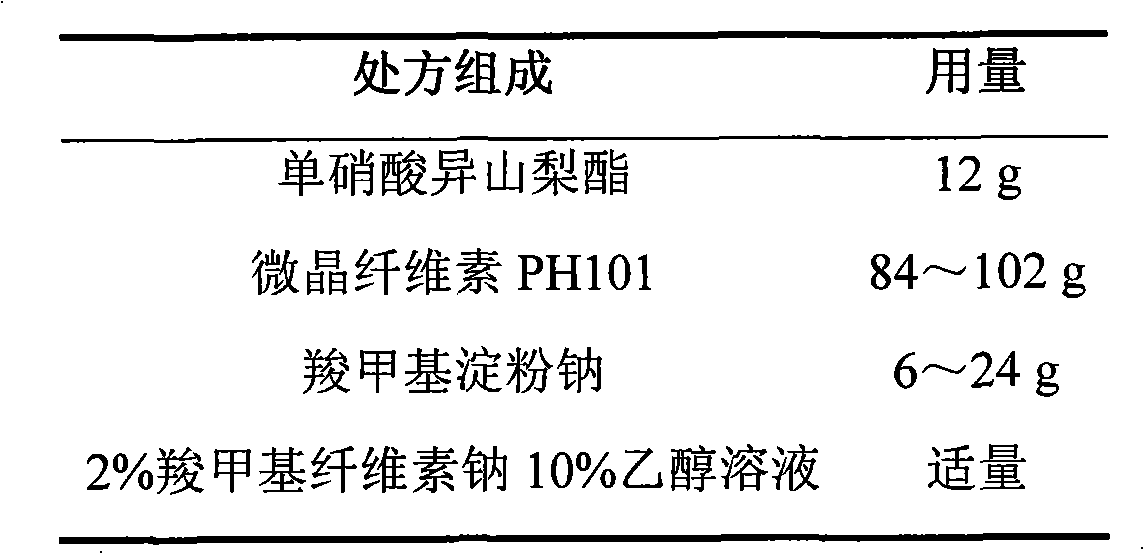
Isosorbide mononitrate controlled release tablet and preparation method thereof
ActiveCN101744783AConstant rate releaseSmooth releasePharmaceutical delivery mechanismHeterocyclic compound active ingredientsControlled Release TabletIsosorbide mononitrate
The invention relates to an isosorbide mononitrate controlled release tablet which consists of a tablet core and a coating. The isosorbide mononitrate controlled release tablet comprises the following components in percentage by weight: 1-20 percent of isosorbide mononitrate, 30-90 percent of first auxiliary material and 9-50 percent of second auxiliary material. The invention also relates to a preparation method of the isosorbide mononitrate controlled release tablet. The preparation method comprises the following steps of: preparing the tablet core of the isosorbide mononitrate controlled release tablet; preparing coating liquid; and coating the tablet core of the isosorbide mononitrate controlled release tablet.
Owner:北京华素制药股份有限公司 +1
Pueraria capsules
InactiveCN104940654AHigh concentration of active ingredientsPromote absorptionMetabolism disorderPharmaceutical non-active ingredientsSalvia miltiorrhizaCarrageenan
The invention discloses pueraria capsules. The pueraria capsules comprise the following components of, by mass, 2-6 parts of pueraria extract, 10-30 parts of astragalus, 15-30 parts of salvia miltiorrhiza, 15-30 parts of gastrodia elata, 10-15 parts of panax notoginseng, 10-15 parts of shells of trichosanthes kirilowii maxim, 4-12 parts of olibanum and 10-15 parts of angelica sinensis. Shells of the pueraria capsules comprise the components of, by mass, 10-15 parts of propolis, 10-19 parts of granulesten, 10-19 parts of carrageenan, 17-28 parts of gelatin, 11-21 parts of thickeners, 5-8 parts of alginate, 0.02-0.05 part of isosorbide dinitrate, 8-13 parts of plasticizers, 2-5 parts of magnesium stearate, 0.1-0.5 part of ethylparaben, 0.2-0.6 part of methylsilicone oil and 70-90 parts of purified water. According to the pueraria capsules, traditional Chinese medicine with the effect of conditioning the heart and blood vessels is adopted after being extracted appropriately, the concentration of effective components is higher, absorption is easier, and the shells of the pueraria capsules have the treating effect of auxiliary powder.
Owner:黄红林
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com