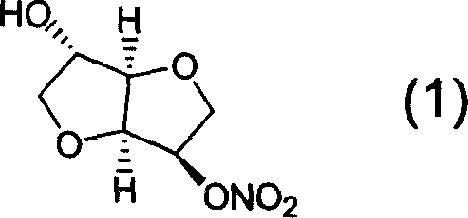
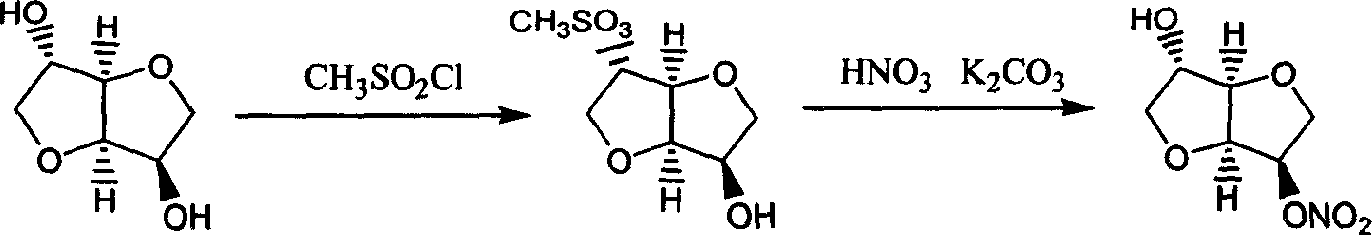
Prepn process of isosorbide mononitrate
A technology of nitrate ester and isosorbide, applied in the field of preparation of the compound isosorbide mononitrate, can solve the problems of long process route and increased cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: Synthesis of intermediate 1,4:3,6-dianhydro-D-sorbitol (3)
[0026] Add 9.5mL of p-toluenesulfonic acid slowly to 310g of 70% sorbitol with stirring and keep warm at 40°C, then slowly raise the temperature under a vacuum of 0.08Mpa to evaporate the water. When the temperature rises to about 117°C, the water is basically evaporated. The vacuum is 0.096Mpa, and the sorbitan is collected below 220°C, with a yield of 91%.
Embodiment 2
[0027] Embodiment 2: 2, the preparation of 5-isosorbide dinitrate (4)
[0028] Dissolve 100g of sorbitan in a mixed solution of 300mL ether, 100mL of acetic acid and 100mL of acetic anhydride. When the internal temperature is controlled at 20-30°C, add a mixed solution of 110g of fuming nitric acid and 30g of concentrated sulfuric acid. After the addition is completed, Continue to react at room temperature for 2.5 h, slowly pour the reaction solution into 1L of ice water, adjust the pH value to 7 with a 30% NaOH solution, let stand to separate the layers, separate the organic phase, and evaporate to dryness under reduced pressure to obtain yellow Concentrate 110g.
Embodiment 3
[0029] Example 3: 1,4; Preparation of 3,6-dianhydro-D-sorbitol-5-nitrate (1)
[0030] Add 100g 2,5-isosorbide dinitrate, 1.7g RuBr to 1000mL isopropanol 2 -(R)-BINAP and 2mL 2,6-lutidine were stirred in 10 atmospheres of hydrogen at room temperature for 2.5h, the resulting solution was filtered, the solvent was evaporated under reduced pressure, and the obtained pale yellow concentrate was dissolved in chloroform and water, Filter out the insoluble matter, separate the chloroform layer, wash with water, anhydrous MgSO 4 Dry, evaporate the solvent by heating to precipitate crystals, and obtain 57 g of 1,4:3,6-dianhydro-D-sorbitol-5-nitrate crystals, yield 70.1%, melting point 91.0-92.0 °C, [α] D 20 +175.6° (ethanol). The infrared spectrum is the same as that of the standard product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com