Isosorbide dinitrate sodium chloride injection
A technology of isosorbide dinitrate and sodium chloride injection, which is applied in directions such as active ingredients of heterocyclic compounds, drug combinations, medical preparations of non-active ingredients, etc. The effect of precipitation of crystals and related substances, improving stability and increasing solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
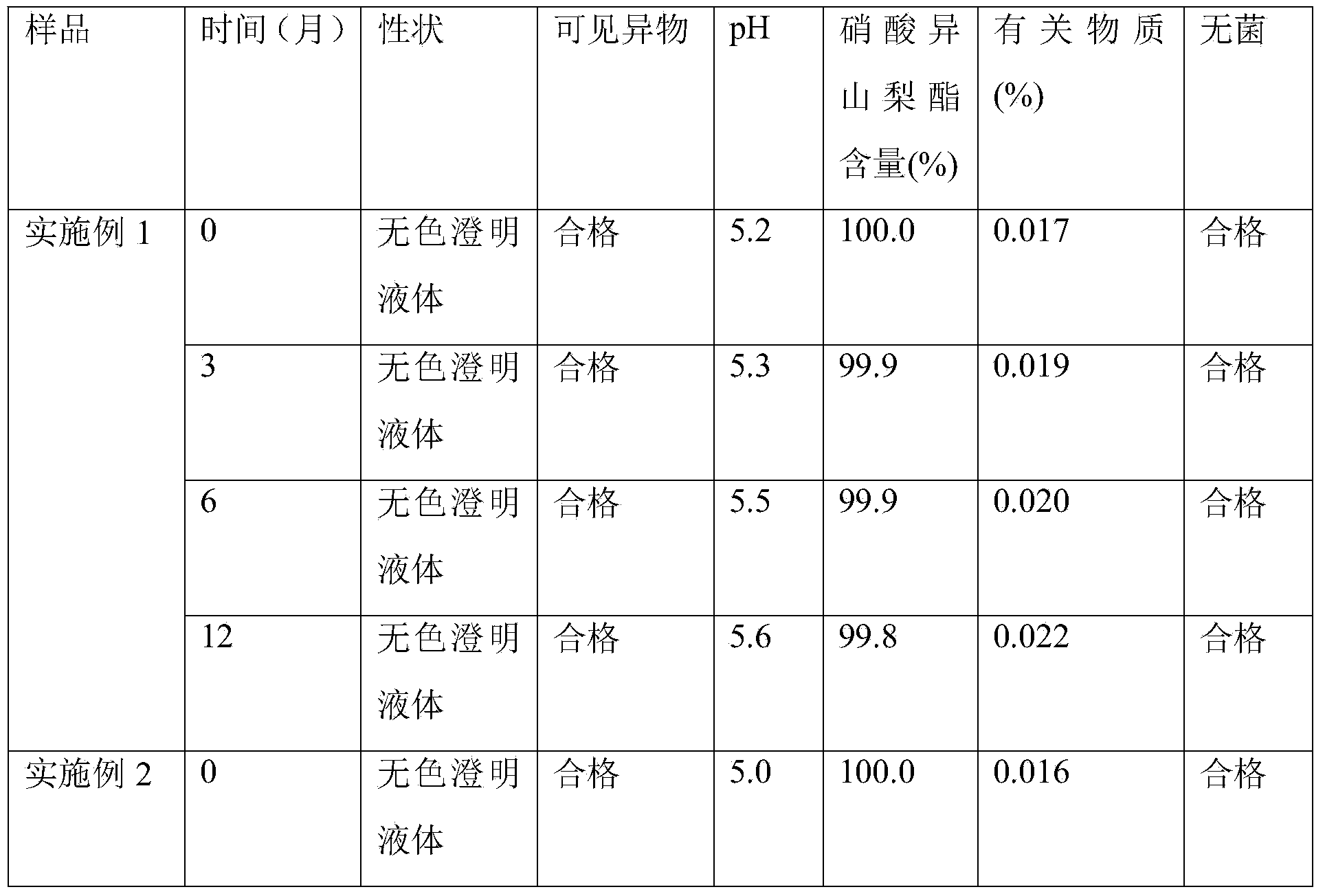
Examples
Embodiment 1
[0025] Embodiment 1 Isosorbide Dinitrate Sodium Chloride Injection
[0026] Isosorbide Dinitrate 1250mg
[0027] Sodium chloride 12000mg
[0028] A mixture of 0.1mol / L sorbic acid and 0.1mol / L ascorbic acid (the molar ratio of the two is 7:1) to adjust the pH to 5.2 L-cysteine hydrochloride 680mg
[0029] Add water for injection to 2000ml.
[0030] Preparation Process:
[0031] The injection preparation process includes two steps: concentrated preparation and diluted preparation. The concentrated preparation is mainly to dissolve the raw materials to minimize the amount of solvent used, and the diluted preparation is the process of diluting and constant volume.
[0032] Concentrated preparation: add an appropriate amount of fresh water for injection to the concentrated preparation tank, add the calculated amount of sodium chloride, stir to dissolve, add 2% (W / W) activated carbon for needles and keep boiling for 15 minutes, then filter to remove carbon, and leave the chlor...
Embodiment 2
[0036] Isosorbide Dinitrate 1200mg
[0037] Sodium chloride 9000mg
[0038] 0.1mol / L sorbic acid to adjust the pH to 5.0
[0039] L-cysteine hydrochloride 650mg
[0040] Add water for injection to 2000ml.
[0041] Preparation process: with embodiment 1.
Embodiment 3
[0043] Isosorbide Dinitrate 1300mg
[0044] Sodium chloride 15000mg
[0045] A mixture of 0.1mol / L sorbic acid and 0.1mol / L ascorbic acid (the molar ratio of the two is 7:1) to adjust the pH to 5.1L-cysteine hydrochloride 700mg
[0046] Add water for injection to 2000ml.
[0047] Preparation process: with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com