Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
39 results about "Dimethyl isosorbide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
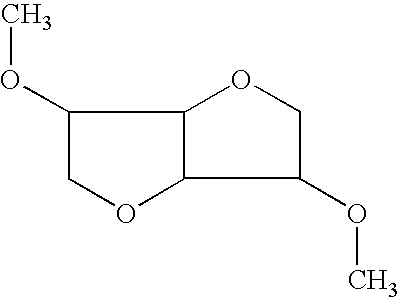
Dimethyl Isosorbide. CAS No.: 5306-85-4. Dimethyl Isosorbide (DMI) is a high purity solvent and carrier which offers a safe, effective delivery enhancement mechanism for active ingredients in personal care products.
Anti-wrinkle composition
ActiveUS20070166267A1Easy to superviseAccurate balanceBiocideOrganic active ingredientsWrinkle skinDimethyl isosorbide
A composition is disclosed for treating the skin comprising an acylated short chain bioactive peptide and Lycium barbarum extract product. Also disclosed is a method for topically administering the composition in an amount therapeutically effective to reduce wrinkles by building the dermal fibroblast matrix. The composition may contain dimethylisosorbide or ethoxydiglycol as solubilizing and penetration enhancers for the hydrophobically modified peptide.
Owner:GRANT INDS
Anti-wrinkle composition
ActiveUS7807625B2Easy to superviseAccurate balanceBiocideCosmetic preparationsEthoxidineLycium barbarum fruit
A composition is disclosed for treating the skin comprising an acylated short chain bioactive peptide and Lycium barbarum extract product. Also disclosed is a method for topically administering the composition in an amount therapeutically effective to reduce wrinkles by building the dermal fibroblast matrix. The composition may contain dimethylisosorbide or ethoxydiglycol as solubilizing and penetration enhancers for the hydrophobically modified peptide.
Owner:GRANT INDS
Betamethasone spray
InactiveUS20080102039A1Organic active ingredientsAerosol deliveryBetamethasone valerateDimethyl isosorbide
A spray foaming dosage form comprising betamethasone valerate, dimethyl isosorbide, propylene glycol, non ionic surfactant, sodium dodecyl sulphate, a buffer, optional preservative, optional further excipients, and water.
Owner:NUPHARM LAB
Diesel cycle fuel compositions containing dianhydrohexitols and related products
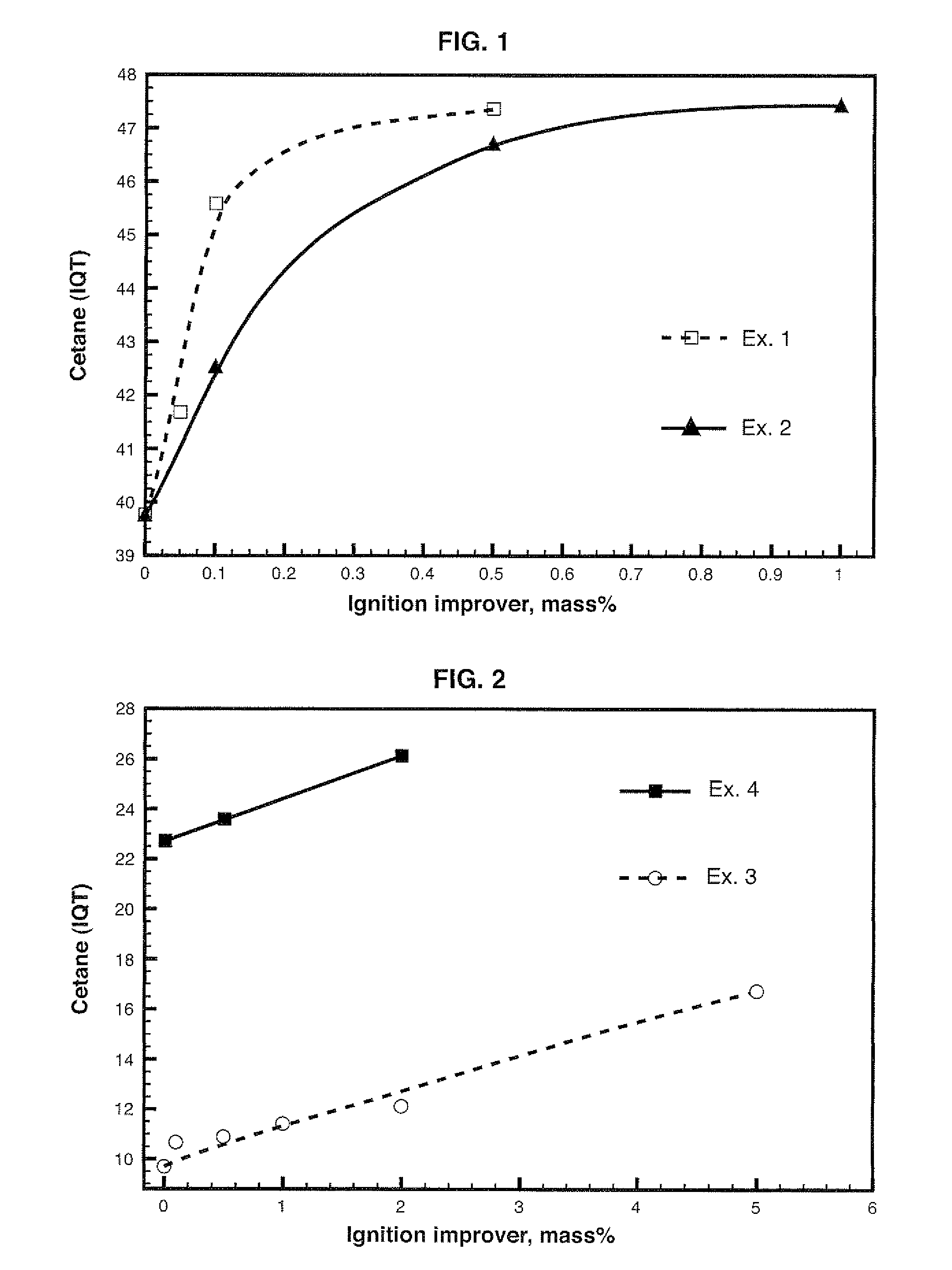
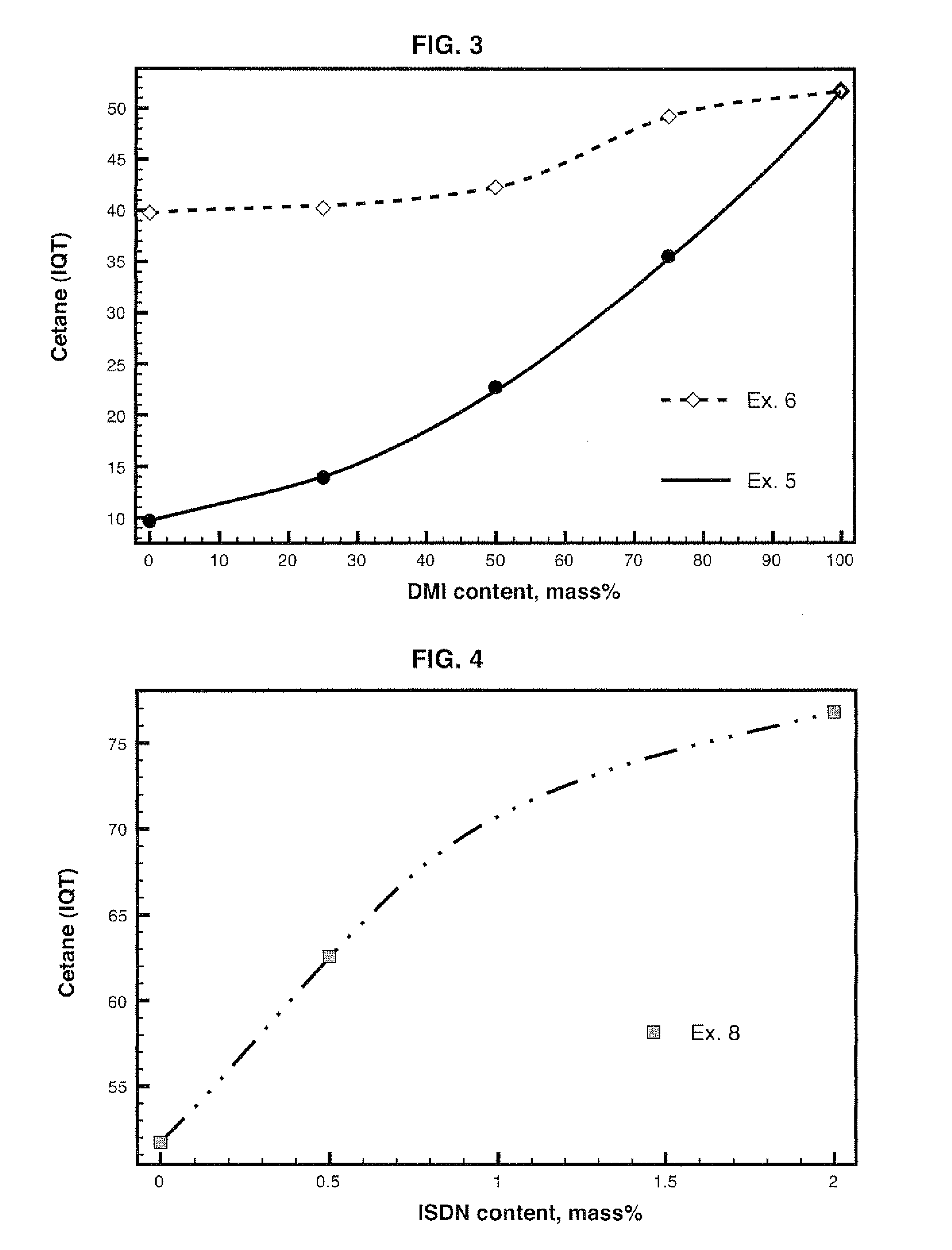
Diesel cycle fuel compositions are described containing at least one dianhydrohexitol compound according to the general formula 2 and / or itsderived hydrocarbyl ethers or nitric ethers compounds, where the R′ and R″ substituents are both H or one or both of R′ and R″ is alkyl, cycloalkyl or phenyl, or one or both are —NO2. A preferred fuel composition is that containing dimethyl isosorbide (DMI) added or not of isosorbide dinitrate (ISDN) as ignition improver. The dianhydrohexitols compounds form compositions with at least one of the components selected among petroleum-derived diesel fuel, biodiesel, ethanol and water. The mixture of DMI and ISDN has excellent cetane number (IQT). Still, the oxygenated nature of the dianhydrohexitols and related compounds of the fuel compositions inhibits soot formation upon burning of the said Diesel cycle fuel compositions.
Owner:PETROLEO BRASILEIRO SA (PETROBRAS)
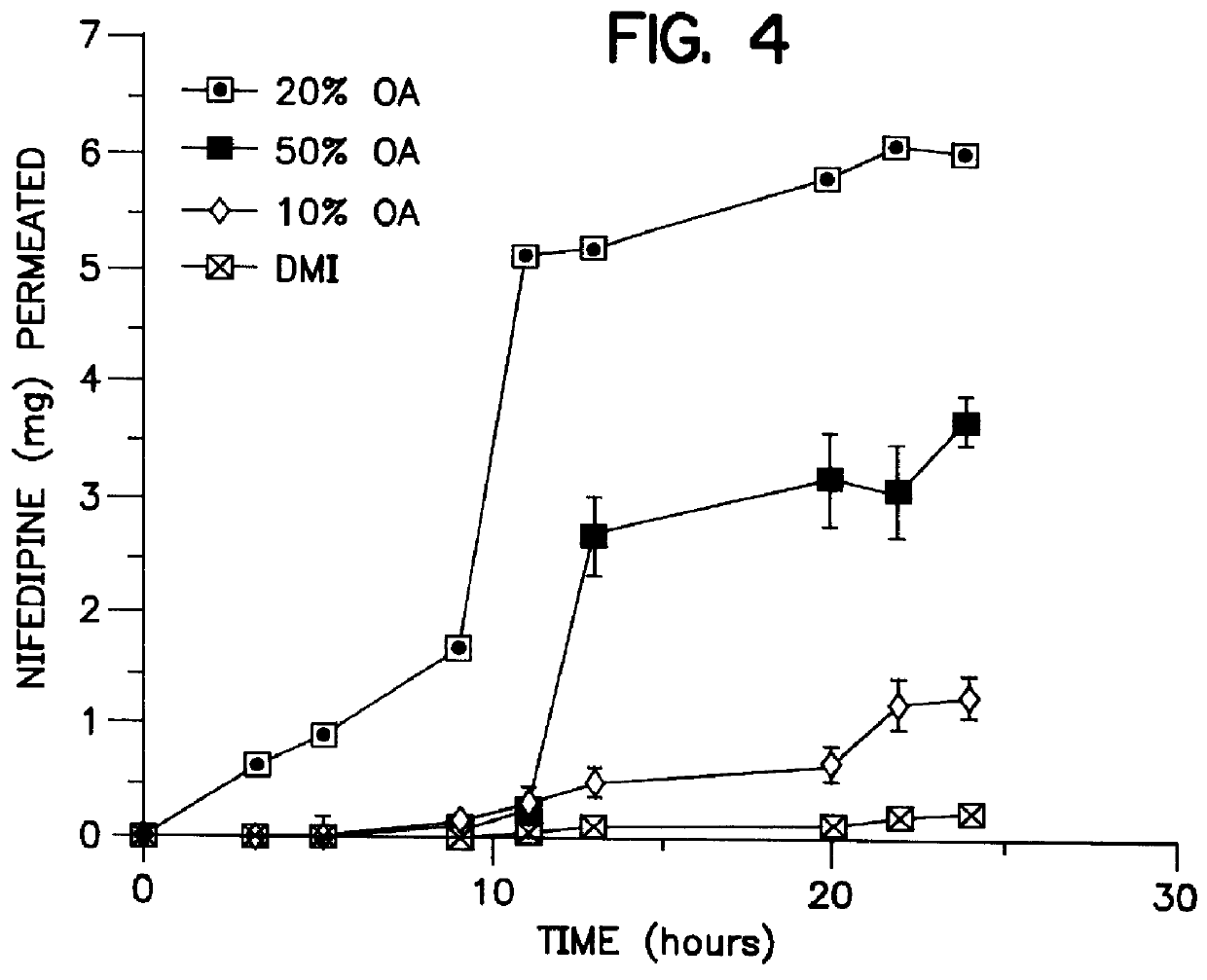
Transdermal delivery of calcium channel blockers, such as nifedipine
InactiveUS6106856AImprove pass rateLittle influenceAdhesive dressingsDrug compositionsDihydropyridinePolypropylene glycol
A transdermal formulation of dihydropyridine calcium antagonists and specifically nifedipine, nimodipine and nitrendipine. The calcium antagonists are dispersed in a mixed liquid. The mixed liquid comprises varying mole fractions of cis-oleic acid and dimethylisosorbide dispersed in a polypropylene glycol base.
Owner:THE BOARD OF GOVERNORS FOR HIGHER EDUCATION STATE OF RHODE ISLAND AND
Lipstick with lip enhancement effect and preparation method thereof
ActiveCN109125188AImprove moisturizingPromote repairCosmetic preparationsMake-upCapric triglyceridesPreservative
The invention discloses a lipstick with a lip enhancement effect and a preparation method thereof, and belongs to the technical field of cosmetics. The lipstick comprises a thickener, a film forming agent, a coloring agent, a filling agent, an emollient, a preservative and a functional additive; the functional additive is composed of a functional additive I, a functional additive II and a functional additive III; the functional additive I is a mixture of jojoba seed oil and a pomegranate flower extract; the functional additive II is a mixture of caprylic / capric triglyceride, dimethyl isosorbide, a sesame seed extract and tocopherol; the functional additive III is a mixture of bisabolol and a ginger root extract; the lipstick contains 0.3 to 5 mass percent of the functional additive I, 1 to5 mass percent of the functional additive II and 0.1 to 0.5 percent of the functional additive III. In addition, the preparation process of the lipstick is simple, and the prepared lipstick has a good lip enhancement effect.
Owner:I&B GUANGZHOU BIOLOGICAL TECH CO LTD
Method for preparing dialkyloxydianhyrohexitol by etherification of dianhydrohexitol using a light alcohol, in the presence of an acidic catalyst
ActiveUS9321783B2Improved and simple and economical processEasy to industrializeOrganic chemistryDimethyl isosorbideMethyl group
Owner:CENT NAT DE LA RECHERCHE SCI +1
Cosmetic compositions comprising acetyl trifluoromethylphenyl valylglycine
ActiveUS10449133B1Difficult to solubilizeReduce the amount of solutionCosmetic preparationsToilet preparationsLauryl sarcosinateDimethyl isosorbide
The present disclosure relates to cosmetic compositions including surprisingly high amounts of trifluoromethylphenyl valylglycine, which provides a variety of beneficial properties to skin. The cosmetic compositions include: (a) about 1 wt. % to about 25 wt. % of acetyl trifluoromethylphenyl valylglycine; (b) about 2 to about 30 wt. % of two or more solubilizing solvents selected from ethoxydiglycol, dimethyl isosorbide, triethyl citrate, isopropyl lauryl sarcosinate, and propylene glycol; and (c) optionally, water; wherein all weight percentages are based on the total weight of the cosmetic composition.
Owner:LOREAL SA
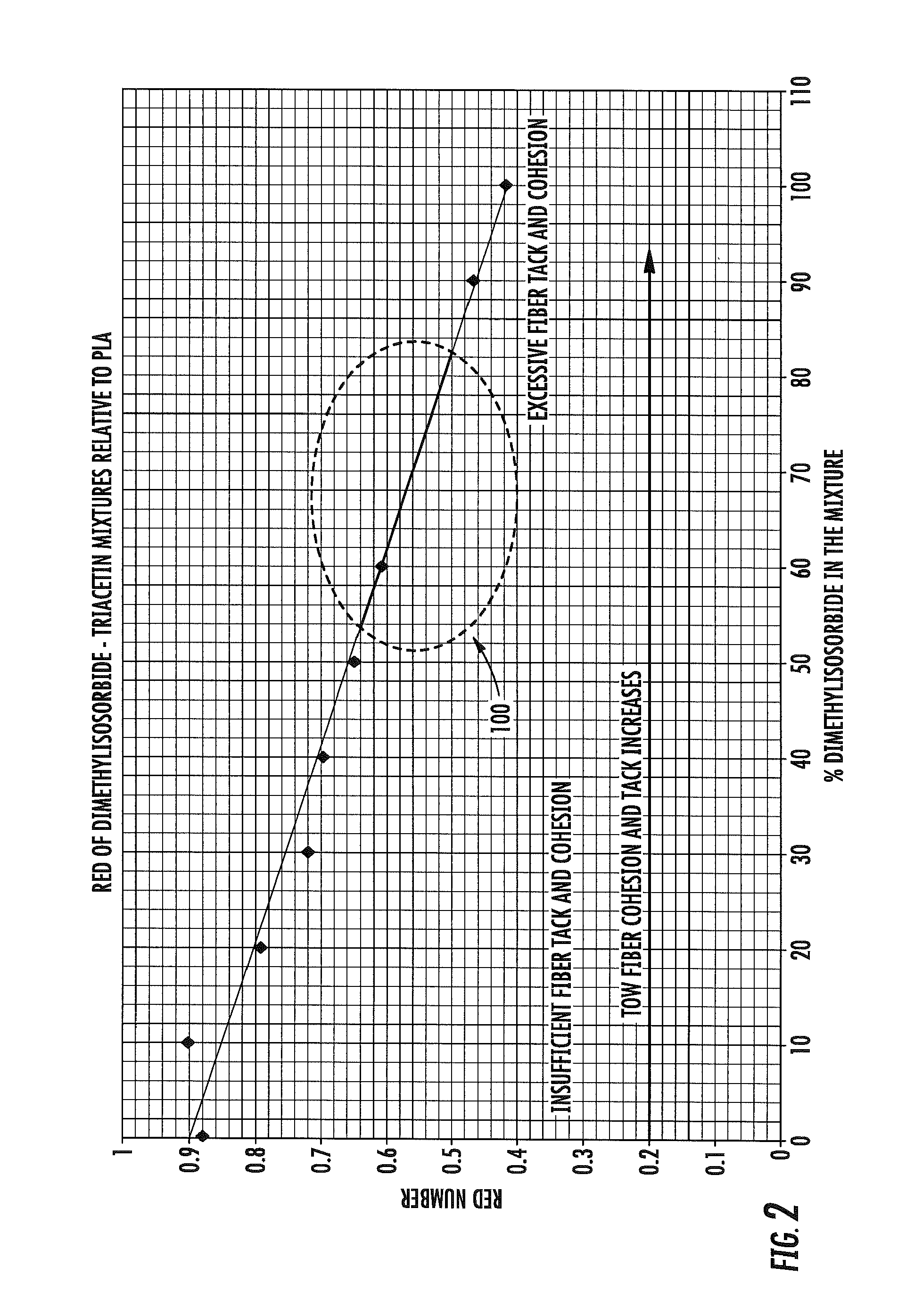
Plasticizer composition for degradable polyester filter tow
A filter material adapted for use as a filter element of a smoking article is provided, the filter material being in the form of a fibrous tow that includes a plurality of filaments of a degradable polyester and a plasticizer composition applied thereto, the plasticizer composition and the degradable polyester having a Relative Energy Difference calculated using Hansen Solubility Parameters of less than about 1.3. Exemplary degradable polyesters include polyglycolic acid, polylactic acid, polyhydroxyalkanoates, polycaprolactone, polybutylene succinate adipate and copolymers or blends thereof. Exemplary plasticizer compositions include one or more of dimethylisosorbide, propylene carbonate, methylbenzyl alcohol, glycerol carbonate acetate, glycerol carbonate ethyl ether, and mixtures thereof, optionally in combination with triacetin. Filter elements and smoking articles, such as cigarettes, that contain the filter material are also provided.
Owner:R J REYNOLDS TOBACCO COMPANY
Clobetasol spray
InactiveUS20080102038A1Reduce stimulationReduce solubilityBiocideOrganic active ingredientsPreservativeDimethyl isosorbide
A spray foaming dosage form comprising clobetasol propionate, dimethyl isosorbide, propylene glycol, polysorbate, sodium dodecyl sulphate, buffer, optional preservative, optional further excipients, and water.
Owner:NUPHARM LAB
Composition and method of treating skin conditions
ActiveUS9399009B1Rid of other cellular debrisEffective keratolytic effectCosmetic preparationsBiocideGlycolic acidSalicylic acid
The present invention is a composition and method of treatment for skin disorders, conditions, and severe skin dryness in general through topical systematic and periodic application of a formulation that generally may include salicylic acid, glycolic acid, urea, dimethyl isosorbide, ethoxydiglycol, barrier repair agents, anti-irritants, humectants, and also has an acidic pH formulation.
Owner:CLARK PHARMA LLC
Solubilized magnolol analogs
InactiveCN104023699ASoap detergents with organic compounding agentsCosmetic preparationsPersonal careDimethyl isosorbide
A composition comprising a solubilized magnolol analog comprising at least one magnolol analog chosen from propyl magnolol, isopropyl magnolol, butyl magnolol, and isobutyl magnolol, and dimethyl isosorbide. These solubilized analogs are useful in personal care, oral care, and home care compositions to provide anti-bacterial activity and reducing the expression of pro-inflammatory mediators.
Owner:COLGATE PALMOLIVE CO
Solubilized magnolol analogs
A composition comprising a solubilized magnolol analog comprising at least one magnolol analog chosen from propyl magnolol, isopropyl magnolol, butyl magnolol, and isobutyl magnolol, and dimethyl isosorbide. These solubilized analogs are useful in personal care, oral care, and home care compositions to provide anti-bacterial activity and reducing the expression of pro-inflammatory mediators.
Owner:COLGATE PALMOLIVE CO
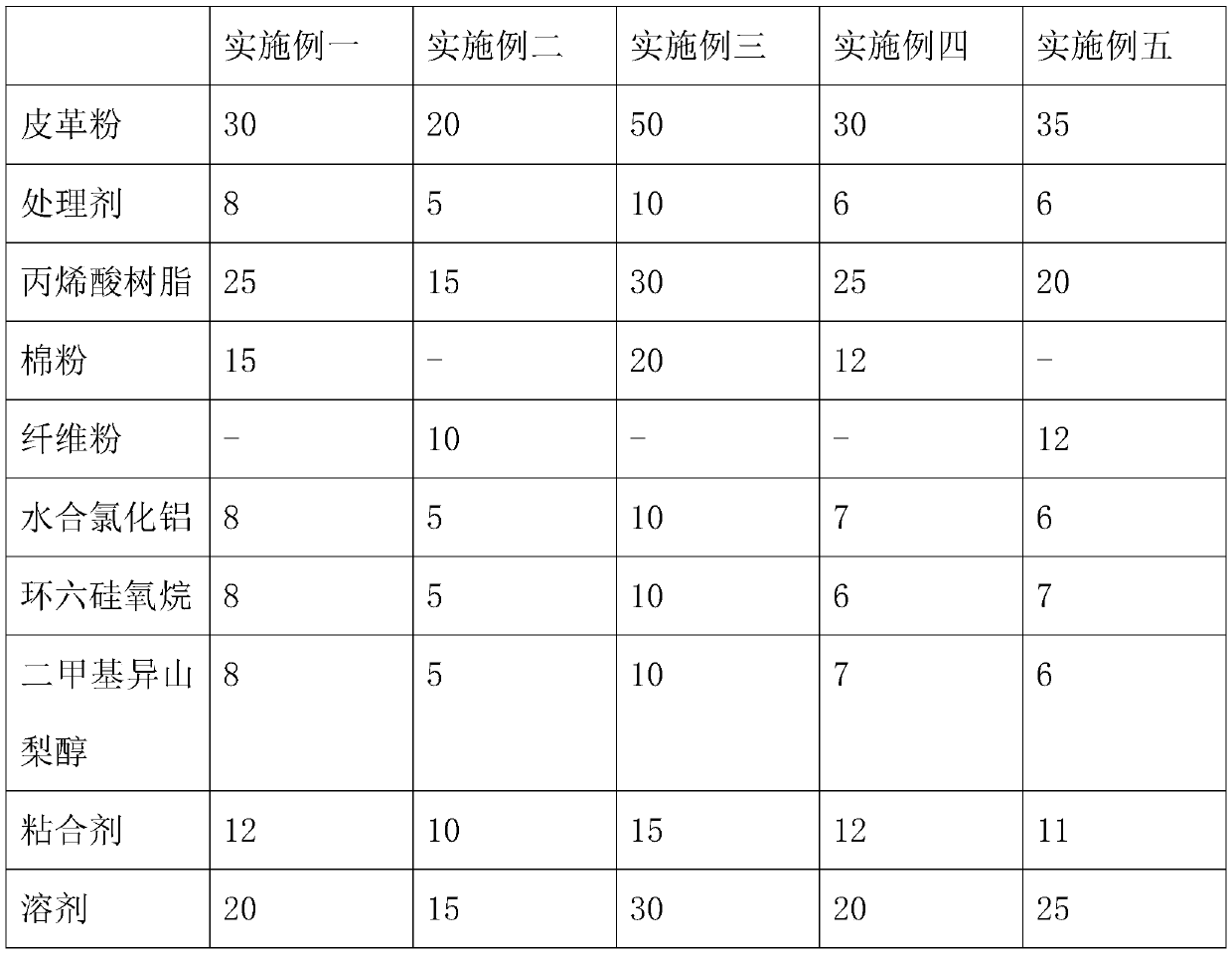
Sweat-resistant leather and preparation method thereof
The invention discloses sweat-resistant leather and a preparation method thereof. The sweat-resistant leather comprises a leather base layer, a bonding layer, a sweat-resistant layer and a silica gelsurface layer which are connected in sequence, and the sweat-resistant layer is prepared from the following components: leather powder, a treating agent, acrylic resin, cotton powder or fiber powder,hydrated aluminum chloride, cyclohexasiloxane, dimethyl isosorbide, an adhesive and a solvent. The preparation method of the sweat-resistant leather comprises the following steps: adding the acrylic resin and the treating agent into the leather powder, performing uniform mixing under stirring, and allowing the mixed material to stand for a period of time to obtain a first intermediate product; adding the cotton powder or the fiber powder, the hydrated aluminum chloride, the cyclohexasiloxane, the dimethyl isosorbide, the adhesive and the solvent into the first intermediate product, performinguniform stirring, and performing drying for a period of time to obtain a second intermediate product; pressing the second intermediate product to obtain the sweat-resistant layer; bonding the sweat-resistant layer to one surface of the leather layer through an adhesive; and coating the surface of the sweat-resistant layer by silica gel, and performing drying to obtain the silica gel layer. The sweat-resistant leather provided by the invention can prevent stink caused by sweat.
Owner:漳州香洲皮革有限公司
Skin-refreshing essence set
InactiveCN111110597APromote absorptionIncrease moisture contentCosmetic preparationsToilet preparationsGlycerolDimethyl isosorbide
The invention provides a skin-refreshing essence set. The skin-refreshing essence set comprises an essence emulsion, and an essence No.1, an essence No.2, an essence No.3 and an essence No.4 which areused in sequence. The essence emulsion comprises the following components: water, glycerol, 1,3-propylene glycol, dimethyl isosorbide, dipropylene glycol, inositol, isoprene glycol, hydroxydecyl ubiquinone, cetyl ethyl hexanoate and the like. The essences comprise the following components: water, 1,3-propylene glycol, dipropylene glycol, lactic acid, 1,2-pentanediol, chondrus crispus, butanediol,hydrolyzed royal jelly protein and the like. The skin-refreshing essence set disclosed by the invention contains the hydroxydecyl ubiquinone (idebenone) and various beautifying and skin-refreshing components, can effectively soften cuticle, improve skin absorption capacity and water content, supplement skin nutrients, strengthen skin protection capacity, endow the skin with elastic and compact feeling, and make the skin smooth, fine, fresh, alive and glossy; and the essence set can effectively repair skin problems, improve various skin troubles such as coarse pores, rough pores and dark skin,and create young and plump skin.
Owner:韩妍蜜化妆品(浙江)有限公司
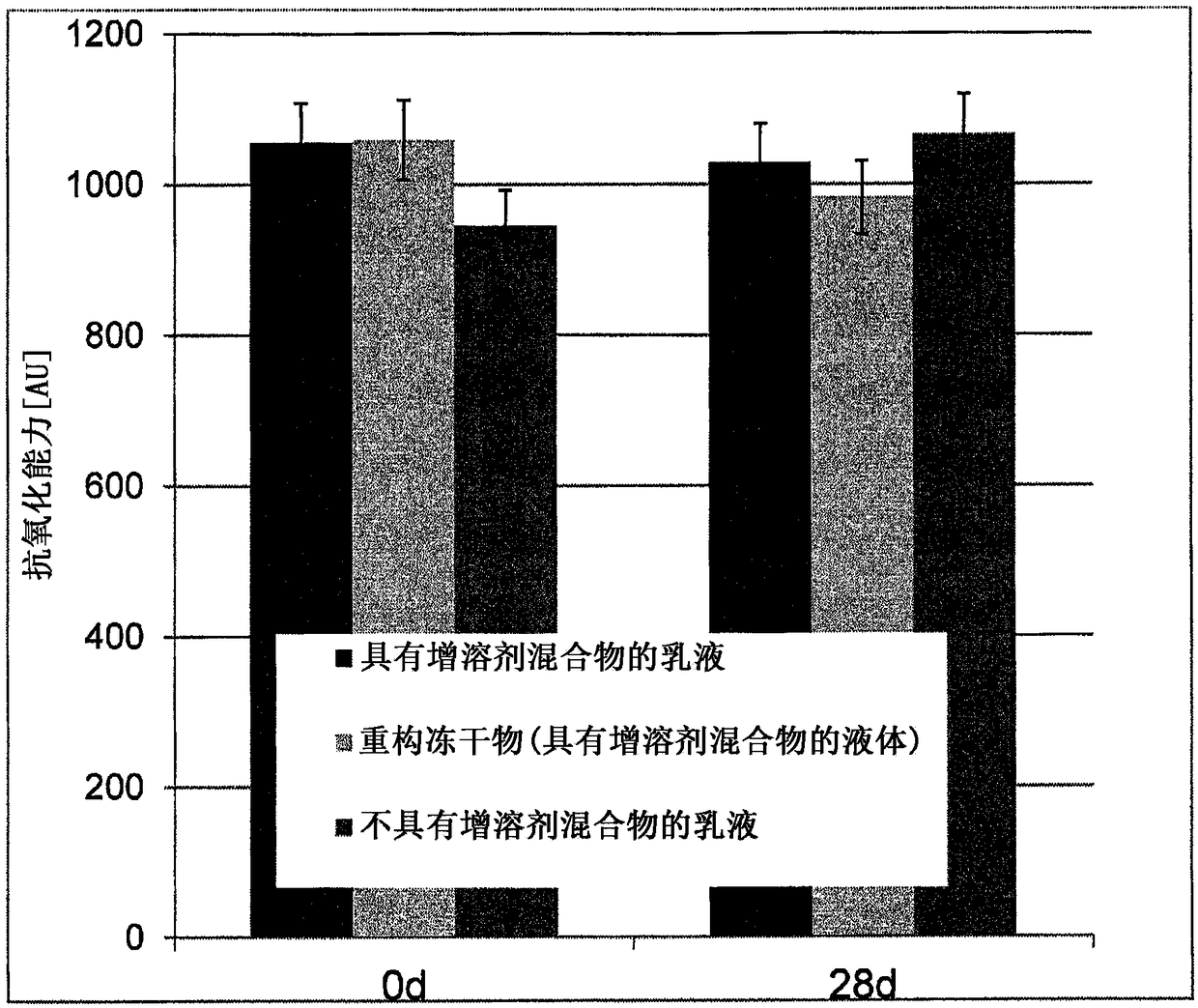
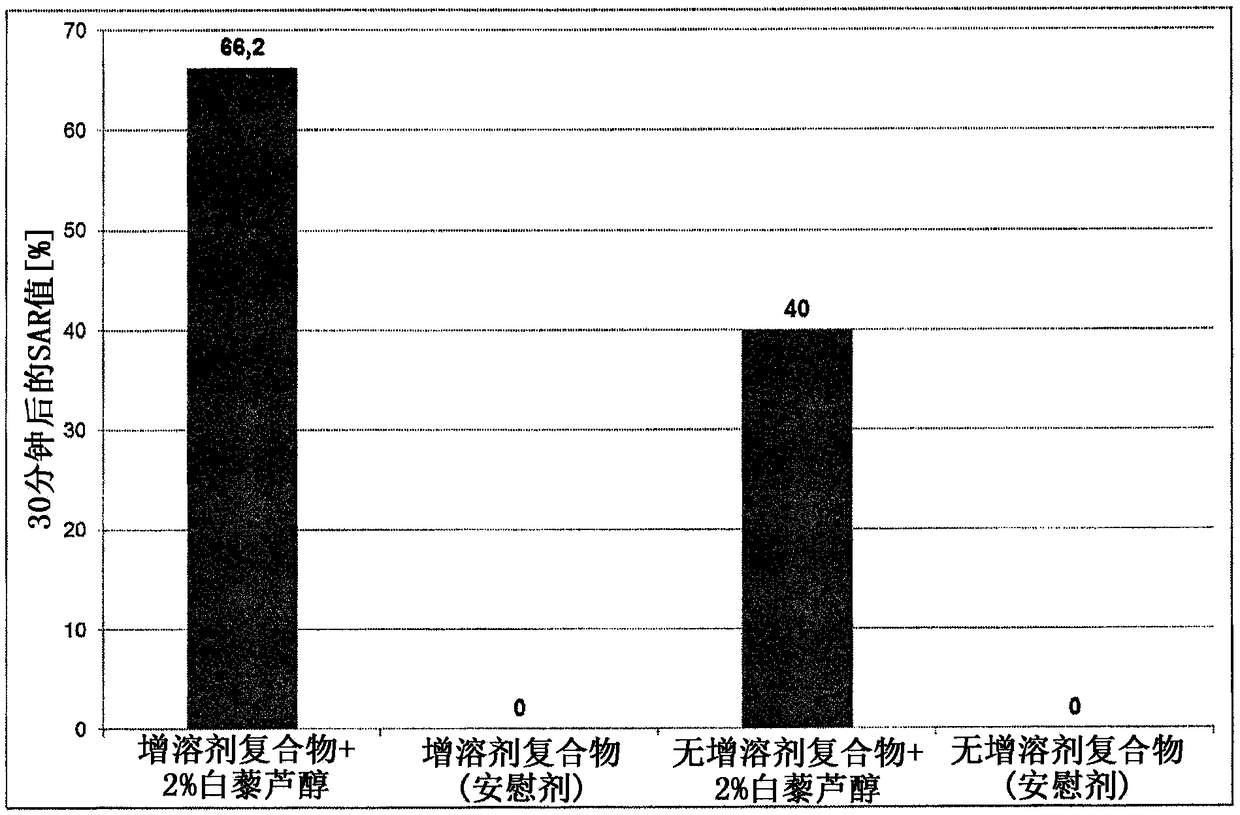
Composition for topical application comprising dimethyl isosorbide, a polyol, and a phenolic or polyphenolic antioxidant
ActiveCN108135816AOptimizing Osmotic KineticsImprove solubilityCosmetic preparationsHydroxy compound active ingredientsPolyolAntioxidant
The invention relates to a composition suitable for topical application comprising water, dimethyl isosorbide, a polyol and a phenolic or polyphenolic antioxidant. A method for producing the composition is also part of the invention, as is a kit for making the composition. Optionally, the phenolic or polyphenolic antioxidant is provided in the form of a lyophilisate for the method and the kit. Theuse of the composition for a treatment by topical application is also within the scope of the invention.
Owner:MEDSKIN SOLUTIONS DR SUWELACK AG
Method for preparing dialkyloxydianhyrohexitol by etherification of dianhydrohexitol using a light alcohol, in the presence of an acidic catalyst
ActiveUS20150203507A1Reduce usageImproved and simple and economical processOrganic chemistryDimethyl isosorbideMethyl group
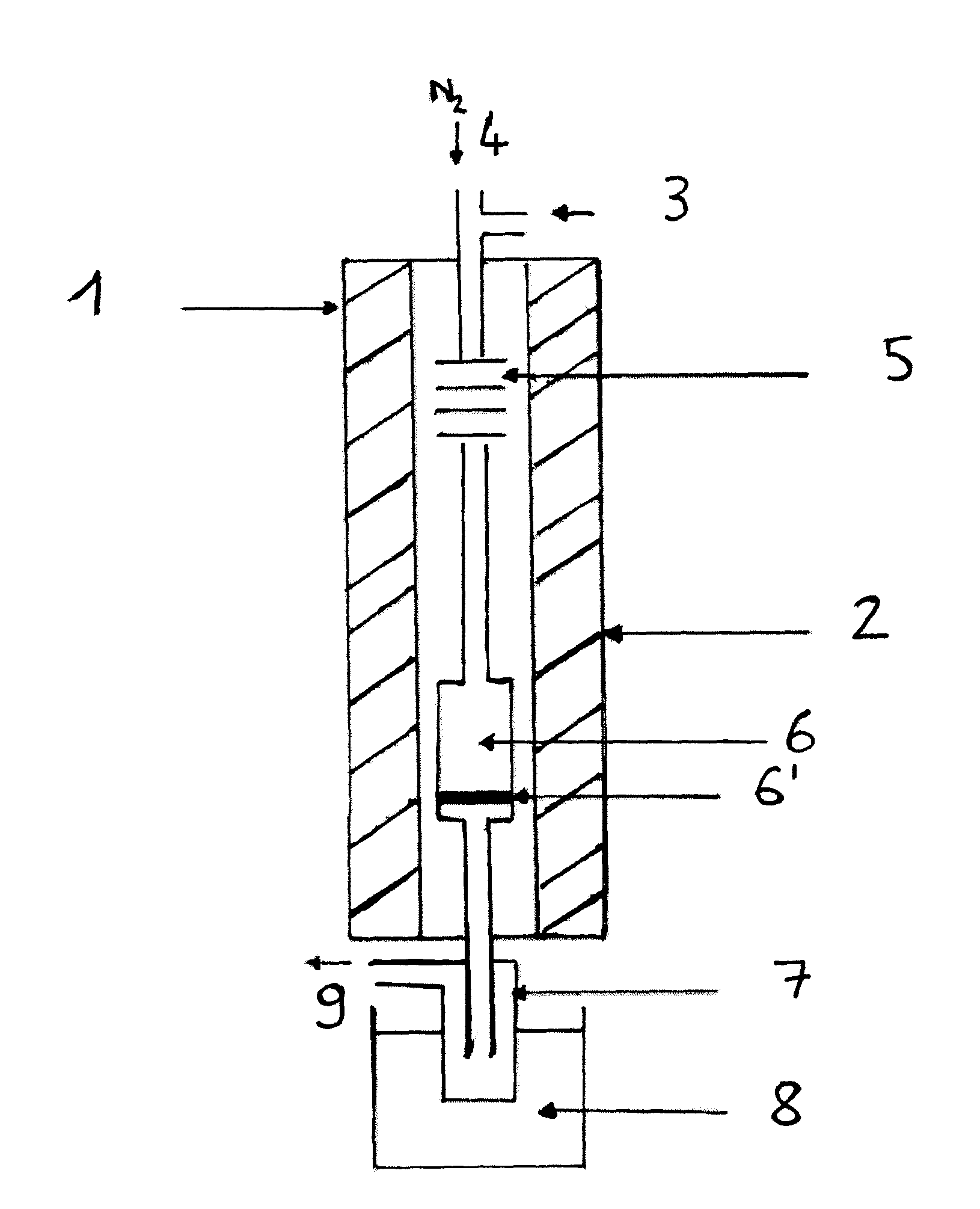
A method for preparing a dialkyloxydianhyrohexitol (dimethylisosorbide) composition by etherification of dianhydrohexitol (isosorbide). The aim is to achieve a “clean” method that avoids the use of a methylation agent such as dimethyl sulfate or methyl chloride, which generates stoechiometric quantities of salts, or expensive dialkyl-carbonates, wherein only one of the two methyl groups participates in the preparation of mixed isosorbide ethers. The method involves using at least one O-alkylation agent and a catalyst including an acid or an acid salt, preferably a catalyst having Lewis or BrØnsted acid properties. A device for carrying out the method wherein the device includes a vaporization oven and a reaction oven is also described.
Owner:CENT NAT DE LA RECHERCHE SCI +1
Plasticizer composition for degradable polyester filter tow
A filter material adapted for use as a filter element of a smoking article is provided, the filter material being in the form of a fibrous tow that includes a plurality of filaments of a degradable polyester and a plasticizer composition applied thereto, the plasticizer composition and the degradable polyester having a Relative Energy Difference calculated using Hansen Solubility Parameters of less than about 1.3. Exemplary degradable polyesters include polyglycolic acid, polylactic acid, polyhydroxyalkanoates, polycaprolactone, polybutylene succinate adipate and copolymers or blends thereof. Exemplary plasticizer compositions include one or more of dimethylisosorbide, propylene carbonate, methylbenzyl alcohol, glycerol carbonate acetate, glycerol carbonate ethyl ether, and mixtures thereof, optionally in combination with triacetin. Filter elements and smoking articles, such as cigarettes, that contain the filter material are also provided.
Owner:R J REYNOLDS TOBACCO COMPANY
Usnic acid topical formulation
InactiveUS20140057976A1Reduce deliveryIncrease delivery rateSalicyclic acid active ingredientsBiocideSalicylic acid esterSkin treatments
Topical skin treatment formulation containing usnic acid or an usnate salt, dissolved in a solvent system comprising (i) dimethyl isosorbide; (ii) a C1 to C9 alkyl salicylate; and (iii) a glyceryl fatty acid ester. The solvent system may also comprise an alcohol, a polyoxyalkylene-based solvent, and / or a C1 to C4 alkyl glucose ester. The formulation may be used in the treatment of microbial conditions, in particular acne. The solvent system assists in the effective dissolution of the usnic acid or usnate and in targeting its delivery to relevant sites on the skin.
Owner:INNOVENN LTD
Quick-drying water-based anti-rust agent and preparation method thereof
InactiveCN104294287AImprove corrosion resistanceImprove high temperature resistanceWater basedTectorial membrane
The invention discloses a quick-drying water-based anti-rust agent which is characterized by being prepared from the following components in parts by weight: 3-5 parts of silicone-acrylic emulsion, 3-5 parts of vinyl acetate-acrylic emulsion, 1-2 parts of xanthan gum, 1-2 parts of dimethyl isosorbide, 1-2 parts of sulfonated caster oil, 0.3-0.5 part of succinic acid, 0.5-1 part of sodium dodecyl benzene sulfonate, 0.2-0.4 part of ethylene glycol monobutyl ether, 1-2 parts of petroleum sodium sulfonate, 3-5 parts of hydroxypropyl methyl cellulose, 1-2 parts of sodium silicate, 0.5-1 part of sodium polyacrylate, 4-6 parts of a modifying additive and 30-40 parts of water. The anti-rust agent can quickly form a hard and tough protection film on the metal surface, and is anti-fouling, anti-static, good in corrosion resistance and high temperature resistance and long in protection time.
Owner:HEFEI DAAN PRINTING
Composition for treating fungal infections in nails
ActiveUS10251858B1Cosmetic preparationsHydroxy compound active ingredientsAlcoholDimethyl isosorbide
A pharmaceutically active composition suitable for topical application to the nails contains undecylenic acid and tolnaftate. The composition also employs a urea-based component, a monohydric alcohol such as isopropanol, a diol such as propylene glycol, dimethyl isosorbide, and a carboxylic acid other than undecylenic acid, preferably lactic acid.
Owner:MARLINZ PHARMA LLC
Salicylic acid topical formulation
InactiveUS20140107081A1Increase speed of onsetImprove durabilityBiocideSalicyclic acid active ingredientsSalicylic acid esterSkin treatments
Owner:INNOVENN LTD
Compositions And Methods For The Preparation And Administration Of Poorly Water Soluble Drugs
InactiveUS20080171687A1BiocidePharmaceutical delivery mechanismDimethyl isosorbideWater soluble drug
Sterile, stable pharmaceutical formulations of poorly water-soluble drugs dissolved in dimethyl isosorbide, a water-miscible solvent, as well as methods for their preparation and administration.
Owner:AMERICAN BIOSCIENCE INC +1
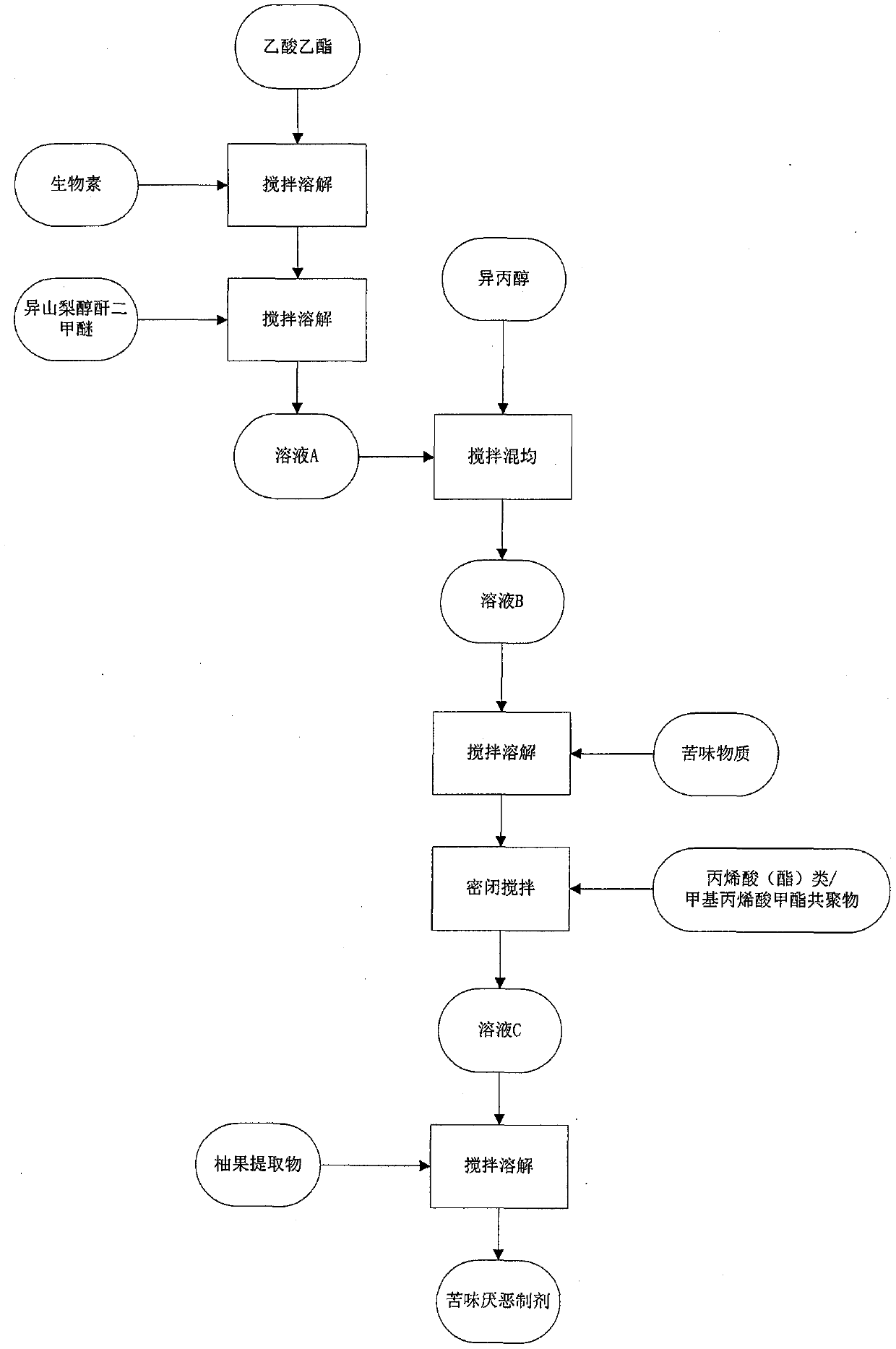
Bitterness aversion preparation and preparation method thereof, and anti-bite pen
InactiveCN110038010APromote infiltrationStrong bitternessOrganic active ingredientsNervous disorderAcetic acidEthyl ester
The present invention discloses a bitterness aversion preparation. The bitterness aversion preparation comprises 85-90 parts of a carrier solvent, 3-4 parts of a bitter substance and 8-12 parts of anadditive. A preparation method of the bitterness aversion preparation comprises the following steps: (1) dissolving dimethyl isosorbide in ethyl acetate in proportion and conducting even stirring to obtain a solution A; (2) adding isopropanol to the solution A and conducting stirring and mixing to obtain a solution B; (3) adding the bitter substance into the solution B, conducting stirring until the bitter substance is completely dissolved, then adding an acrylic acid (acrylate) / methyl methacrylate copolymer, and conducting sealed stirring to obtain a solution C; and (4) adding a pomelo fruitextract while stirring to the solution C, and conducting even mixing to obtain the bitterness aversion preparation. An anti-bite pen is provided with a water absorbent cotton pen core and the bitterness aversion preparation is filled into the water absorbent cotton pen core. The bitterness aversion preparation, the preparation method thereof, and the anti-bite pen disclosed by the invention havethe following beneficial effects: 1, a bitterness effect is good; and 2, cross-contamination is not caused.
Owner:瑞雅科医药(北京)有限公司
Diesel cycle fuel compositions containing dianhydrohexitols and related products
Diesel cycle fuel compositions are described containing at least one dianhydrohexitol compound according to the general formula 2 and / or itsderived hydrocarbyl ethers or nitric ethers compounds, where the R′ and R″ substituents are both H or one or both of R′ and R″ is alkyl, cycloalkyl or phenyl, or one or both are —NO2. A preferred fuel composition is that containing dimethyl isosorbide (DMI) added or not of isosorbide dinitrate (ISDN) as ignition improver. The dianhydrohexitols compounds form compositions with at least one of the components selected among petroleum-derived diesel fuel, biodiesel, ethanol and water. The mixture of DMI and ISDN has excellent cetane number (IQT). Still, the oxygenated nature of the dianhydrohexitols and related compounds of the fuel compositions inhibits soot formation upon burning of the said Diesel cycle fuel compositions.
Owner:PETROLEO BRASILEIRO SA (PETROBRAS)
Plasticizer composition for degradable polyester filter tow
A filter material adapted for use as a filter element of a smoking article is provided, the filter material being in the form of a fibrous tow that includes a plurality of filaments of a degradable polyester and a plasticizer composition applied thereto, the plasticizer composition and the degradable polyester having a Relative Energy Difference calculated using Hansen Solubility Parameters of less than about 1.3. Exemplary degradable polyesters include polyglycolic acid, polylactic acid, polyhydroxyalkanoates, polycaprolactone, polybutylene succinate adipate and copolymers or blends thereof. Exemplary plasticizer compositions include one or more of dimethylisosorbide, propylene carbonate, methylbenzyl alcohol, glycerol carbonate acetate, glycerol carbonate ethyl ether, and mixtures thereof, optionally in combination with triacetin. Filter elements and smoking articles, such as cigarettes, that contain the filter material are also provided.
Owner:R J REYNOLDS TOBACCO COMPANY
Method for treating fungal infections in nails
ActiveUS10206962B1Poor adhesionCosmetic preparationsHydroxy compound active ingredientsDiseaseDimethyl isosorbide
A method of treating nail diseases by topically applying to the nails a composition containing undecylenic acid and tolnaftate, a urea-based component, a monohydric alcohol such as isopropanol, a diol such as propylene glycol, dimethyl isosorbide, and a carboxylic acid, preferably lactic acid.
Owner:MARLINZ PHARMA LLC
Acne removing, skin tendering and whitening essence and preparation method thereof
InactiveCN112120959AImprove smoothnessIncrease moist feelingCosmetic preparationsAntipyreticGlycerolDimethyl isosorbide
The invention discloses acne removing, skin tendering and whitening essence and a preparation method thereof, and relates to the technical field of cosmetics. The essence comprises the following components in percentage by weight of a phase A including 0.05-0.1% of EDTA disodium, 0.15-0.4% of hydrolyzed sclerotium gum, 0.15-0.25% of xanthan gum, 0.2-1% of allantoin, 0.1-1% of methylparaben, 5-10%of glycerol, 5-8% of butanediol and 0.005-0.1% of polyquaternium-73; a phase B including 1-3% of carnosine, 2-4% of nicotinamide, 1-5% of acetylchitosamine, 2-6% of alpha-arbutin and 0.04-0.2% of sodium hyaluronate; and a phase C including 1-4% of dimethyl isosorbide, 0.3-1% of phenoxyethanol, 0.005-1% of essence and 82-100% of water. According to the acne removing, skin tendering and whitening essence and the preparation method thereof, acne-removing and skin-whitening essence cream is prepared through reasonable use and matching of various components and a special process; the synergistic effect among the components is very outstanding; the acne-removing and skin-whitening essence cream not only can efficiently inhibit formation of melanin to achieve an excellent whitening effect, but also has the prominent blackhead-removing, acne-removing and anti-inflammatory effects to achieve the skin-whitening and acne-removing effects of comprehensive conditioning; and the essence has advantages of simple preparation process, easiness in control and easiness in operation.
Owner:清远市华宝生物科技有限公司
Compositions comprising dimethylisosorbide, polyols and phenolic or polyphenolic antioxidants for topical application
ActiveCN108135816BOptimizing Osmotic KineticsImprove solubilityCosmetic preparationsHydroxy compound active ingredientsPolyolAlcohol
The present invention relates to a composition suitable for topical application comprising water, dimethylisosorbide, a polyol and a phenolic or polyphenolic antioxidant. The methods for producing the compositions of the invention are also part of the invention, as are the kits for preparing the compositions. Optionally, for the methods and kits, the phenolic or polyphenolic antioxidants are provided in the form of a lyophilizate. The use of a composition according to the invention for therapy by topical application is also within the scope of the invention.
Owner:MEDSKIN SOLUTIONS DR SUWELACK AG
Uracil dermatological formulations
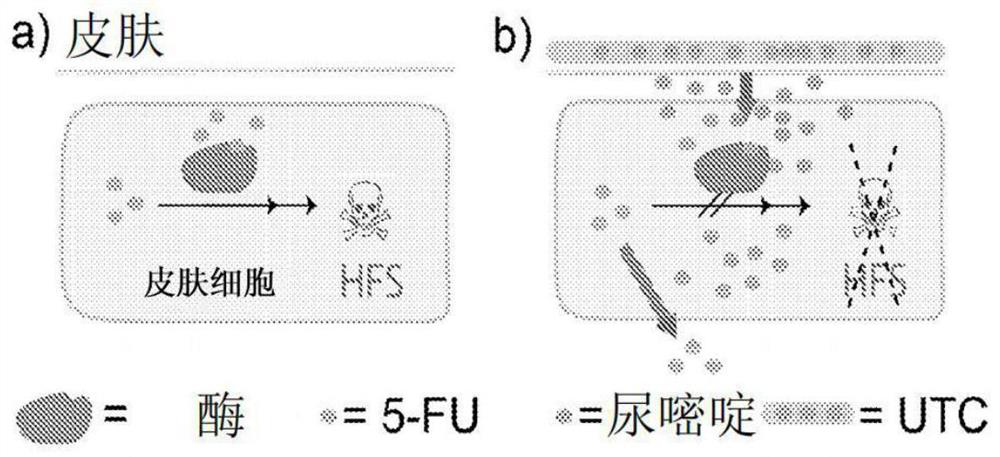
PendingCN114423431ACapable of mass productionOrganic active ingredientsOrganic chemistryTG - TriglyceridePyrrolidinones
The present invention provides a topical pharmaceutical formulation comprising uracil and a penetration enhancer and a method of administering the same. Also provided is a method of treating or preventing a skin disease associated with the administration of 5-fluorouracil or a precursor or prodrug thereof, such as capecitabine. The penetration enhancer is selected from the group consisting of dimethyl isosorbide, isopropyl myristate, isopropyl palmitate, octyldodecanol, oleic acid, oleyl alcohol, polyoxyglyceride, pyrrolidone, thymol, trioctyl extract, triolein, myristic acid, medium chain triglyceride, linoleic acid, lauric acid, sugar furfuryl alcohol, glyceryl monooleate, ethyl oleate and dimethyl sulfoxide; and dibutyl sebacate and mixtures thereof.
Owner:纳诺麦缇科斯有限责任公司(经营别称为PHD生物科学
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com