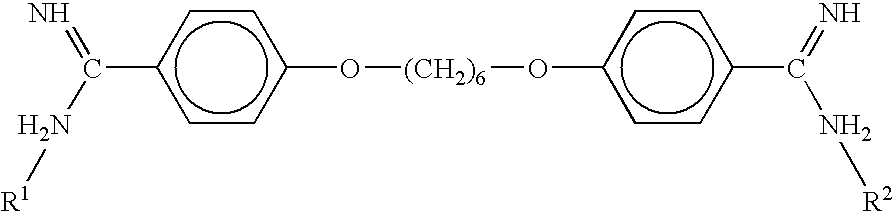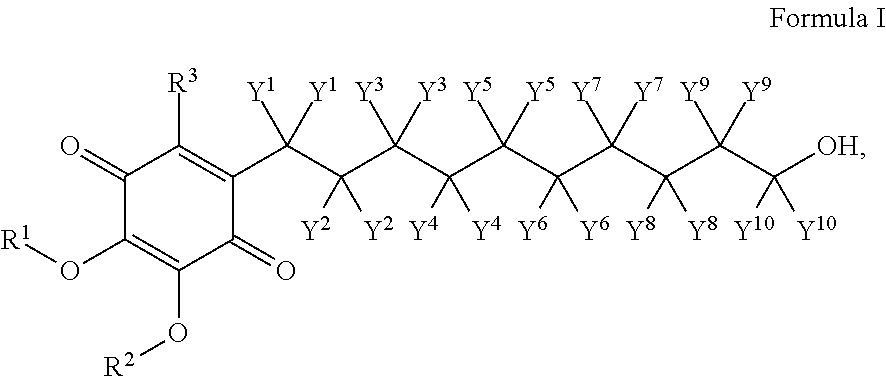Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
54 results about "Idebenone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
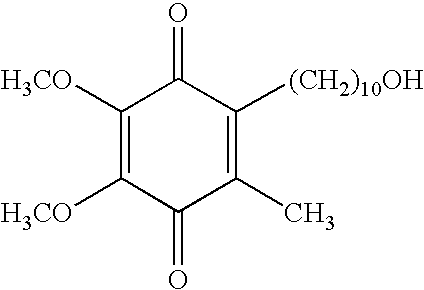
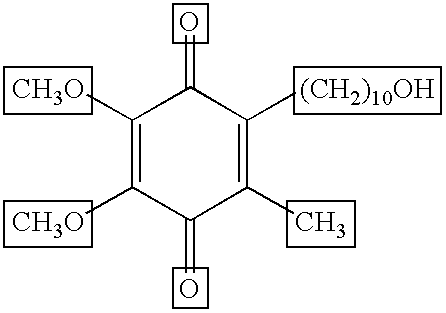
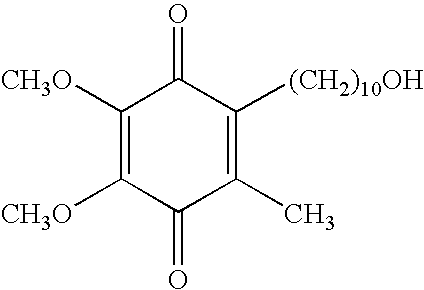
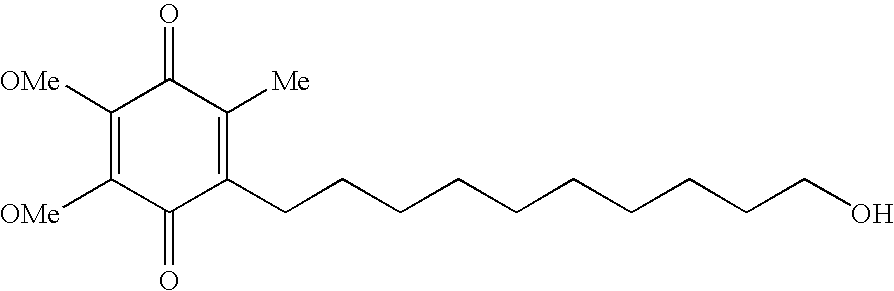
Idebenone (pronounced eye-deb-eh-known, trade names Catena, Raxone, Sovrima, among others) is a drug that was initially developed by Takeda Pharmaceutical Company for the treatment of Alzheimer's disease and other cognitive defects. This has been met with limited success. The Swiss company Santhera Pharmaceuticals has started to investigate it for the treatment of neuromuscular diseases. In 2010, early clinical trials for the treatment of Friedreich's ataxia and Duchenne muscular dystrophy have been completed. As of December 2013 the drug is not approved for these indications in North America or Europe. It is approved by the European Medicines Agency (EMA) for use in Leber's hereditary optic neuropathy (LHON) and was designated an orphan drug in 2007.
Skin care compositions containing idebenone
InactiveUS20060275237A1Effective supervisionMultiple skin care benefitBiocideCosmetic preparationsIdebenoneDermatology
Owner:THE PROCTER & GAMBLE COMPANY
Method and preparation for reducing skin hyperpigmentation
InactiveUS20050175559A1Reduce generationReduce hyperpigmentationBiocideCosmetic preparationsIdebenoneTopical preparation
A method for reducing an occurrence of hyperpigmentation in human skin includes applying to the skin a topical preparation including an amount of an agent effective to reduce an occurrence of hyperpigmentation in human skin. The agent includes idebenone or a derivative of idebenone.
Owner:WIELAND EBERHARD +2
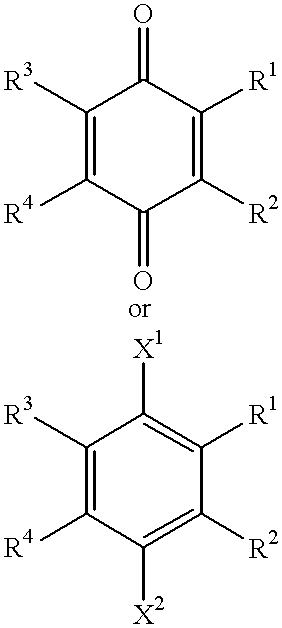
Use of idebenone and analogues against beta amyloid induced cytotoxicity
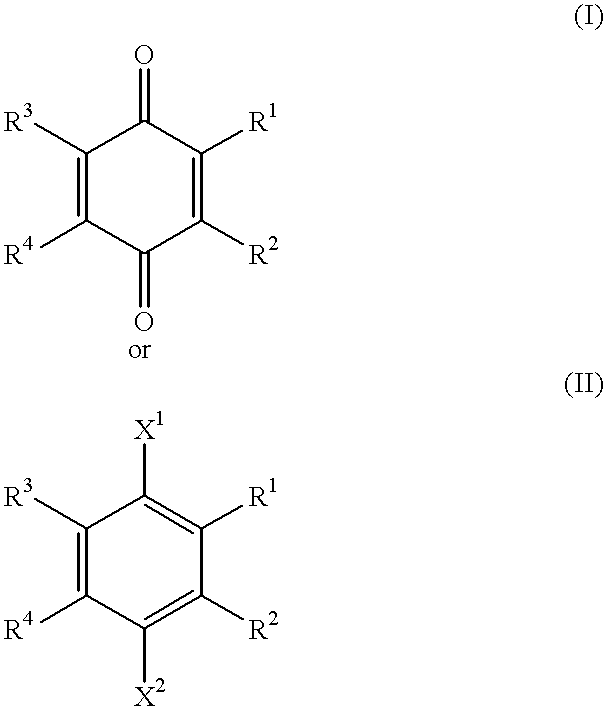
A compound of the formula:wherein R1 represents a lower alkyl; R2 represents H, an optionally substituted alkyl or an optionally substituted alkenyl; R3 and R4 each represents an optionally substituted lower alkyl or a lower alkoxy, or R3 and R4 form, taken together, an optionally substituted butadienylene; and X1 and X2 each represents an optionally esterified or etherified hydroxy, or a salt thereof is useful for protecting cells from the cytotoxicity of beta-amyloid protein.
Owner:TAKEDA PHARMA CO LTD
Method and preparation for reducing irritation and/or inflammatory reaction in human skin
InactiveUS20050197407A1Reducing of inflammatory reactionLess irritatingBiocideCosmetic preparationsHuman skinIdebenone
A method for reducing an occurrence of irritation and / or inflammatory reaction in human skin includes applying a topical preparation to the skin. The preparation includes an amount of an agent effective to reduce an occurrence of irritation and / or inflammatory reaction in human skin. The agent includes idebenone or a derivative of idebenone.
Owner:WIELAND EBERHARD +2
Pharmaceutical composition for parenteral administration of idebenone
InactiveUS20100130619A1Formula stableHigh shear mixingBiocideNervous disorderParenteral nutritionIdebenone
Owner:ALPHARX
Skin care compositions containing idebenone
InactiveUS20060275228A1Enhanced skin care benefitImprove skin feelBiocideCosmetic preparationsIdebenoneDermatology
Owner:THE PROCTER & GAMBLE COMPANY
Regulation of mammalian keratinous tissue using personal care compositions comprising cetyl pyridinium chloride
InactiveUS20070020221A1Tactile feel is improvedImprove efficiencyCosmetic preparationsCationic surface-active compoundsDehydroacetic acidRetinoid
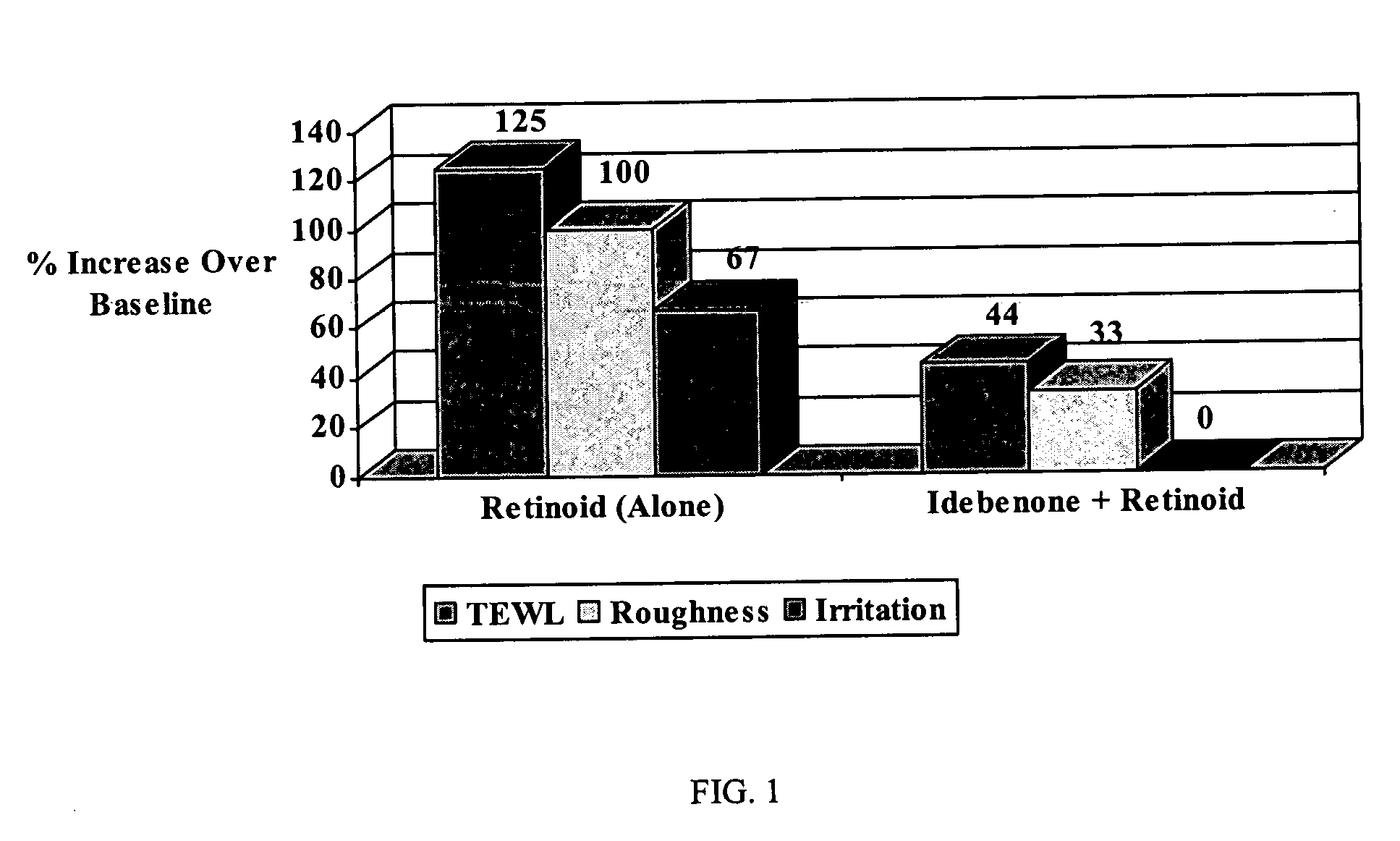
Personal care composition including a first skin and / or hair care active cetyl pyridinium chloride; and at least one additional skin and / or hair care active selected from the group consisting of tetrahydrocurcumin, sugar amine, vitamin B3, retinoids, hydroquinone, peptides, phytosterol, dialkanoyl hydroxyproline, hexamidine, salicylic acid, n-acyl amino acid compounds, sunscreen actives, water soluble vitamins, oil soluble vitamins, hesperedin, mustard seed extract, glycyrrhizic acid, glycyrrhetinic acid, carnosine, Butylated Hydroxytoluene (BHT) and Butylated Hydroxyanisole (BHA), ergothioneine, vanillin or its derivatives, diethylhexyl syrinylidene malonate, melanostatine, sterol esters, idebenone, dehydroacetic acid, Licohalcone A, creatine, creatinine, feverfew extract, yeast extract, beta glucans, alpha glucans, their salts, their derivatives, their precursors, and / or combinations thereof; and a dermatologically acceptable carrier. The invention further relates to methods for regulating the condition of mammalian keratinous tissue wherein the methods each comprise the step of topically applying to the keratinous tissue of a mammal needing such treatment, a safe and effective amount of the personal care composition of the invention.
Owner:THE PROCTER & GAMBLE COMPANY
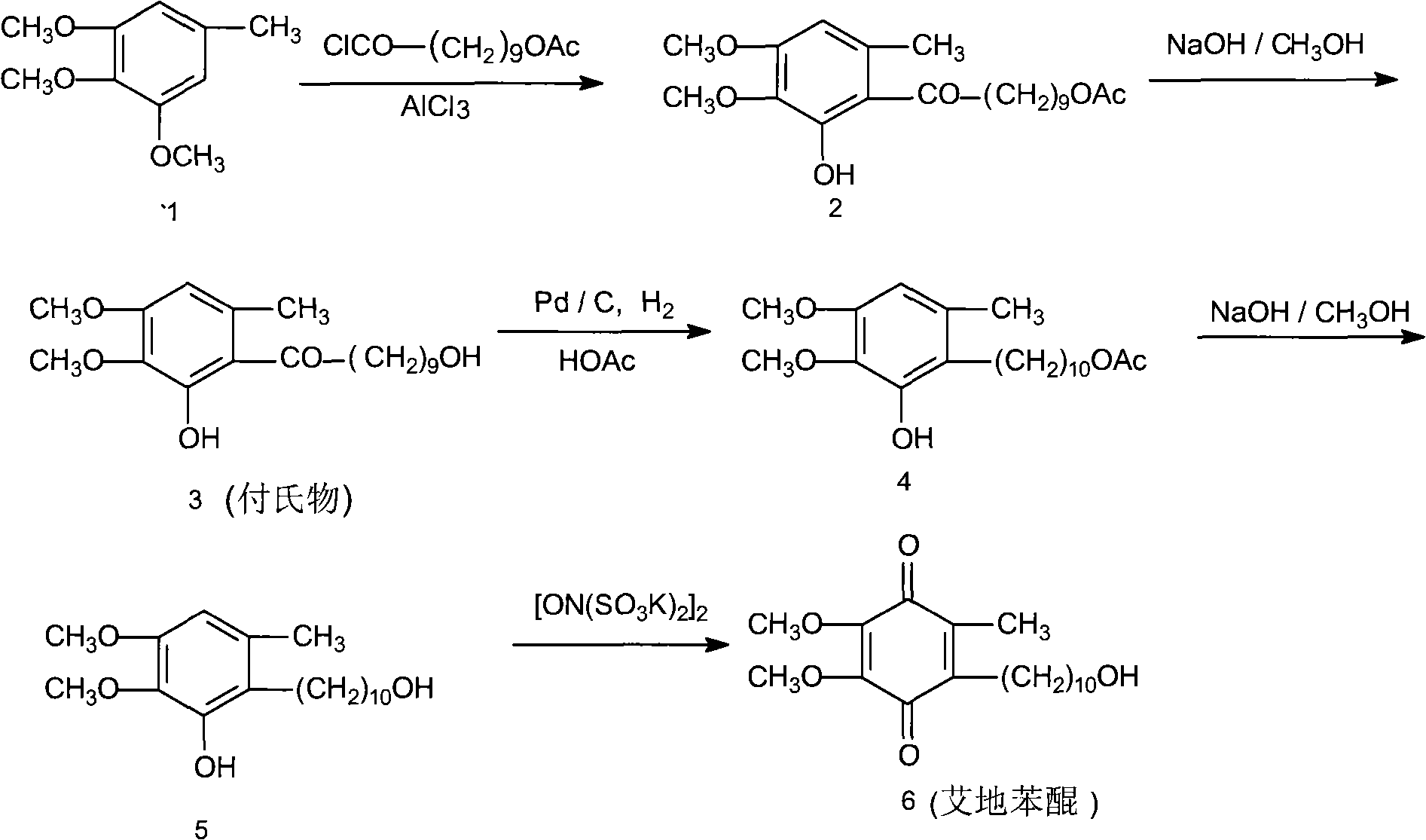
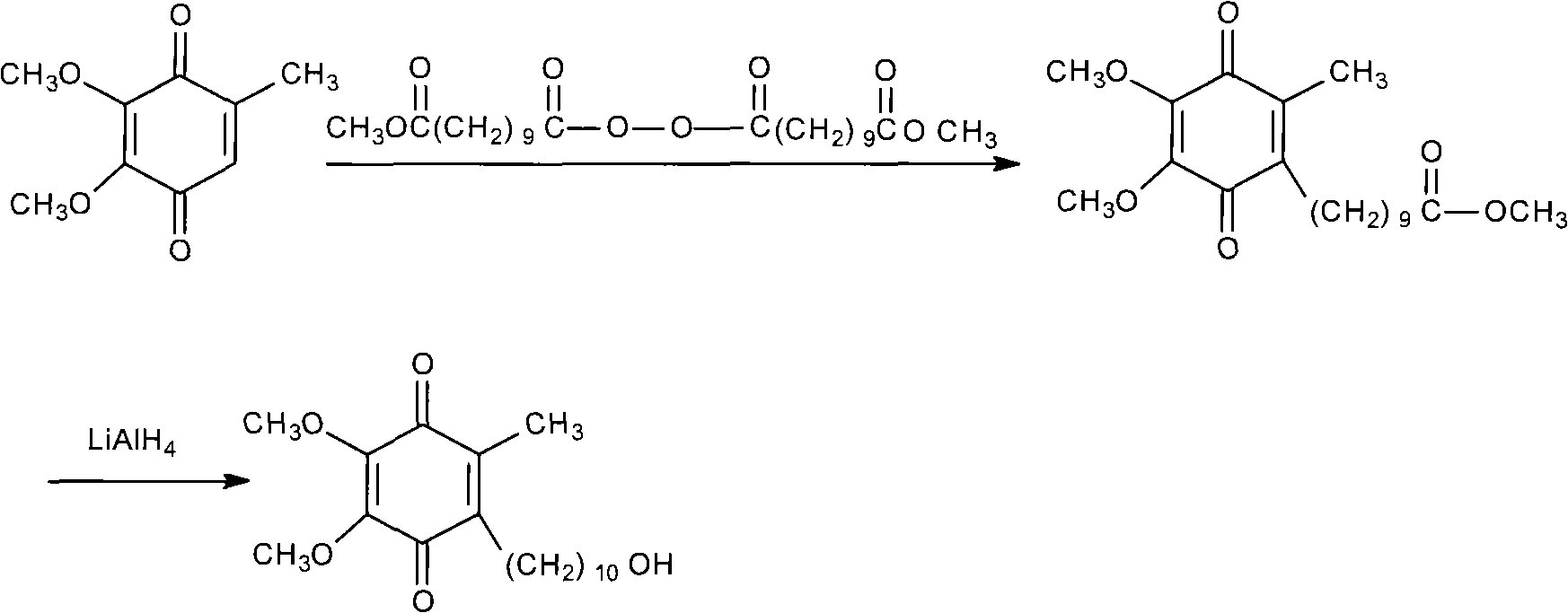
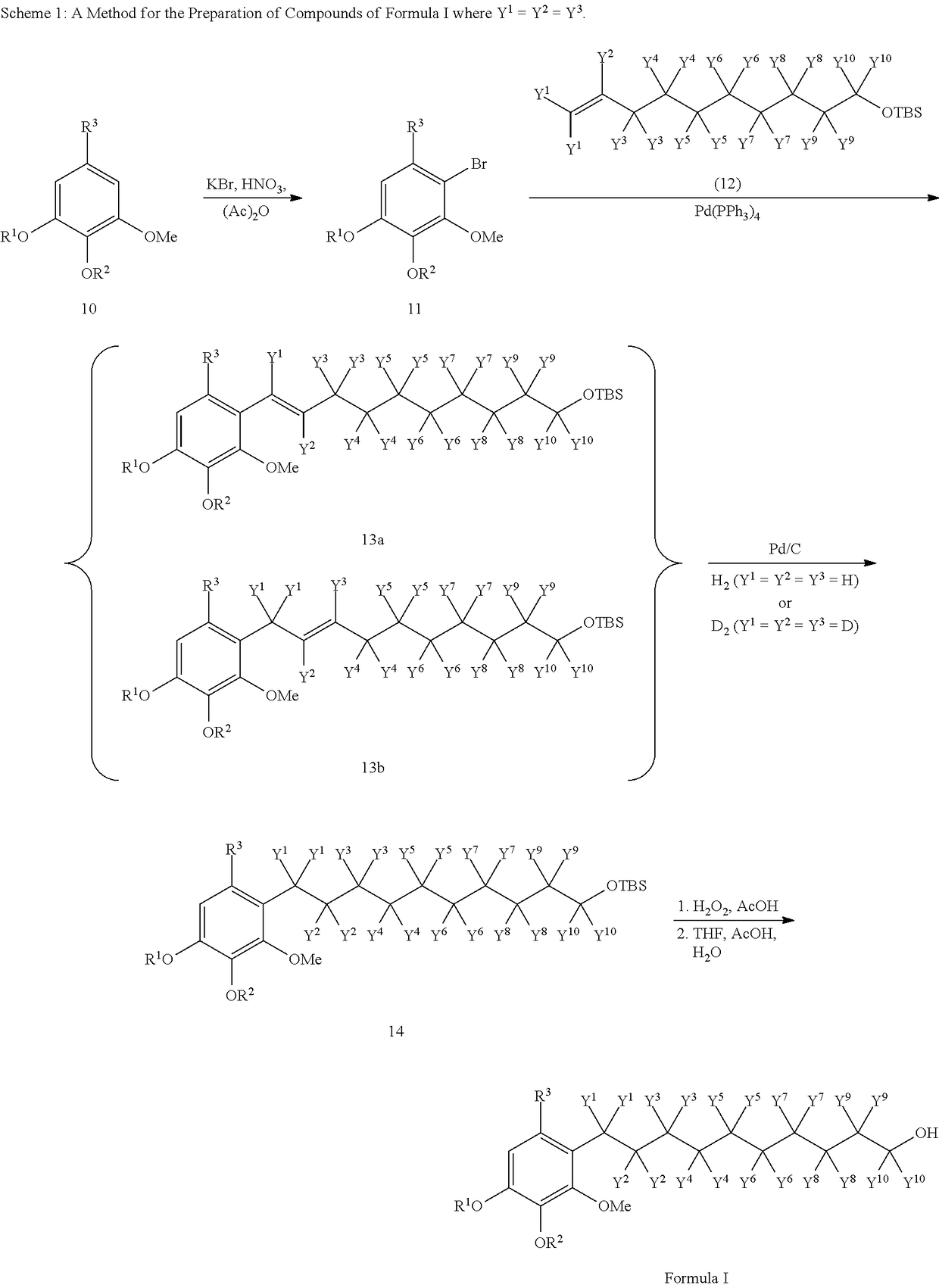
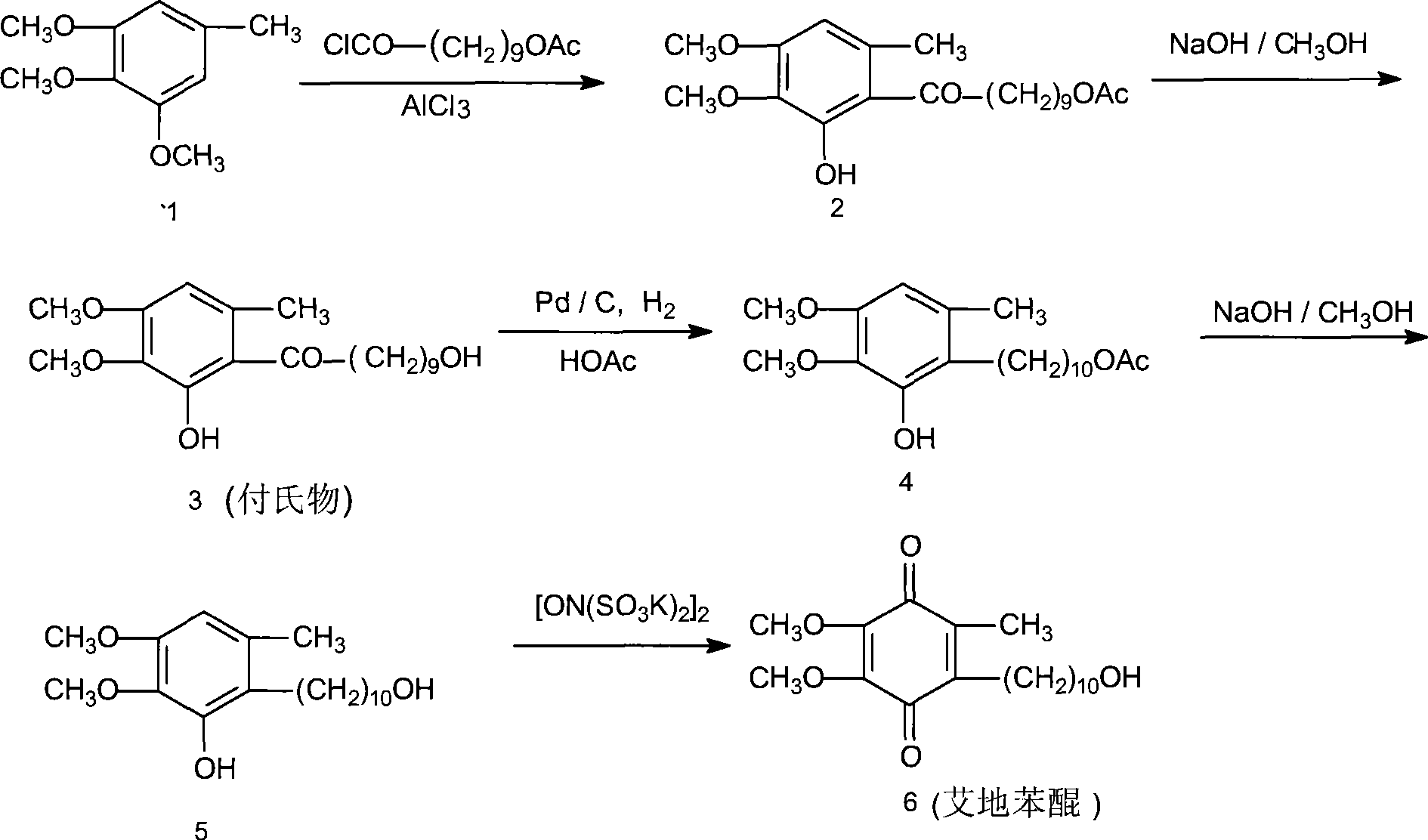
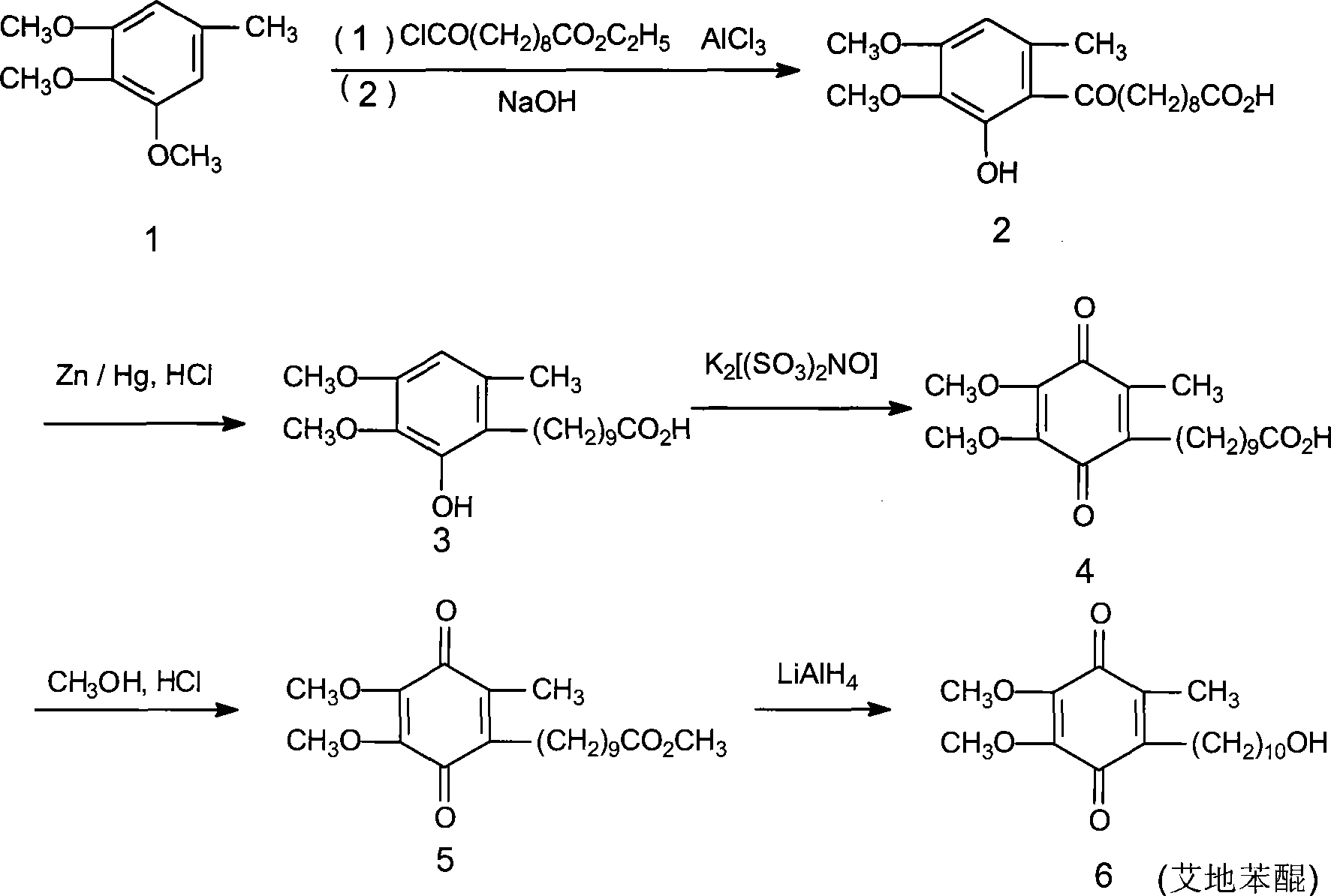
Method for synthesizing Idebenone
InactiveCN1696096AEasy to operateNot dangerousOrganic compound preparationQuinone preparationIdebenoneRetro-Diels–Alder reaction
Owner:SHANGHAI RECORD PHARM CO LTD +1
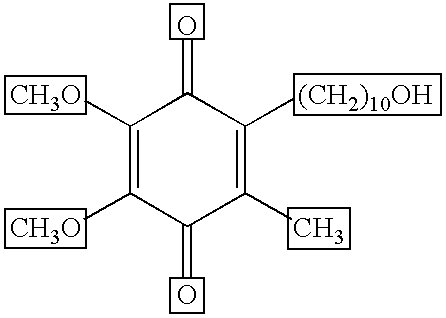
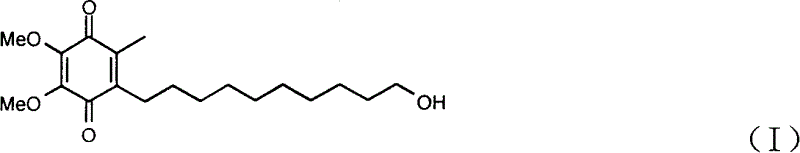
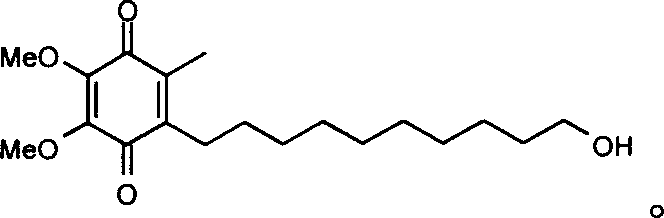
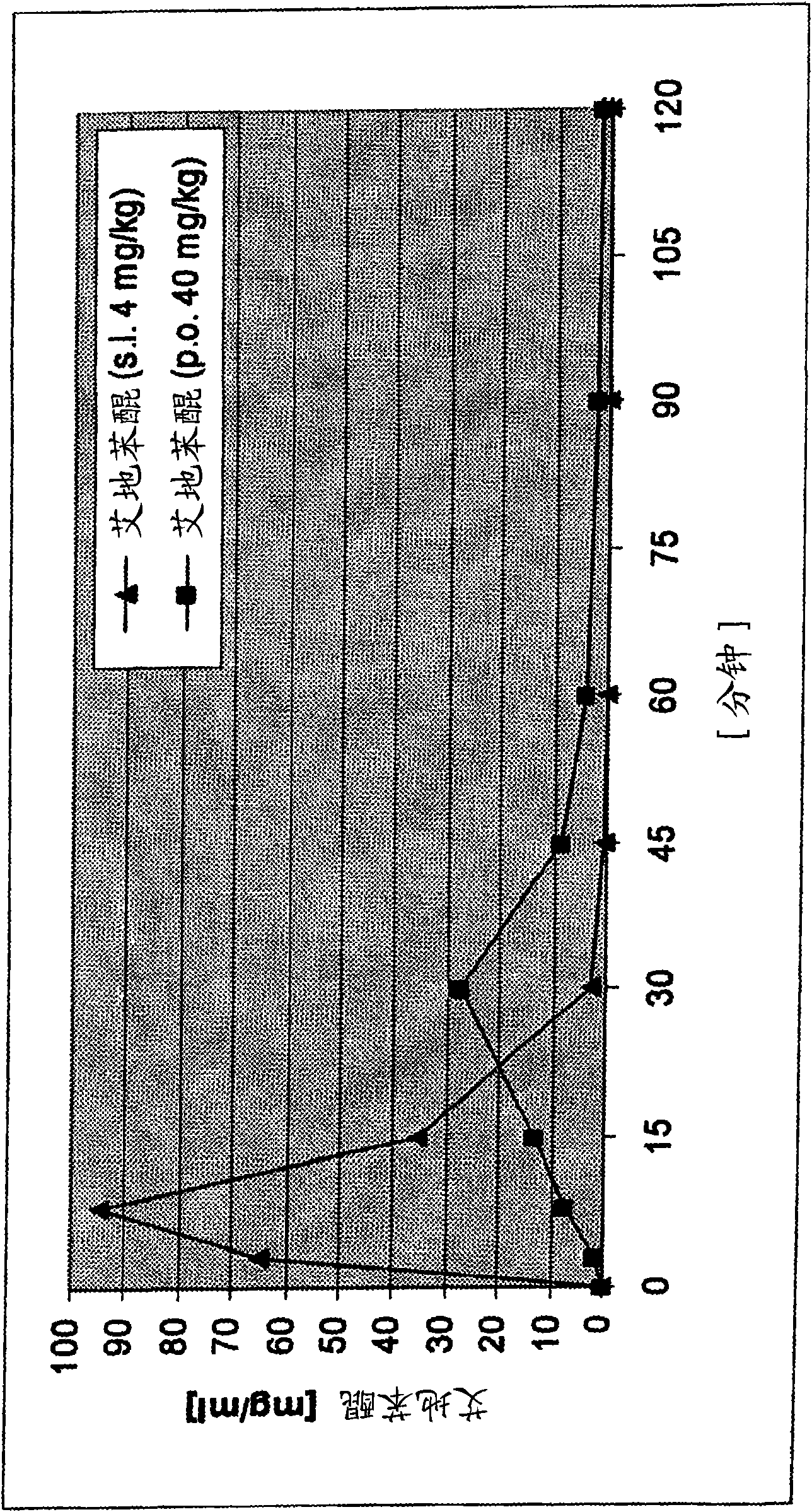
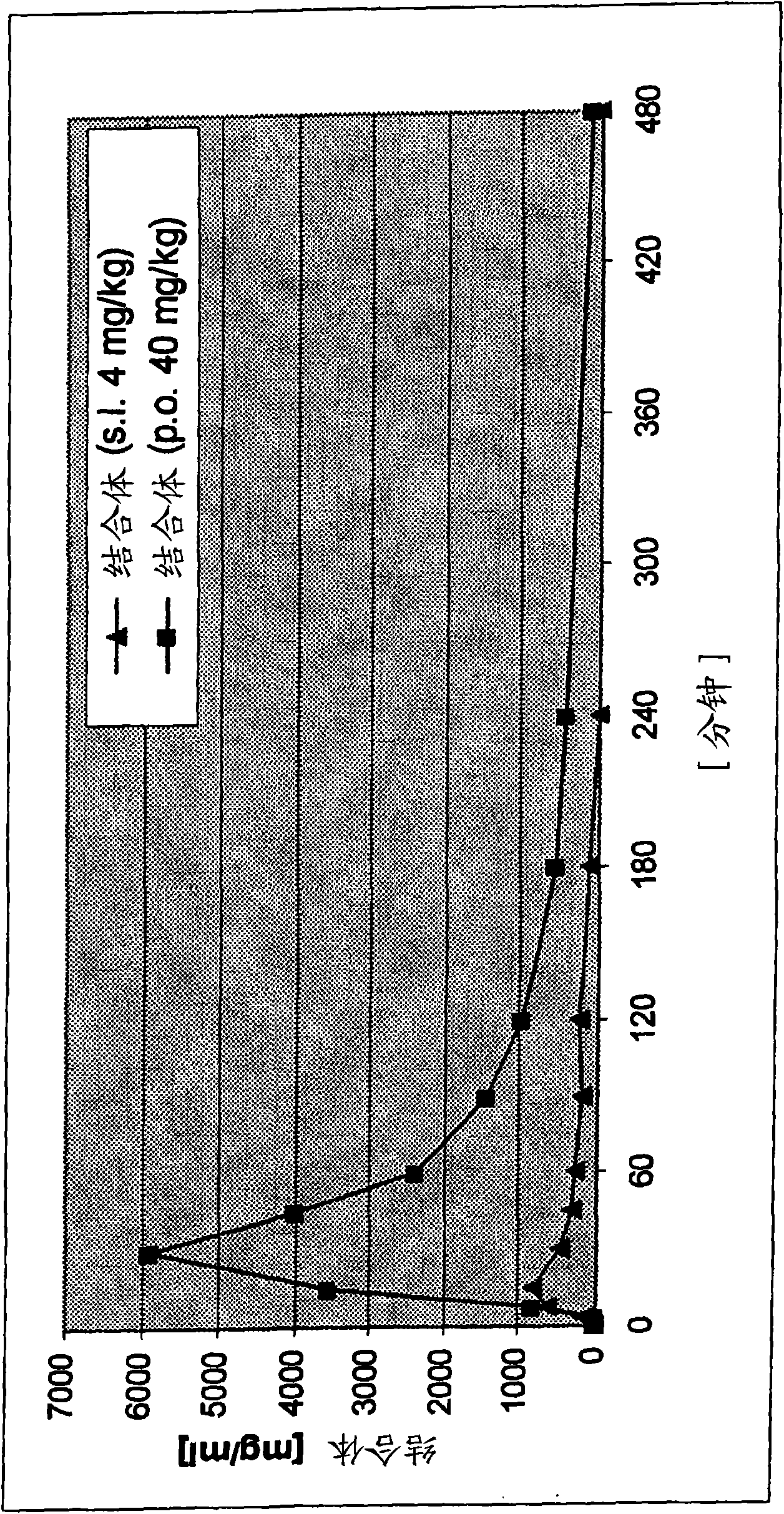
Transmucosal administration of 2,3-dimethoxy-5-methyl-6-(10- hydroxydecy l)-1,4-benzoquinone
Owner:SANTHERA PHARMA SCHWEIZ
Idebenone nanometer lipid carrier transdermal absorption preparation and preparation method thereof
InactiveCN102091038APrevent crystallizationImprove stabilityOrganic active ingredientsNervous disorderIdebenoneActive agent
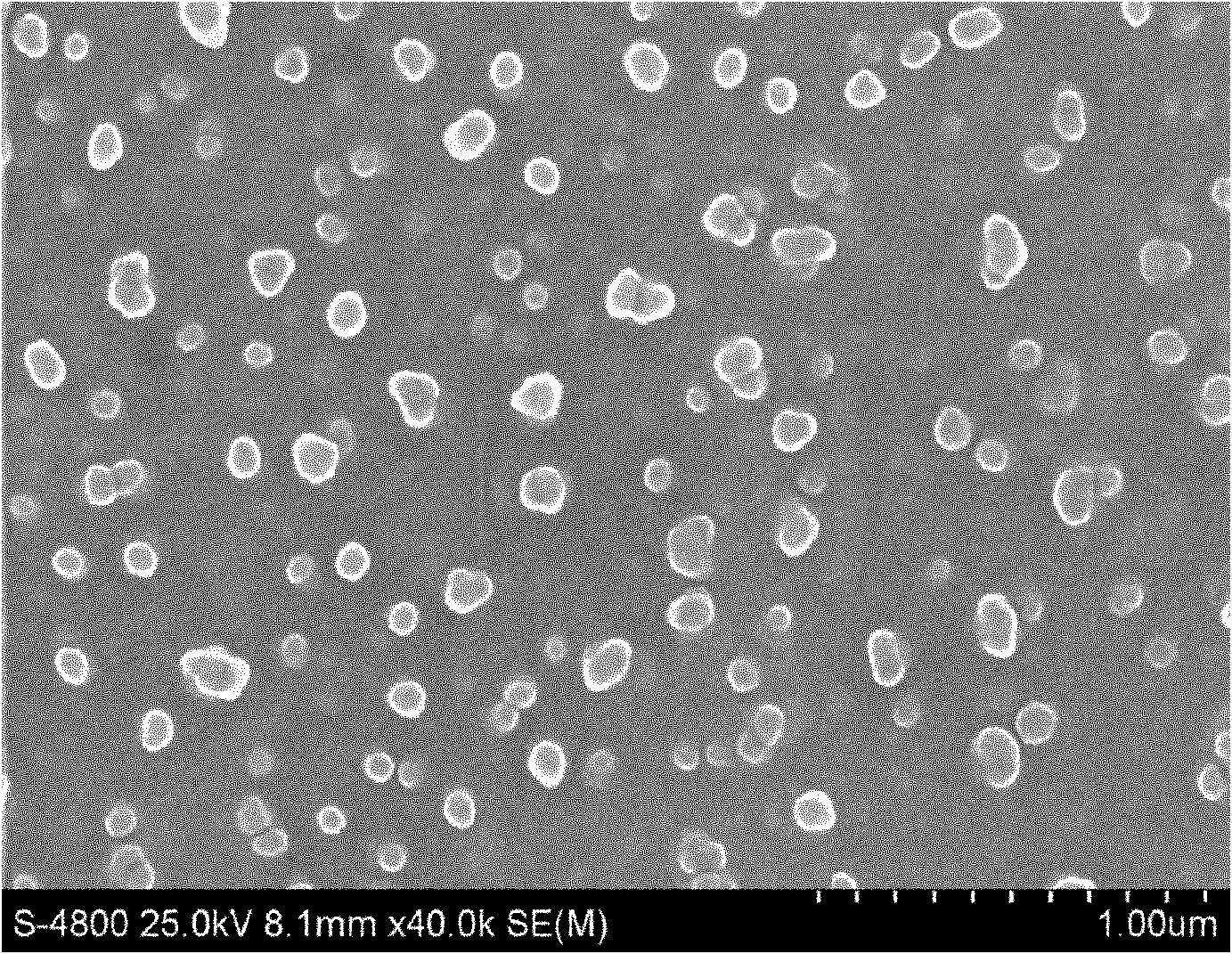
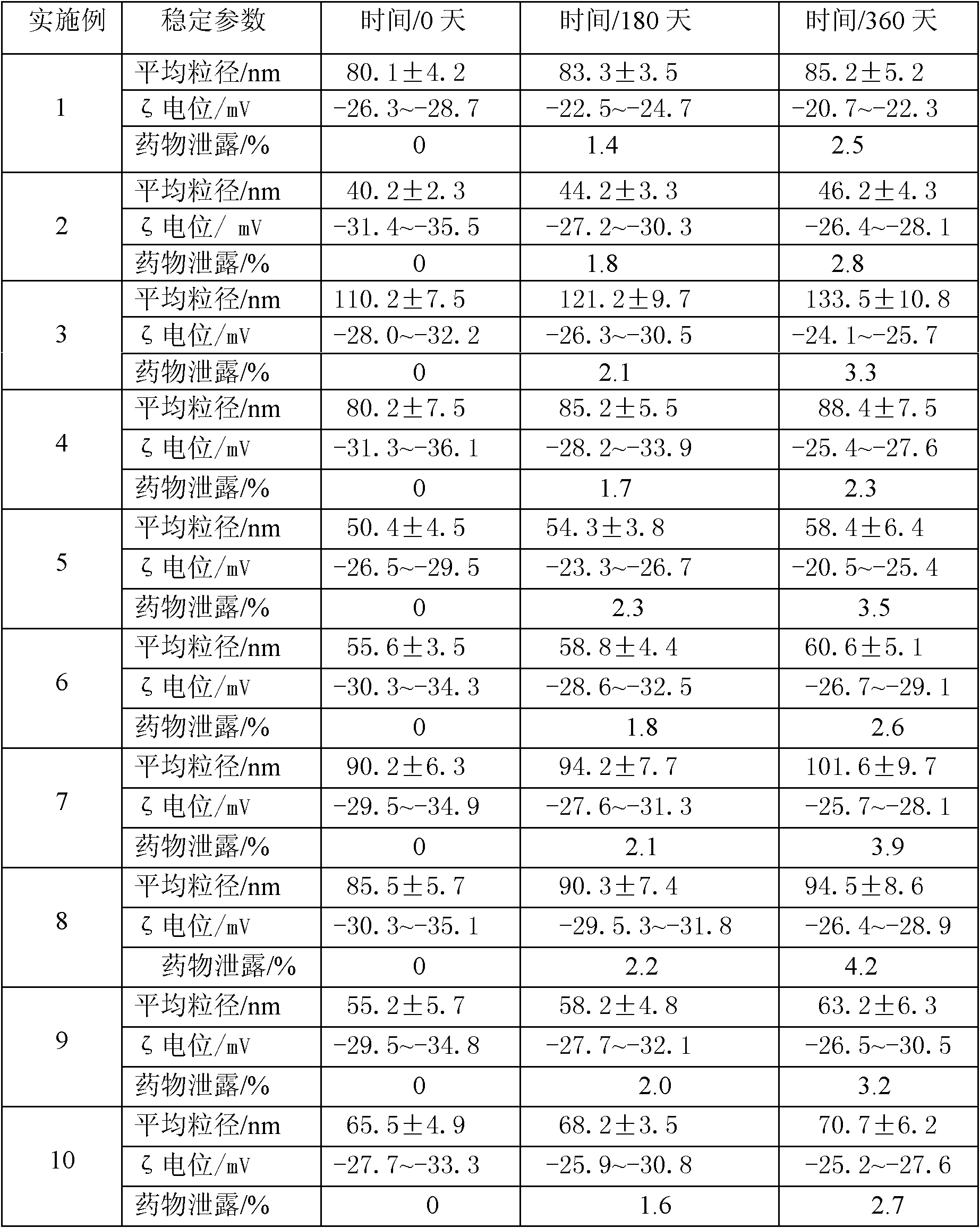
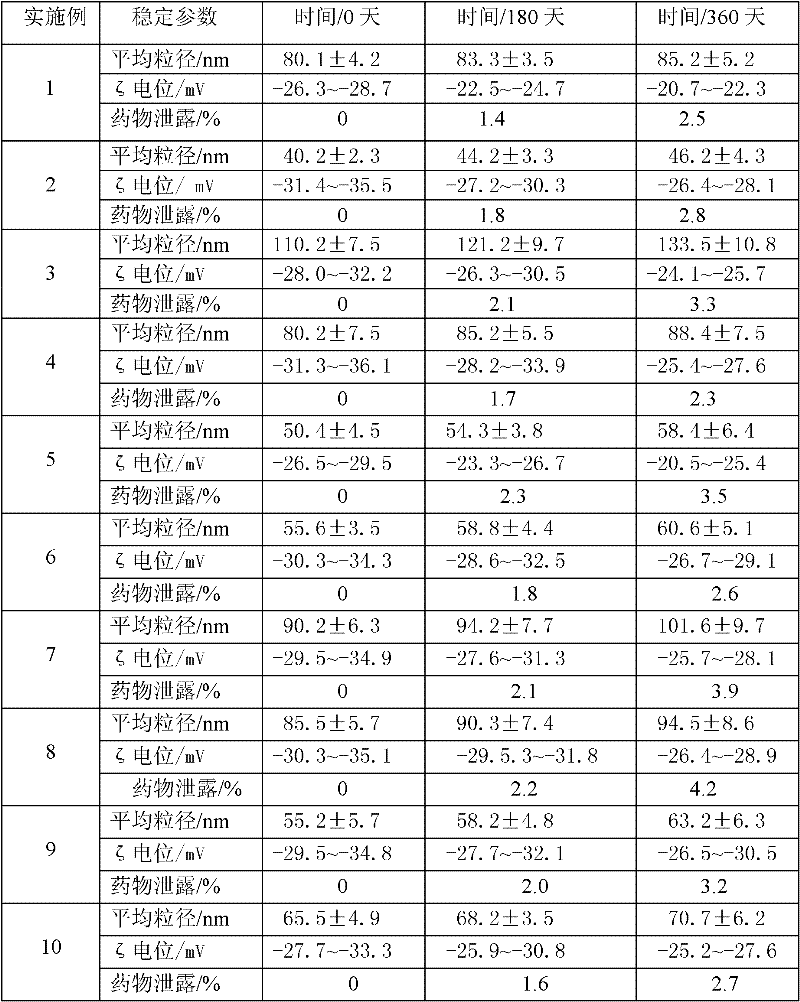
The invention discloses an idebenone nanometer lipid carrier transdermal absorption preparation and a preparation method thereof. The preparation method comprises the following steps: mixing idebenone, solid lipid materials, liquid lipid materials and a surface active agent 1 uniformly, and heating until the mixture is turned into a lipid phase; mixing a surface active agent 2 with ultrapure water uniformly so that the mixture is turned into a water phase; adding the lipid phase into the water phase slowly under the condition of keeping the temperature of the lipid phase and the water phase consistent while stirring; homogenizing rapidly, cutting, carrying out primary emulsification, carrying out ultrasonication again, and cooling down, so that a lipid carrier of a nanometer structure loading idebenone is prepared; and adding pharmaceutically acceptable auxiliary materials, so that the idebenone nanometer lipid carrier transdermal absorption preparation is prepared. The particle diameter of the idebenone nanometer lipid carrier transdermal absorption preparation provided by the invention is 40.2-110.2nm, the entrapment rate is 80.5%-99.1%, the zeta potential is minus 25.5-minus 34.4mV, the morphology is stable, an organic solvent is not contained, the idebenone nanometer lipid carrier transdermal absorption preparation has good compatibility with skin and has no irritation, the first pass effect is avoided, and the bioavailability is improved.
Owner:TIANJIN UNIV
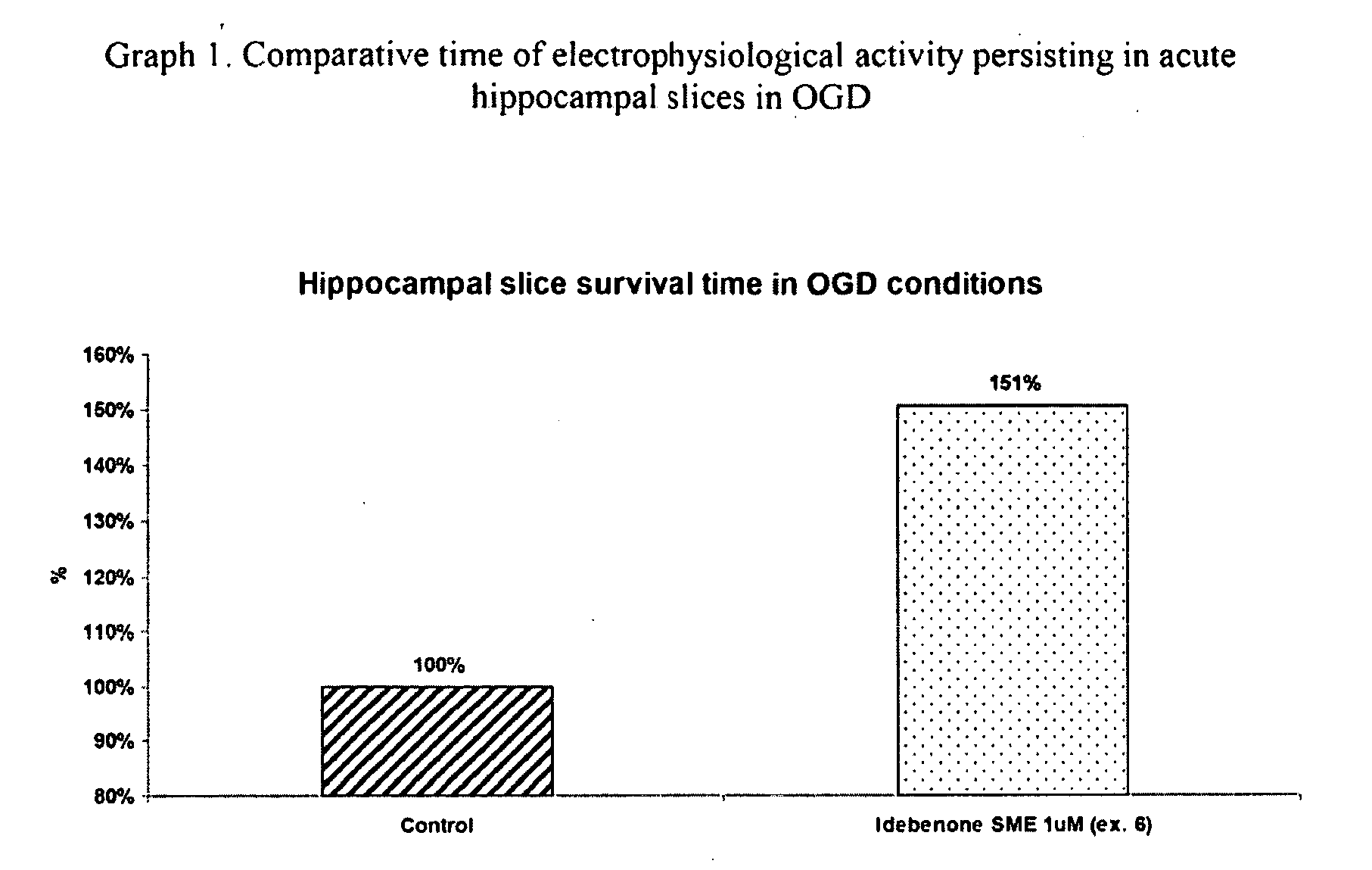
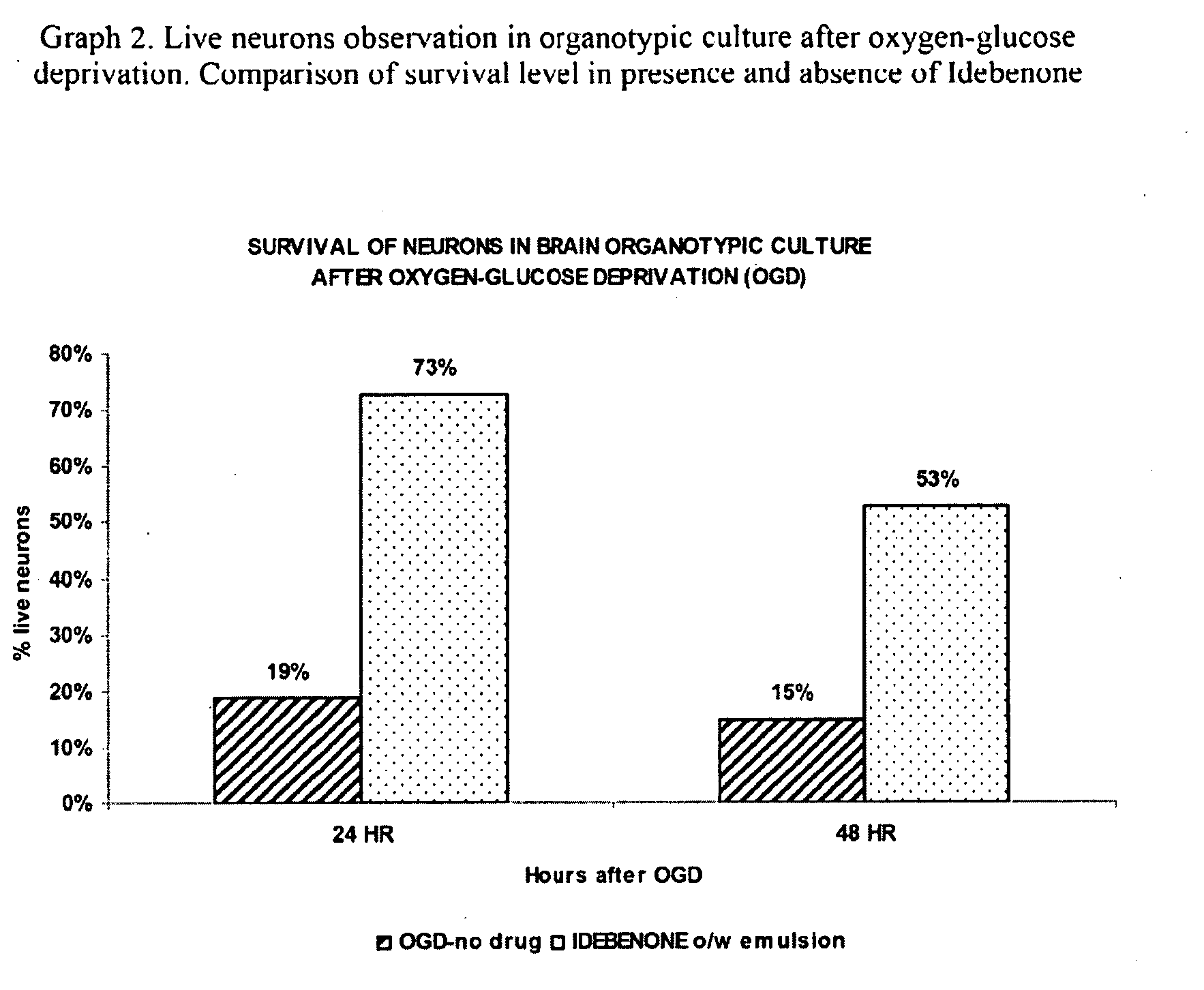
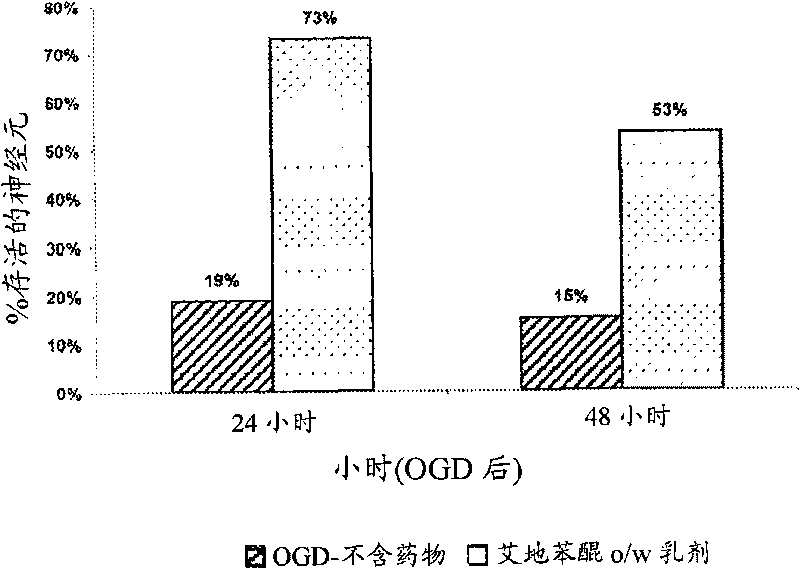
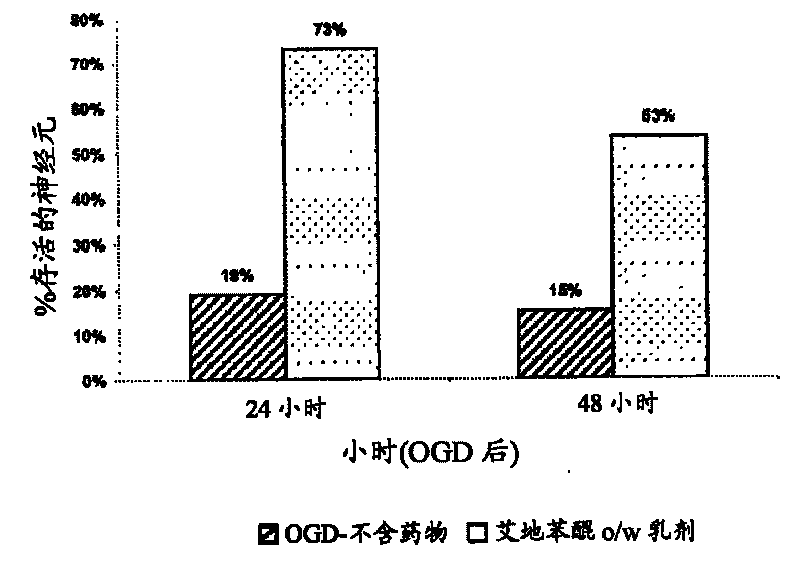
Idebenone composition for the treatment of neurological disorders
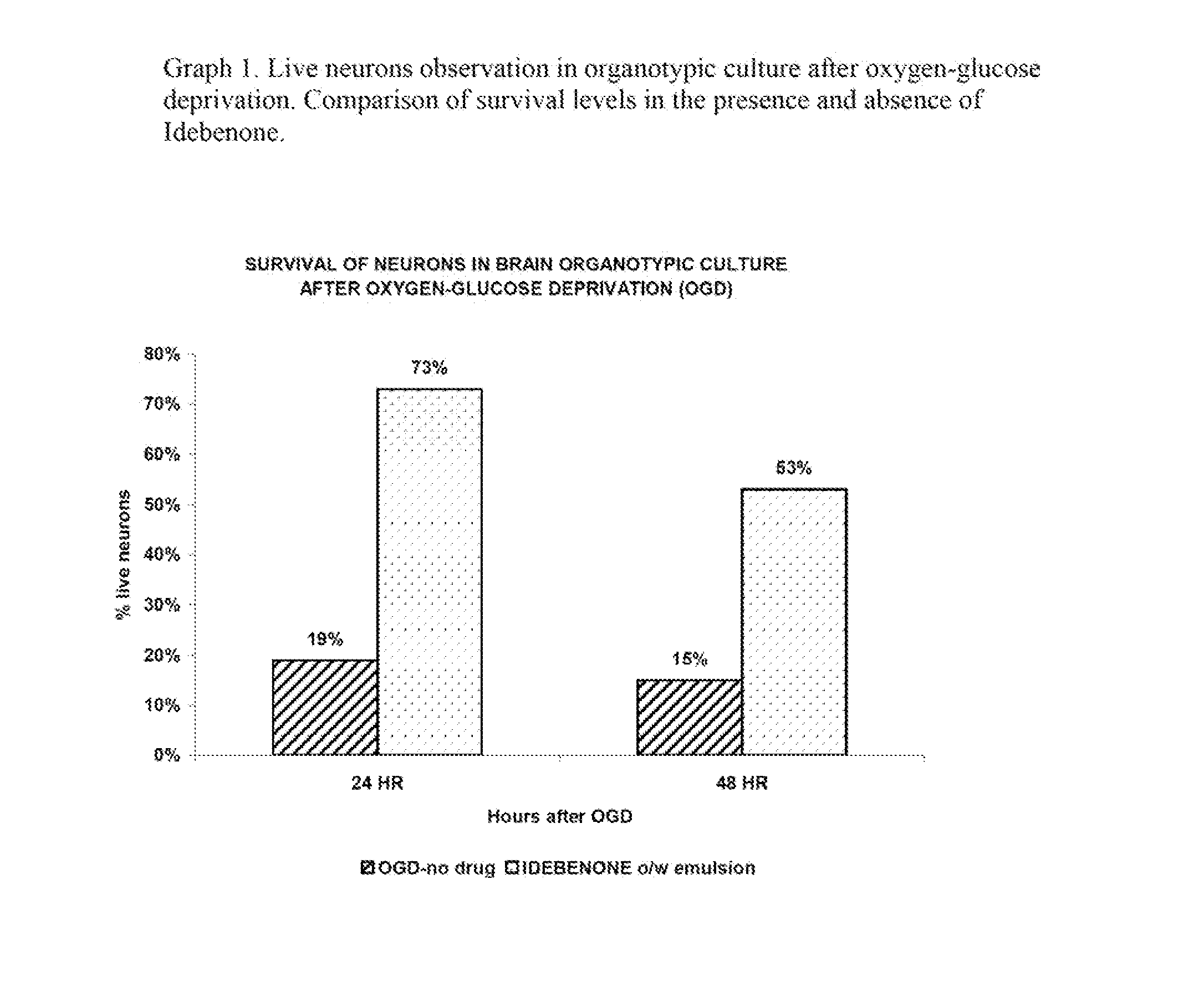
The invention describes the use of an injectable form of Idebenone to induce protect from neuronal damage, improve recovery after brain trauma; stroke, intoxication, neurodegenerative diseases, memory loss or neuropathology associated with neuroinflammation or infection damage and to restore cognitive function, suppress disorientation, alcoholic and drug abuse associated syndromes and other signs of neuronal damage.
Owner:ALPHARX
Weight management composition
A method for promoting weight management in a mammal is provided, comprising administering to the mammal a composition comprising at least one weight loss agent and at least one mitochondria enhancingagent. The weight loss agent may be selected from the group consisting of psyllium, guar gum, capsaicin, chitosan, caffeine, garcinia cambogia, Pinus densiflora, capsaicin, yohimbre, hoodia, glucomannan, African mango, guarana, pyruvate, carnitine, beta-glucan, fucoxanthin, raspberry ketone, white kidney bean, kola nut, chromium, ginseng, psyllium, St. John's wort, dandelion, hydroxycitric acid,conjugated linoleic acid, green tea, black tea, green coffee beans extract, forskolin and bitter orange. The mitochondria enhancing agent may be selected from the group consisting of beetroot extract,nitrate, idebenone, nicotinamide riboside, elamepratide, vitamin C, vitamin D, vitamin E, thiamine, riboflavin, magnesium, calcium, phosphate, phospholipid, creatine, pyruvate, coenzyme Q10, NADH, nicotinic acid, L-camitine, dicholoracetate, curcumin, schisandrin and resveratrol. Compositions for weight management are also provided.
Owner:ASERKIN CO LTD
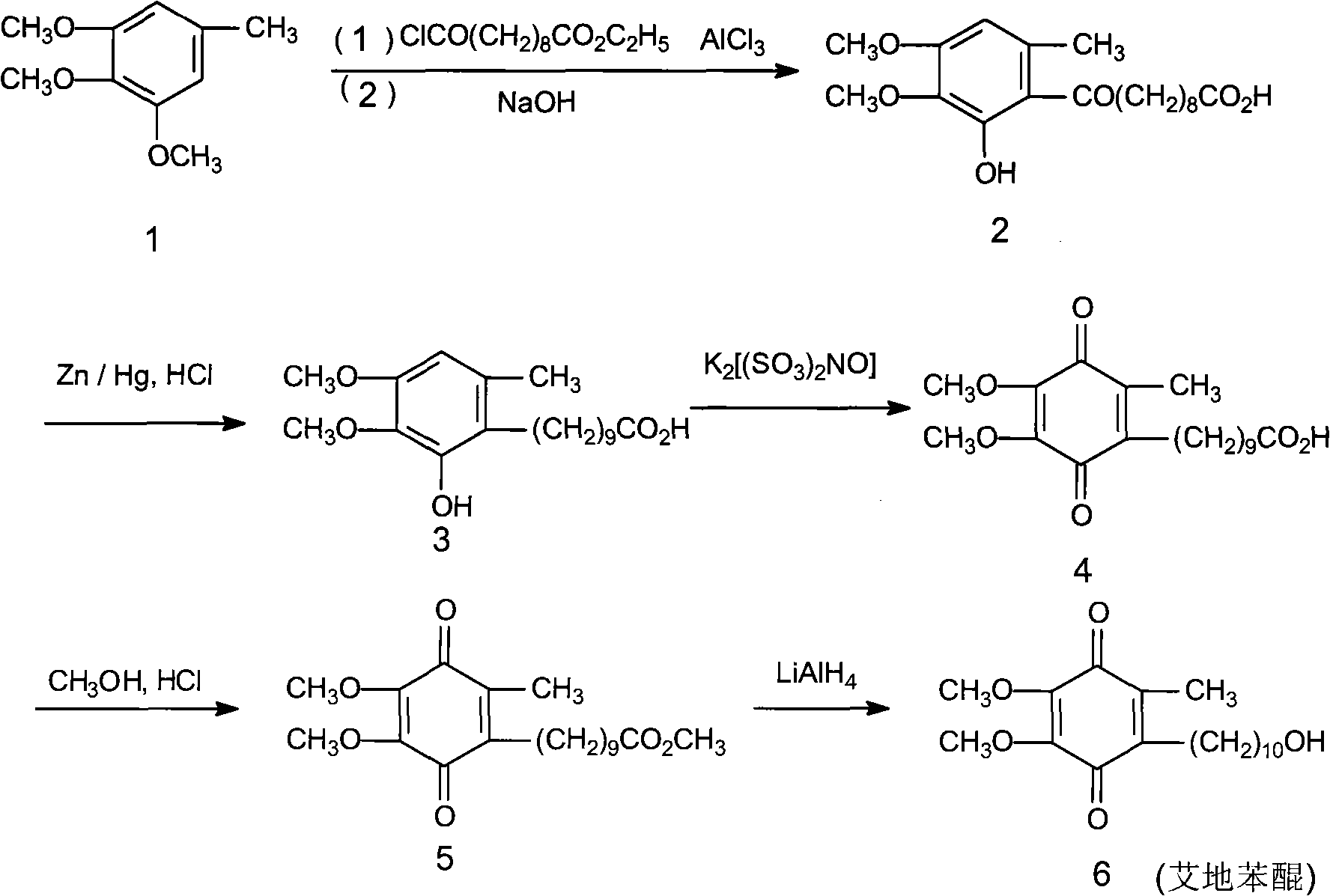
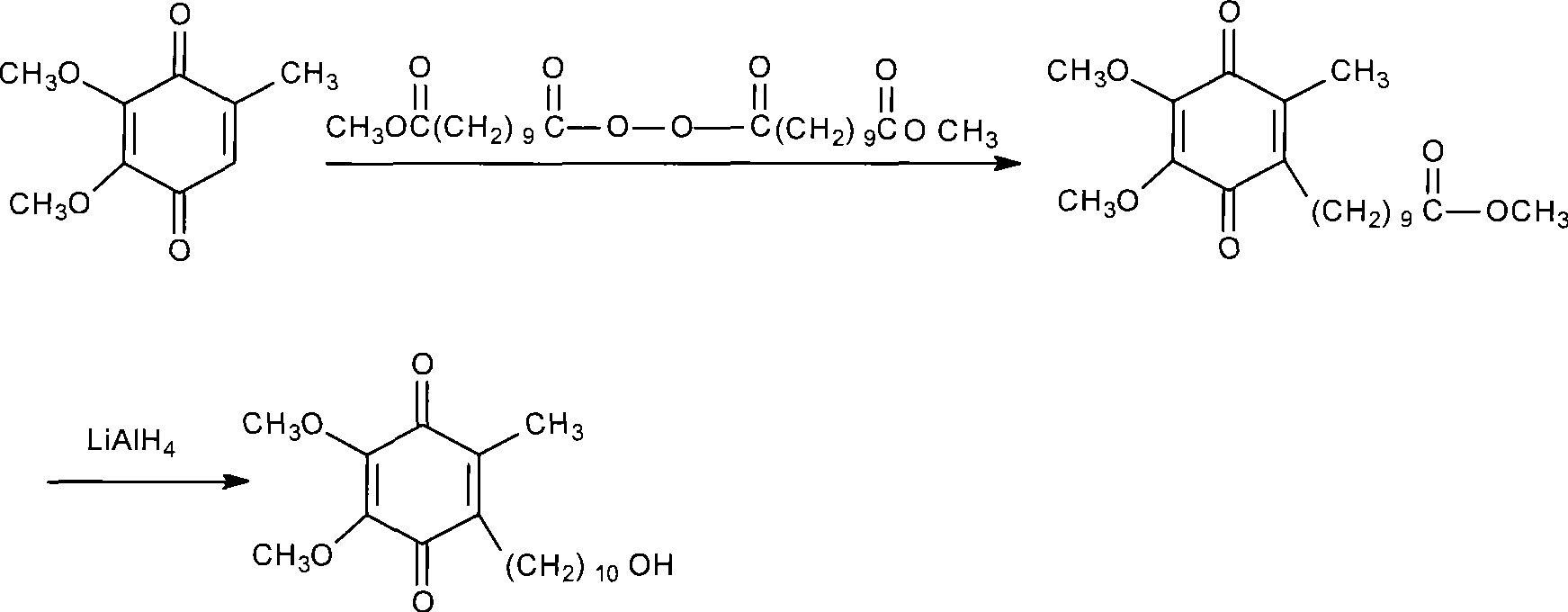
Method for preparing idebenone
The invention relates to a preparation method for idebenone, comprising the following steps: (1), 3,4,5-trimethoxy-toluene has friedel-crafts reaction with 10-acetoxy-decane chloride or 10-acetoxy-decanoyl- bromine to generate 6-(10-acetoxy-1-oxo-decane yl)-2,3-dimethoxy-5-methyl phenol; (2), the 6-(10-acetoxy-1-oxo-decane yl)-2,3-dimethoxy-5-methyl phenol obtained in the step (1) is reacted with hydrogen under the joint effect of palladium-carbon catalyst and dehydrating agent DCC to generate 6-(10-acetoxy-decyl)-2,3-dimethoxy-5-methyl phenol; (3), the6-(10-acetoxy-decyl)-2,3-dimethoxy-5-methyl phenol prepares 6-(10-hydroxy decyl) -2,3-dimethoxy-5-methyl phenol through hydrolysis; (4), the 6-(10-hydroxy decyl) -2,3-dimethoxy-5-methyl phenol has oxidation reaction with an oxygen-supply body under the effect of catalytic oxidizer Cu (Salen) so as to generate the idebenone. The preparation method has low cost and high yield, which is suitable for large-scale industrial idebenone production.
Owner:SUZHOU LIXIN PHARMA
Method for ameliorating of post-anesthetic recovery
InactiveUS20100099775A1Formula stableSufficient protectionBiocideNervous disorderNeuronal damageWhole body
The invention describes use of injectable form of Idebenone to improve recovery after general anesthesia and of cognitive functions, suppress disorientation and other signs of neuronal damage.
Owner:ALPHARX
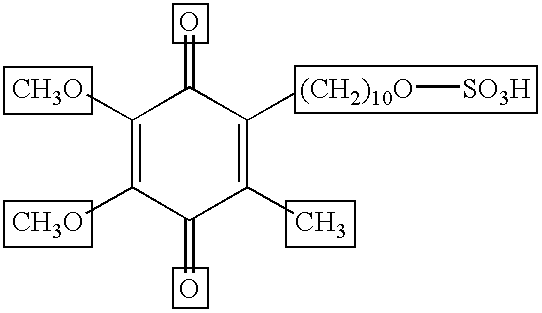
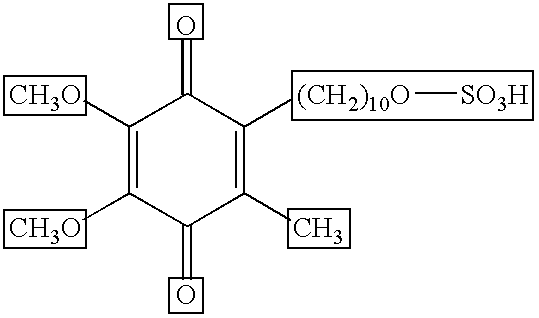
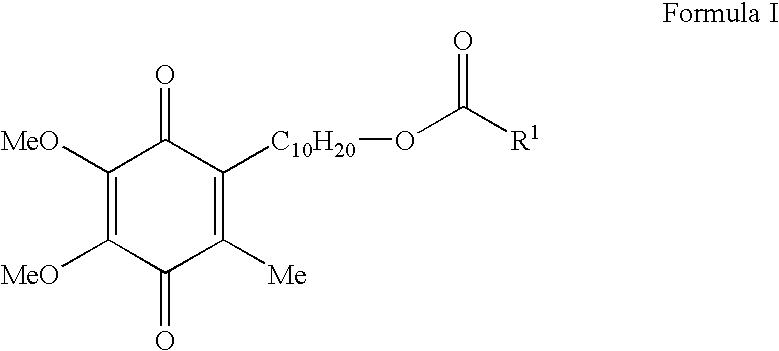
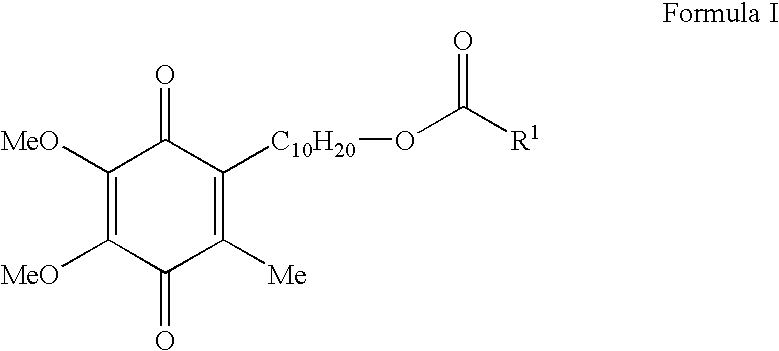
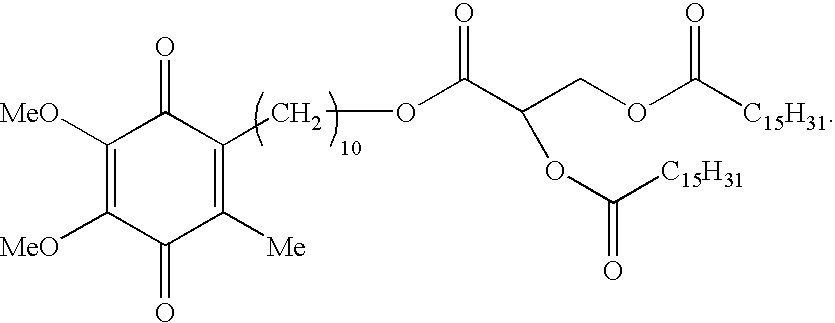
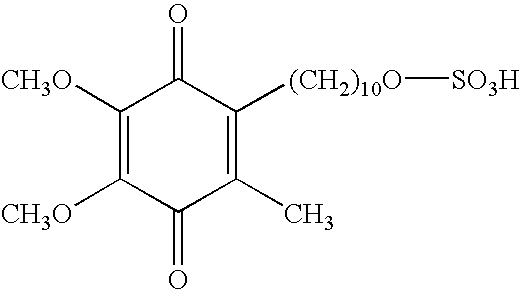
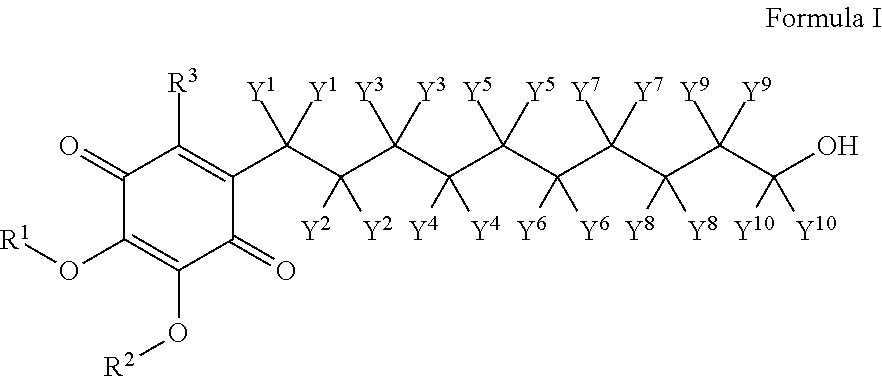
Skin treatments containing carboxylic acid-substituted idebenone derivatives and methods of preparation and use thereof
The present invention relates to novel carboxylic acid-substituted idebenone derivatives, skin treatment compositions containing these carboxylic acid-substituted idebenone derivatives, methods of treating skin changes by topical application of these carboxylic acid-substituted idebenone derivatives, and their methods of synthesis. The carboxylic acid-substituted idebenone derivatives of the present invention are unexpectedly effective in treating skin, particularly with respect to skin tolerance. When included in a topical composition, the carboxylic acid-substituted idebenone derivatives of the present invention have an antioxidant effect that is useful in treating a skin change.
Owner:BRANDCO ELIZABETH ARDEN 2020 LLC
Idebenone composition for the treatment of neurological disorders
The invention describes the use of an injectable form of Idebenone to induce protect from neuronal damage, improve recovery after brain trauma; stroke, intoxication, neurodegenerative diseases, memory loss or neuropathology associated with neuroinflammation or infection damage and to restore cognitive function, suppress disorientation, alcoholic and drug abuse associated syndromes and other signs of neuronal damage.
Owner:ALPHARX
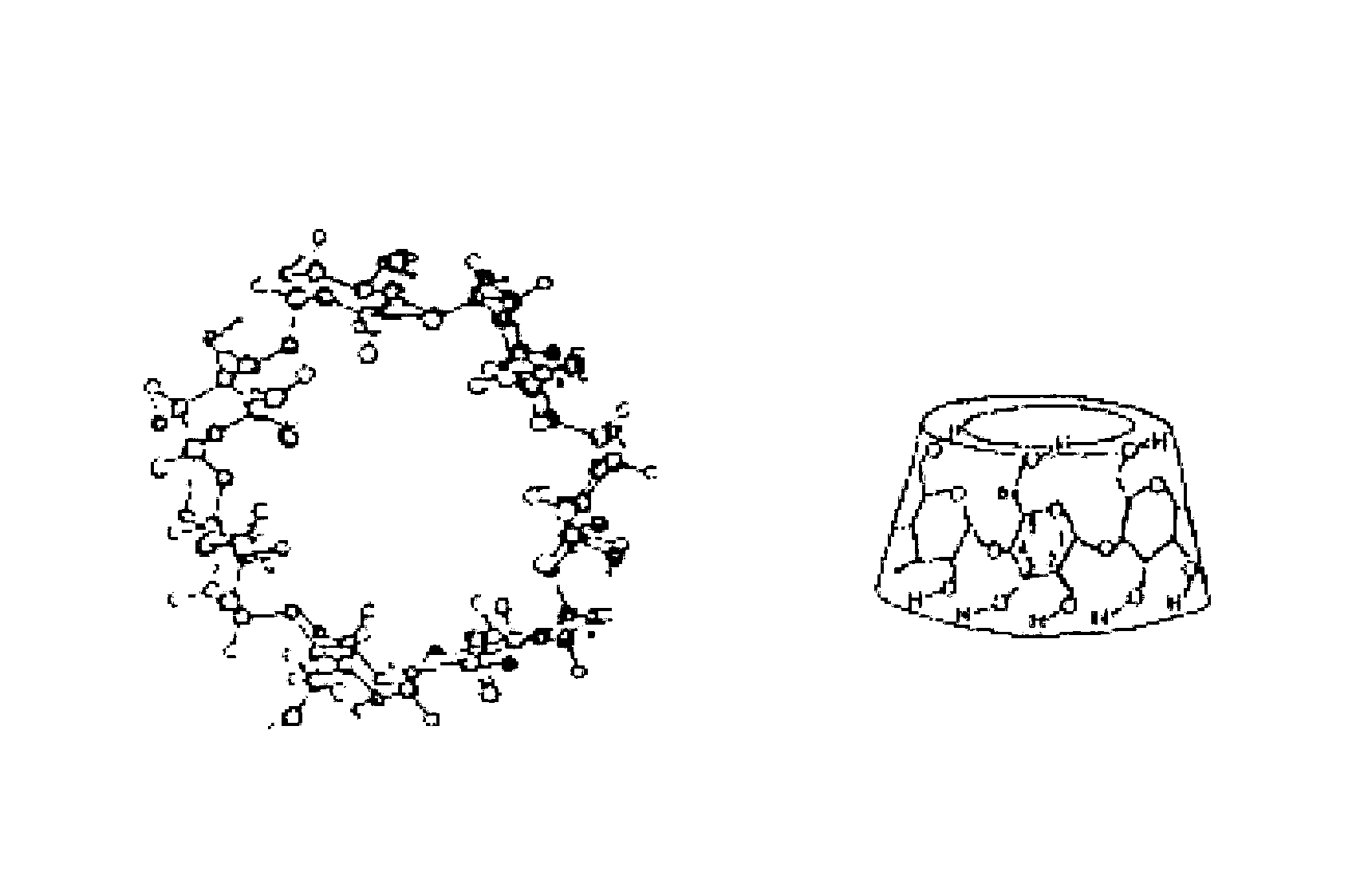
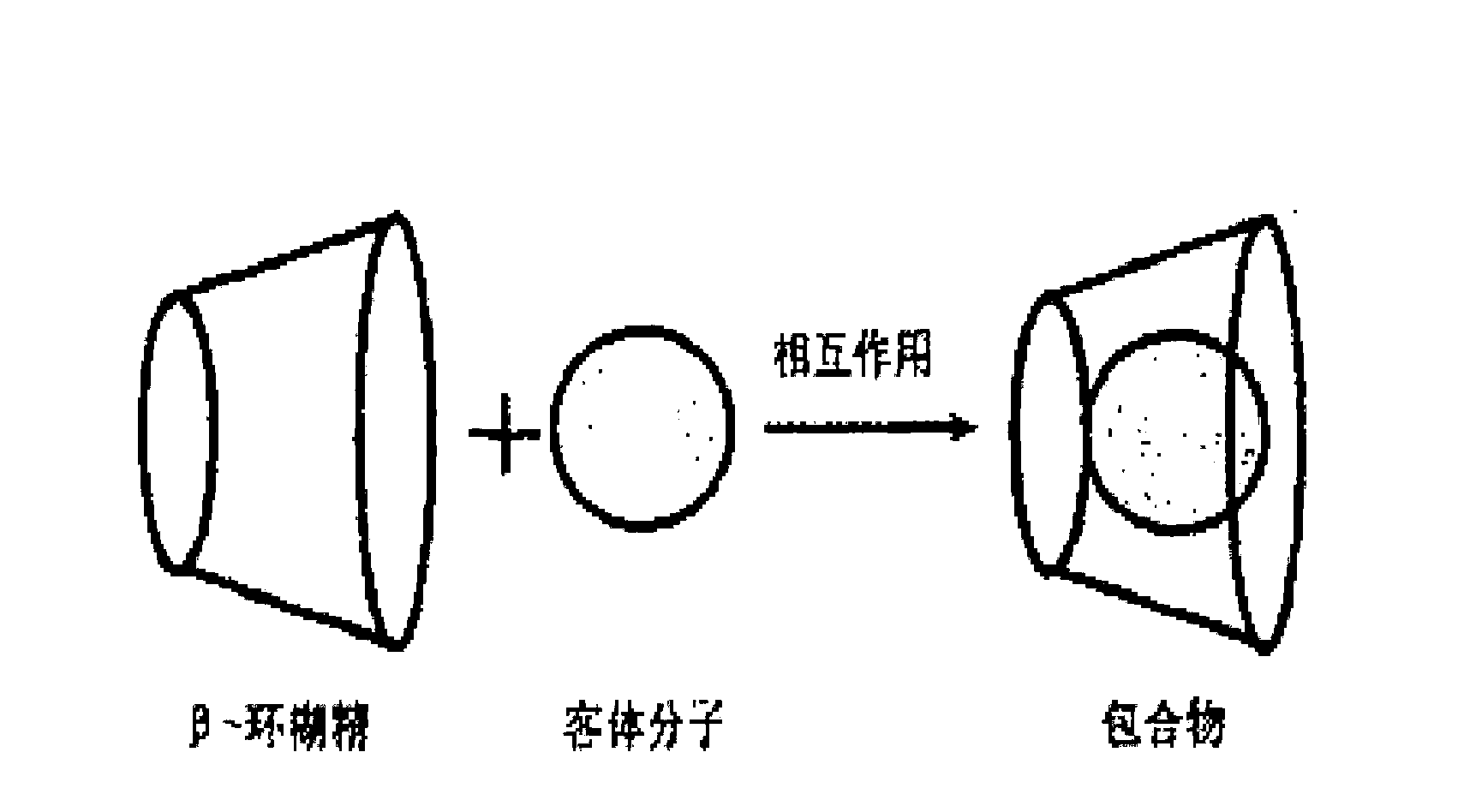
Preparation method for idebenone microcapsule through ultrasonic inclusion by beta-cyclodextrin
InactiveCN103251570ASolve the problem of water solubilityReduce colorOrganic active ingredientsAntinoxious agentsSolubilityWater baths
The invention discloses a preparation method for an idebenone microcapsule through ultrasonic inclusion by beta-cyclodextrin. The preparation method comprises the following steps: dissolving beta-cyclodextrin in distilled water to prepare a saturated solution, heating the saturated solution of beta-cyclodextrin to a temperature of 30 to 80 DEG C in a water bath and carrying out isothermal stirring for 15 to 50 min; adding idebenone under the condition of isothermal stirring, continuing stirring to realize uniform mixing so as to obtain a mixed solution and subjecting the mixed solution to ultrasonic inclusion so as to obtain a beta-cyclodextrin-idebenone inclusion solution; and standing the beta-cyclodextrin-idebenone inclusion solution at a temperature of 0 to 8 DEG C for 24 h, carrying out pumping filtration and drying to obtain solid powder, grinding the solid powder into fine powder and carrying out sieving so as to obtain the beta-cyclodextrin-idebenone microcapsule. The preparation method provided by the invention overcomes the problem of water-solubility of idebenone and substantially weakens the inherent color of idebenone, so idebenone is compatible with other components in a formula for a cosmetic without influencing the appearance of the cosmetic, thereby greatly enlarging the application scope of IDBN.
Owner:广州保税区雅兰国际化妆品有限公司
Crystallization method for preparing high-purity idebenone
The utility model relates to a crystallization method for preparing high-purity idebenone, belonging to the technical field of compound separation and purification. The crystallization method includes the steps as follows: firstly, dissolving crude idebenone in big-polarity good solvent dichloromethane, thus obtaining clear solution; adding a certain amount of small-polarity antisolvent normal hexane in the clear solution, thus making the solution to become turbid; increasing temperature until the burbid solution becomes clear; cooling the clear solution by slowing reducing the temperature; and crystallizing the idebenone product out from the solvent and leaving impurities in mother solution. In the invention, a two-step method of antisolvent crystallization and cooling crystallization is adopted and the phenomenon of oil separation is avoided by controlling the types and the proportioning of mixed solvent as well as the crystallization starting concentration, the temperature and the cooling rate. The invention firstly discloses a new crystallization method for purifying idebenone by controlling oil separation; by the adoption of the method, the crystallization product of the idebenone is up to above 99.5% in purity and above 78% in yield.
Owner:JIANGNAN UNIV
Method and preparation for reducing sunburn cell formation in skin
InactiveUS20050152857A1Reduce formationHigh sensitivityCosmetic preparationsToilet preparationsIdebenoneTopical preparation
A method for reducing sunburn cells in human skin includes applying a topical preparation comprising an amount of an agent effective to reduce the formation of sunburn cells in human skin, and exposing the skin to ultraviolet radiation. The agent includes idebenone or a derivative of idebenone.
Owner:PCR TECH HLDG LC
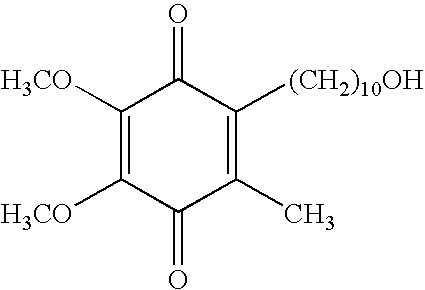
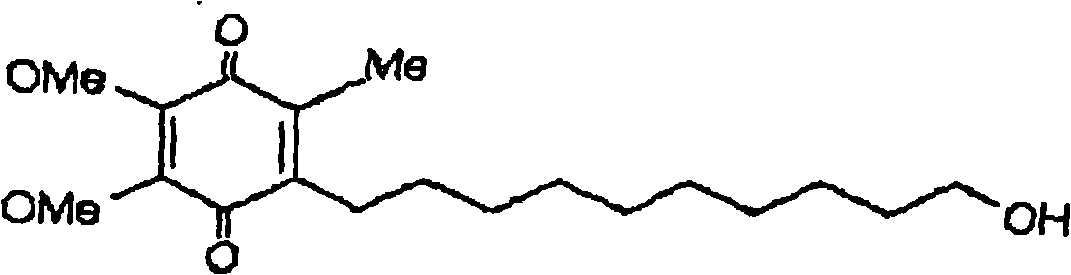
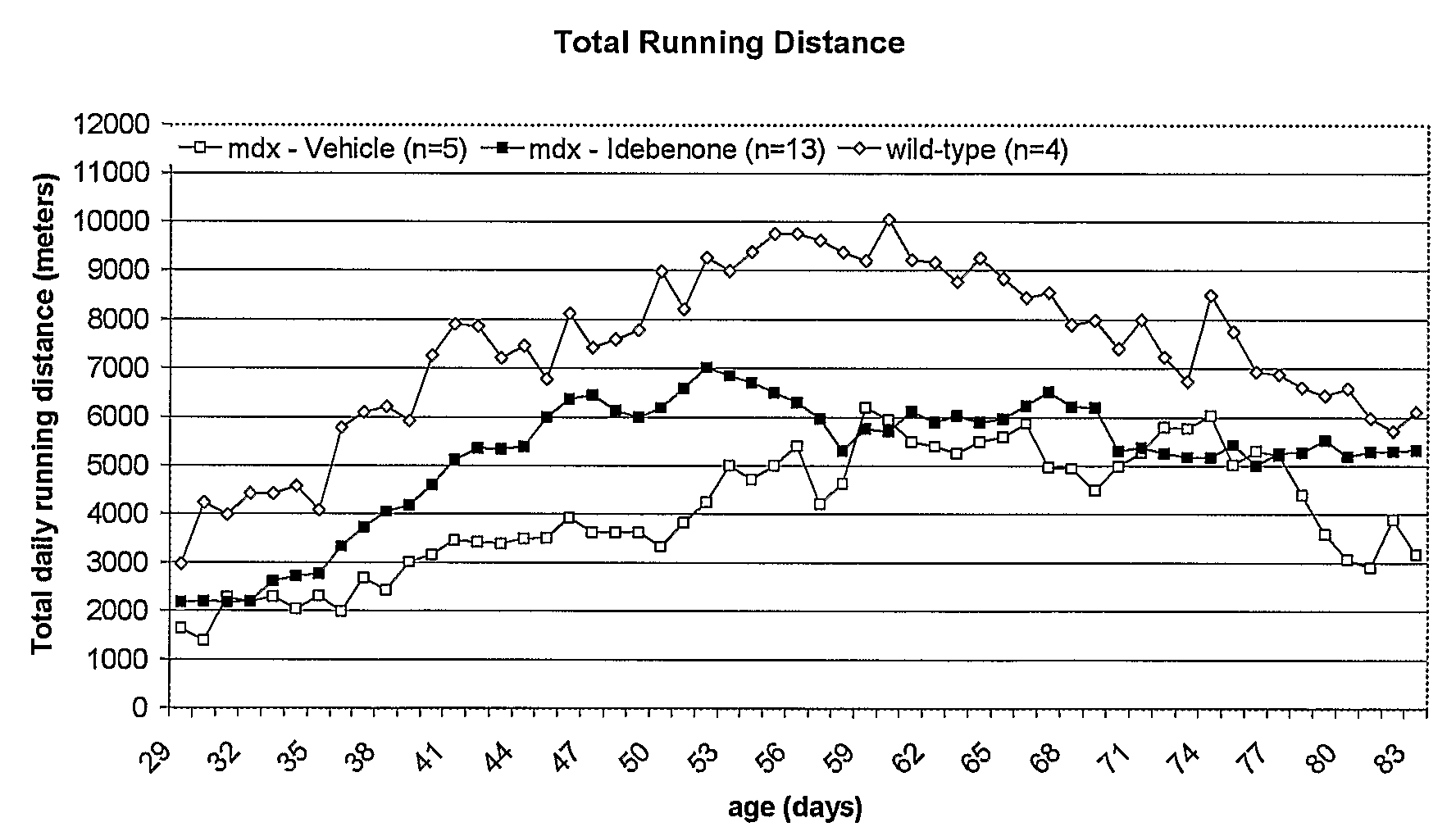
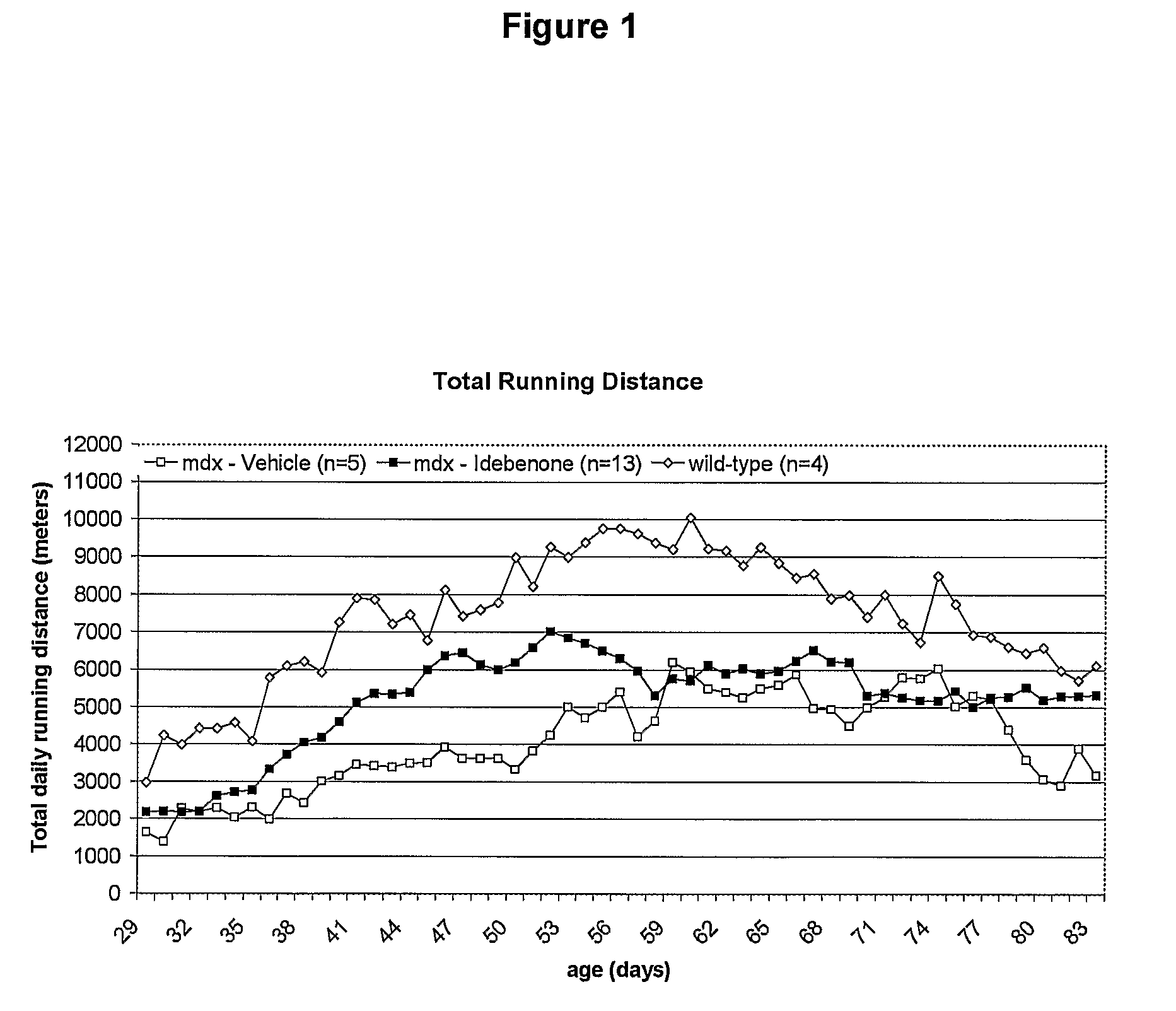
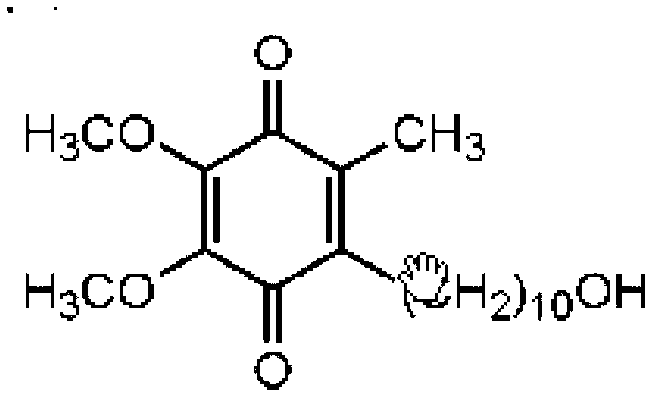
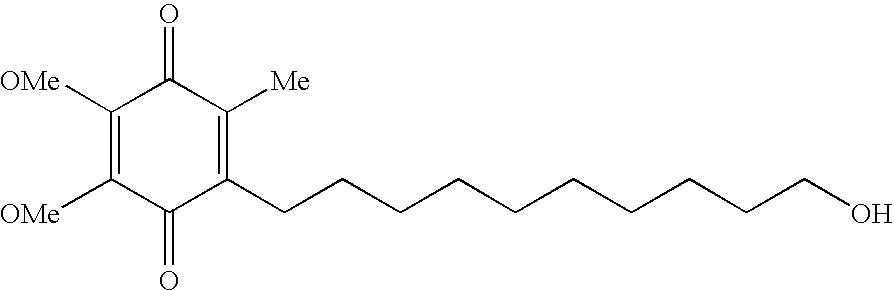
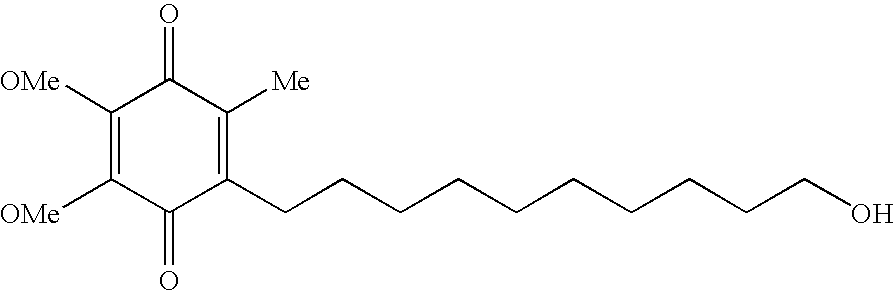
Quinone Derivative 2,3-Dimethoxy-5-Methyl-6-(10-Hydroxydecyl)-1,4-Benzoquinone for the Treatment of Muscular Dystrophies
Use of idebenone for the preparation of a medicament for the treating of a muscular dystrophy in particular for treating and / or preventing weakness and / or loss of skeletal muscle tissue and / or cardiomyopathy associated with a muscular dystrophy.
Owner:SANTHERA PHARMA SCHWEIZ
Deuterated idebenone
Owner:SUN PHARMA IND INC
A complex coacervation - spray drying method for preparing idebenone microcapsules
InactiveCN103230344AGood moisturizing effectMoisturizingCosmetic preparationsToilet preparationsSolubilityEmulsion
The present invention discloses a complex coacervation - spray drying method for preparing idebenone (IDBQ) microcapsules. The method comprises the steps of: dissolving wall materials separately in deionized water by heating, mixing according to a ratio to a wall material solution, adding in a core material idebenone, mixing and stirring uniformly for homogeneous emulsification to obtain an emulsion; and then spray drying the emulsion to be powder to obtain the idebenone microcapsules. Particles of the idebenone microcapsules provided by the invention have characteristics such as smooth outer walls, an uniform particle size, and few air holes and fissures, and have good hydrophilicity; the core material is completely wrapped in the wall materials, thereby diluting the original color of IDBN and solving problems such as water-solubility in the application of the IDBN in cosmetics; and the preparation method is simple and feasible, easy for industrial production.
Owner:广州保税区雅兰国际化妆品有限公司
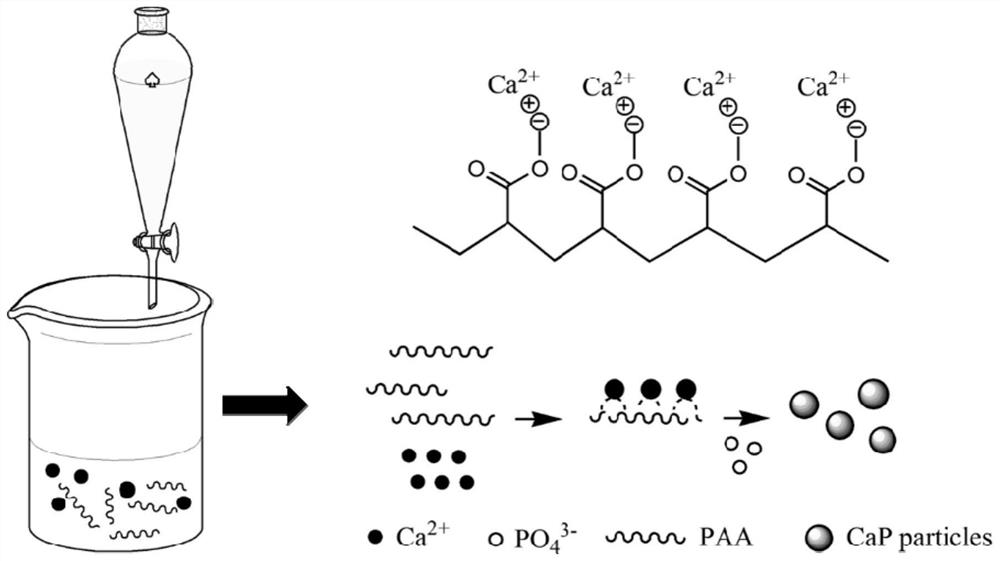
Nano calcium phosphate-loaded idebenone particles as well as preparation method and cosmetics thereof
ActiveCN111631970ASlow reaction rateGood dispersionCosmetic preparationsToilet preparationsCalcium biphosphateControl release
The invention relates to nano calcium phosphate-loaded idebenone particles as well as a preparation method and cosmetics thereof. The preparation method of the nano calcium phosphate-loaded idebenoneparticles comprises the following steps: mixing sodium polyacrylate and a calcium ion solution, and carrying out complexation reaction to obtain a first mixed solution; mixing the first mixed solutionwith a phosphate solution, controlling the molar ratio of calcium atoms in the first mixed solution to phosphorus atoms in the phosphate solution to be (1.5-1.8):1, and performing reaction for 12-24hours at a temperature of 25-90 DEG C to obtain a second mixed solution containing calcium phosphate particles; and mixing an ethanol solution of idebenone with the second mixed solution for loading to obtain the nano calcium phosphate-loaded idebenone particles. The nano calcium phosphate-loaded idebenone particles prepared by the method can be well dispersed in water, are good in stability, andcan realize controlled release under the weakly acidic condition of skin.
Owner:HUNAN YUJIA COSMETICS MFG CO LTD
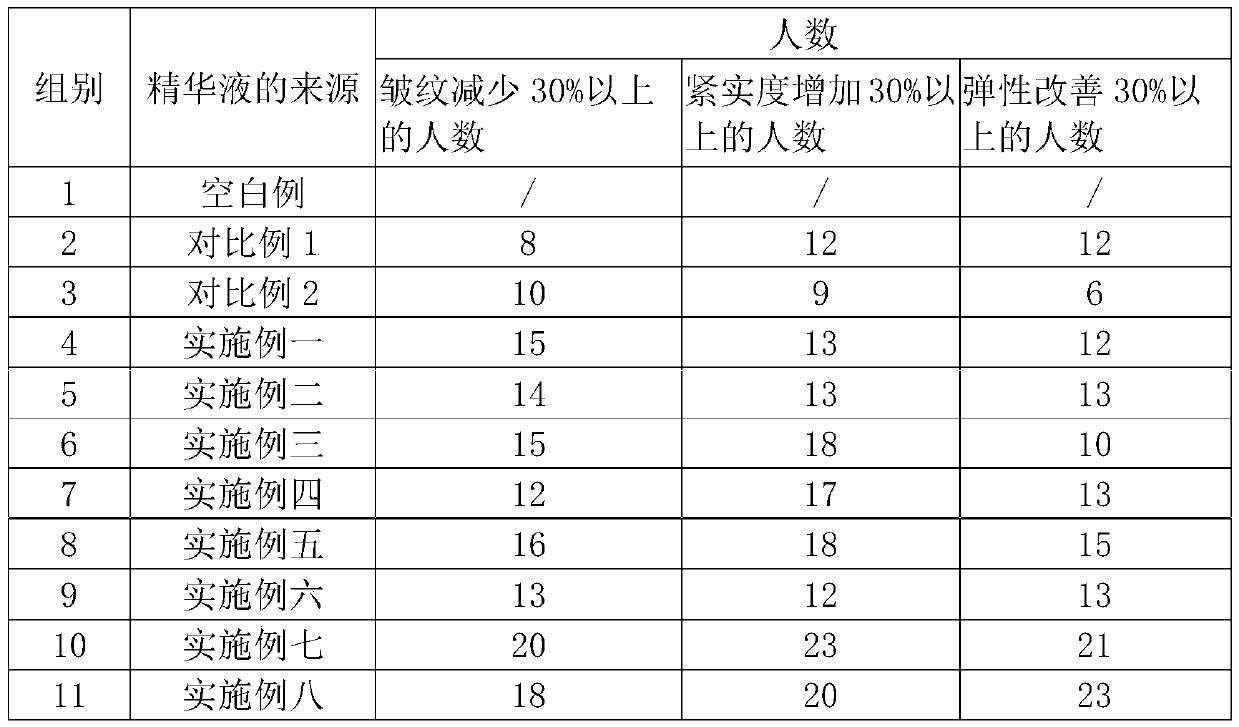
Wrinkle-removing essence and preparation method thereof
PendingCN111544330AProtection against injuryReduce churnCosmetic preparationsToilet preparationsBiotechnologyIdebenone
The invention discloses wrinkle-removing essence and a preparation method thereof. The wrinkle-removing essence comprises the following components in parts by weight: 0.3-1 part of astaxanthin, 0.2-1.5 parts of a green tea extract, 0.1-2 parts of idebenone, 0.2-2 parts of procyanidine, and 0.5-5 parts of collagen. The astaxanthin, the green tea extract, the idebenone, the procyanidine, the collagen and other materials are compounded, and the wrinkle-removing essence has strong effects of resisting oxidation, scavenging free radicals, resisting aging and the like on the skin, supplements necessary nutritional ingredients for the skin, promotes metabolism of the skin, enables the skin to be moisturized and glossy, and emits healthy luster, thereby enabling the skin to have a good anti-wrinkle effect.
Owner:广州市茗妍化妆品有限公司
Quinone derivative 2,3-dimethoxy-5-methyl-6-(10-hydroxydecyl)-1,4-benzoquinone for the treatment of primary progressive multiple sclerosis
InactiveUS20100280130A1Treatment safetyRelieve symptomsBiocideNervous disorderProgressive multiple sclerosisMS multiple sclerosis
The present invention relates to approaches, methods, pharmaceuticals and uses directed to the curative treating or preventing of Primary Progressive Multiple Sclerosis (PP-MS), by using 2,3-dimethoxy-5-methyl-6-(10-hydroxydecyl)-1,4-benzoquinone (Idebenone) as the active agent.
Owner:UNITED STATES OF AMERICA +1
Idebenone-embedded nanolipid carrier as well as preparation method and application thereof
InactiveCN111249185AImprove stabilityHigh embedding rateCosmetic preparationsToilet preparationsPolyolIdebenone
The invention provides an idebenone-embedded nanolipid carrier as well as a preparation method and application thereof, and relates to the technical field of cosmetics. The nanolipid carrier providedby the invention includes the following components in percentages by mass: 10-30% of macadamia nut oil, 5-15% of palm wax, 1-5% of idebenone, 6-10% of a surfactant, 1-5% of polyol, 0.5-1.5% of lecithin, and the balance of water. According to the carrier, the macadamia nut oil, the palm wax, the surfactant, the polyol and the lecithin are used together to coat the idebenone, so that the provided idebenone-embedded nanolipid carrier has the advantages of good stability, a high embedding rate, a high drug loading amount and good skin absorption, and can be well applied in cosmetics. The inventionprovides a preparation method of the idebenone-embedded nanolipid carrier. The preparation method provided by the invention adopts a heat high-pressure homogenization technology, and can effectivelyrealize the stable embedding of the idebenone by the nano-lipid carrier.
Owner:上海格兰化妆品有限公司
Composite plant whitening and moisturizing essence and preparation technology thereof
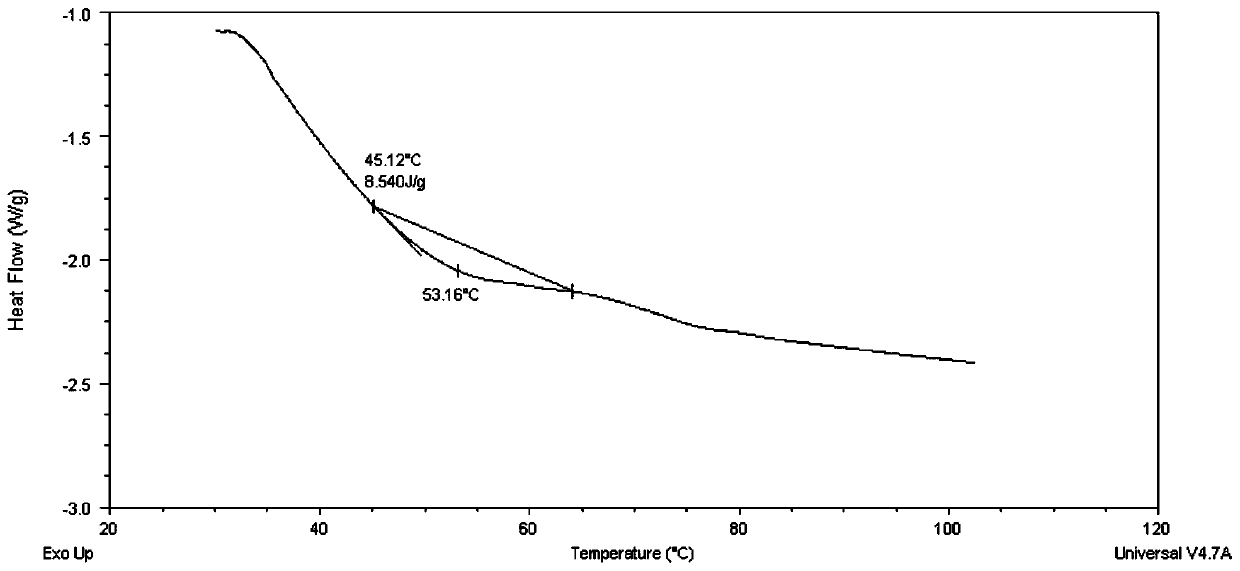
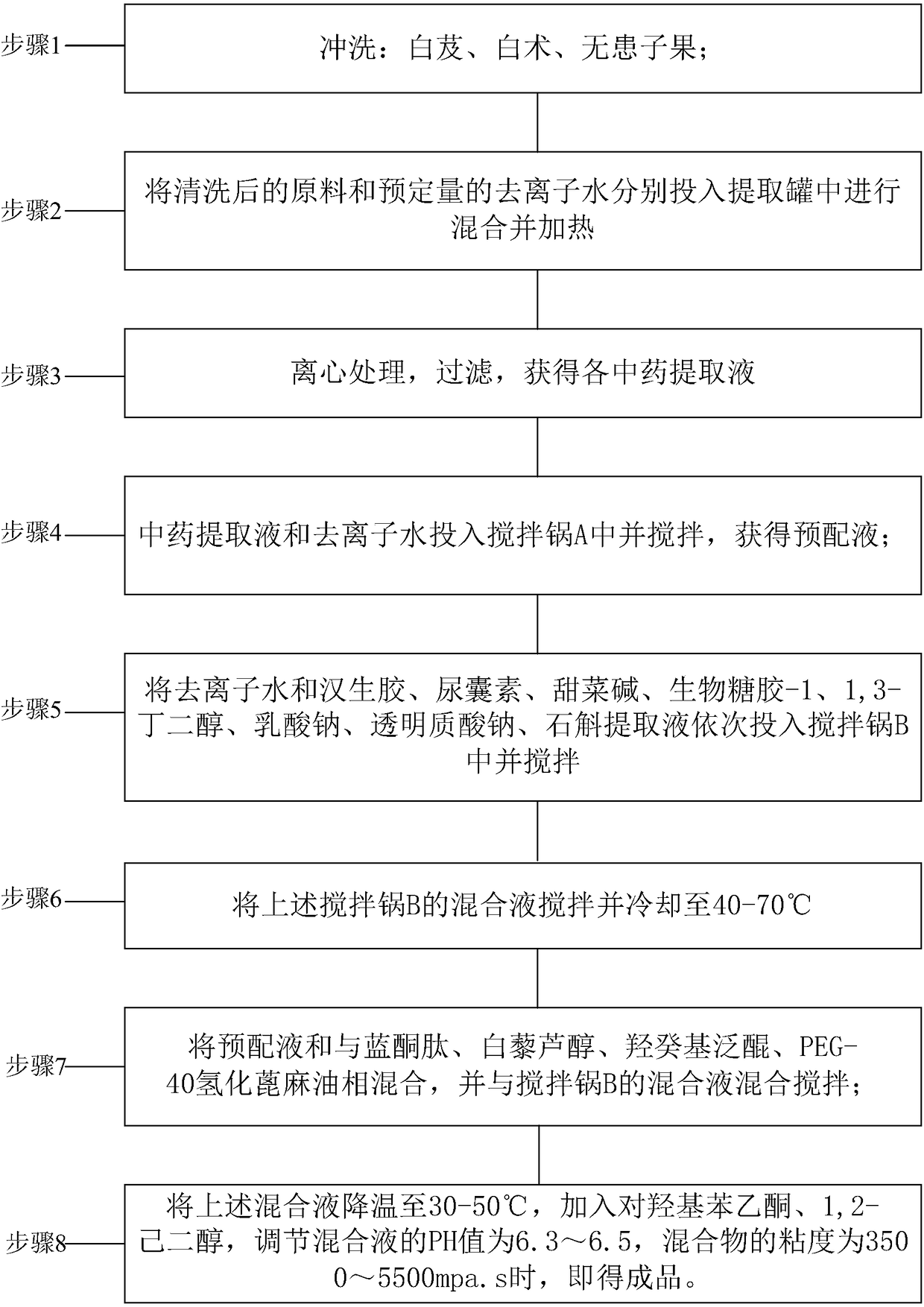
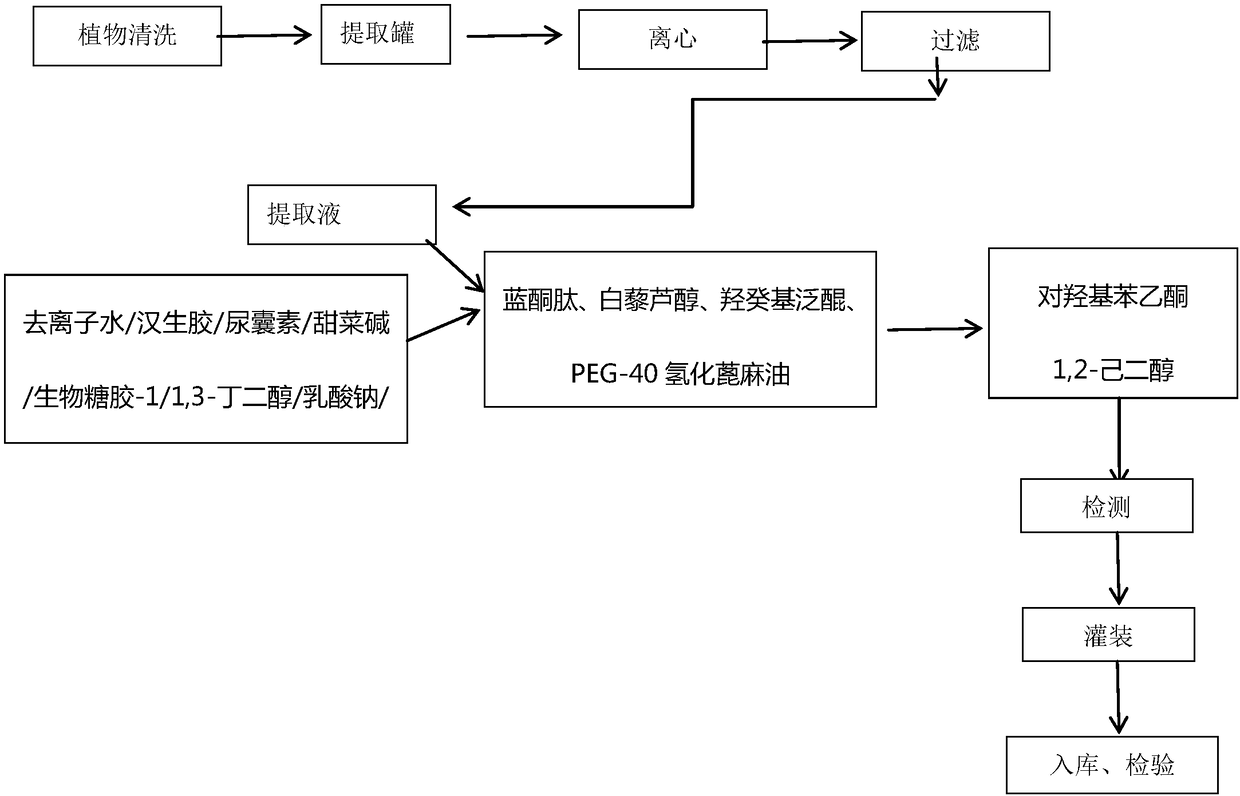
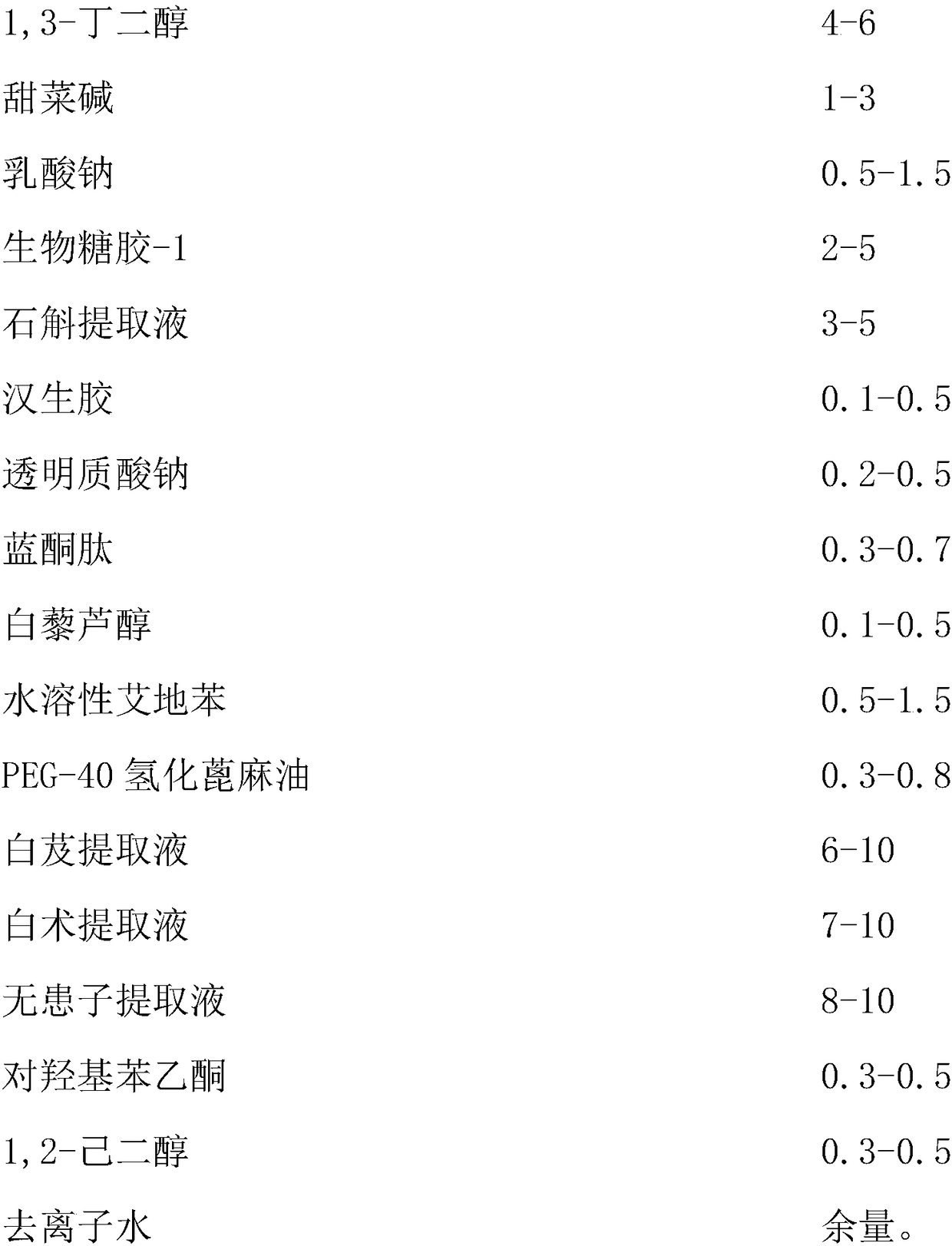
InactiveCN108261366AAvoid irritationInhibit synthesisCosmetic preparationsToilet preparationsSodium lactateBletilla striata
The invention discloses a composite plant whitening and moisturizing essence and a preparation technology thereof. The composite plant whitening and moisturizing essence comprises: Chinese herb extracted liquid: bletilla striata extracted liquid, bighead atractylodes rhizome extracted liquid and sapindus extracted liquid, and excipients: 1, 3-butanediol, betaine, sodium lactate, bio-saccharide gum-1, dendrobium extracted liquid, xanthan gum, sodium hyaluronate, blue copper peptide, resveratrol, water soluble idebenone and the like. The composite plant whitening and moisturizing essence adoptsbletilla striata extracted liquid, bighead atractylodes rhizome extracted liquid, sapindus extracted liquid and other Chinese herbal plants, avoids irritation to human skin, achieves facial and body moisturizing and whitening care, inhibits the synthesis of melanin, keeps the face and body skin young, permeable and fair-skinned, makes the skin soft, smooth and elastic, and at the same time can meet the water replenishment requirement of skin.
Owner:广州重生化妆品实业有限公司
Idebenone nanometer lipid carrier transdermal absorption preparation and preparation method thereof
InactiveCN102091038BAvoid residueEnsure safetyOrganic active ingredientsNervous disorderIdebenoneActive agent
The invention discloses an idebenone nanometer lipid carrier transdermal absorption preparation and a preparation method thereof. The preparation method comprises the following steps: mixing idebenone, solid lipid materials, liquid lipid materials and a surface active agent 1 uniformly, and heating until the mixture is turned into a lipid phase; mixing a surface active agent 2 with ultrapure water uniformly so that the mixture is turned into a water phase; adding the lipid phase into the water phase slowly under the condition of keeping the temperature of the lipid phase and the water phase consistent while stirring; homogenizing rapidly, cutting, carrying out primary emulsification, carrying out ultrasonication again, and cooling down, so that a lipid carrier of a nanometer structure loading idebenone is prepared; and adding pharmaceutically acceptable auxiliary materials, so that the idebenone nanometer lipid carrier transdermal absorption preparation is prepared. The particle diameter of the idebenone nanometer lipid carrier transdermal absorption preparation provided by the invention is 40.2-110.2nm, the entrapment rate is 80.5%-99.1%, the zeta potential is minus 25.5-minus 34.4mV, the morphology is stable, an organic solvent is not contained, the idebenone nanometer lipid carrier transdermal absorption preparation has good compatibility with skin and has no irritation, the first pass effect is avoided, and the bioavailability is improved.
Owner:TIANJIN UNIV
Quinone derivative 2,3-dimethoxy-5-methyl-6-(10-hydroxydecyl)-1,4-benzoquinone for the treatment of muscular dystrophies
Owner:SANTHERA PHARMA SCHWEIZ
Method for preparing idebenone
The invention relates to a preparation method for idebenone, comprising the following steps: (1), 3,4,5-trimethoxy-toluene has friedel-crafts reaction with 10-acetoxy-decane chloride or 10-acetoxy-decanoyl- bromine to generate 6-(10-acetoxy-1-oxo-decane yl)-2,3-dimethoxy-5-methyl phenol; (2), the 6-(10-acetoxy-1-oxo-decane yl)-2,3-dimethoxy-5-methyl phenol obtained in the step (1) is reacted withhydrogen under the joint effect of palladium-carbon catalyst and dehydrating agent DCC to generate 6-(10-acetoxy-decyl)-2,3-dimethoxy-5-methyl phenol; (3), the6-(10-acetoxy-decyl)-2,3-dimethoxy-5-methyl phenol prepares 6-(10-hydroxy decyl) -2,3-dimethoxy-5-methyl phenol through hydrolysis; (4), the 6-(10-hydroxy decyl) -2,3-dimethoxy-5-methyl phenol has oxidation reaction with an oxygen-supply body under the effect of catalytic oxidizer Cu (Salen) so as to generate the idebenone. The preparation method has low cost and high yield, which is suitable for large-scale industrial idebenone production.
Owner:SUZHOU LIXIN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com