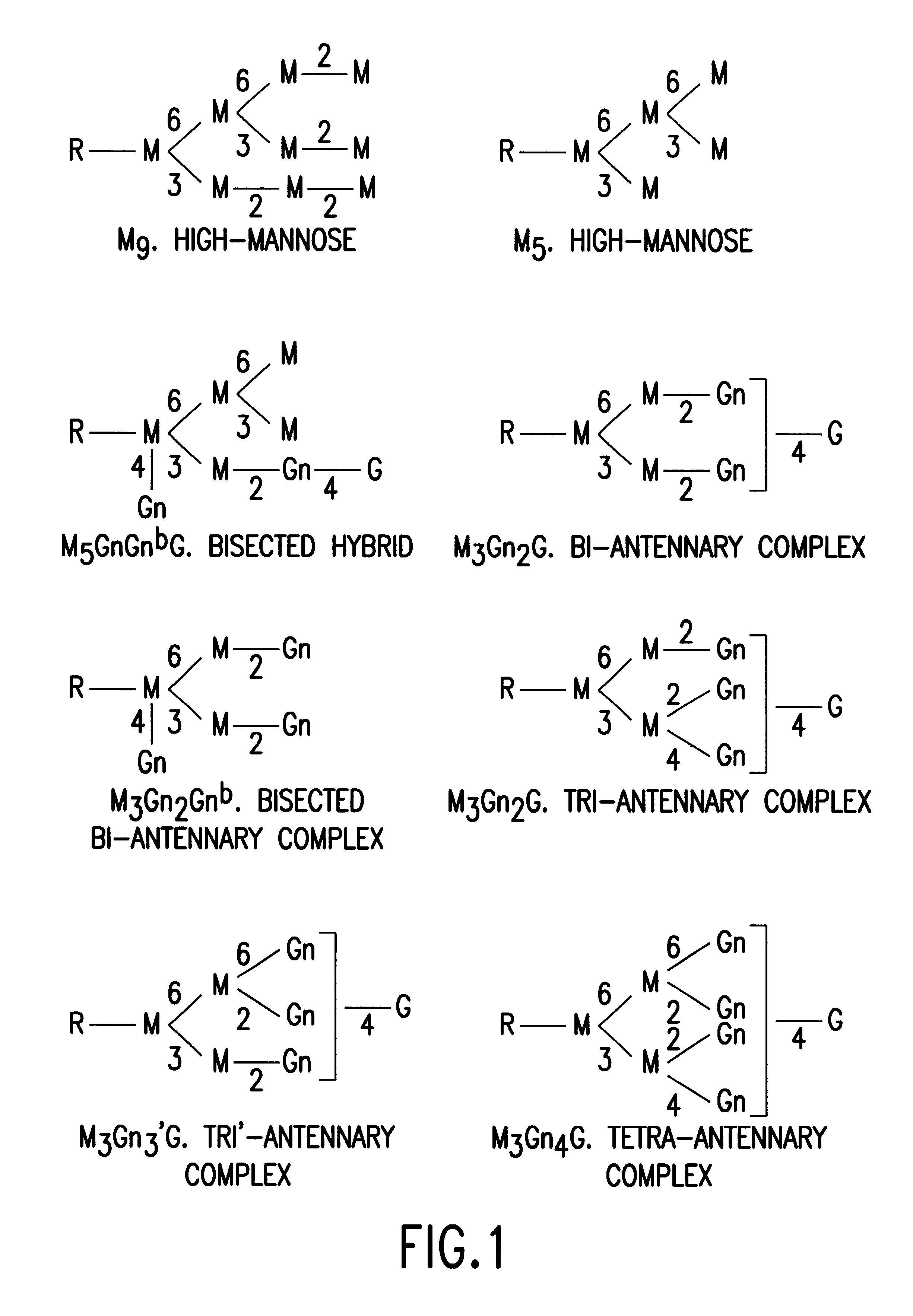
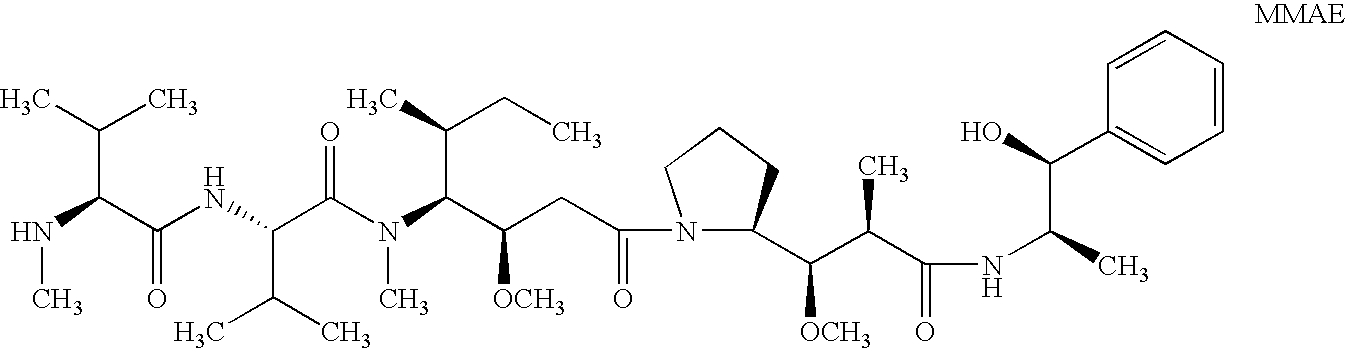
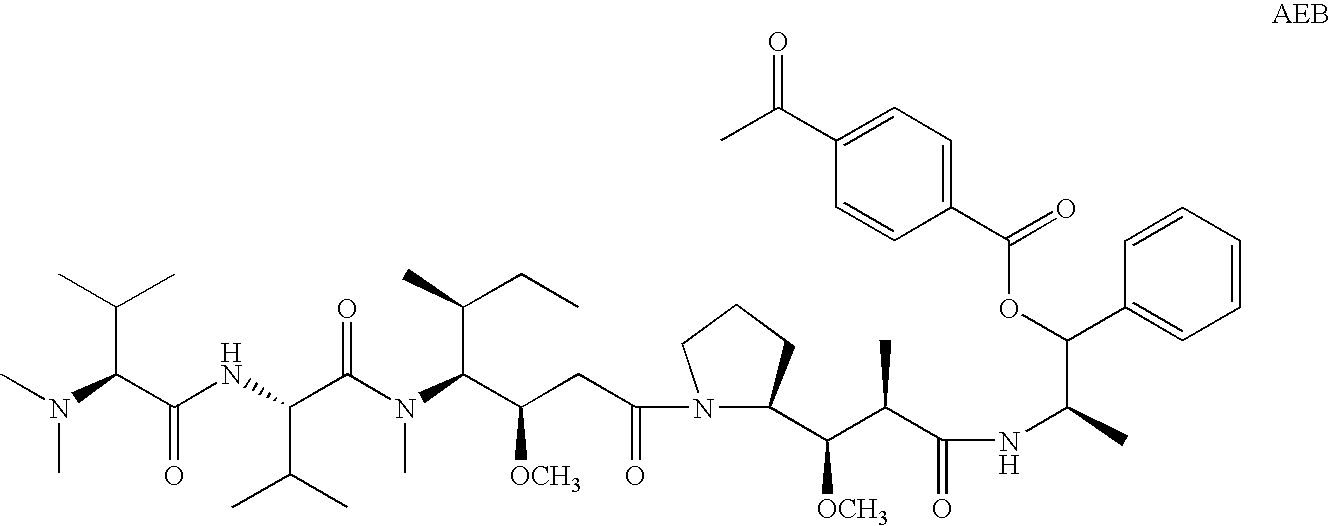
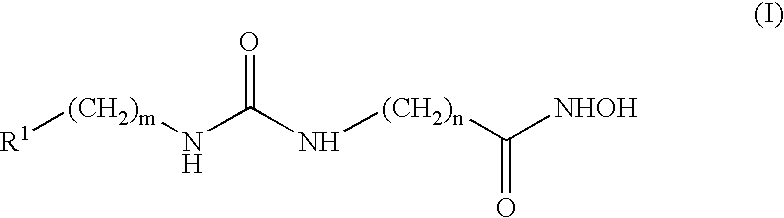
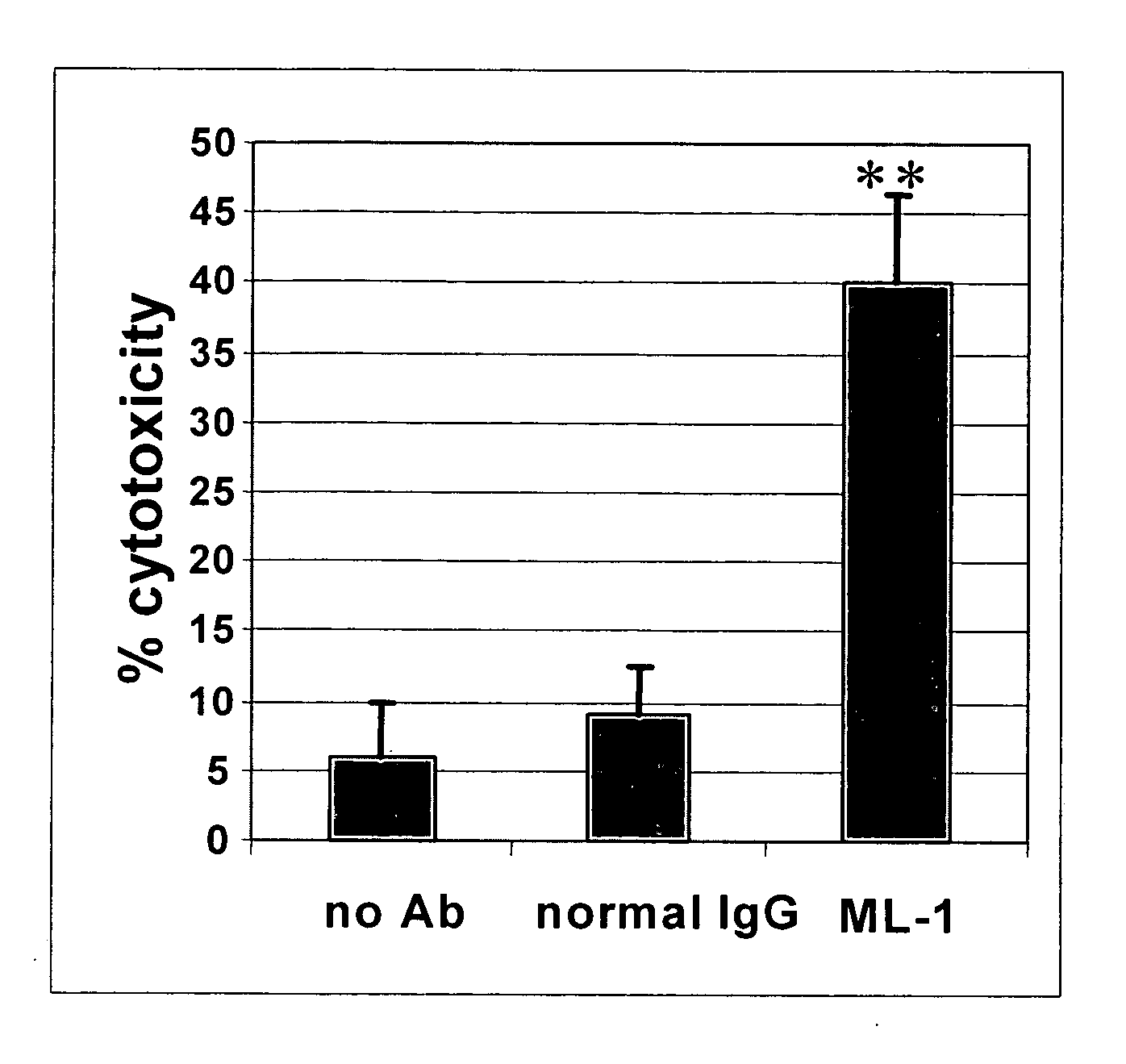
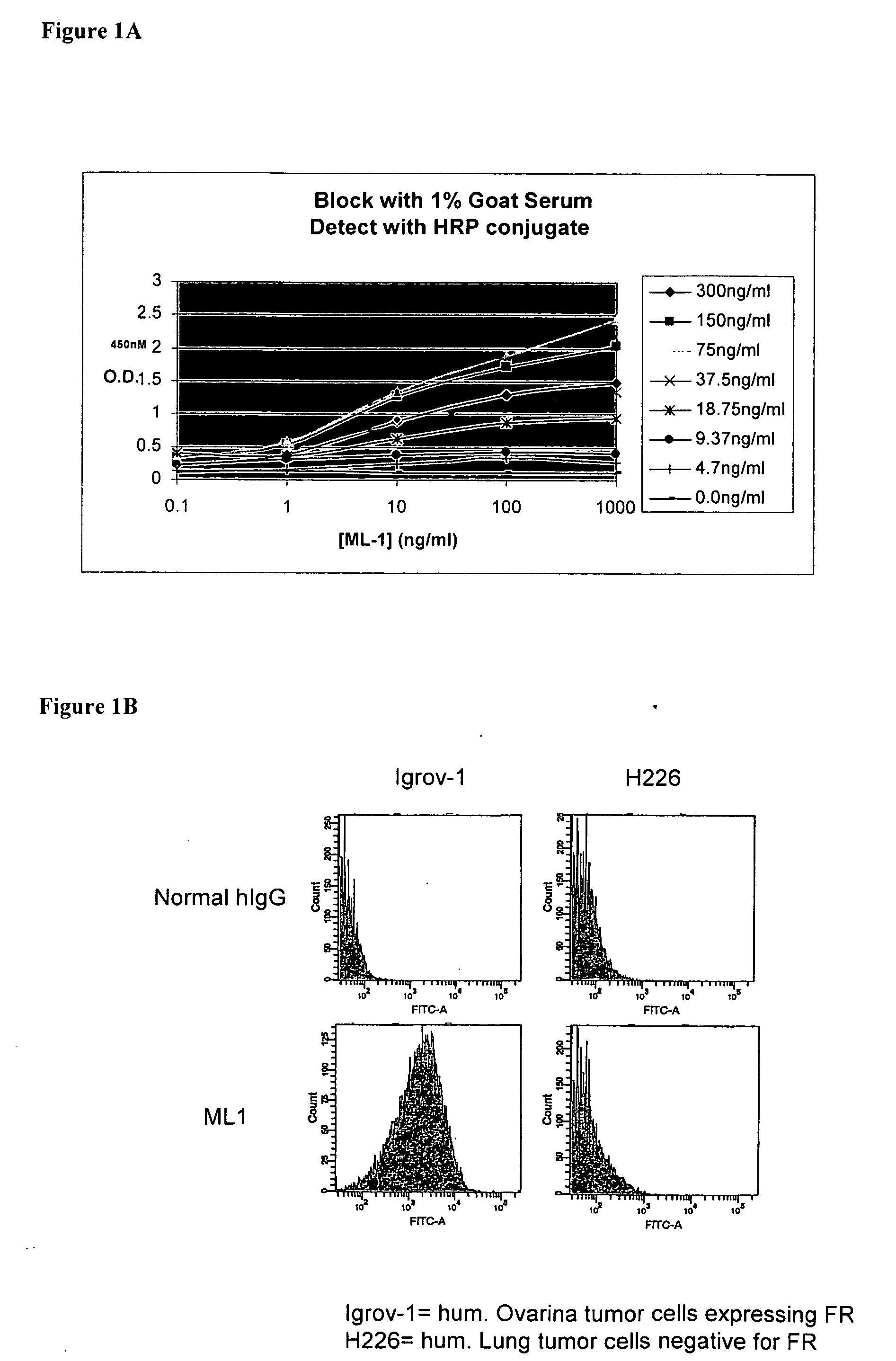
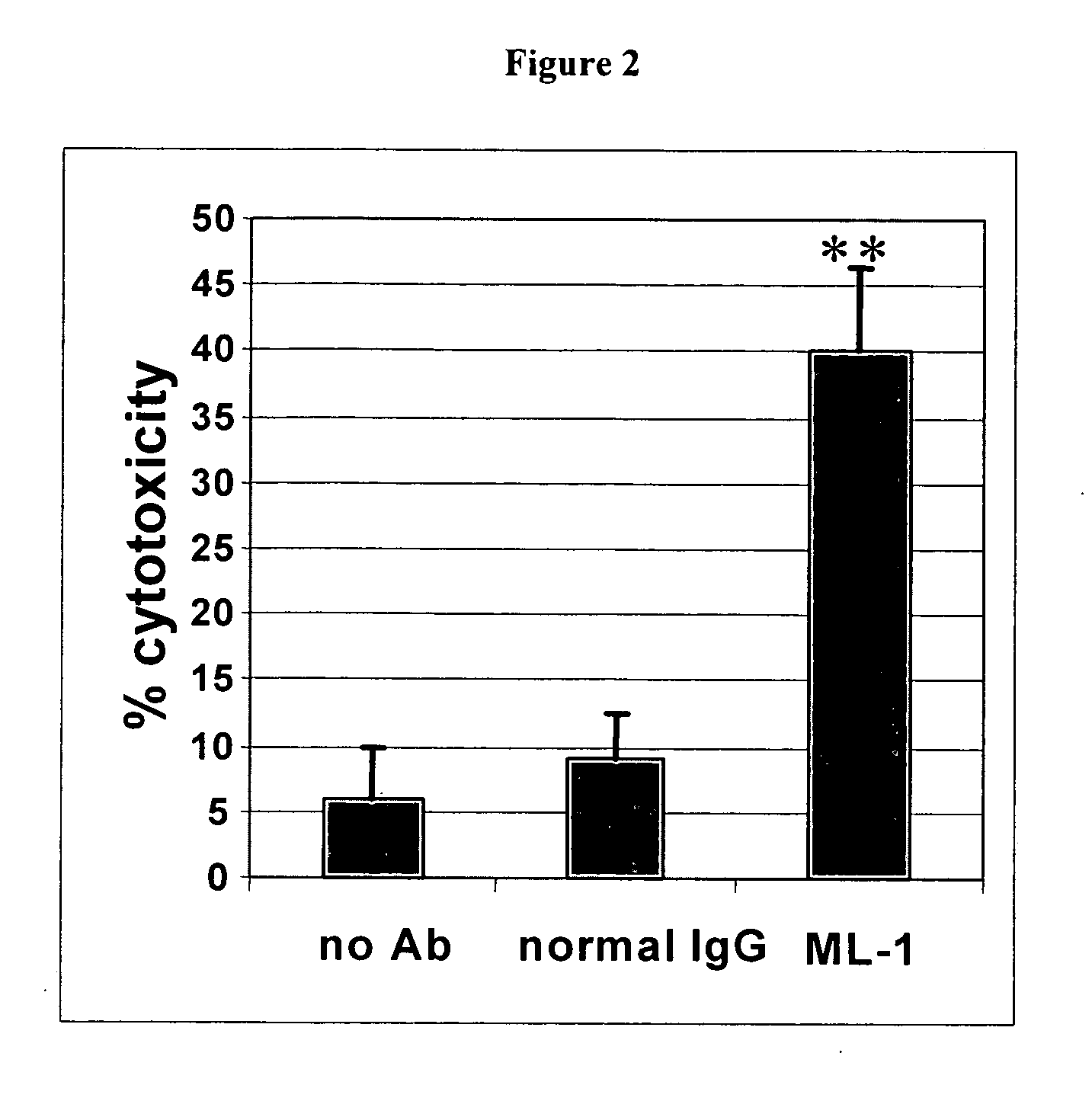
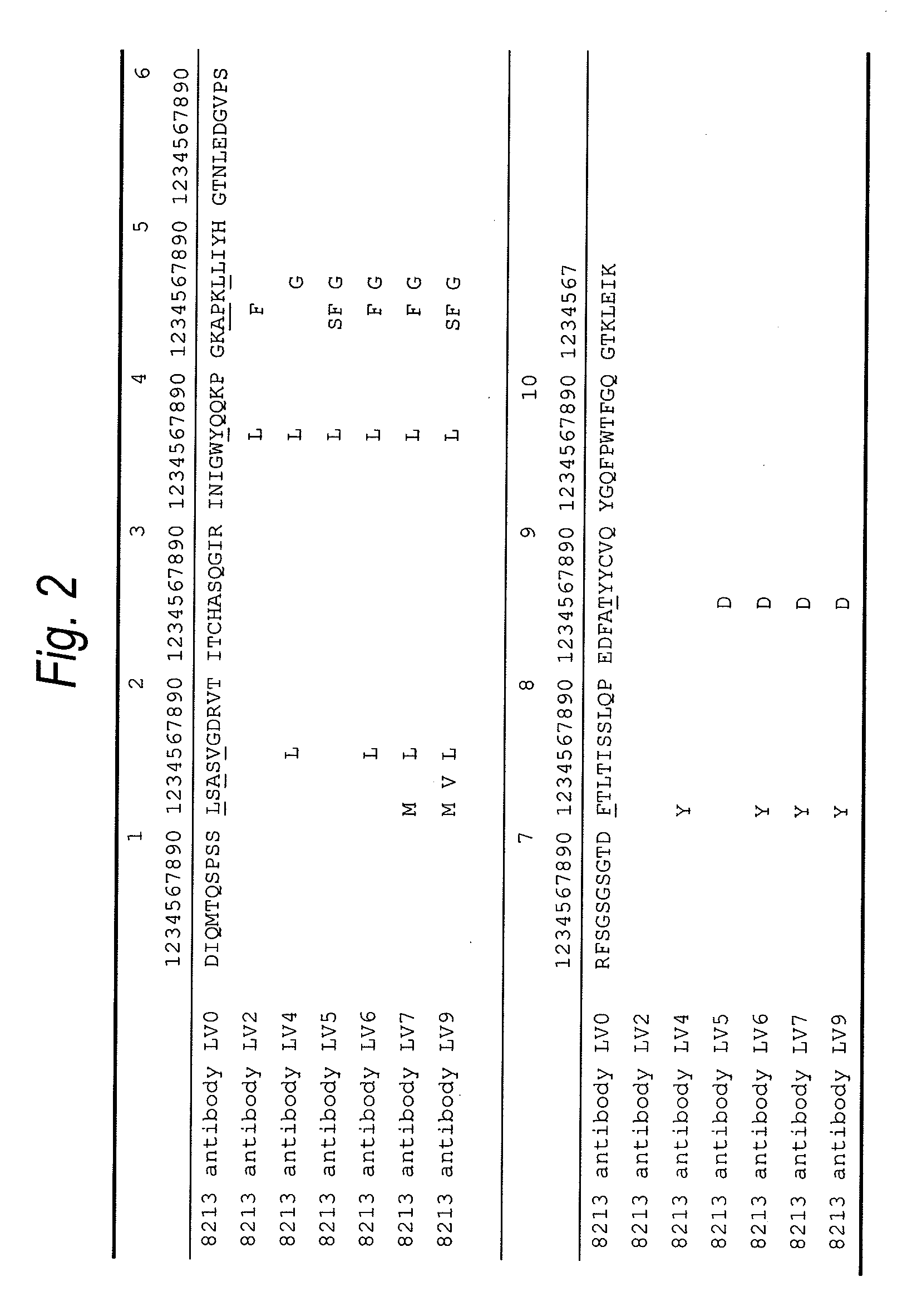
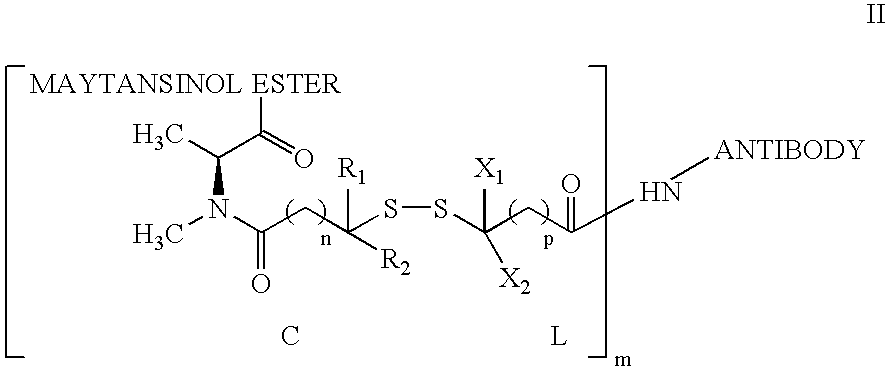
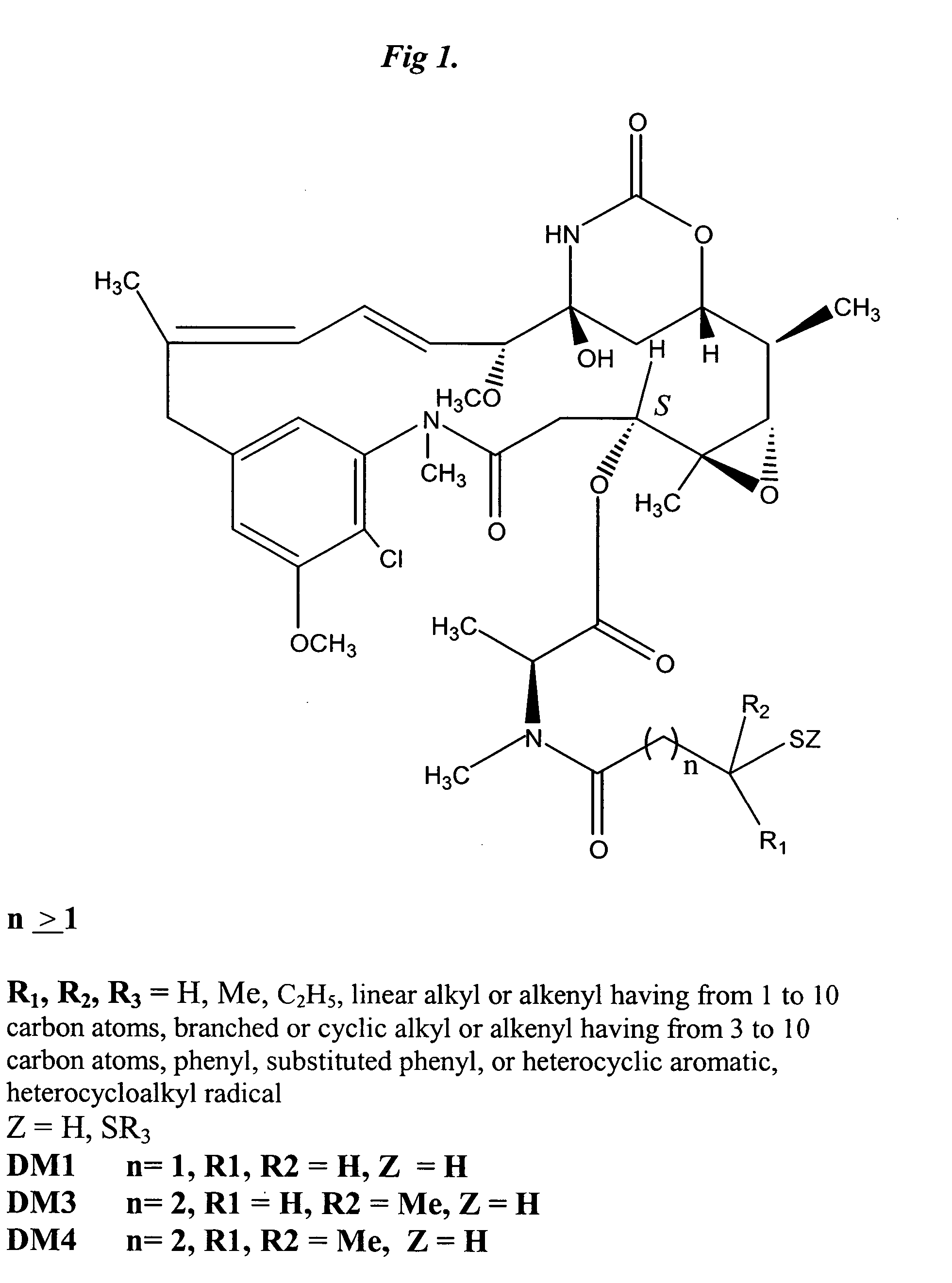
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1698 results about "Cellular Cytotoxicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Antibody-dependent cell-mediated cytotoxicity (ADCC) (antibody-dependent cellular cytotoxicity) lysis of target cells coated with antibody by effector cells with cytolytic activity and specific immunoglobulin receptors called Fc receptors, including K cells, macrophages, and granulocytes.
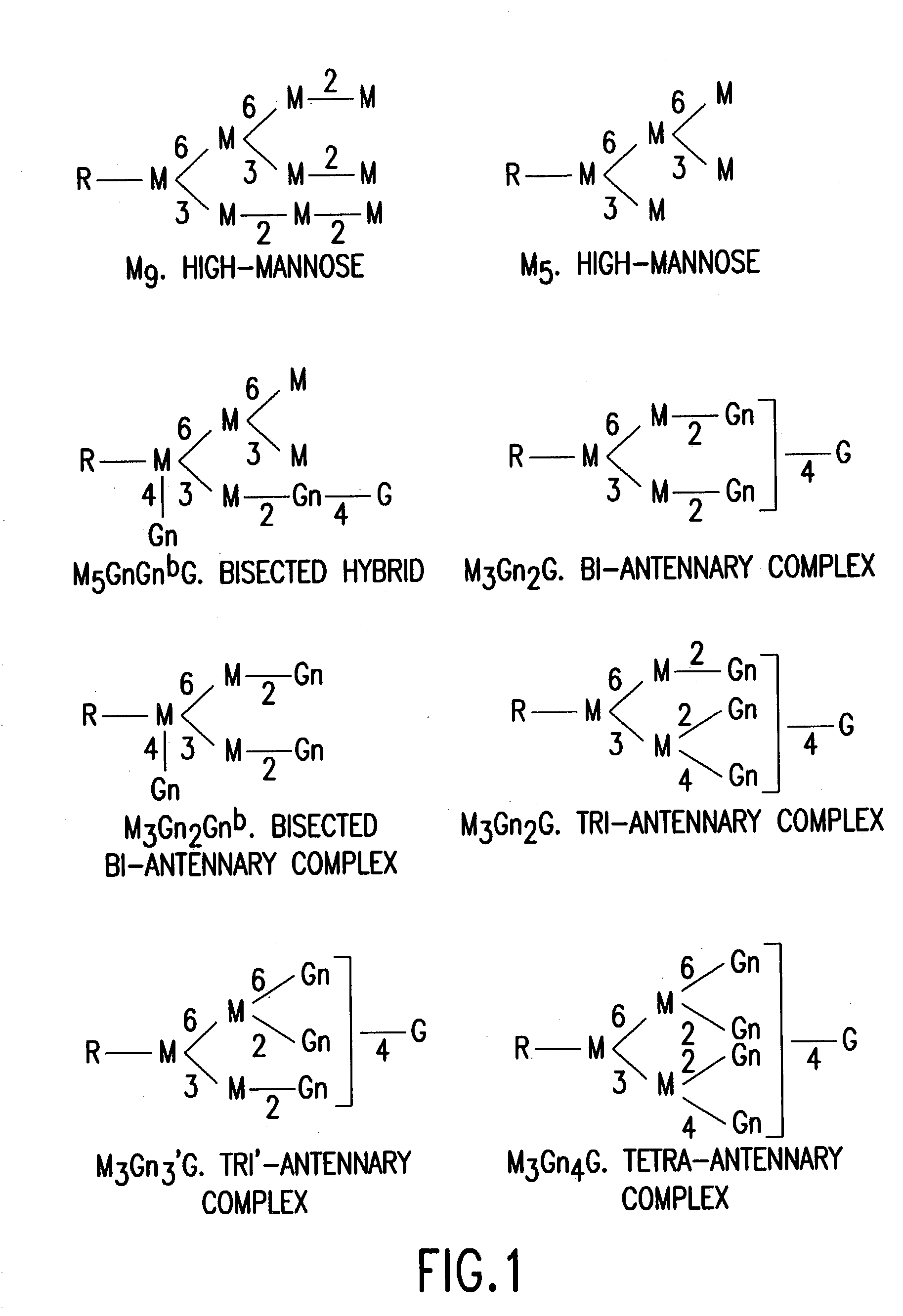
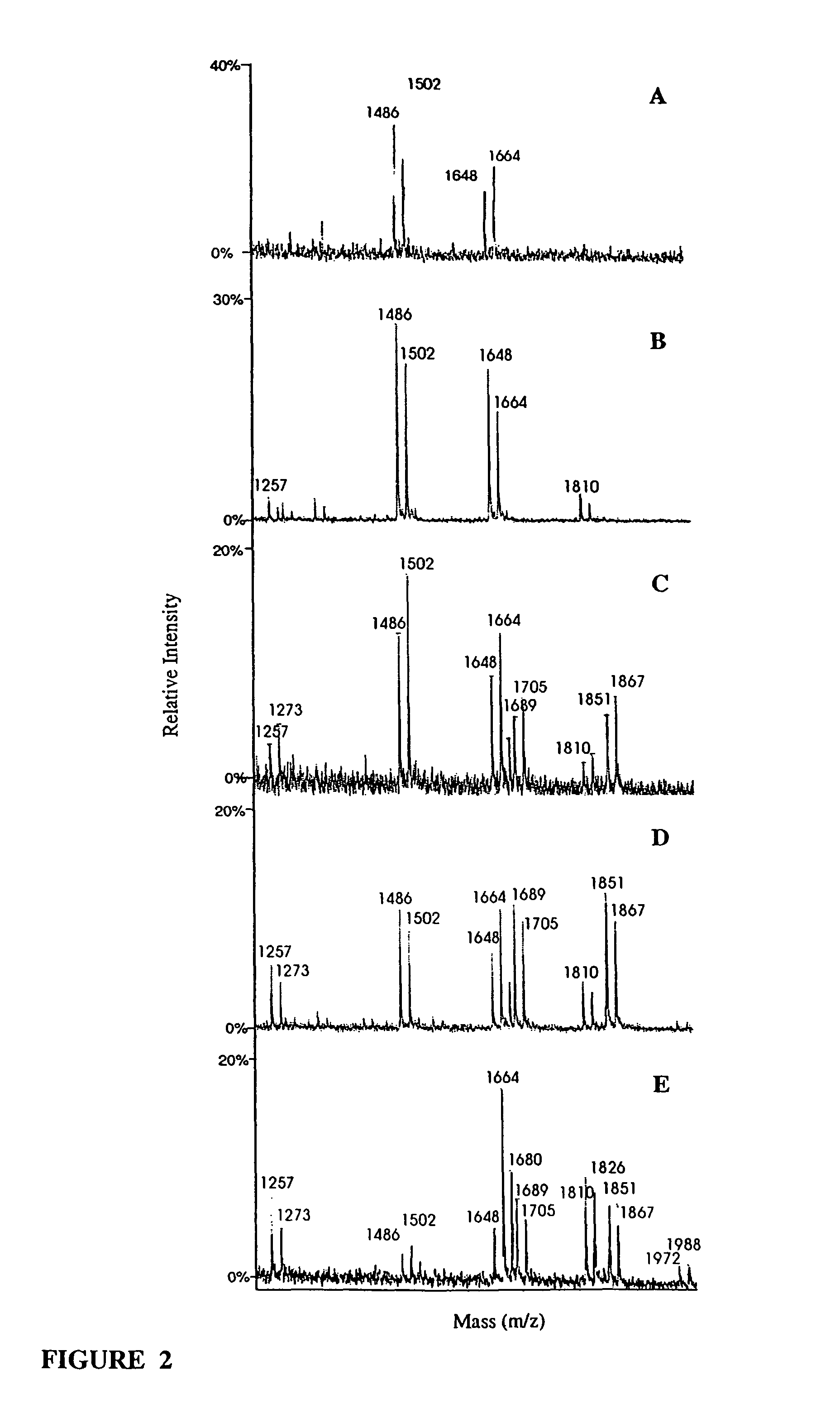
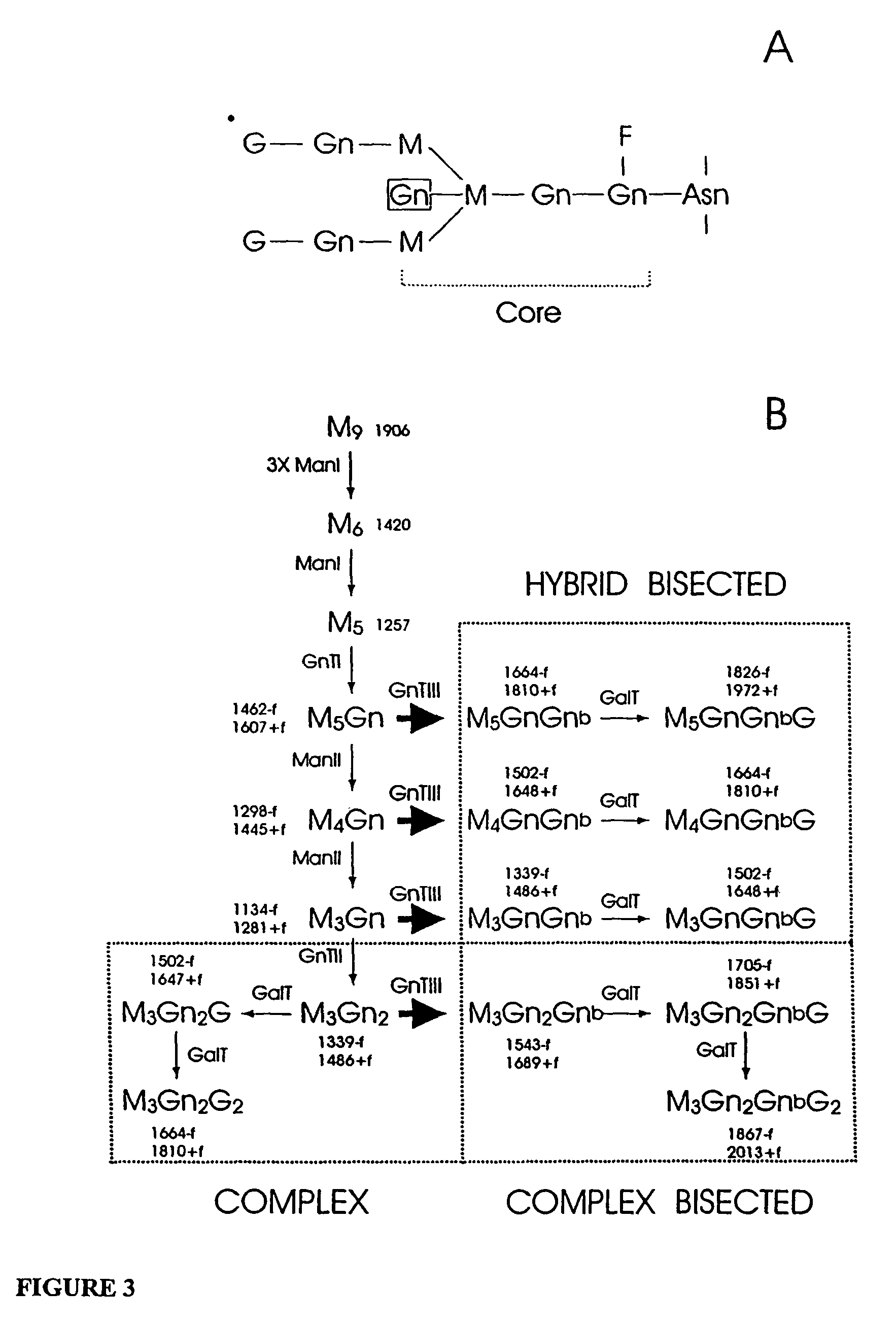
Glycosylation engineering of antibodies for improving antibody-dependent cellular cytotoxicity
InactiveUS6602684B1Increase healing valueEnhanced Fc-mediated cellular cytotoxicityNanotechFungiAntibody fragmentsADAMTS Proteins
The present invention relates to the field glycosylation engineering of proteins. More particular, the present invention is directed to the glycosylation engineering of proteins to provide proteins with improved therapeutic properties, e.g., antibodies, antibody fragments, or a fusion protein that includes a region equivalent to the Fc region of an immunoglobulin, with enhanced Fc-mediated cellular cytotoxicity.
Owner:ROCHE GLYCART AG
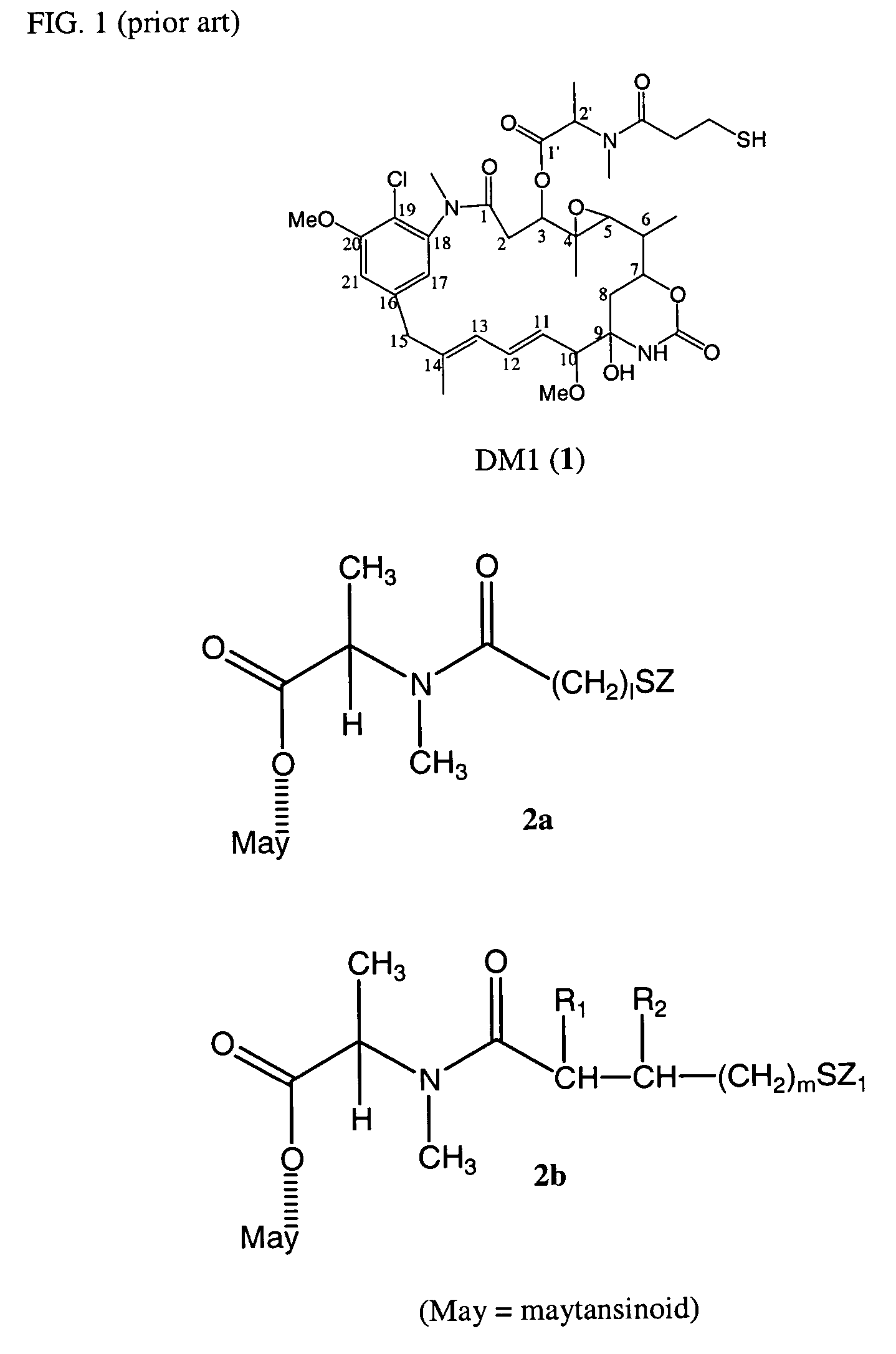
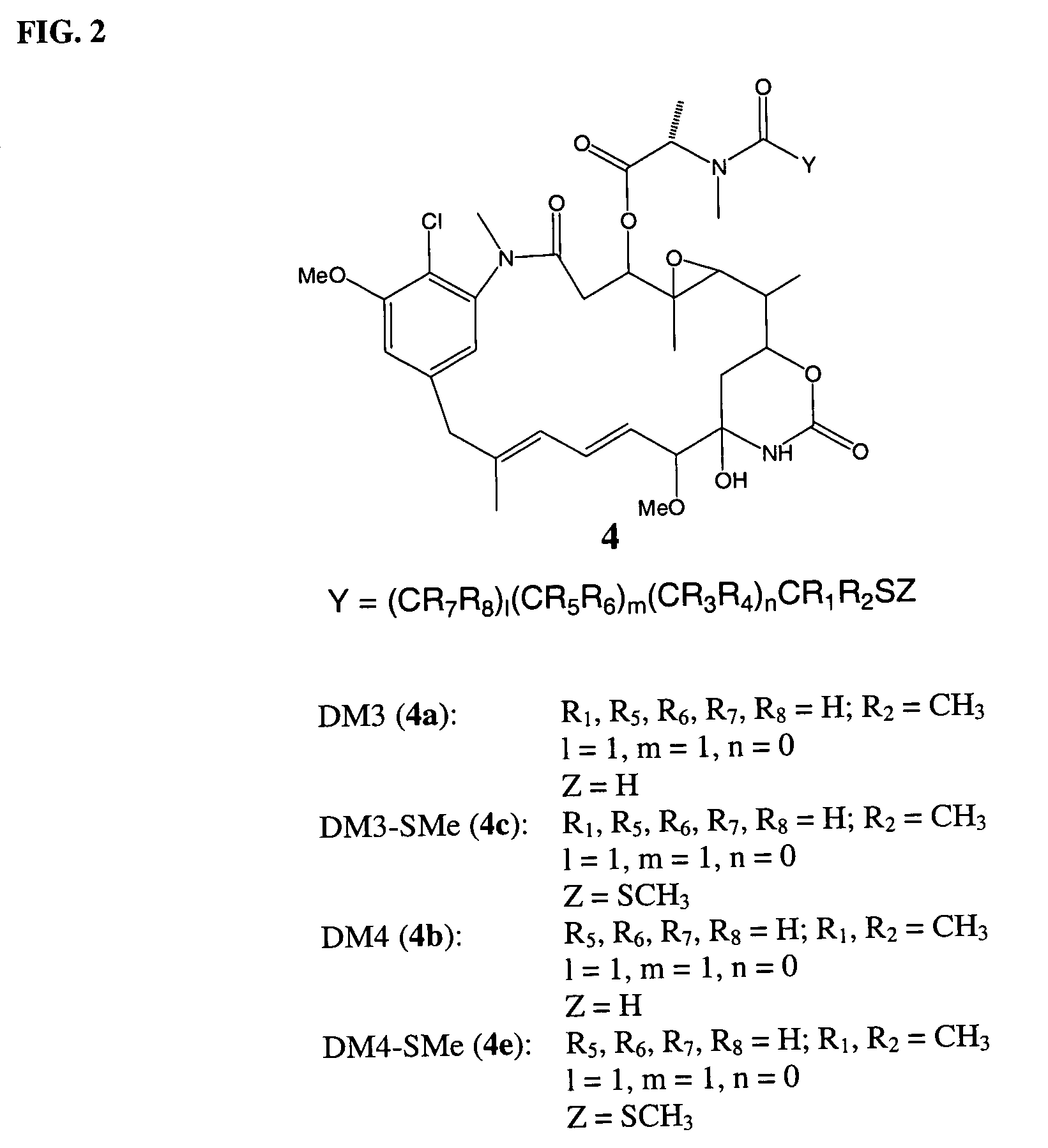
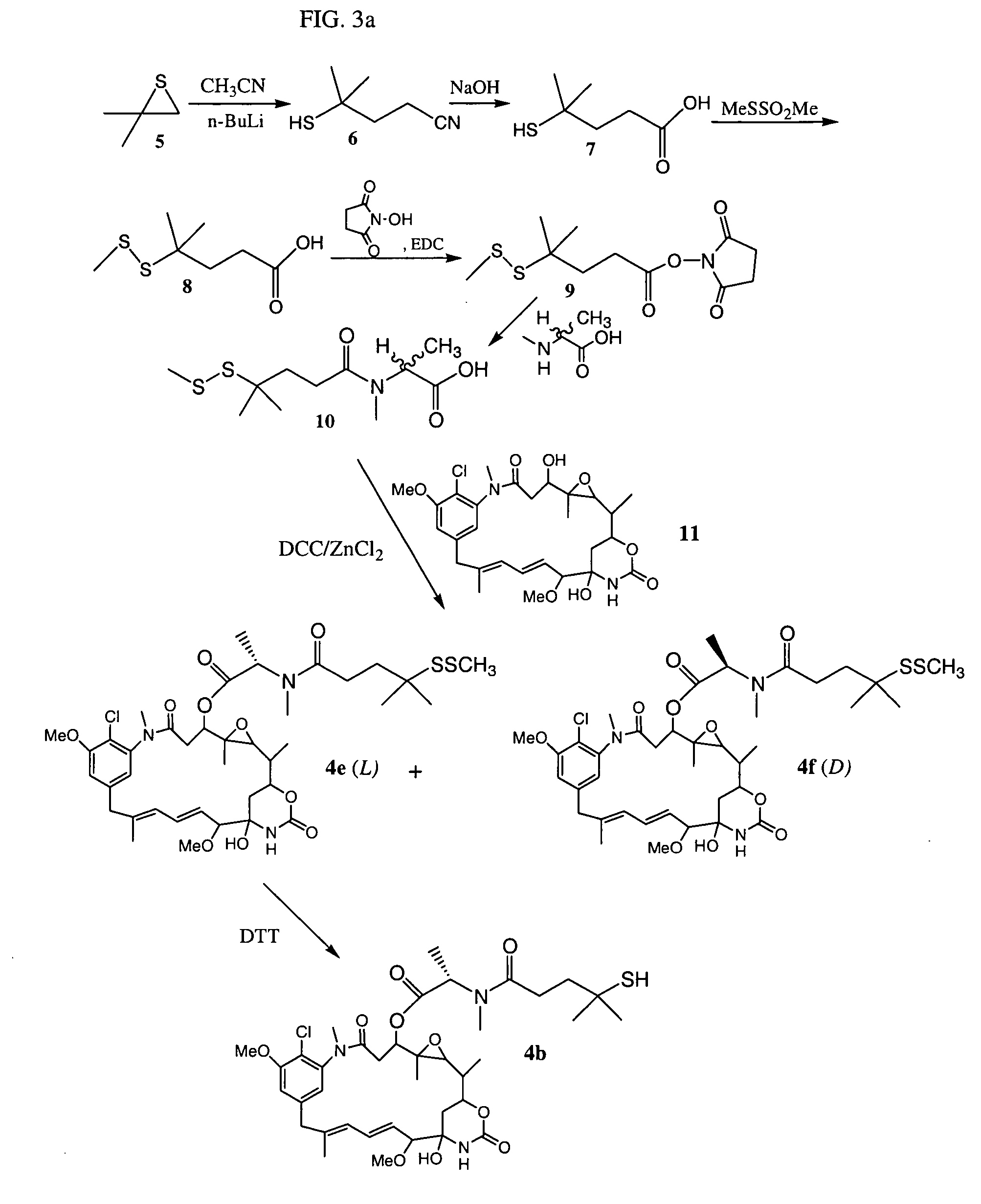
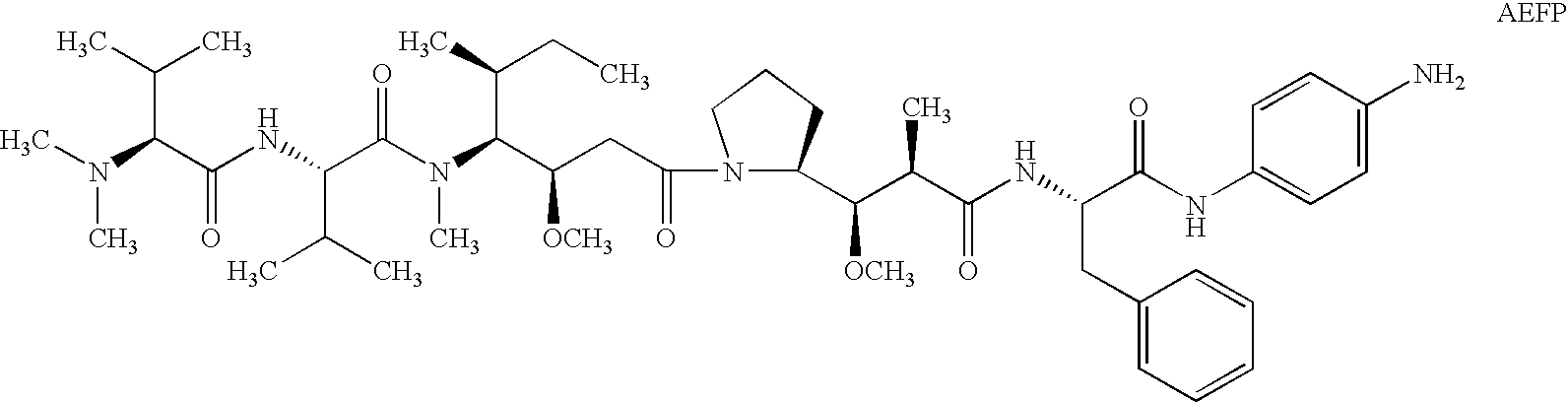
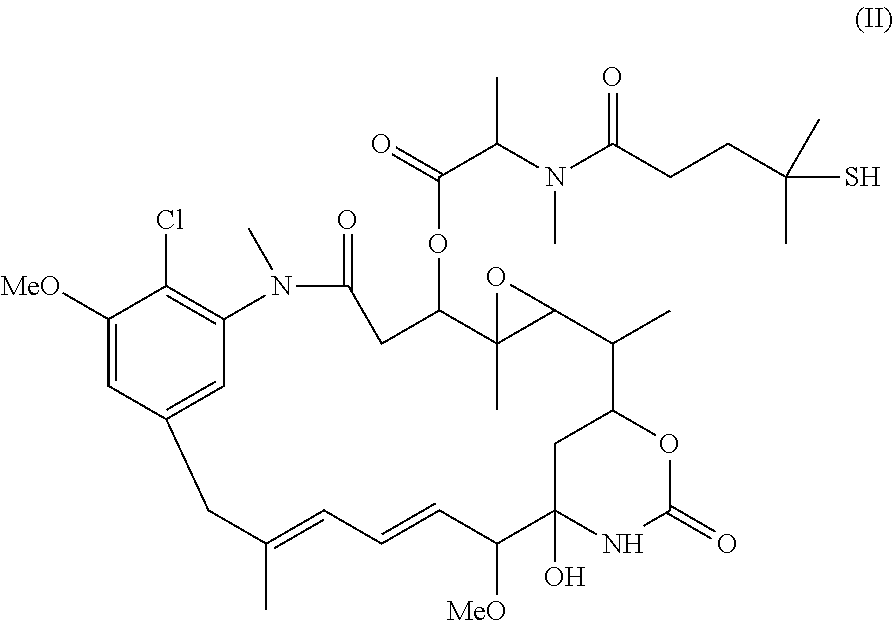
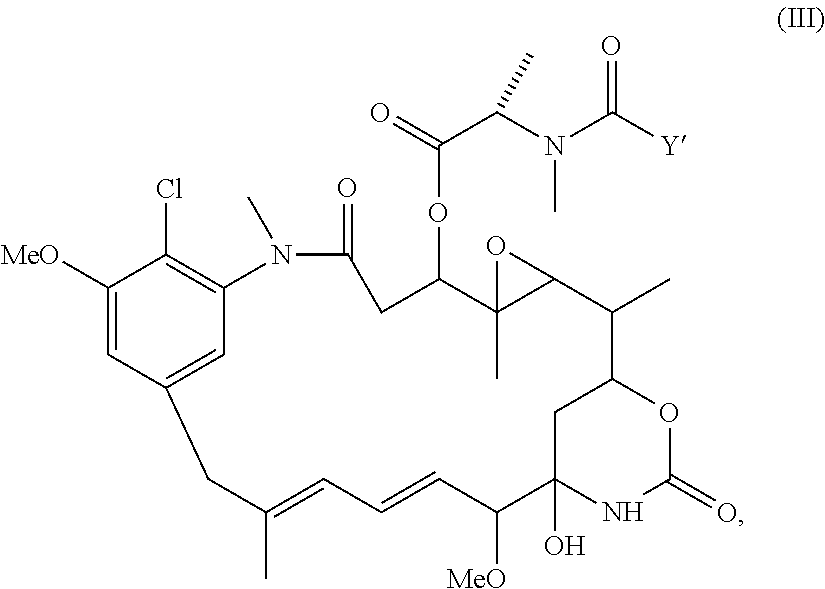
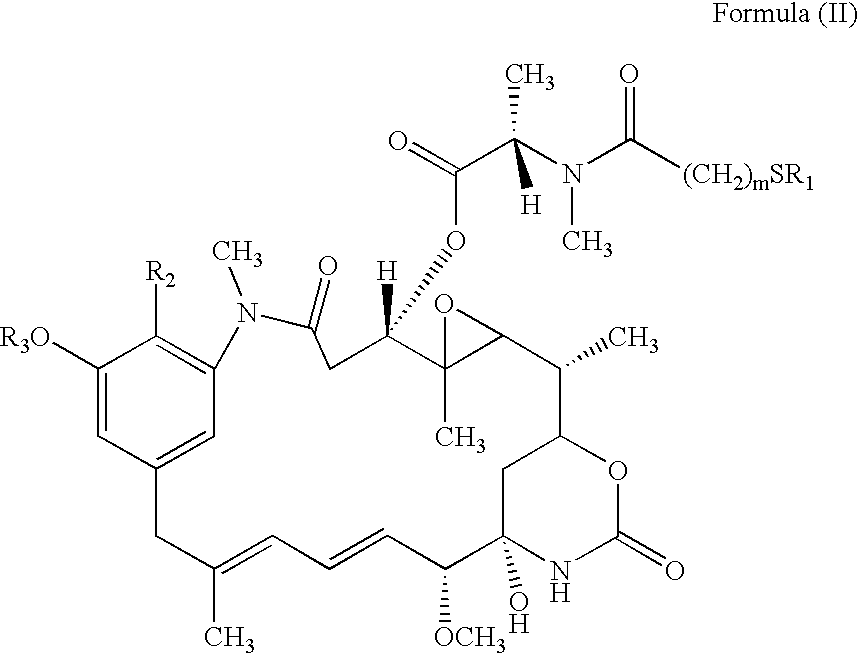
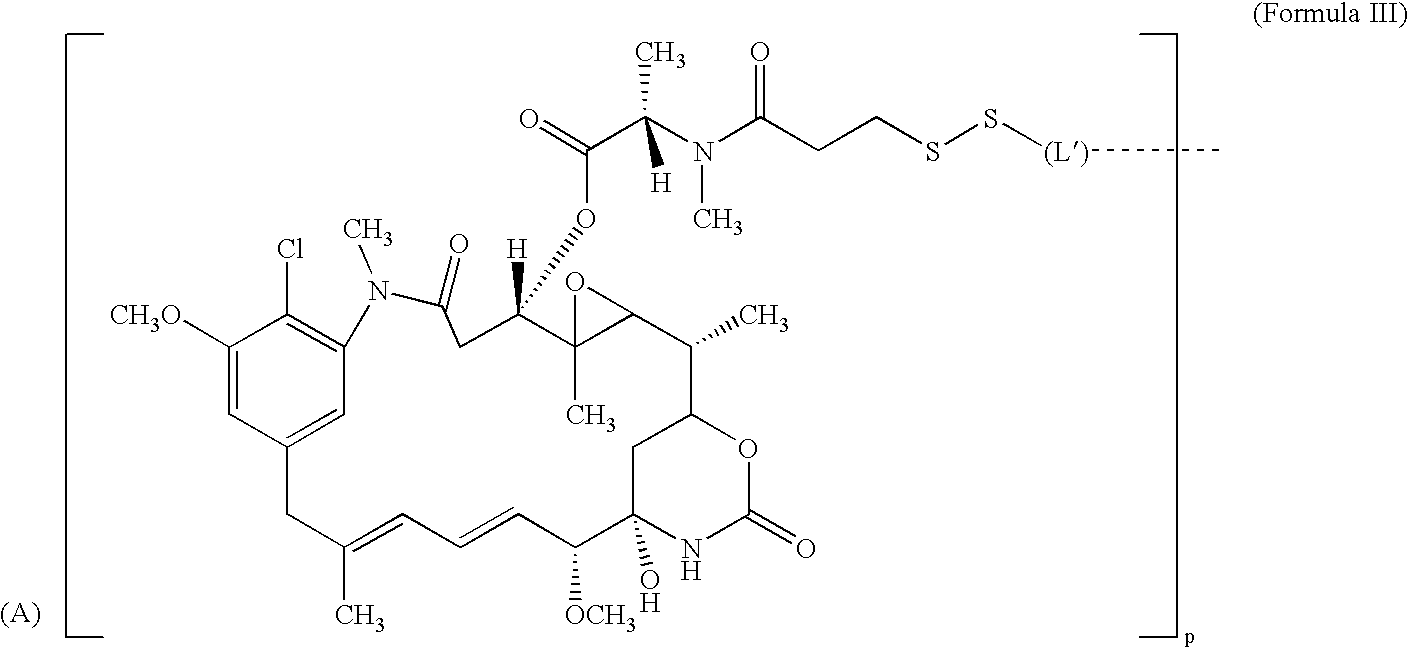
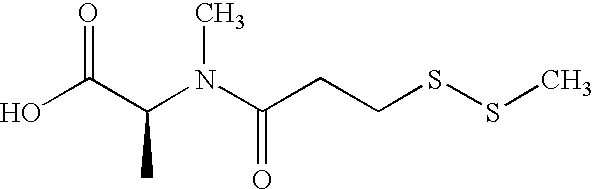
Cytotoxic agents comprising new maytansinoids
ActiveUS7276497B2Improve anti-tumor activityImprove biological activityOrganic active ingredientsOrganic chemistryAnimal tumorEfficacy
New thiol and disulfide-containing maytansinoids bearing a mono or di-alkyl substitution on the α-carbon atom bearing the sulfur atom are disclosed. Also disclosed are methods for the synthesis of these new maytansinoids and methods for the linkage of these new maytansinoids to cell-binding agents. The maytansinoid-cell-binding agent conjugates are useful as therapeutic agents, which are delivered specifically to target cells and are cytotoxic. These conjugates display vastly improved therapeutic efficacy in animal tumor models compared to the previously described agents.
Owner:IMMUNOGEN INC
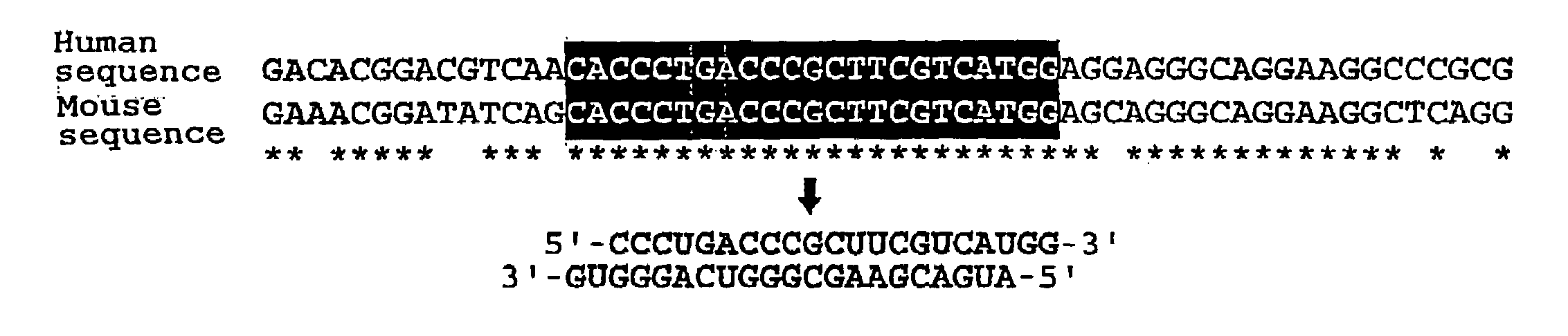
Polynucleotides for causing RNA interference and method for inhibiting gene expression using the same
InactiveUS20080113351A1High RNA interference effectLittle riskOrganic active ingredientsNervous disorderBase JNucleotide
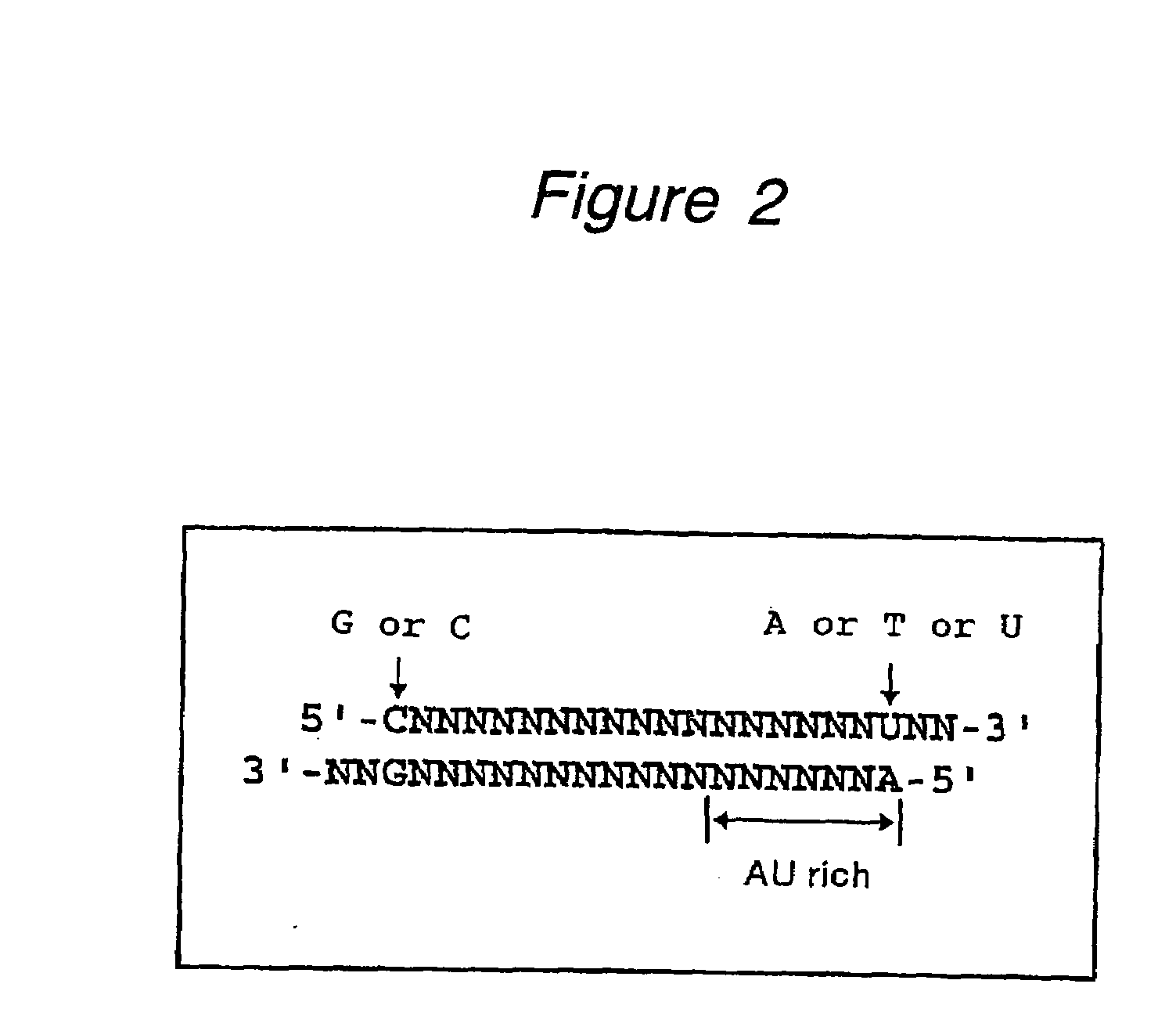
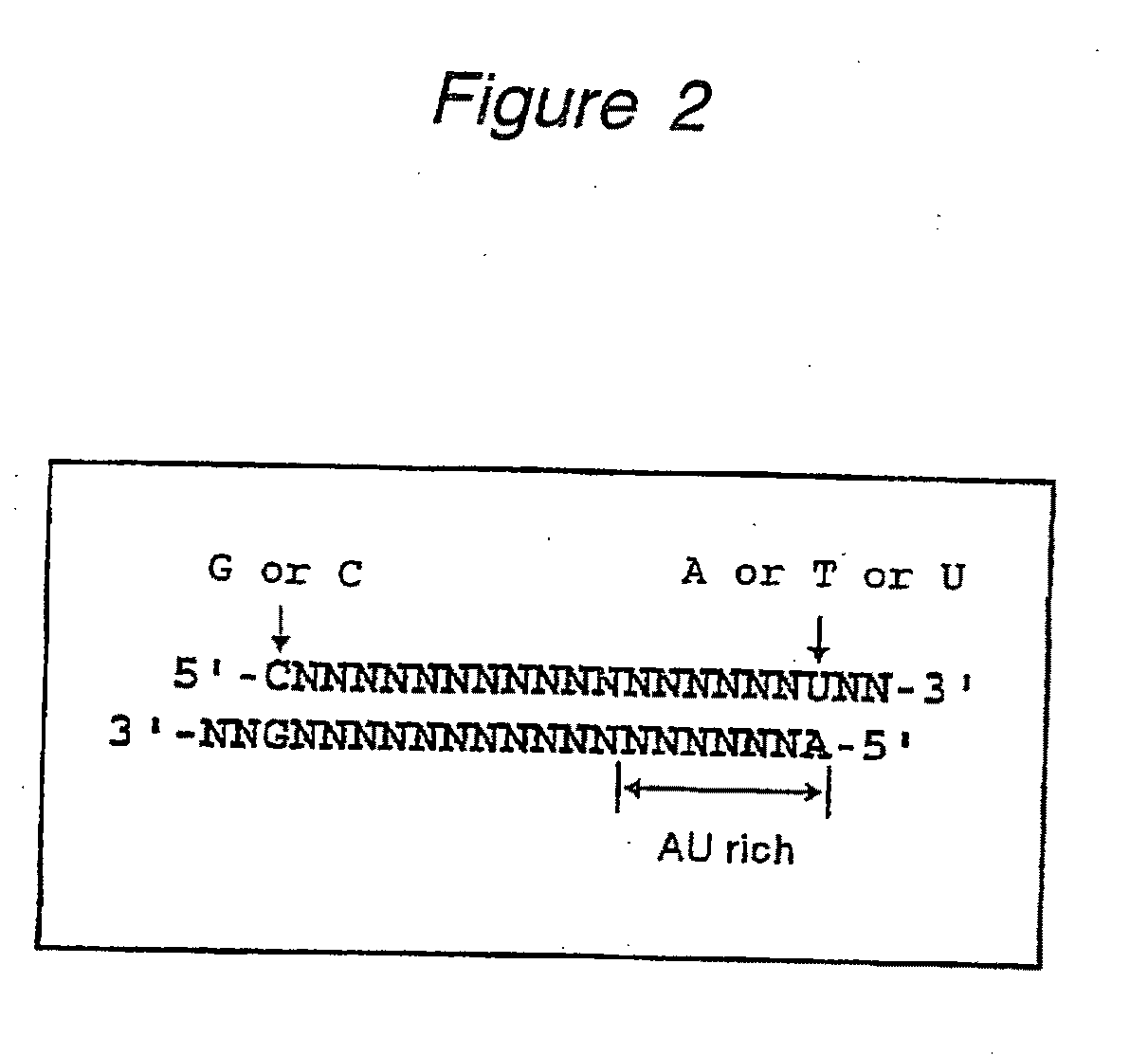
The present invention provides a polynucleotide that not only has a high RNA interference effect on its target gene, but also has a very small risk of causing RNA interference against a gene unrelated to the target gene. A sequence segment conforming to the following rules (a) to (d) is searched from the base sequences of a target gene for RNA interference and, based on the search results, a polynucleotide capable of causing RNAi is designed, synthesized, etc.:(a) The 3′ end base is adenine, thymine, or uracil,(b) The 5′ end base is guanine or cytosine,(c) A 7-base sequence from the 3′ end is rich in one or more types of bases selected from the group consisting of adenine, thymine, and uracil, and(d) The number of bases is within a range that allows RNA interference to occur without causing cytotoxicity.
Owner:ALPHAGEN
Novel Anti-cd38 antibodies for the treatment of cancer
ActiveUS20090304710A1Improve propertiesLess immunogenicSenses disorderAntipyreticComplement-dependent cytotoxicityAntibody fragments
Antibodies, humanized antibodies, resurfaced antibodies, antibody fragments, derivatized antibodies, and conjugates of same with cytotoxic agents, which specifically bind to CD38, are capable of killing CD38+ cells by apoptosis, antibody-dependent cell-mediated cytotoxicity (ADCC), and / or complement-dependent cytotoxicity (CDC). Said antibodies and fragments thereof may be used in the treatment of tumors that express CD38 protein, such as multiple myeloma, chronic lymphocytic leukemia, chronic myelogenous leukemia, acute myelogenous leukemia, or acute lymphocytic leukemia, or the treatment of autoimmune and inflammatory diseases such as systemic lupus, rheumatoid arthritis, multiple sclerosis, erythematosus, and asthma. Said derivatized antibodies may be used in the diagnosis and imaging of tumors that express elevated levels of CD38. Also provided are cytotoxic conjugates comprising a cell binding agent and a cytotoxic agent, therapeutic compositions comprising the conjugate, methods for using the conjugates in the inhibition of cell growth and the treatment of disease, and a kit comprising the cytotoxic conjugate. In particular, the cell binding agent is a monoclonal antibody, and epitope-binding fragments thereof, that recognizes and binds the CD38 protein.
Owner:SANOFI AVENTIS US LLC
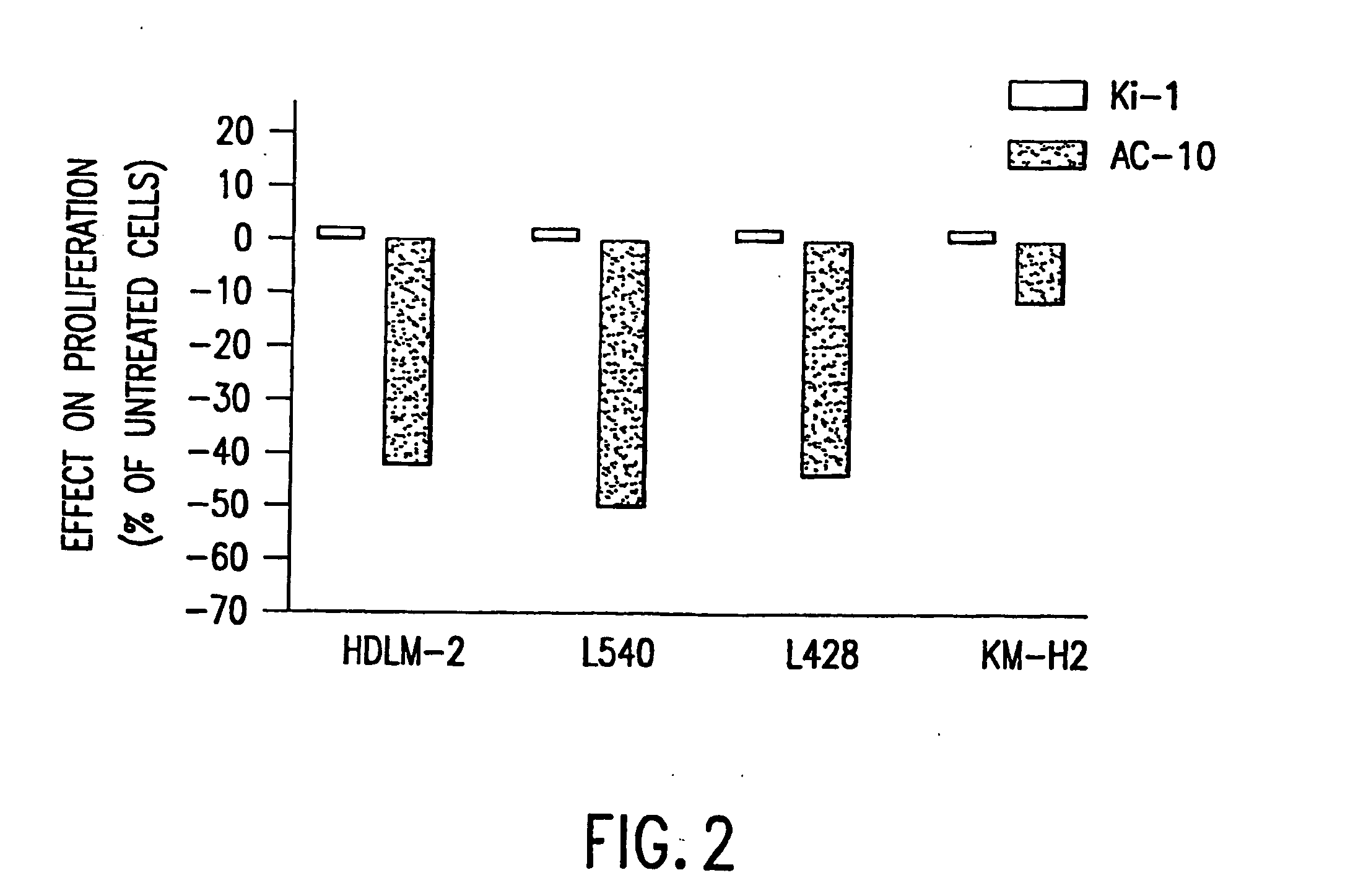
Recombinant anti-CD30 antibodies and uses thereof
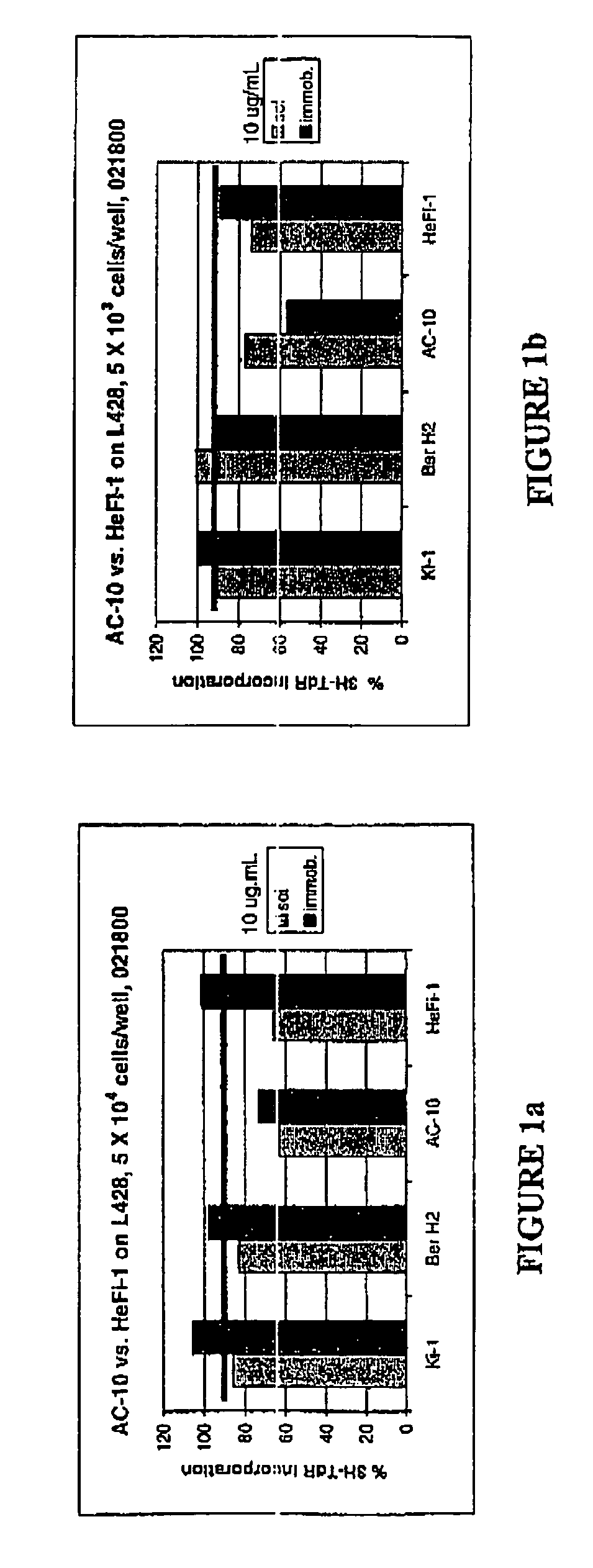
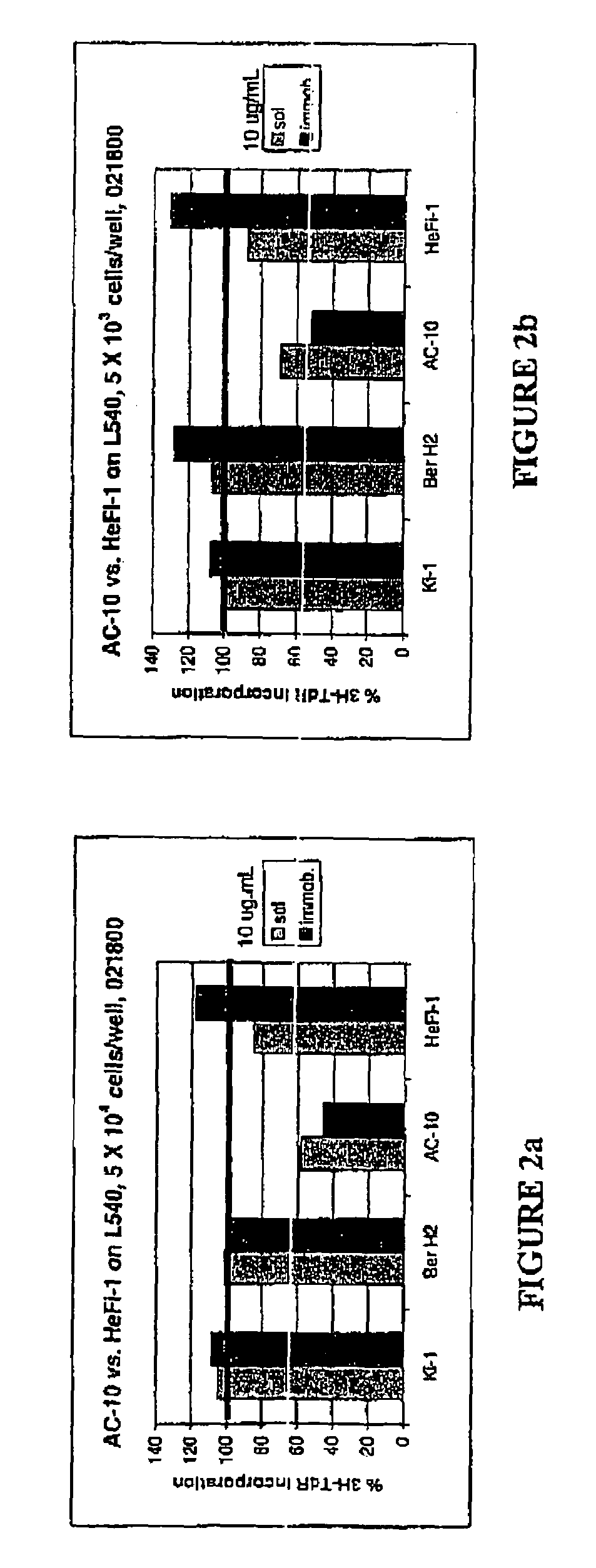
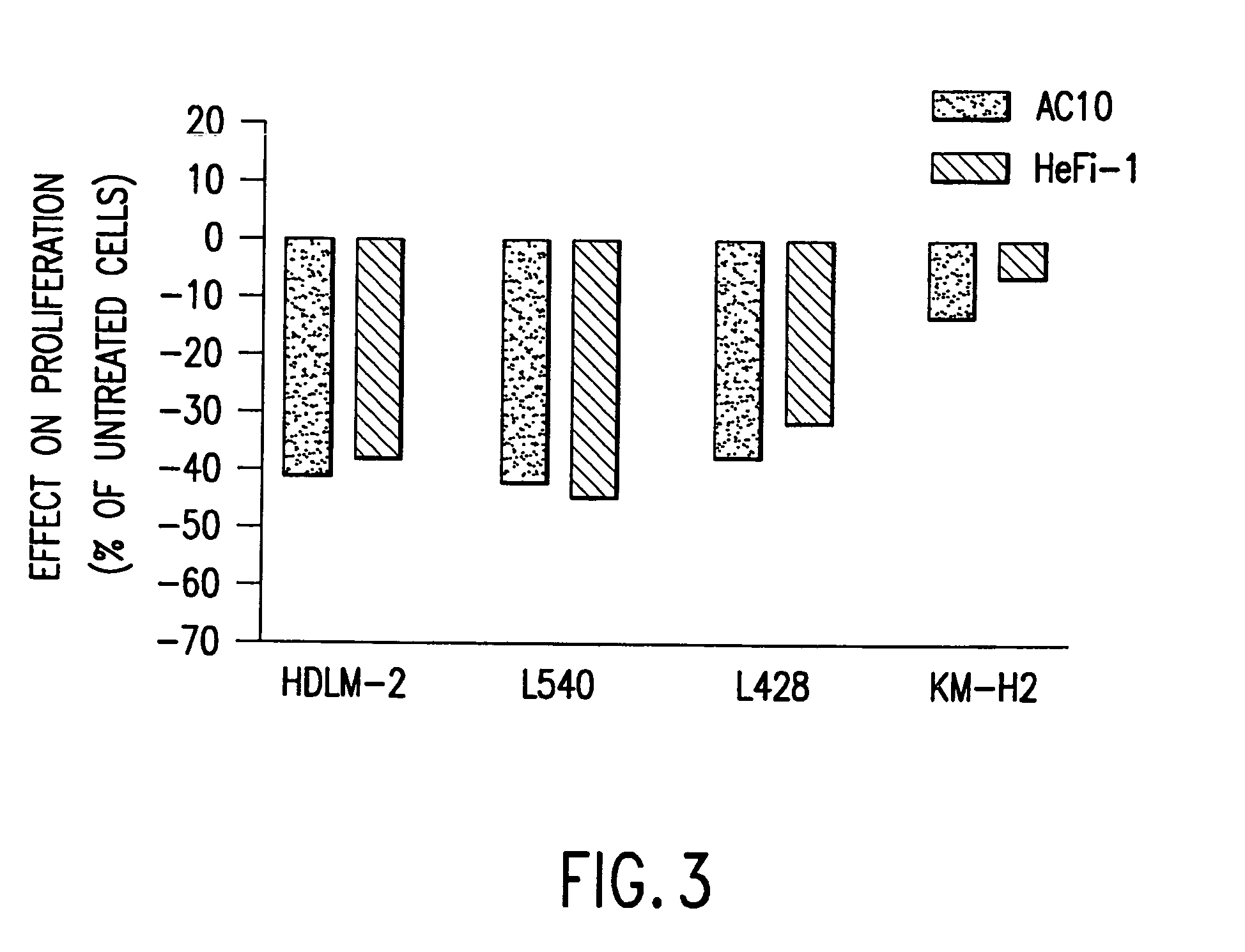
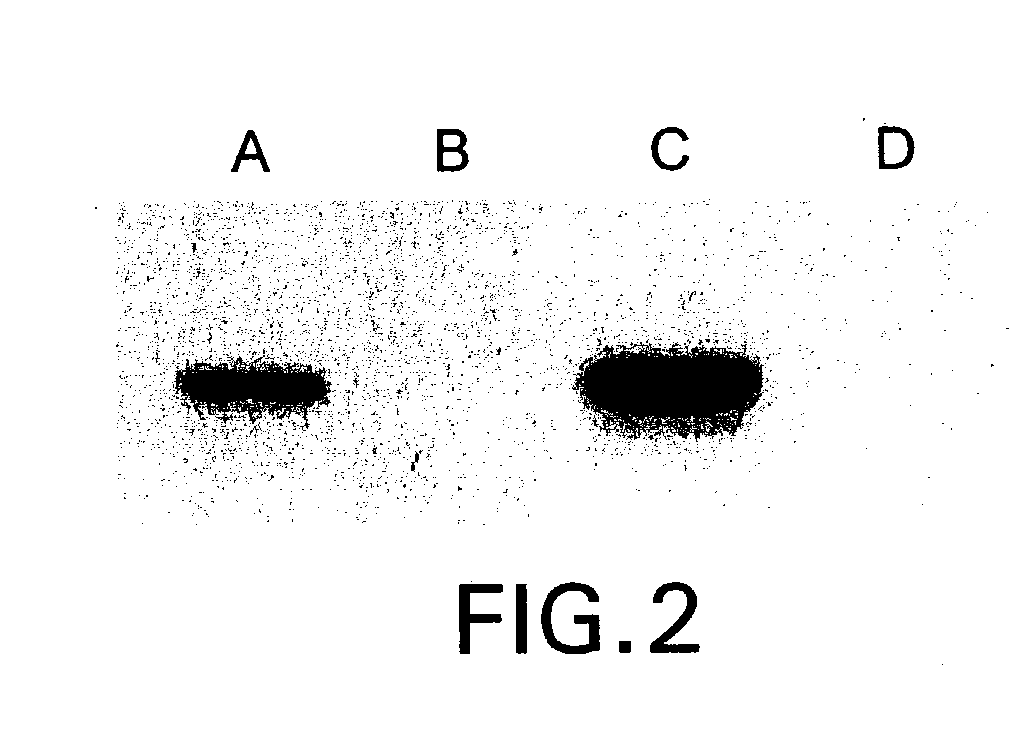
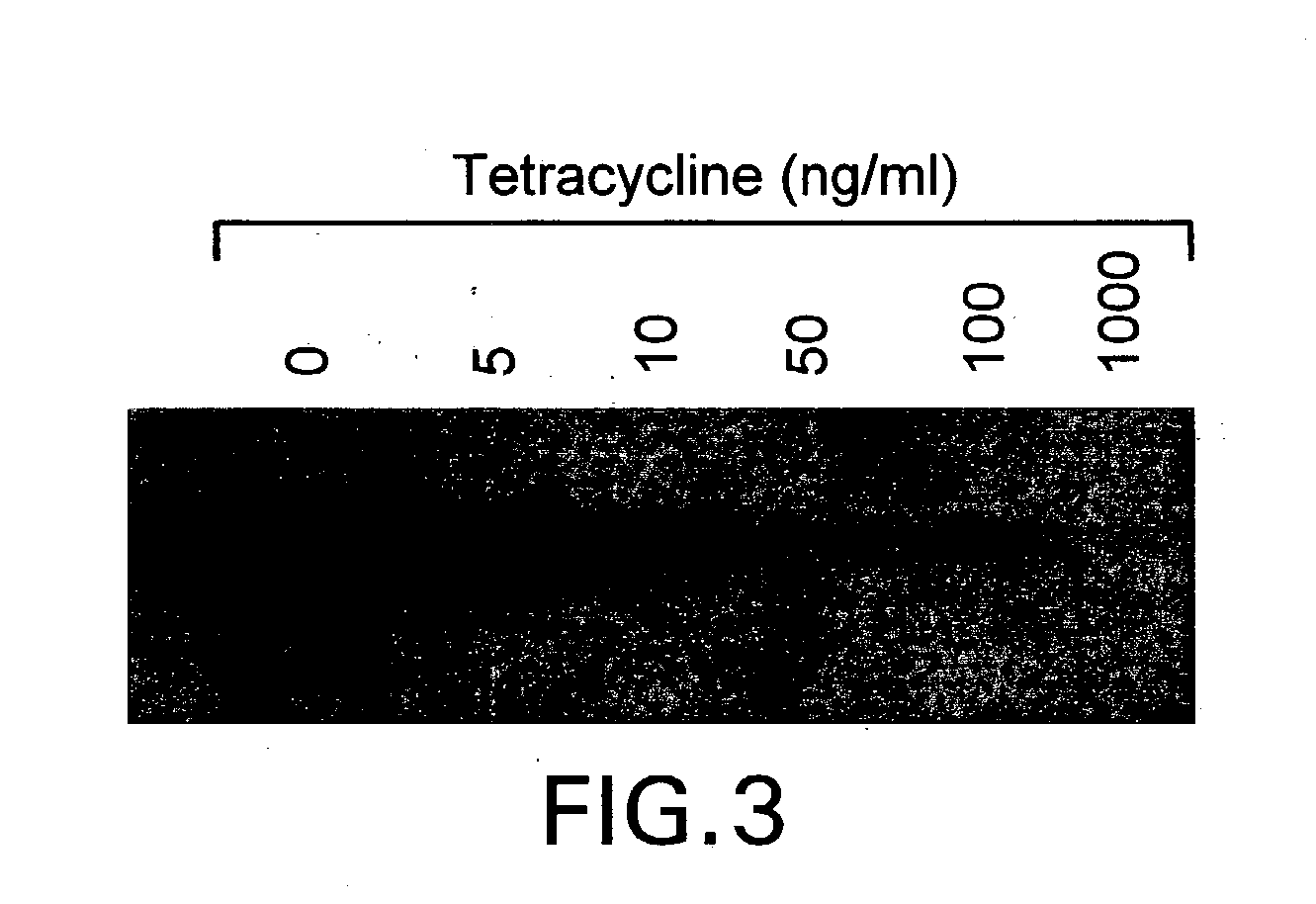
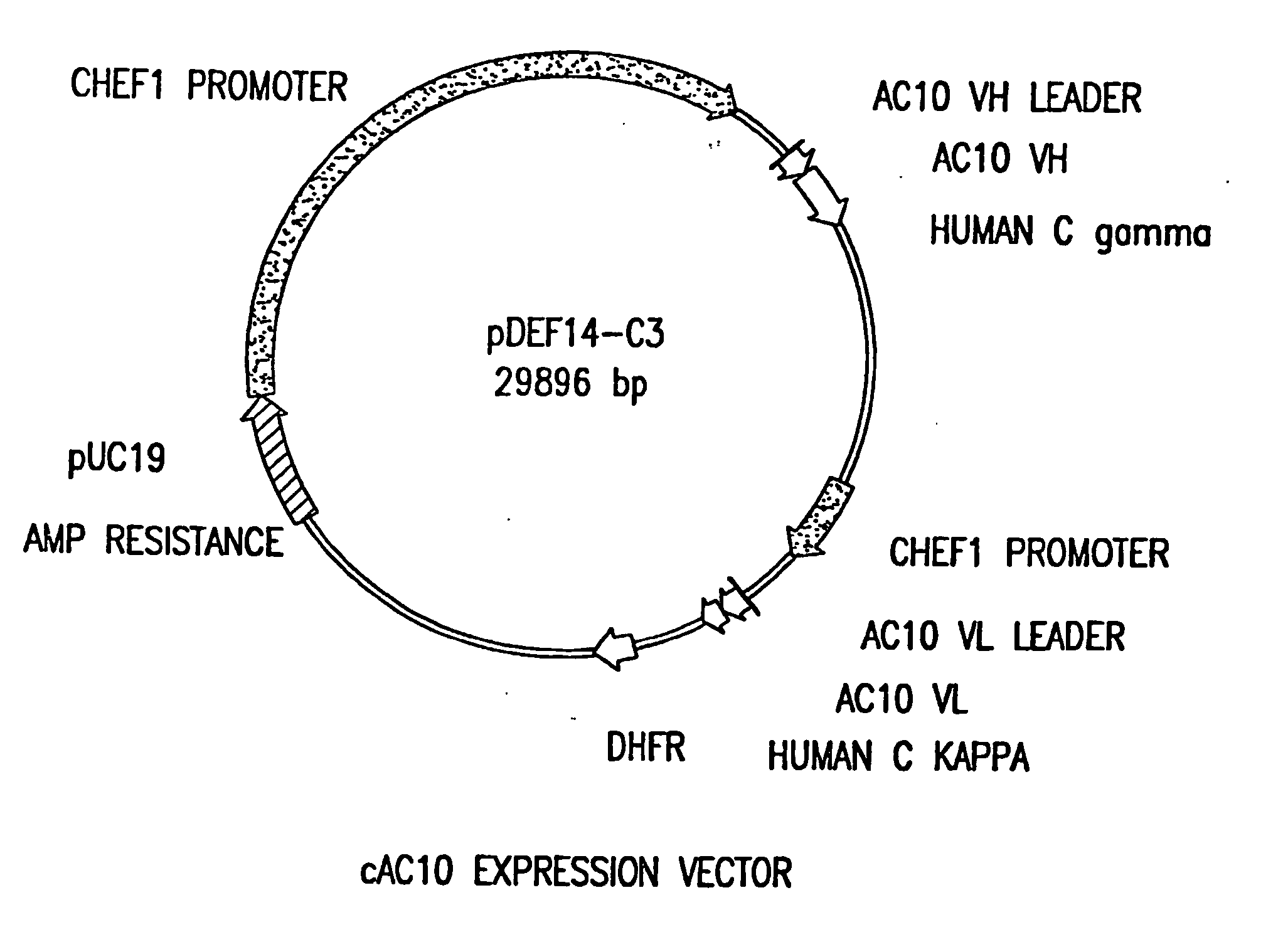
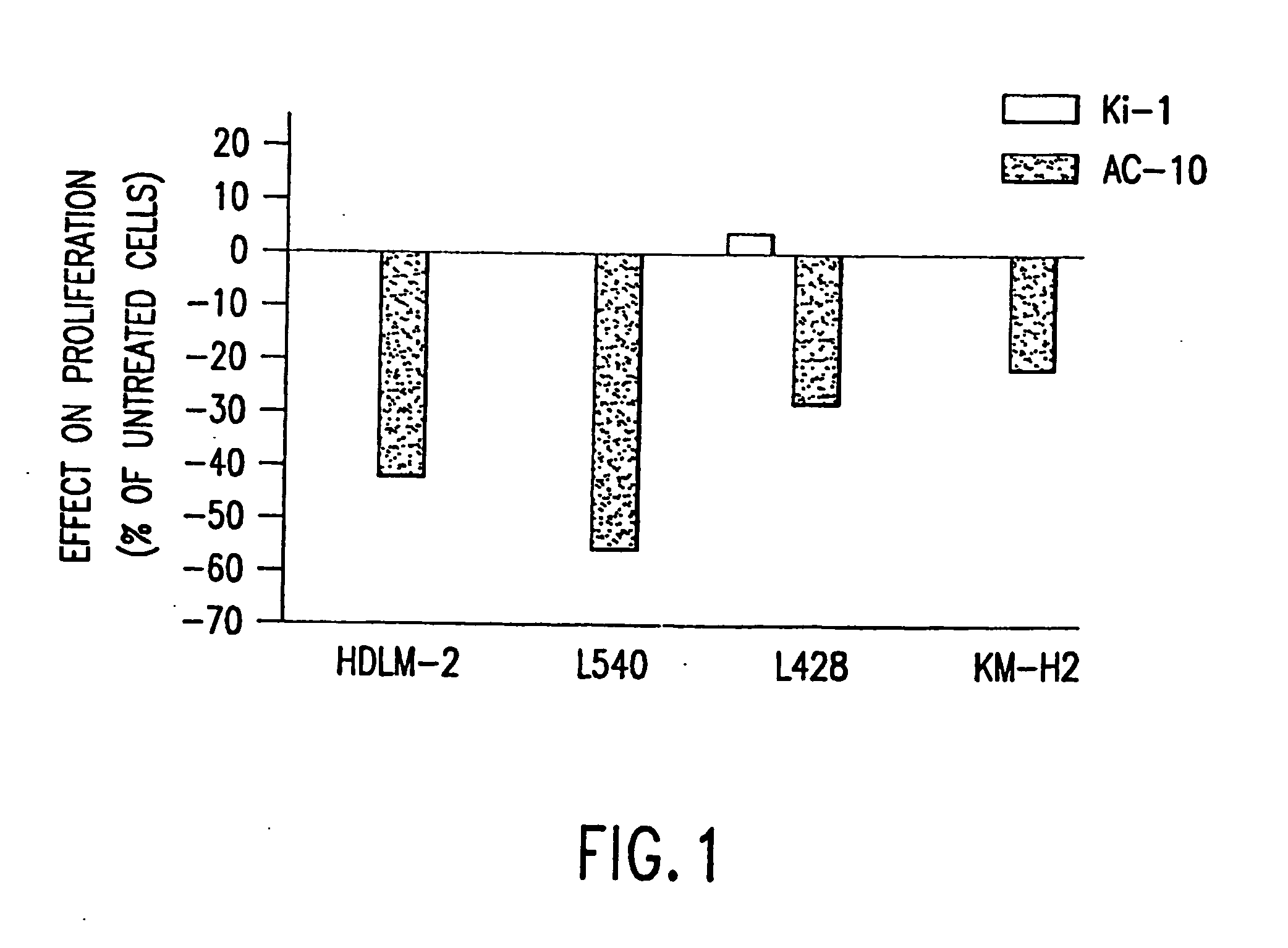
The present invention relates to methods and compositions for the treatment of Hodgkin's Disease, comprising administering proteins characterized by their ability to bind to CD30, or compete with monoclonal antibodies AC10 or HeFi-1 for binding to CD30, and exert a cytostatic or cytotoxic effect on Hodgkin's Disease cells. Such proteins include derivatives of monoclonal antibodies AC10 and HeFi-1. The proteins of the invention can be human, humanized, or chimeric antibodies; further, they can be conjugated to cytotoxic agents such as chemotherapeutic drugs. The invention further relates to nucleic acids encoding the proteins of the invention. The invention yet further relates to a method for identifying an anti-CD30 antibody useful for the treatment or prevention of Hodgkin's Disease.
Owner:SEAGEN INC
Glycosylation engineering of antibodies for improving antibody-dependent cellular cytotoxicity
InactiveUS20040072290A1Increase healing valueStrong cytotoxicityFungiNanotechAntibody fragmentsADAMTS Proteins
The present invention relates to the field glycosylation engineering of proteins. More particular, the present invention is directed to the glycosylation engineering of proteins to provide proteins with improved therapeutic properties, e.g., antibodies, antibody fragments, or a fusion protein that includes a region equivalent to the Fc region of an immunoglobulin, with enhanced Fc-mediated cellular cytotoxicity.
Owner:ROCHE GLYCART AG
Recombinant Anti-Cd30 Antibodies and Uses Thereof
The present invention relates to methods and compositions for the treatment of Hodgkin's Disease, comprising administering proteins characterized by their ability to bind to CD30, or compete with monoclonal antibodies AC10 or HeFi-1 for binding to CD30, and exert a cytostatic or cytotoxic effect on Hodgkin's disease cells in the absence of effector cells or complement. Such proteins include derivatives of monoclonal antibodies AC10 and HeFi-1. The proteins of the invention can be human, humanized, or chimeric antibodies; further, they can be conjugated to cytotoxic agents such as chemotherapeutic drugs. The invention further relates to nucleic acids encoding the proteins of the invention. The invention yet further relates to a method for identifying an anti-CD30 antibody useful for the treatment or prevention of Hodgkin's Disease.
Owner:SEATTLE GENETICS INC
Monoclonal antibodies against prostate specific membrane antigen (PSMA) lacking in fucosyl residues
ActiveUS7875278B2Inhibit cell growthStrong cytotoxicityAnimal cellsAntibody ingredientsAntigenFucosylation
The invention pertains to anti-PSMA antibodies that lack fucosyl residues. The antibodies of the invention exhibit increased antibody-dependent cellular cytotoxicity (ADCC) activity as compared to the fucosylated form of the antibodies. The invention also provides host cells that express the anti-PSMA antibodies that lack fucosyl residues, wherein the host cells are deficient for a fucosyl transferase. Methods of using the antibodies to inhibit the growth of PSMA+ cells, such as tumor cells, are also provided.
Owner:ER SQUIBB & SONS INC +1
Treatment of immunological disorders using anti-dc30 antibodies
InactiveUS20050123536A1Enhancing cytotoxicEnhancing cytostatic effectOrganic active ingredientsSenses disorderDiseaseAntibody conjugate
The present invention relates to methods for the treatment of immunological disorders other than cancer, comprising administering proteins characterized by their ability to bind to CD30 and exert a cytostatic or cytotoxic effect on an activated lymphocyte. Such proteins include monoclonal antibodies AC10 and IleFi1. AC10 and HeFi-1 derivatives, and antibodies that compete with AC10 and HeFi-1 for binding to CD30. Other such proteins include multivalent anti-CD30 antibodies and anti-CD30 antibodies conjugated to cytotoxic agents. Treatment modalities with antibodies of the invention are also provided.
Owner:SEATTLE GENETICS INC
Drug conjugate composition
ActiveUS7374762B2Snake antigen ingredientsAntibody ingredientsDrug conjugationAntiendomysial antibodies
The invention provides a liquid composition and a lyophilized composition comprising a therapeutically effective amount of a conjugate comprising an antibody chemically coupled to a maytansinoid. The invention further provides a method for killing a cell in a human comprising administering to the human either of the compositions such that the antibody binds to the surface of the cell and the cytotoxicity of the maytansinoid is activated, whereby the cell is killed.
Owner:IMMUNOGEN INC
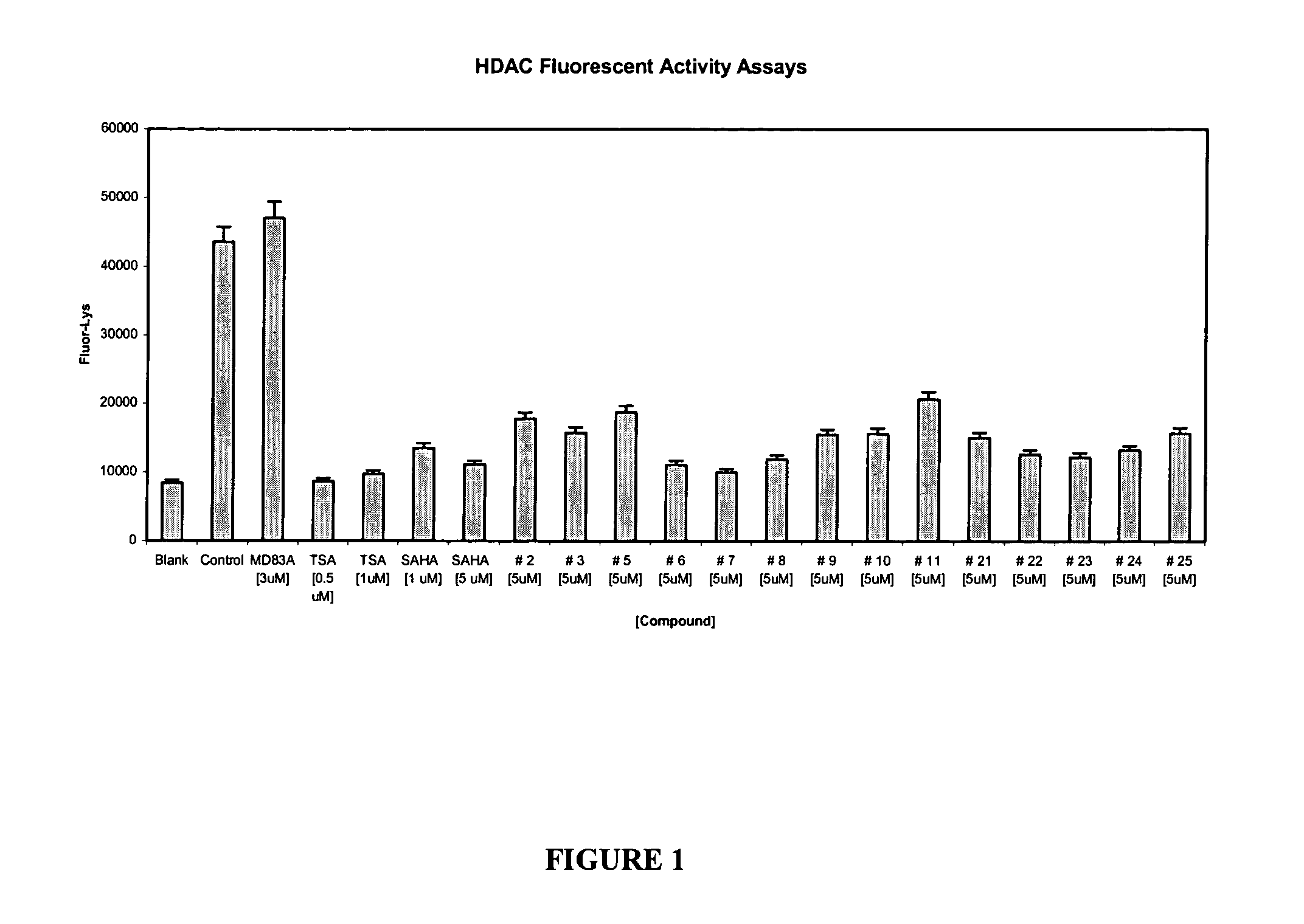
Histone deacetylase inhibitors and methods of use thereof
The invention provides novel classes of HDAC inhibitors. Methods of sensitizing a cancer cell to the cytotoxic effects of radiotherapy are also provided as well as methods for treating cancer and methods for treating neurological diseases. Additionally, the invention further provides pharmaceutical compositions comprising an HDAC inhibitor of the invention, and kits comprising a container containing an HDAC inhibitor of the invention.
Owner:GEORGETOWN UNIV
Mutated anti-CD22 antibodies with increased affinity to CD22-expressing leukemia cells
ActiveUS7355012B2Snake antigen ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenBacteroides
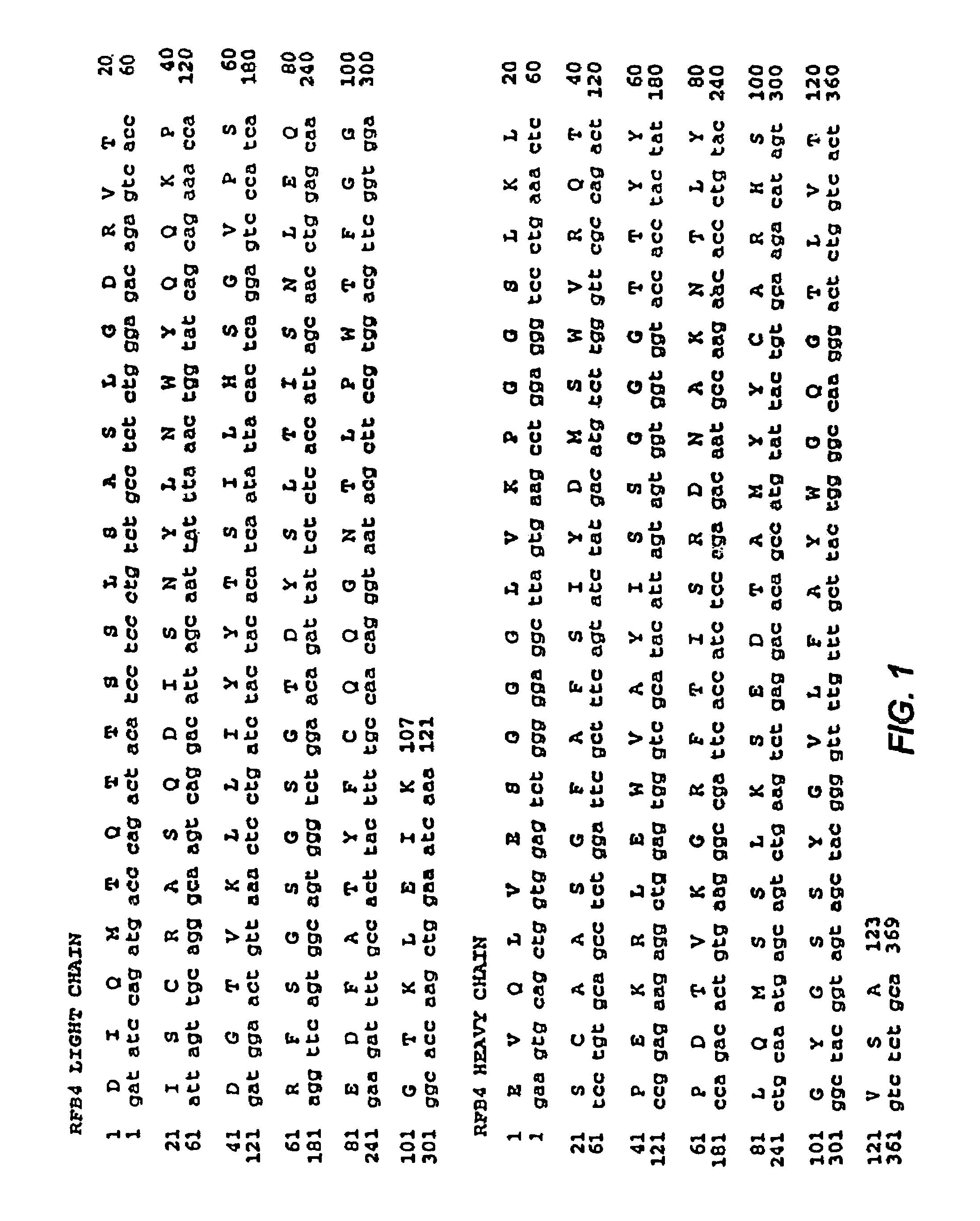
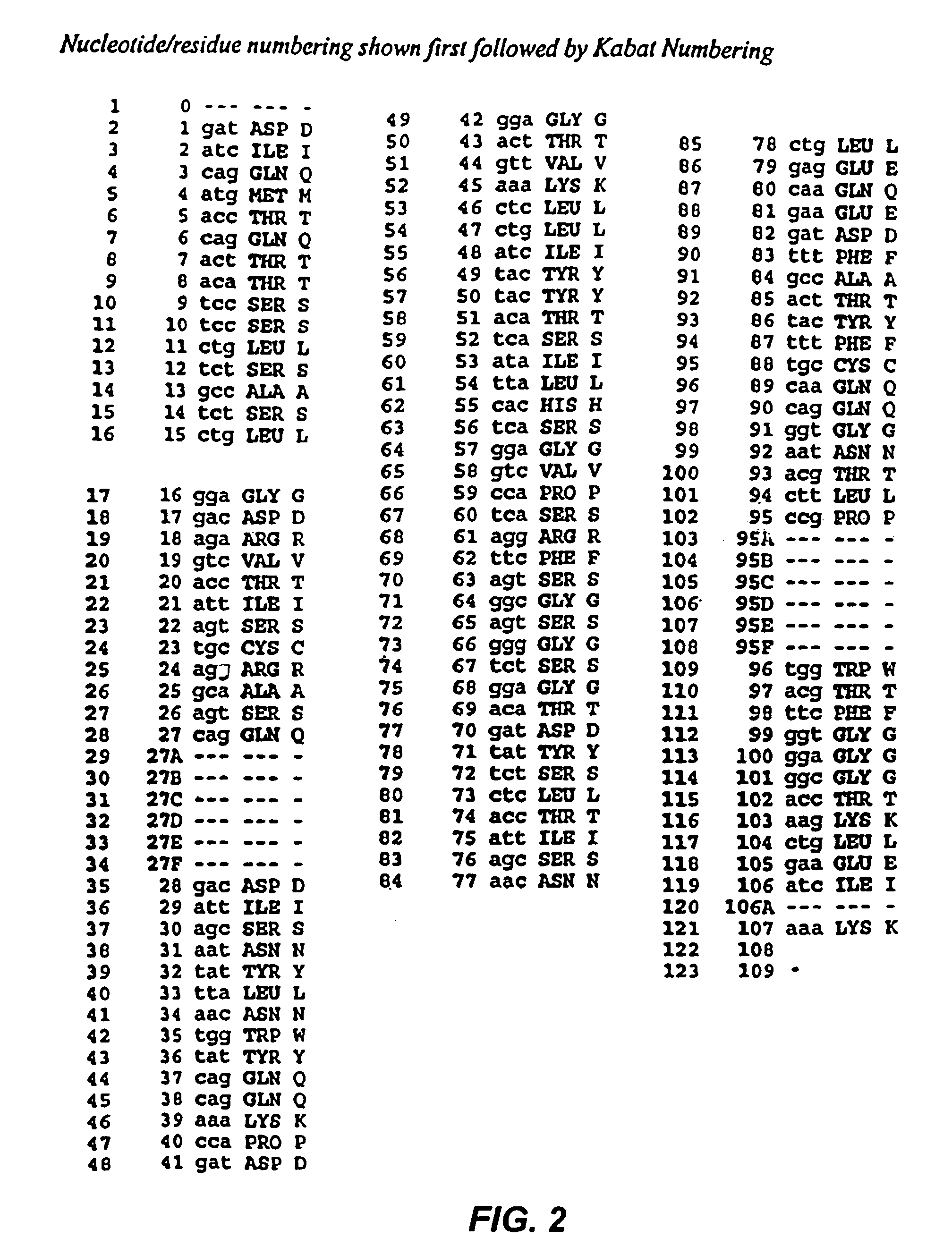
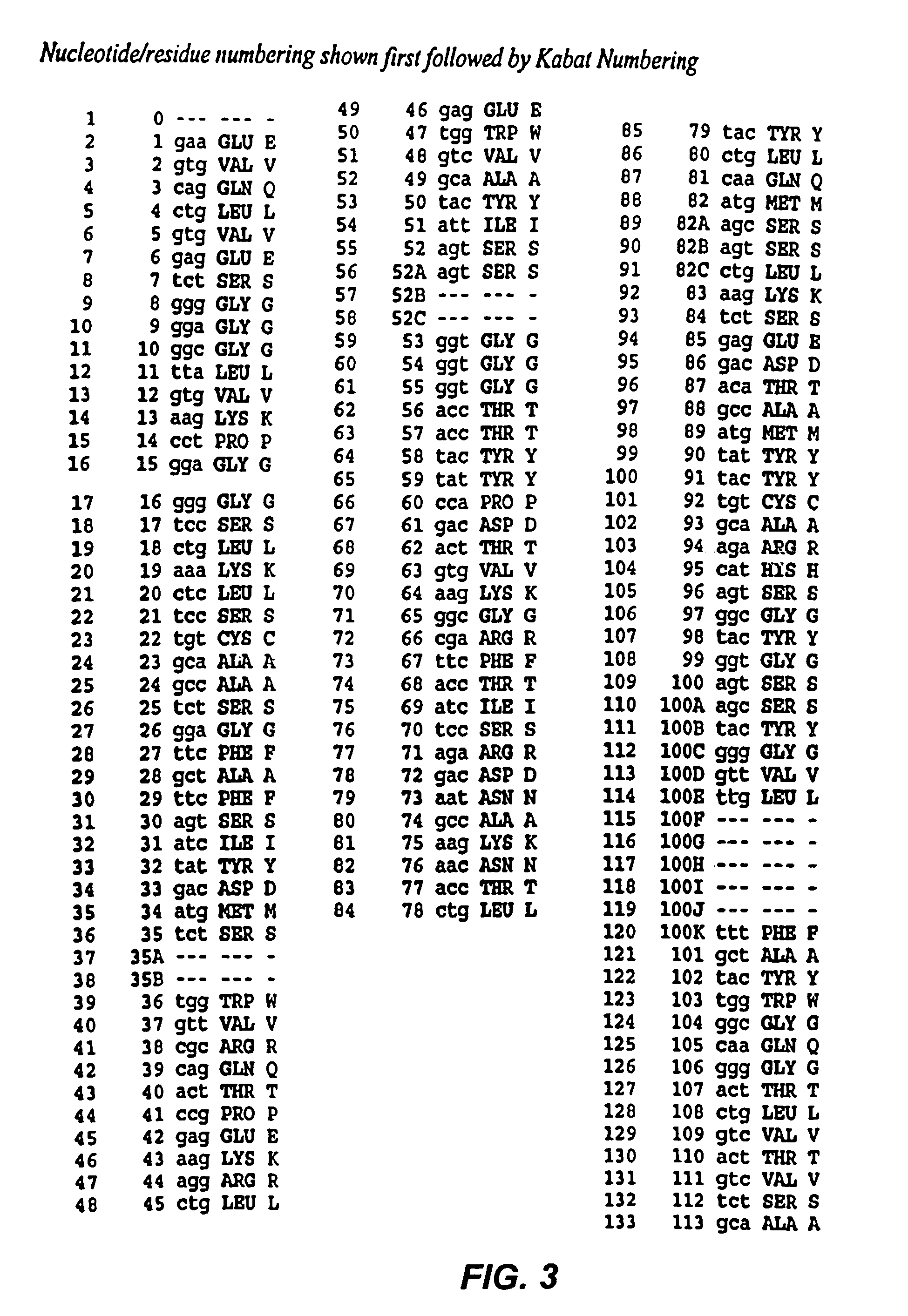
Recombinant immunotoxins are fusion proteins composed of the Fv domains of antibodies fused to bacterial or plant toxins. RFB4 (Fv)-PE38 is an immunotoxin that targets CD22 expressed on B cells and B cell malignancies. The present invention provides antibodies and antibody fragments that have improved ability to bind the CD22 antigen of B cells and B cell malignancies compared to RFB4. Immunotoxins made with the antibodies and antibody fragments of the invention have improved cytotoxicity to CD22-expressing cancer cells. Compositions that incorporate these antibodies into chimeric immunotoxin molecules that can be used in medicaments and methods for inhibiting the growth and proliferation of leukemia and lymphoma cells.
Owner:GOVERNMENT OF THE UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES
Antibodies with immune effector activity and that internalize in folate receptor alpha-positive cells
InactiveUS20060239910A1Improve anti-tumor activityEnhanced antibody-dependent cellular cytotoxicityOrganic active ingredientsNanomedicineDrug specific IgEFolate Receptor Alpha
This invention relates to the use of monoclonal and polyclonal antibodies that specifically bind to and have the ability in the alternative to become internalized by cells expressing folate receptor alpha (FRA) and to induce an immune effector activity such as antibody-dependent cellular cytotoxicity. The antibodies are useful in specific delivery of pharmacologic agents to FRA-expressing cells as well as in eliciting an immune-effector activity particularly on tumor cells and precursors. The invention is also related to nucleotides encoding the antibodies of the invention, cells expressing the antibodies; methods of detecting cancer cells; and methods of treating cancer using the antibodies.
Owner:EISAI INC
Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity
The present invention relates to the field of glycosylation engineering of proteins. More particularly, the present invention relates to glycosylation engineering to generate proteins with improved therapeutic properties, including antibodies with increased antibody-dependent cellular cytotoxicity.
Owner:ROCHE GLYCART AG
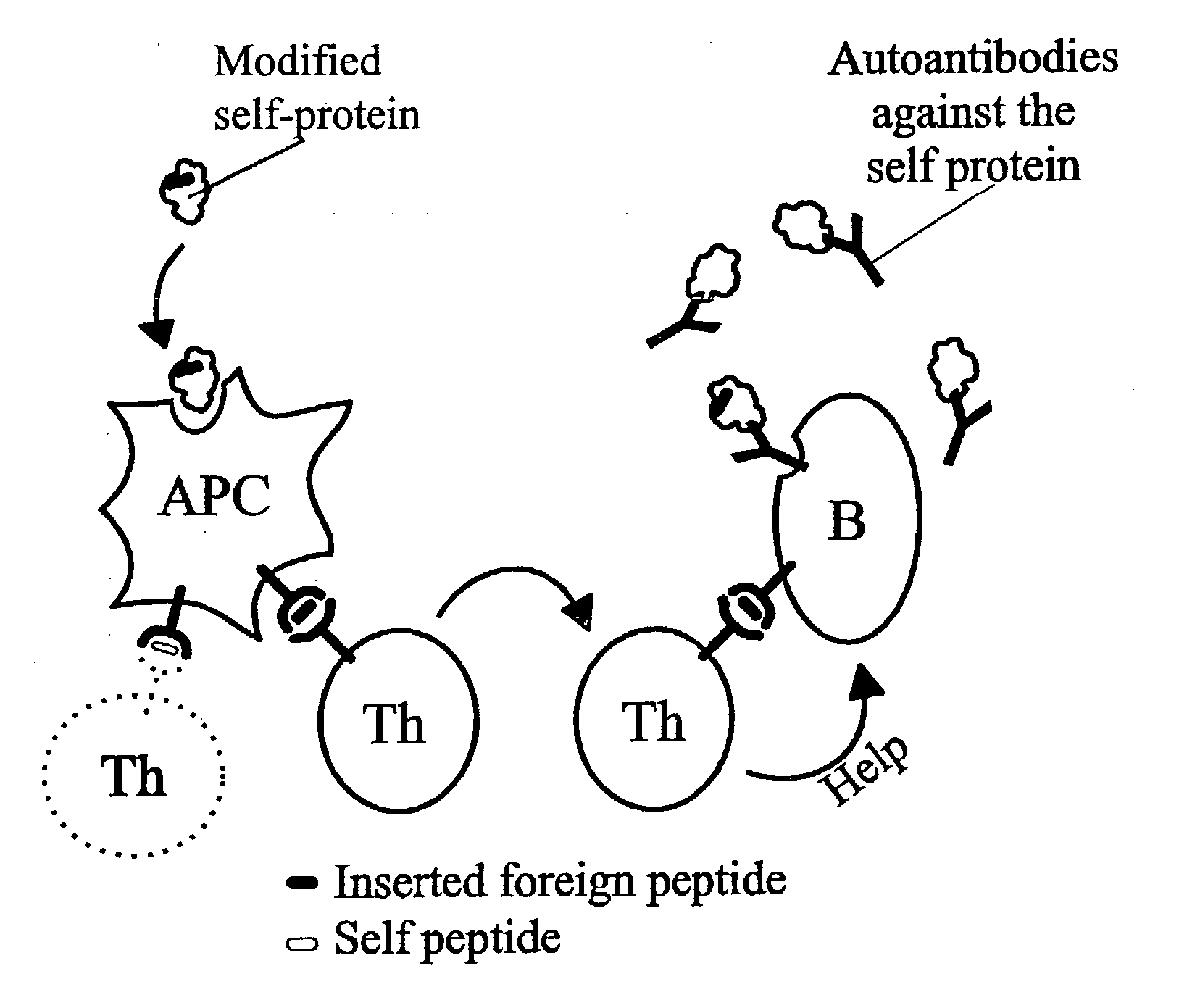
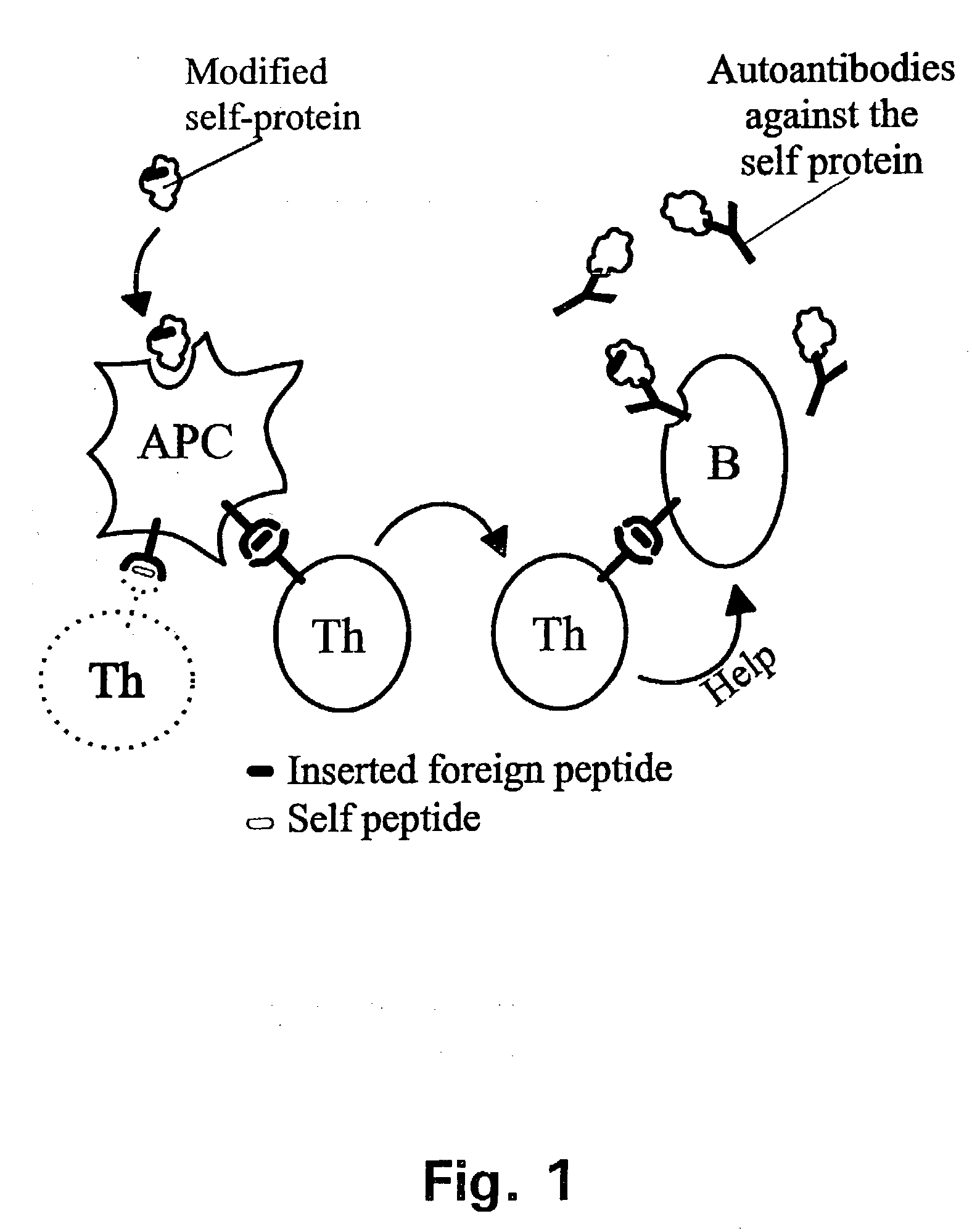
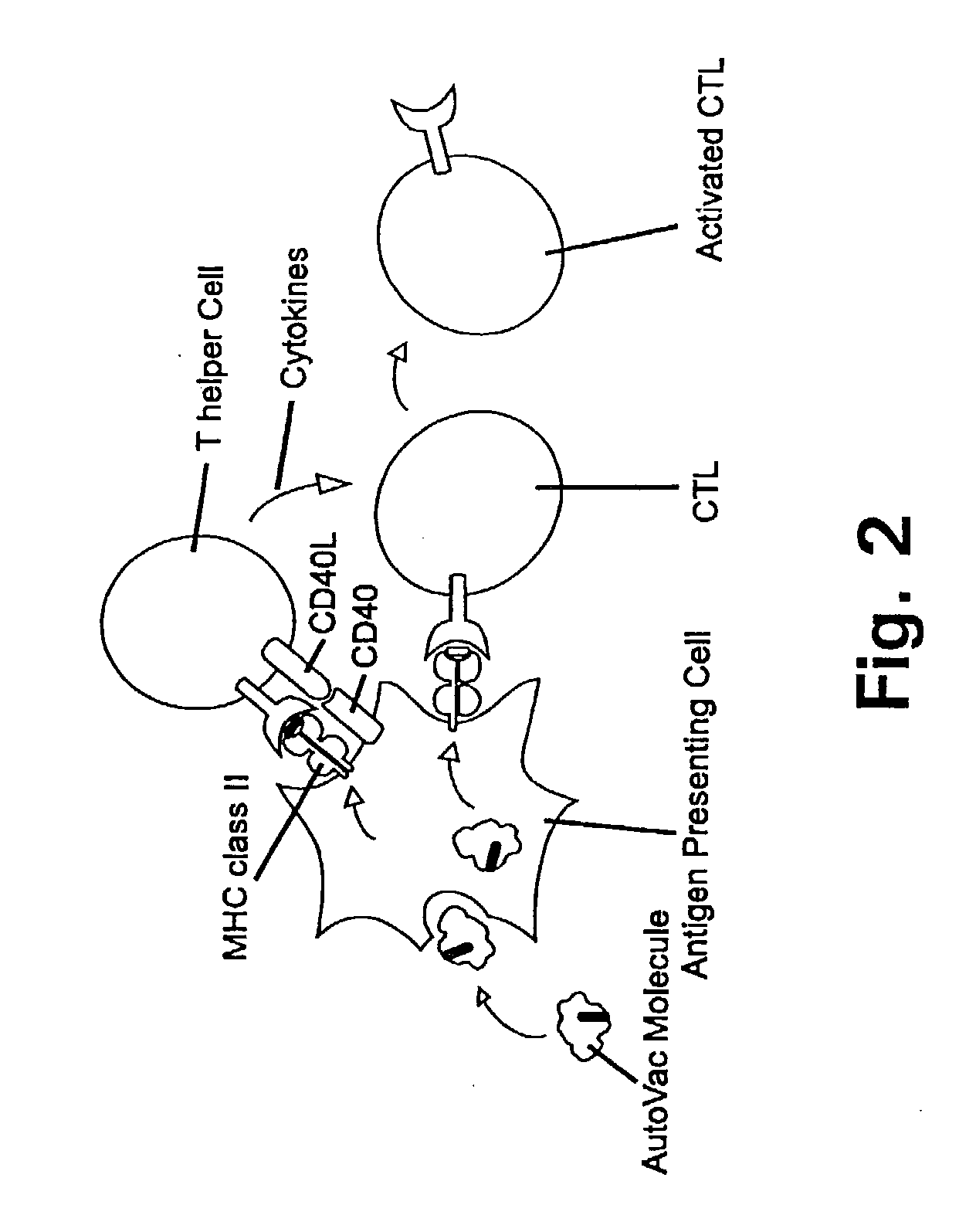
Novel methods for therapeutic vaccination
A method is disclosed for inducing cell-mediated immunity against cellular antigens. More specifically, the invention provides for a method for inducing cytotoxic T-lymphocyte immunity against weak antigens, notably self-proteins. The method entails that antigen presenting cells are induced to present at least one CTL epitope of the weak antigen and at the same time presenting at least one foreign T-helper lymphocyte epitope. In a preferred embodiment, the antigen is a cancer specific antigen, e.g. PSM, Her2, or FGF8b. The method can be exercised by using traditional polypeptide vaccination, but also by using live attenuated vaccines or nucleic acid vaccination. The invention furthermore provides immunogenic analogues of PSM, Her2 and FGF8b, as well as nucleic acid molecules encoding these analogues. Also vectors and transformed cells are disclosed. The invention also provides for a method for identification of immunogenic analogues of weak or non-immunogenic antigens.
Owner:BAVARIAN NORDIC AS
Mutated anti-cd22 antibodies and immunoconjugates
Recombinant immunotoxins are fusion proteins composed of the Fv domains of antibodies fused to bacterial or plant toxins. RFB4 (Fv)-PE38 is an immunotoxin that targets CD22 expressed on B cells and B cell malignancies. The present invention provides antibodies and antibody fragments that have improved ability to bind the CD22 antigen compared to RFB4. Immunotoxins made with the antibodies and antibody fragments of the invention have improved cytotoxicity to CD22-expressing cancer cells. Compositions that incorporate these antibodies into chimeric immunotoxin molecules that can be used in medicaments and methods for inhibiting the growth and proliferation of such cancers. Additionally, the invention provides a method of increasing the cytotoxicity of forms of Pseudomonas exotoxin A (“PE”) with the mutation of a single amino acid, as well as compositions of such mutated PEs, nucleic acids encoding them, and methods for using the mutated PEs.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
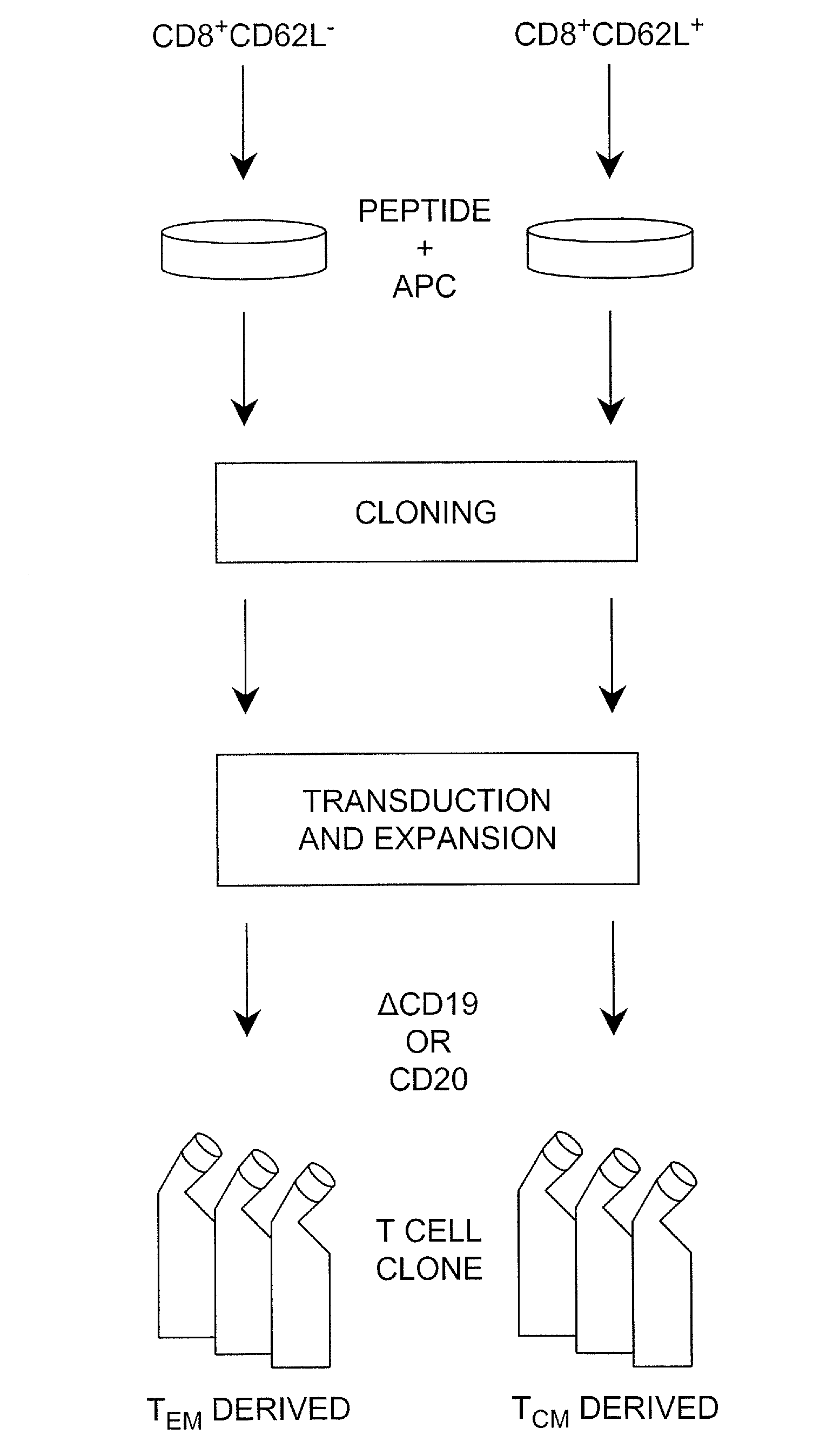
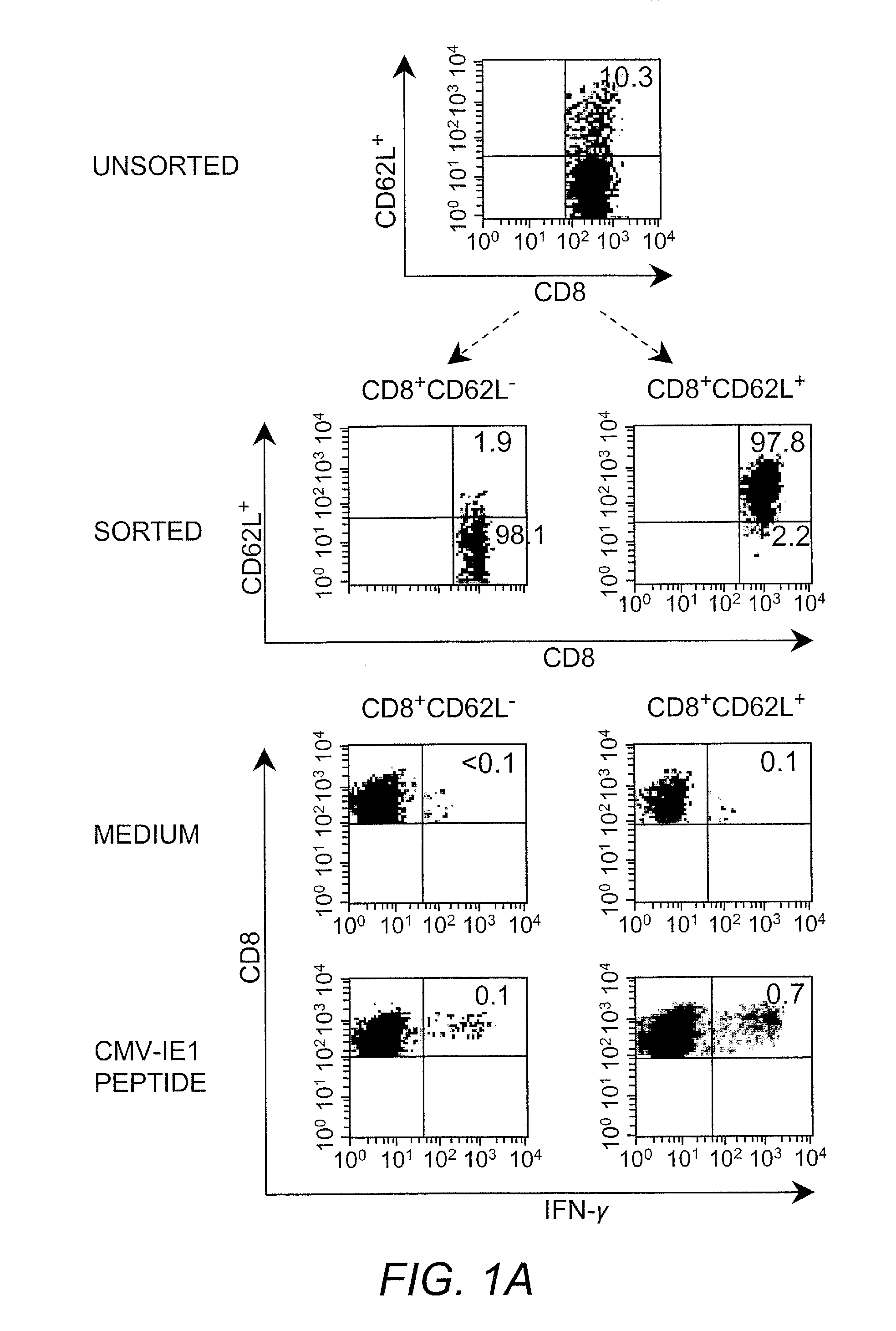
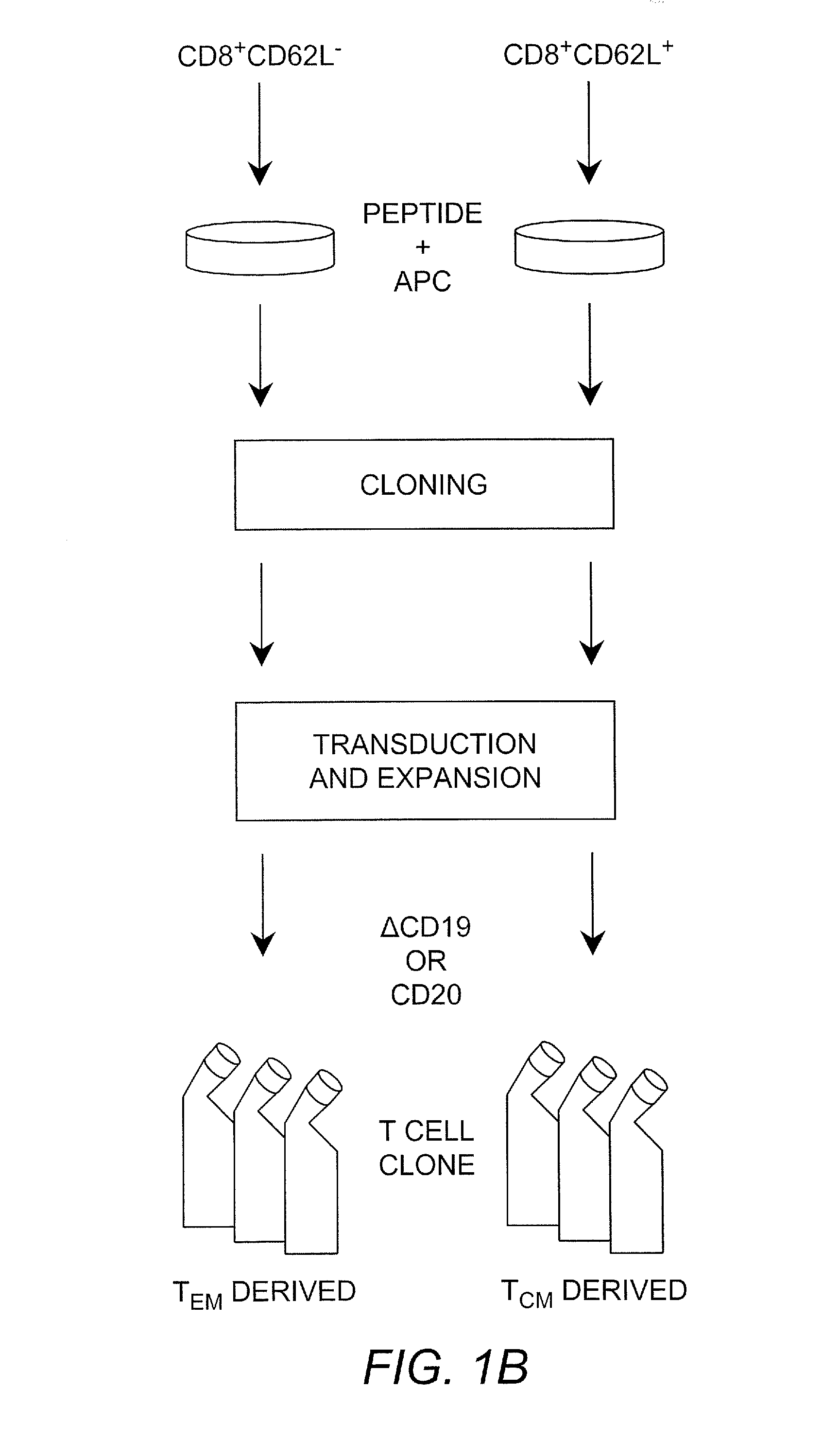
Adoptive transfer of cd8 + t cell clones derived from central memory cells
InactiveUS20080131415A1Increased proliferationBiocideArtificial cell constructsWhite blood cellPharmaceutical formulation
The present invention provides a method of carrying out adoptive immunotherapy in a primate subject in need thereof by administering the subject a cytotoxic T lymphocytes (CTL) preparation in a treatment-effective amount. The method comprises administering as the CTL preparation a preparation consisting essentially of an in vitro expanded primate CTL population, the CTL population enriched prior to expansion for central memory T lymphocytes, and depleted prior to expansion of effector memory T lymphocytes. In some embodiments, the method may further comprise concurrently administering Interleukin-15 to the subject in an amount effective to increase the proliferation of the central memory T cells in the subject. Pharmaceutical formulations produced by the method, and methods of using the same, are also described.
Owner:CITY OF HOPE +1
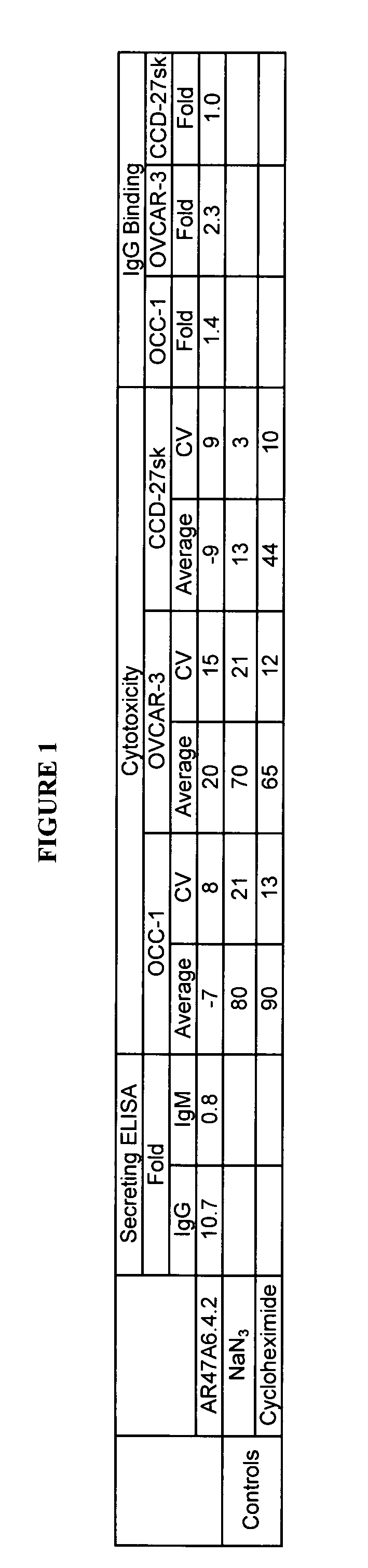
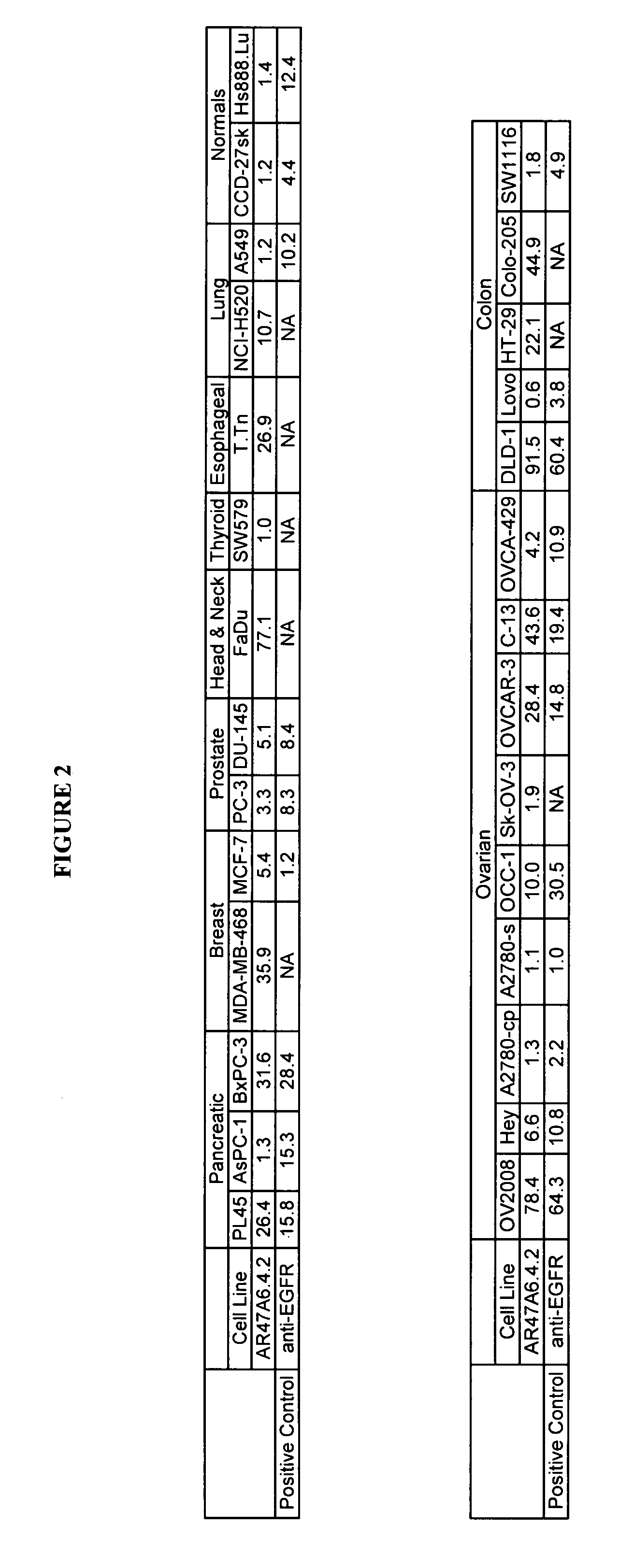
Cytotoxicity mediation of cells evidencing surface expression of TROP-2
InactiveUS7420040B2Reduce the likelihood of problemsProlong survival timeImmunoglobulins against cell receptors/antigens/surface-determinantsFermentationDiseaseHematopoietic cell
The present invention relates to a method for producing cancerous disease modifying antibodies using a novel paradigm of screening. By segregating the anti-cancer antibodies using cancer cell cytotoxicity as an end point, the process makes possible the production of anti-cancer antibodies for therapeutic and diagnostic purposes. The antibodies can be used in aid of staging and diagnosis of a cancer, and can be used to treat primary tumors and tumor metastases. The anti-cancer antibodies can be conjugated to toxins, enzymes, radioactive compounds, cytokines, interferons, target or reporter moieties and hematogenous cells.
Owner:F HOFFMANN LA ROCHE & CO AG
Preparation of maytansinoid antibody conjugates by a one-step process
ActiveUS20120253021A1High purityImprove stabilityOrganic active ingredientsPeptide preparation methodsChemistryBy-product
The invention provides a one-step process for preparing a cell-binding agent cytotoxic agent conjugate comprising contacting a cell-binding agent with a cytotoxic agent to form a first mixture comprising the cell-binding agent and the cytotoxic agent and contacting the first mixture comprising the cell-binding agent and the cytotoxic agent with a bifunctional crosslinking reagent, which provides a linker, in a solution having a pH of about 4 to about 9 to provide a second mixture comprising the cell-binding agent cytotoxic agent conjugate, wherein the cell-binding agent is chemically coupled through the linker to the cytotoxic agent, free cytotoxic agent, and reaction by-products. The second mixture is then optionally subjected to purification to provide a purified cell-binding agent cytotoxic agent conjugate.
Owner:IMMUNOGEN INC
Anti-tim-3 antibody
ActiveUS20120189617A1High ADCC activityHigh activityBacteriaPeptide/protein ingredientsDiseaseAntibody fragments
The present invention provides an anti-human TIM-3 antibody which binds to the amino acid sequence of the extracellular region of TIM-3 or its three-dimensional structure thereof and exhibits higher effector activity such as an antibody-dependent cellular cytotoxicity (ADCC activity) for diseases relating to a human TIM-3 expressing cell. The present invention provides a monoclonal antibody or antibody fragment thereof which binds to the amino acid sequence of the extracellular region of TIM-3 or its three-dimensional structure and exhibits ADCC activity; a hybridoma which produces the antibody; a DNA encoding the antibody; a vector comprising the DNA; a transformant which is obtainable by introducing the vector; a method for producing the antibody or the antibody fragment thereof which comprises using the hybridoma or the transformant; a therapeutic agent and a diagnostic agent comprising the antibody or the antibody fragment thereof as an active ingredient. In addition, the present invention provides an anti-human TIM-3 antibody having high ADCC activity by screening an anti-human TIM-3 antibody which competes with the monoclonal antibody or the antibody fragment thereof.
Owner:KYUSHU UNIV +1
Anti-integrin immunoconjugates, methods and uses
The invention relates to conjugates of anti-integrin specific antibodies with cytotoxic compounds, the synthesis, selection, and use of such conjugates for use in cancer therapy or other diseases mediated by cell proliferation, cell migration, or inflammation and which pathology involves angiogenesis or neovascularization of new tissue. In addition the invention relates to combination therapy of such diseases wherein the treatment comprises use of said conjugates in combination with one or more other treatment modalities including but not limited to: chemotherapy, surgery or radiation therapy. The preferred conjugates contain maytansinoid compounds linked to the antibody by a disulfide linkage, and preferred chemotherapeutic agents are doxorubicin, a taxane, a camptothecin, a podophyllotoxin, a nucleoside analog, or a pyrimidine analog.
Owner:IMMUNOGEN INC +1
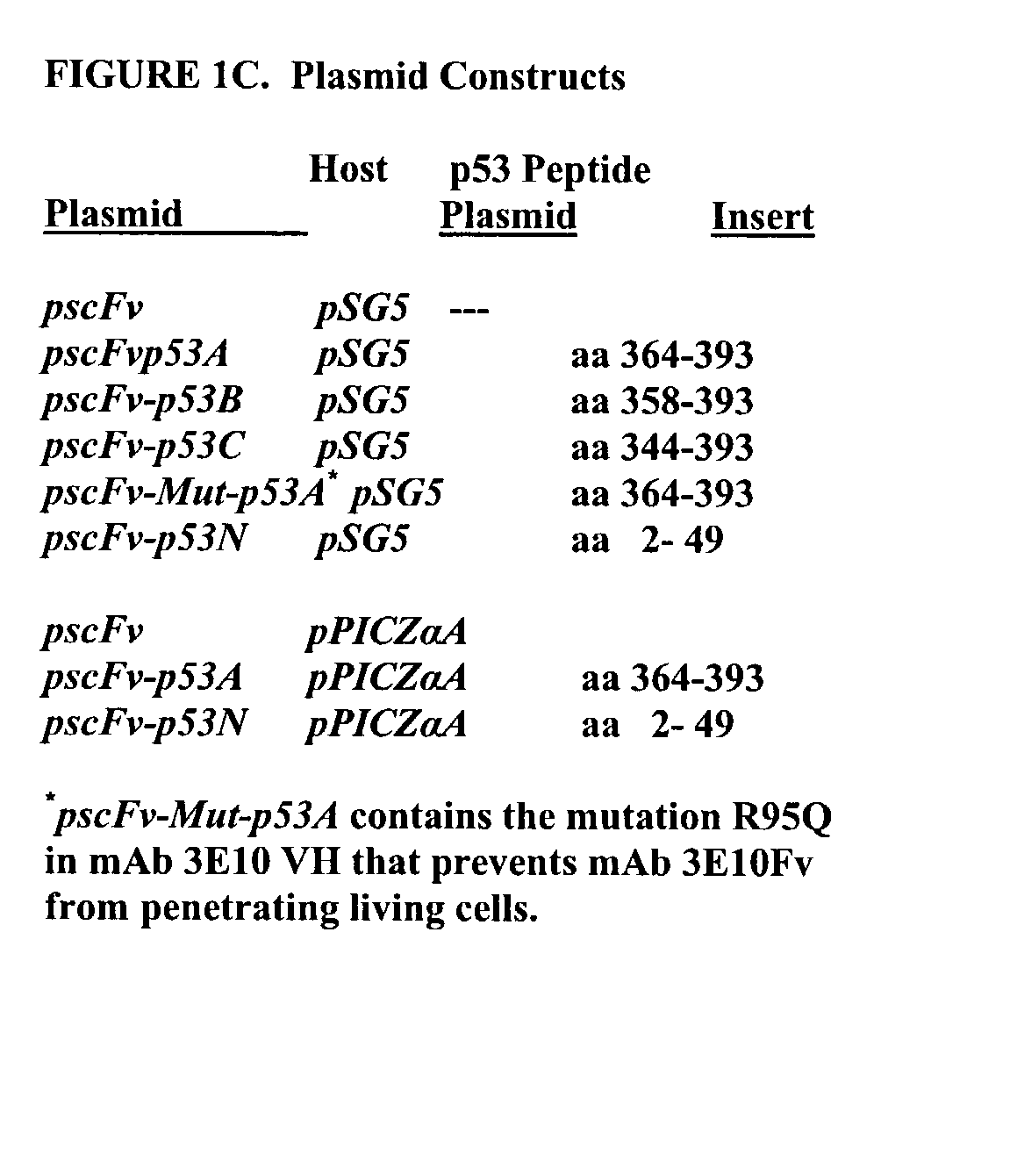
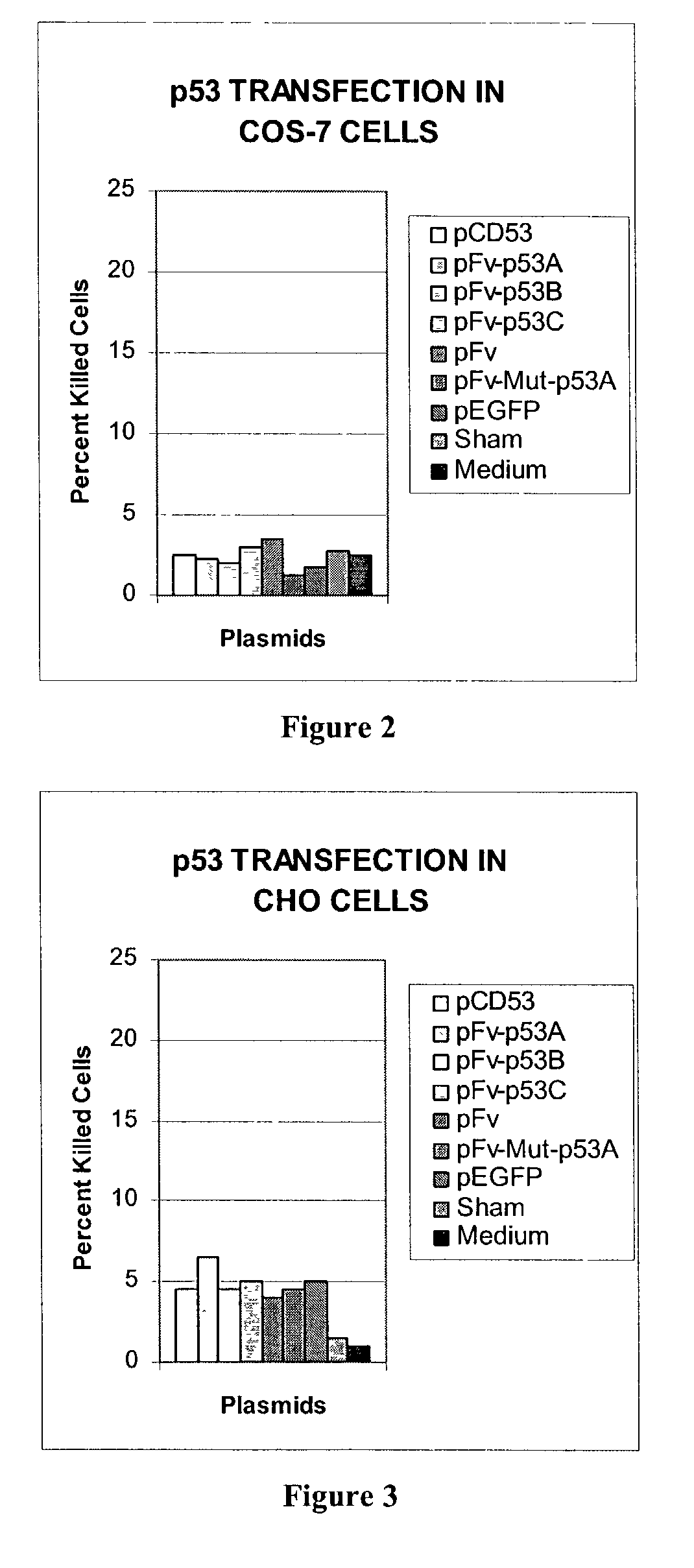
Delivery system using mAb 3E10 and mutants and/or functional fragments thereof
InactiveUS7189396B1Peptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsCancer cellCytotoxicity
A monoclonal antibody, 3E10, and active fragments thereof that selectively are transported in vivo to the nucleus of mammalian cells without cytotoxic effect are provided. The antibody and other molecules that bind to a variant of myosin IIb heavy chain found in the nucleus of skeletal muscle cells are useful as a non-viral delivery vector to target skeletal muscle in vivo. By contrast, in vitro the monoclonal antibody penetrates and is transported to the nucleus of multiple cell lines derived from different tissue types and can be used in screening tests to identify molecules that modulate growth of cells, such as cancer cells. Non-cytotoxic vectors for delivering a drug, polynucleotide or polypeptide selectively to skeletal muscle cells are also provided.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +1
Cytotoxic compounds and conjugates
Owner:ER SQUIBB & SONS INC
A cell therapy method for the treatment of tumors
T cell responses are often diminished in humans with a compromised immune system. We have developed a method to isolate, stimulate and expand naïve cytotoxic T lymphocyte precursors (CTLp) to antigen-specific effectors, capable of lysing tumor cells in vivo. This ex vivo protocol produces fully functional effectors. Artificial antigen presenting cells (AAPCs; Drosophila melanogaster) transfected with human HLA class I and defined accessory molecules, are used to stimulate CD8+ T cells from both normal donors and cancer patients. The class I molecules expressed to a high density on the surface of the Drosophila cells are empty, allowing for efficient loading of multiple peptides that results in the generation of polyclonal responses recognizing tumor cells endogenously expressing the specific peptides. The responses generated are robust, antigen-specific and reproducible if the peptide epitope is a defined immunogen. This artificial antigen expression system can be adapted to treat most cancers in a significant majority of the population.
Owner:JANSSEN PHARMA INC
Cytotoxic CD44 antibody immunoconjugates
The invention relates to novel conjugates of CD44 antibodies with cytotoxic compounds, pharmaceutical compositions comprising such compounds, and their use in tumor therapy.
Owner:BOEHRINGER INGELHEIM INT GMBH
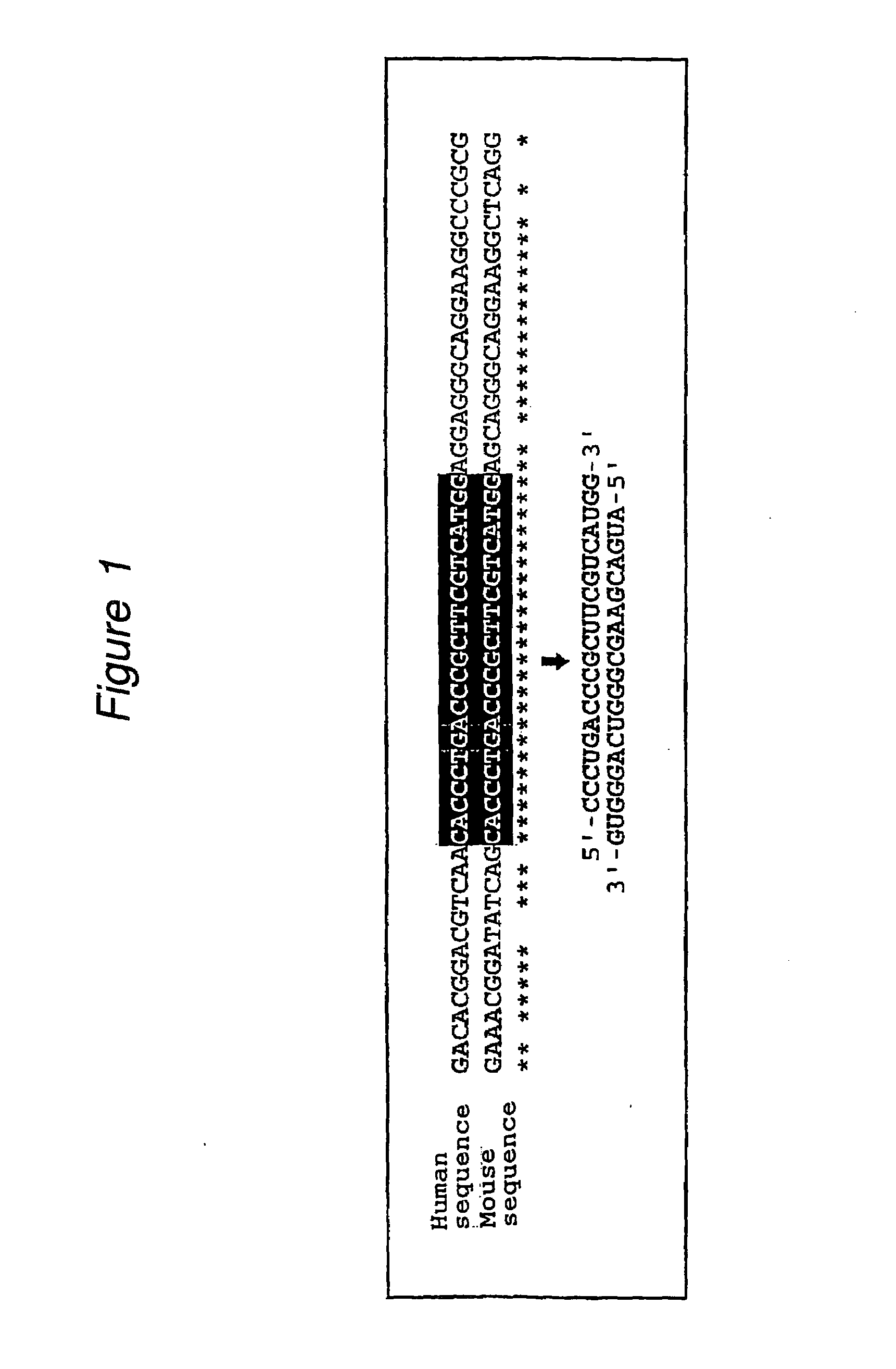
Polynucleotides for causing RNA interference and method for inhibiting gene expression using the same
InactiveUS20110054005A1High RNA interference effectLittle riskOrganic active ingredientsNervous disorderBase JNucleotide
The present invention provides a polynucleotide that not only has a high RNA interference effect on its target gene, but also has a very small risk of causing RNA interference against a gene unrelated to the target gene. A sequence segment conforming to the following rules (a) to (d) is searched from the base sequences of a target gene for RNA interference and, based on the search results, a polynucleotide capable of causing RNAi is designed, synthesized, etc.:(a) The 3′ end base is adenine, thymine, or uracil,(b) The 5′ end base is guanine or cytosine,(c) A 7-base sequence from the 3′ end is rich in one or more types of bases selected from the group consisting of adenine, thymine, and uracil, and(d) The number of bases is within a range that allows RNA interference to occur without causing cytotoxicity.
Owner:BIO THINKTANK
Tolerogenic synthetic nanocarriers to reduce cytotoxic t lymphocyte responses
Disclosed are synthetic nanocarrier compositions, and related methods, comprising MHC Class I-restricted and / or MHC Class II-restricted epitopes associated with undesired CD8+ T cell responses and immunosuppressants that provide tolerogenic immune responses against antigens that comprise the epitopes.
Owner:SELECTA BIOSCI
Real time electronic cell sensing system and applications for cytotoxicity profiling and compound assays
ActiveUS7560269B2Bioreactor/fermenter combinationsBiological substance pretreatmentsCytotoxicityEngineering
Owner:AGILENT TECH INC
Cd3 binding polypeptides
InactiveUS20150232557A1Reduce pointsReduced immunogenicity riskImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntibody fragmentsSingle-domain antibody
The present invention relates to mono-specific and multi-specific polypeptides that specifically bind or interact with CD3. These polypeptides can be, but are not limited to, antibodies, fragments thereof, scFvs, Fabs, di-scFvs single domain antibodies, diabodies, dual variable domain binding proteins and polypeptides containing an antibody or antibody fragments. In one embodiment, a multi-specific polypeptide binds both a T-cell receptor complex on T-cells and a tumor antigen to induce target-dependent T-cell cytotoxicity, activation and proliferation.
Owner:APTEVO RES & DEV LLC
Human anti-KIR antibodies
ActiveUS8119775B2Antibacterial agentsSugar derivativesNatural Killer Cell Inhibitory ReceptorsMonoclonal antibody
Compositions and methods for regulating an immune response in a subject are described. More particularly, described are human antibodies that regulate the activity of NK cells and allow a potentiation of NK cell cytotoxicity in mammalian subjects, and antibodies having antigen-binding properties similar to those of human monoclonal antibody 1-7F9 or 1-4F1. Described also are also fragments and derivatives of such antibodies, as well as pharmaceutical compositions comprising the same and their uses, particularly for use in therapy, to increase NK cell activity or cytotoxicity in subjects.
Owner:UNIV DEGLI STUDI DI GENOVA GENOVA IT +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com