Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
167 results about "Adoptive immunotherapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
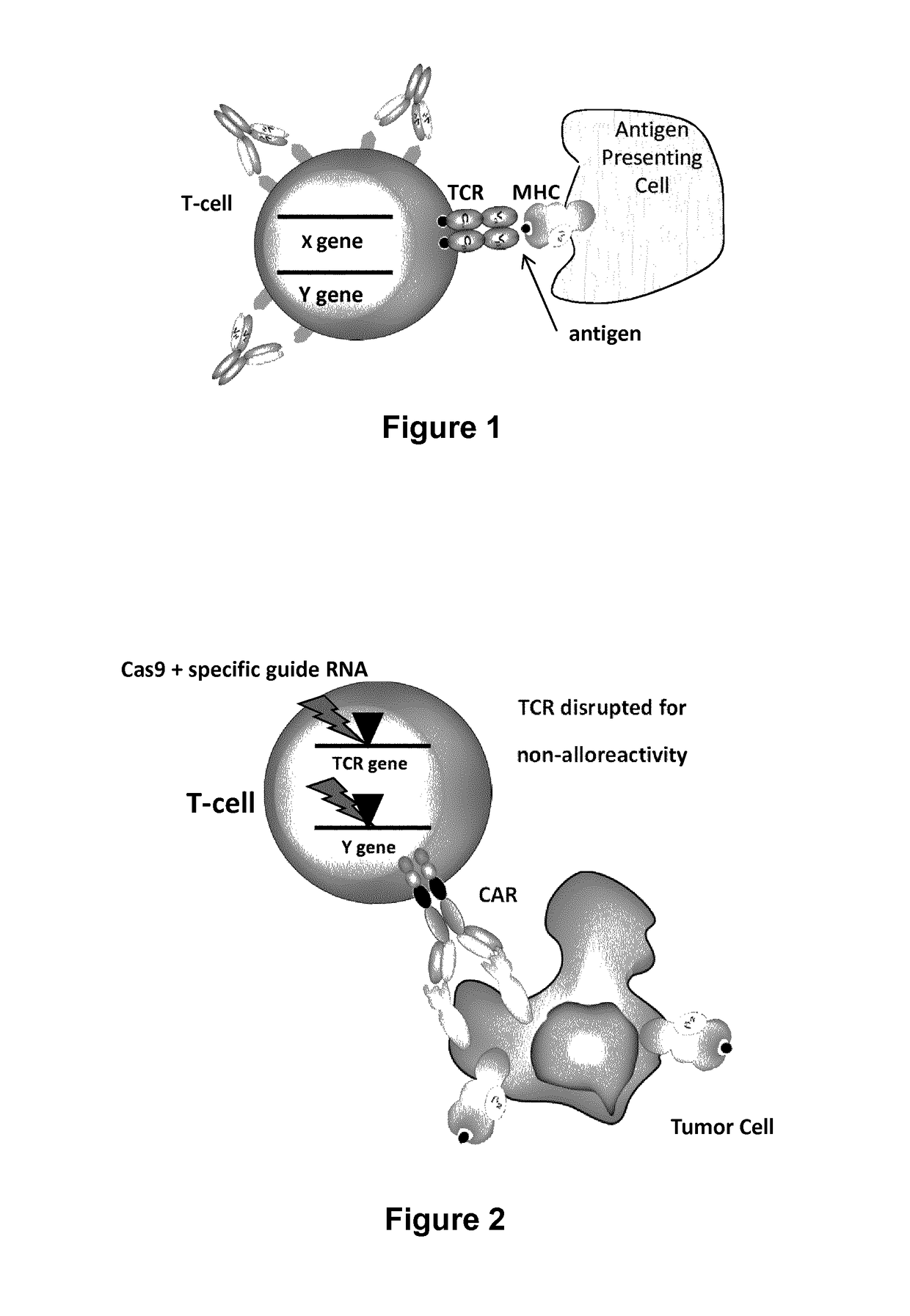
Definition of adoptive immunotherapy : the transfer of immune cells with antitumor activity into a patient to mediate tumor regression especially : treatment typically for cancer in which lymphocytes removed from a patient are cultured with interleukin-2 and are returned to the patient's body
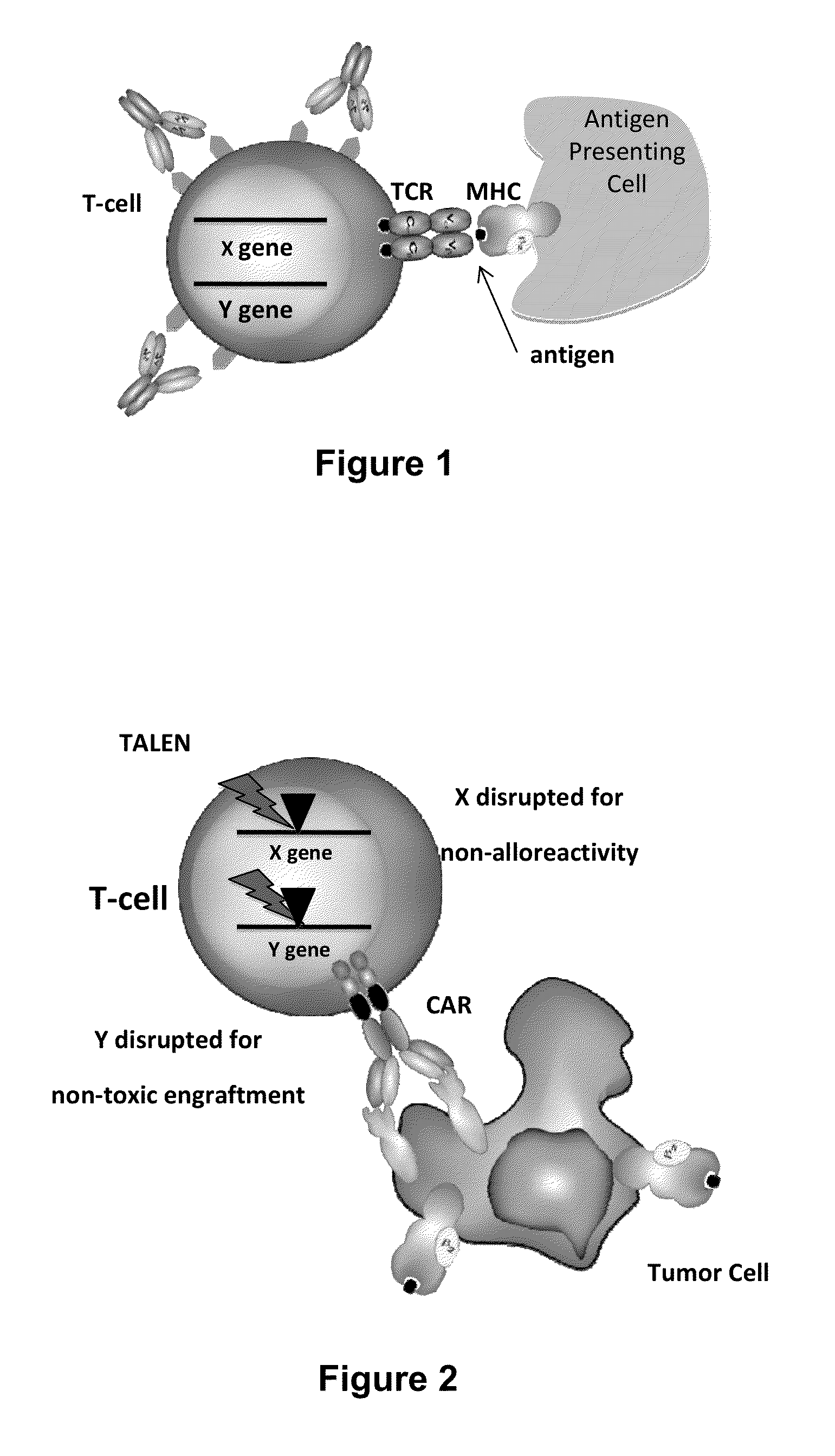
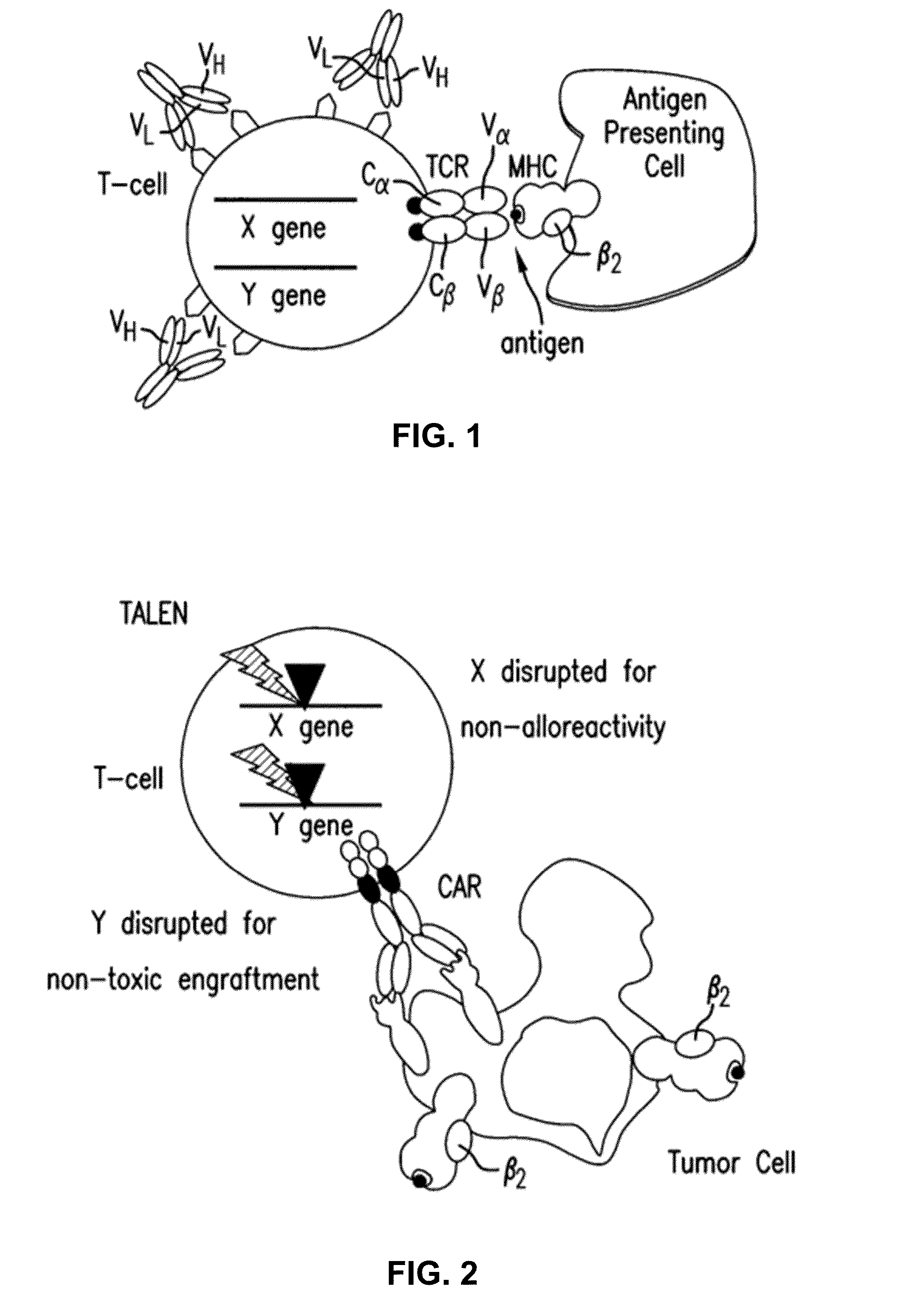
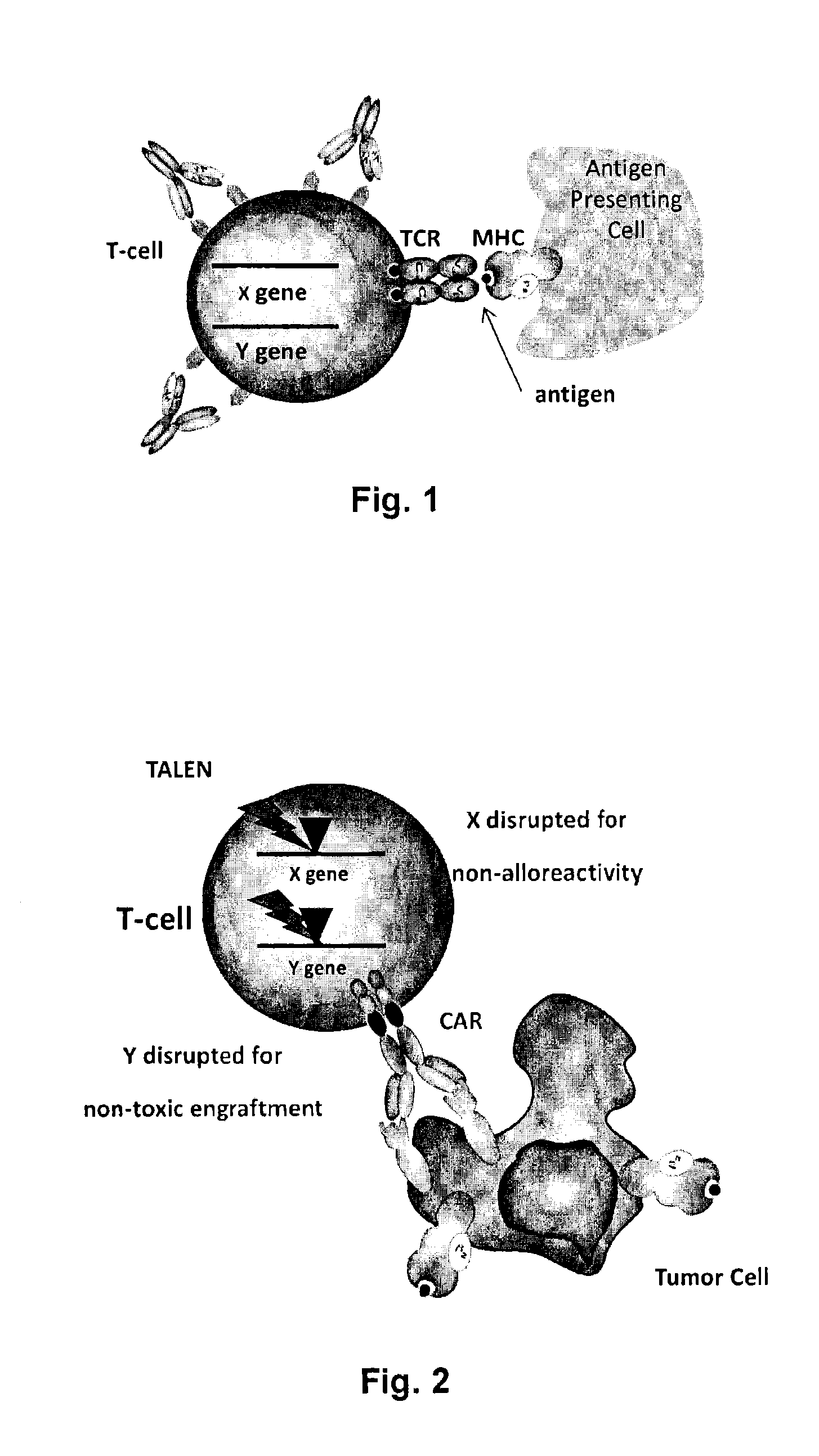
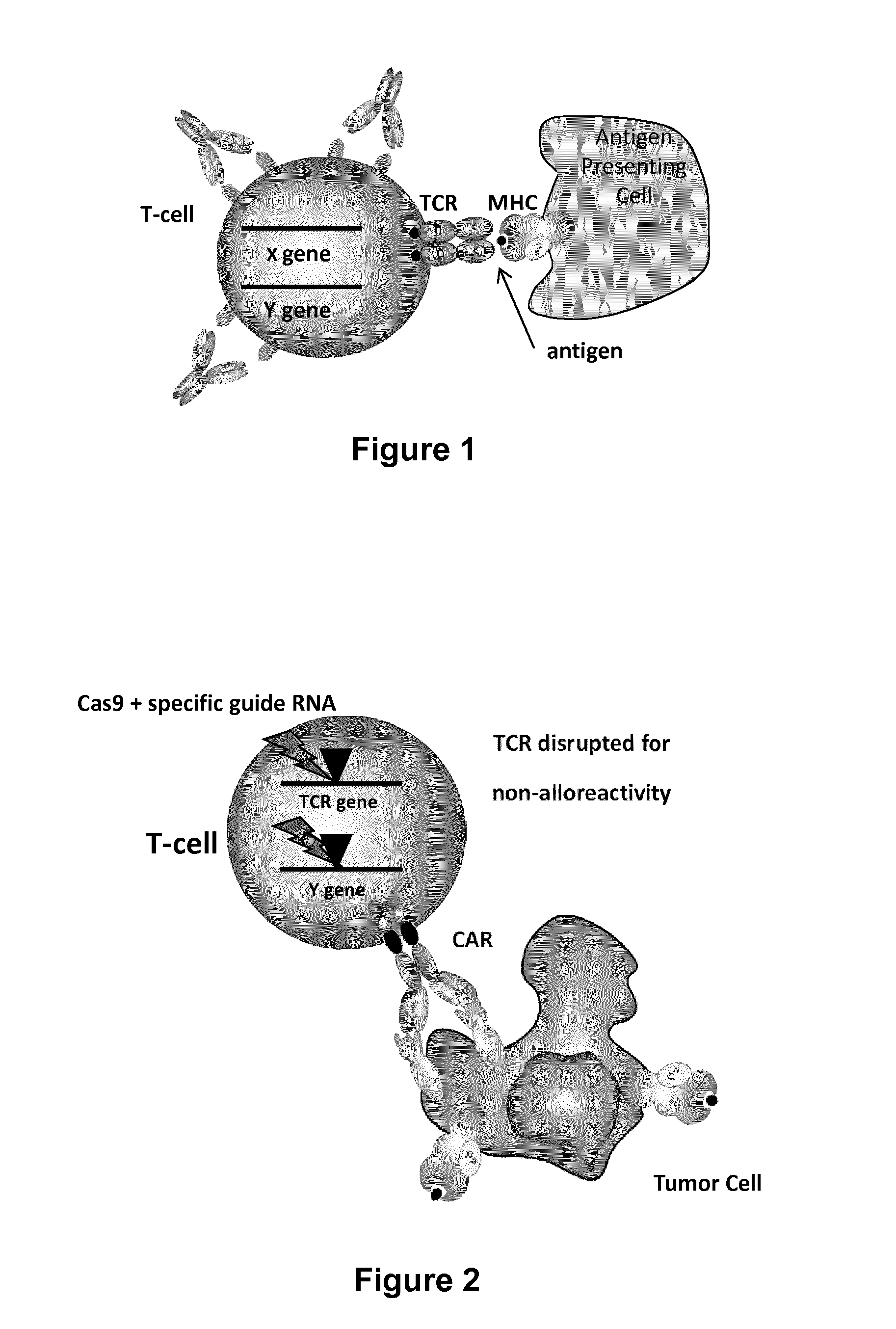
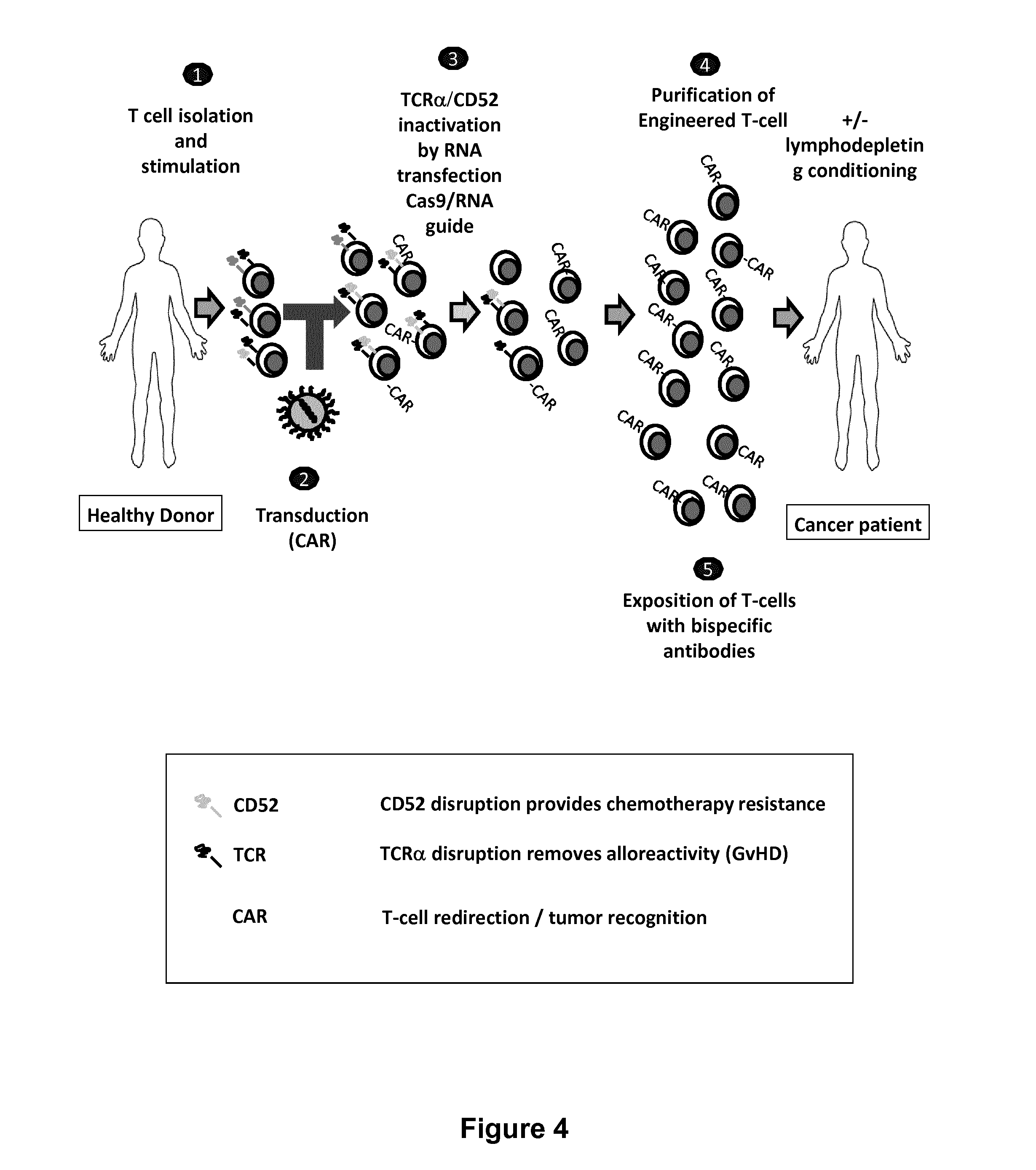
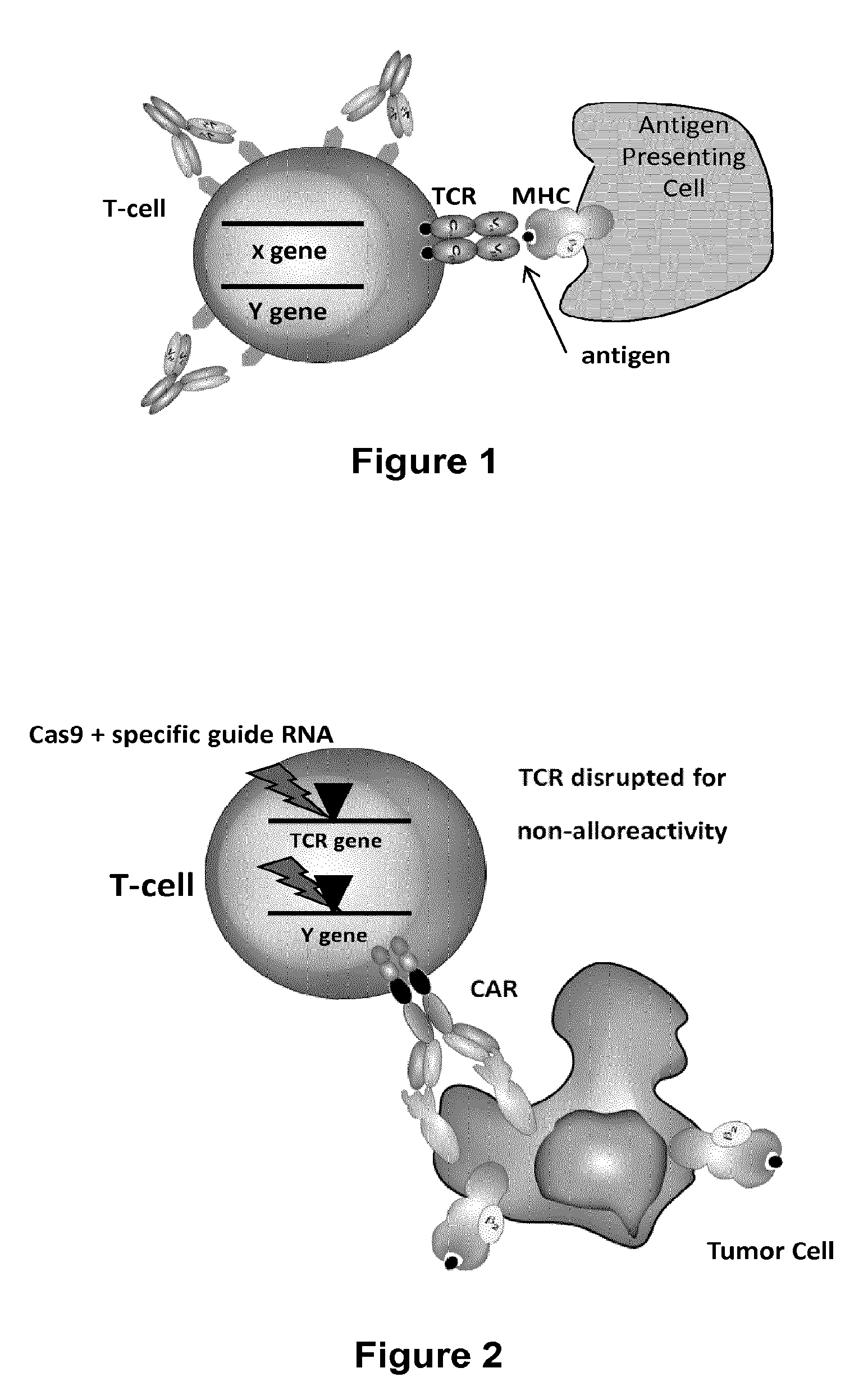
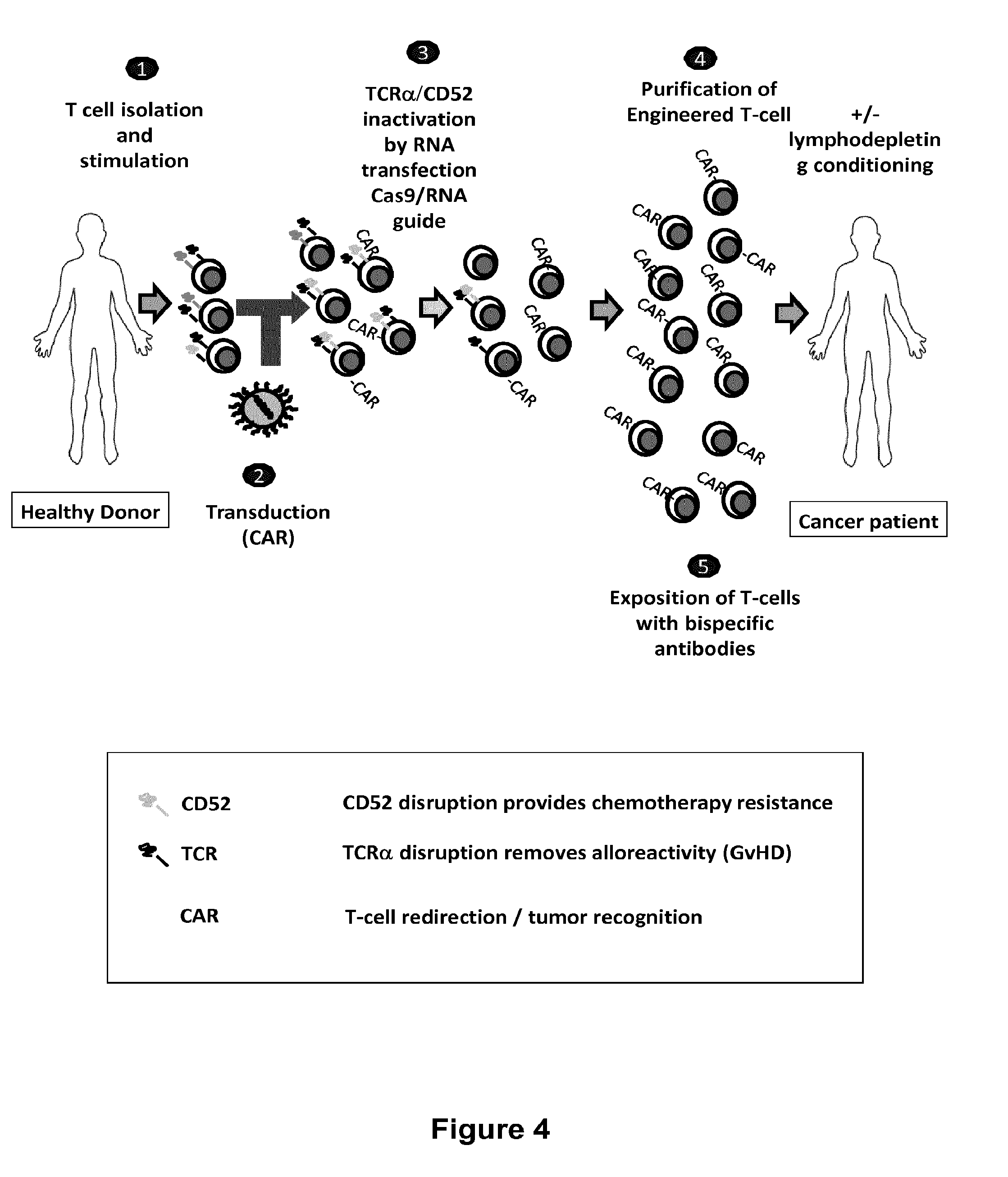
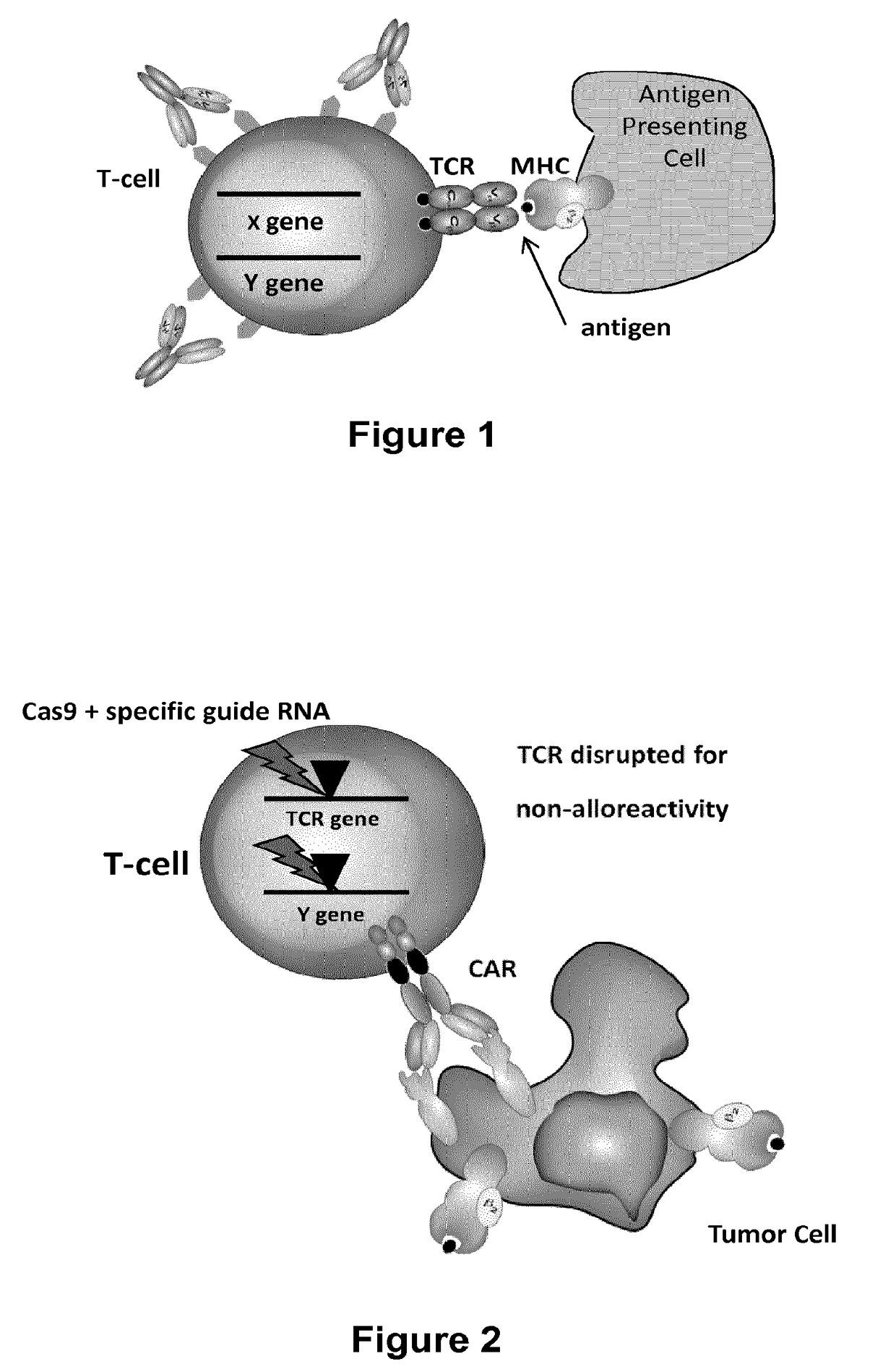
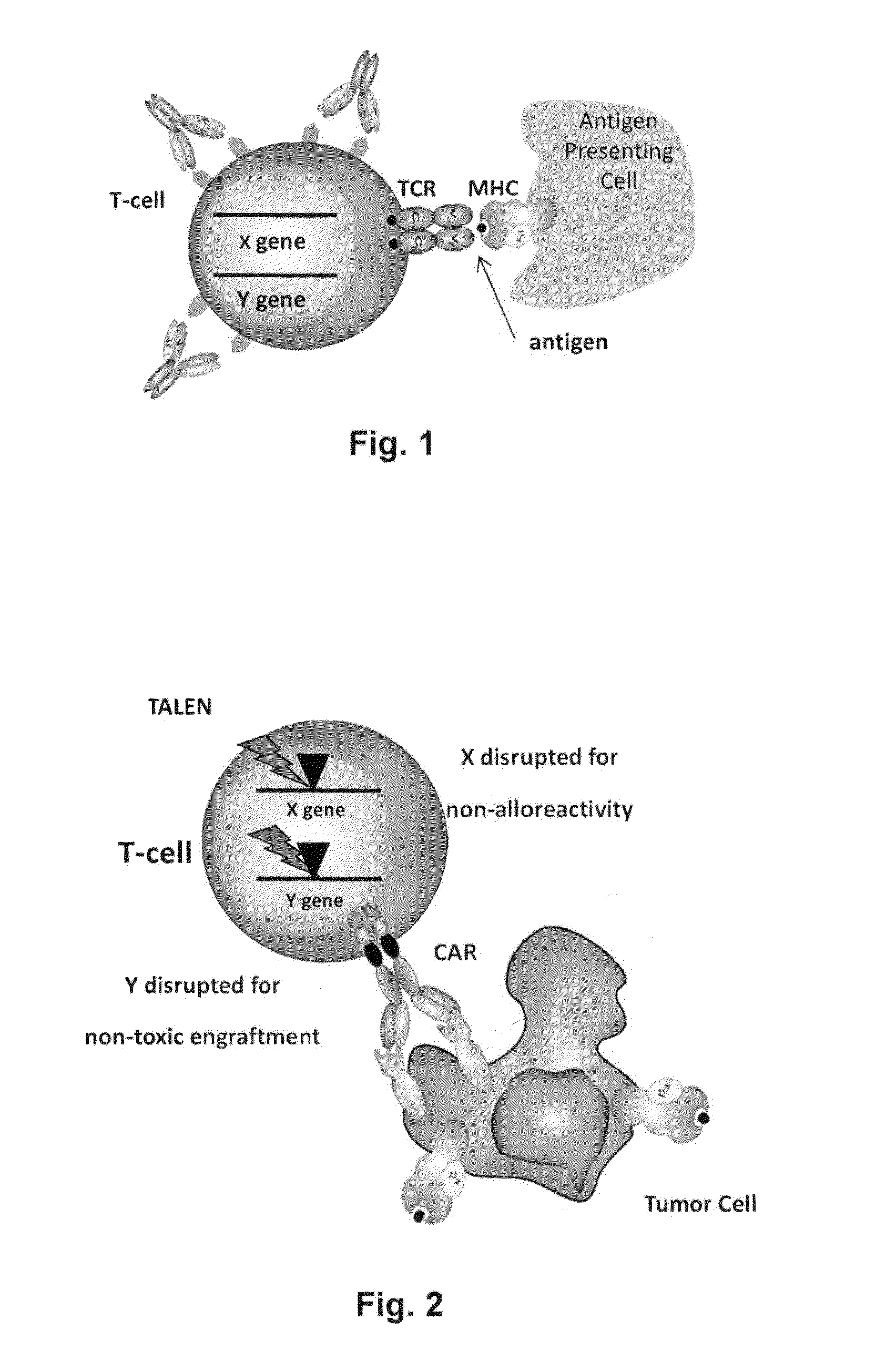
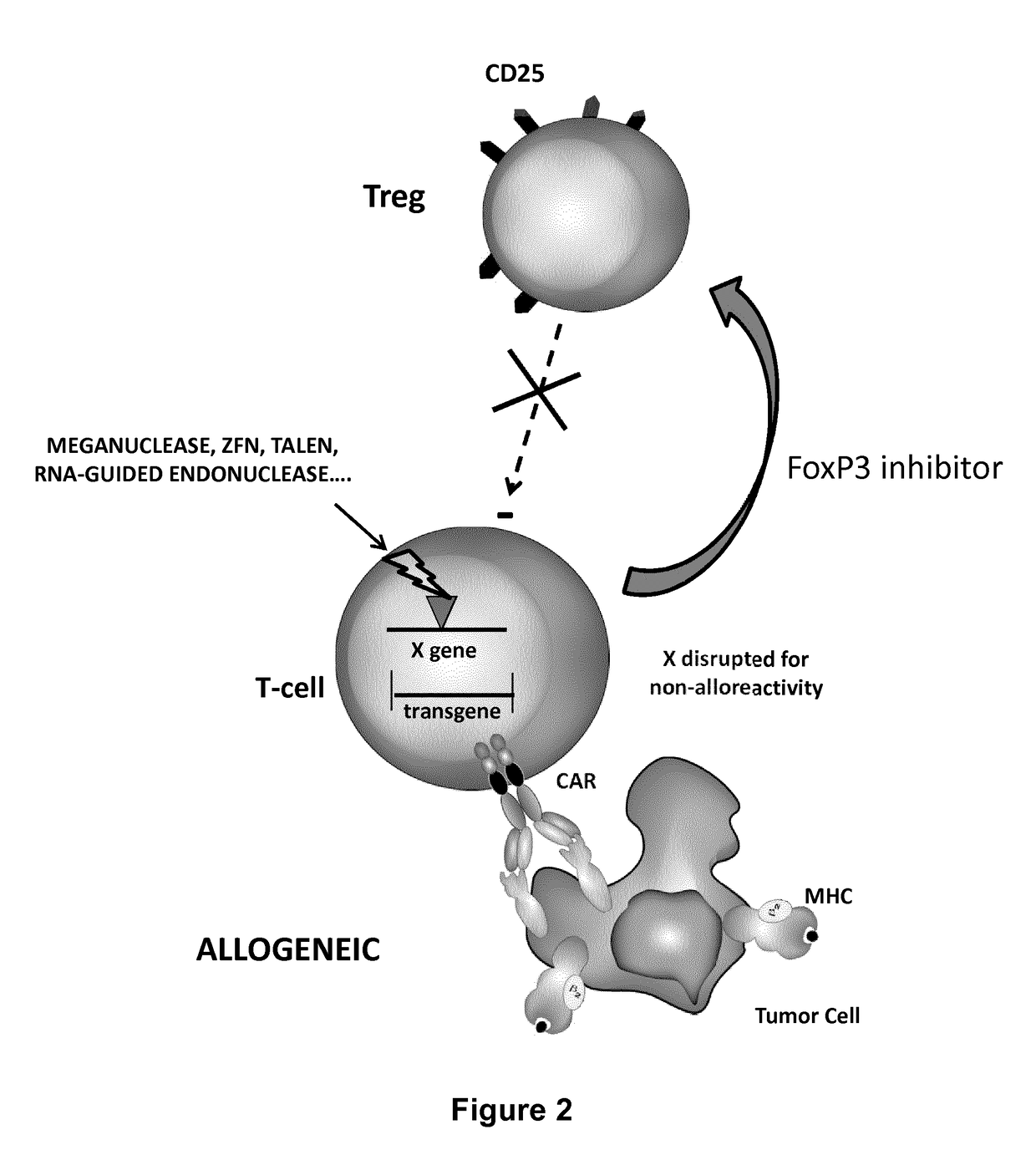
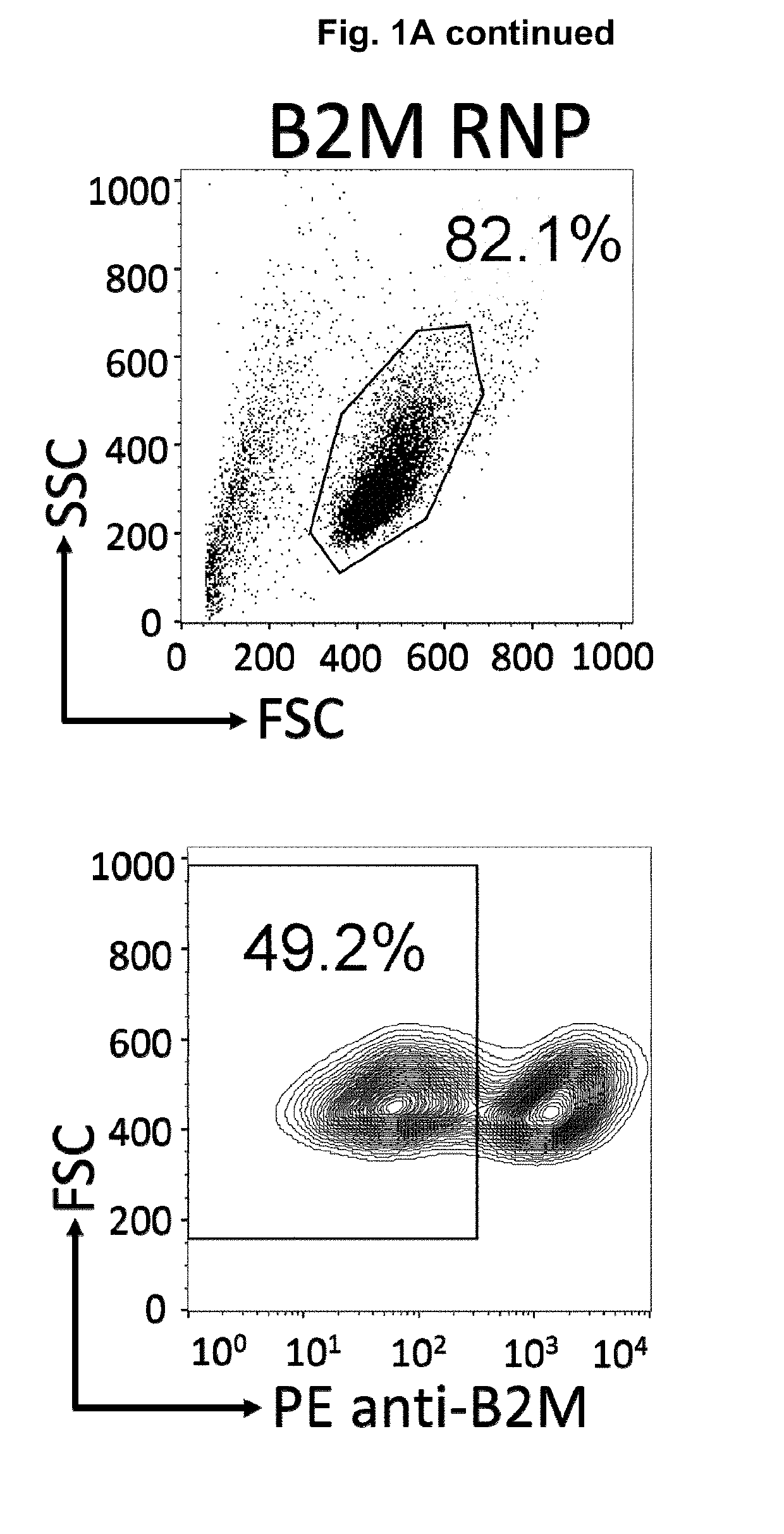
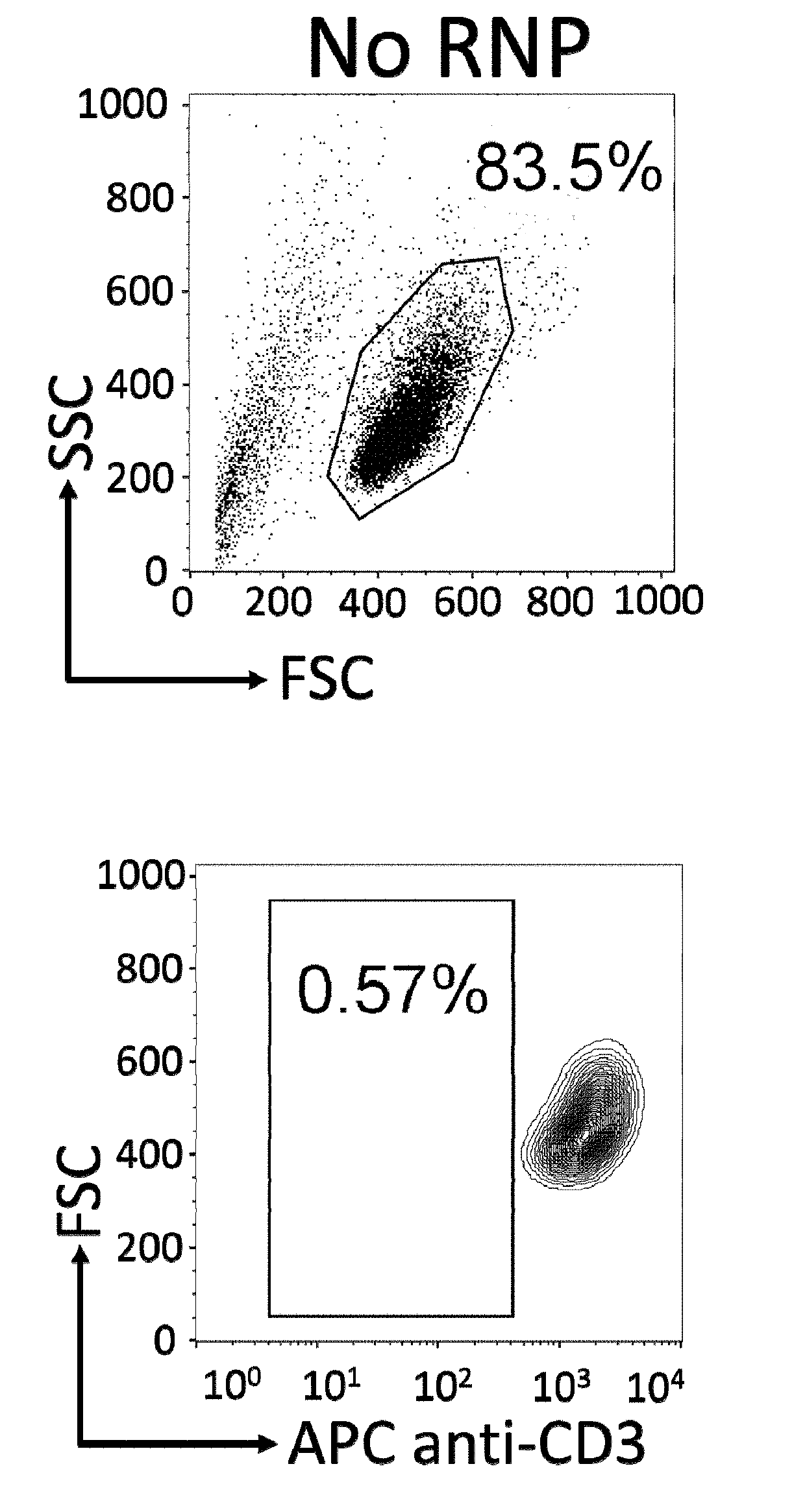
Methods for engineering allogeneic and immunosuppressive resistant t cell for immunotherapy
ActiveUS20130315884A1Precise positioningPeptide/protein ingredientsAntibody mimetics/scaffoldsImmunosuppressive drugPrimary cell
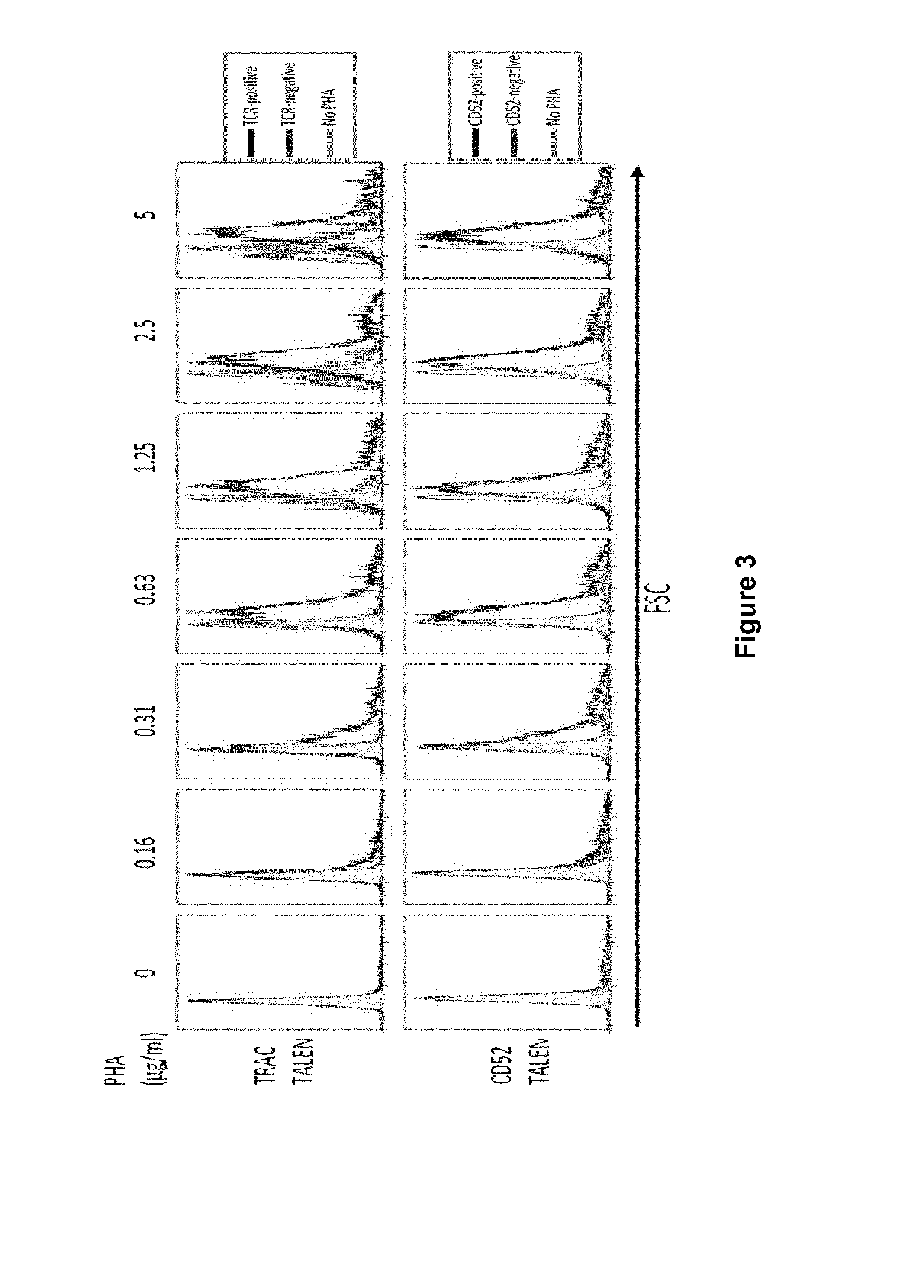
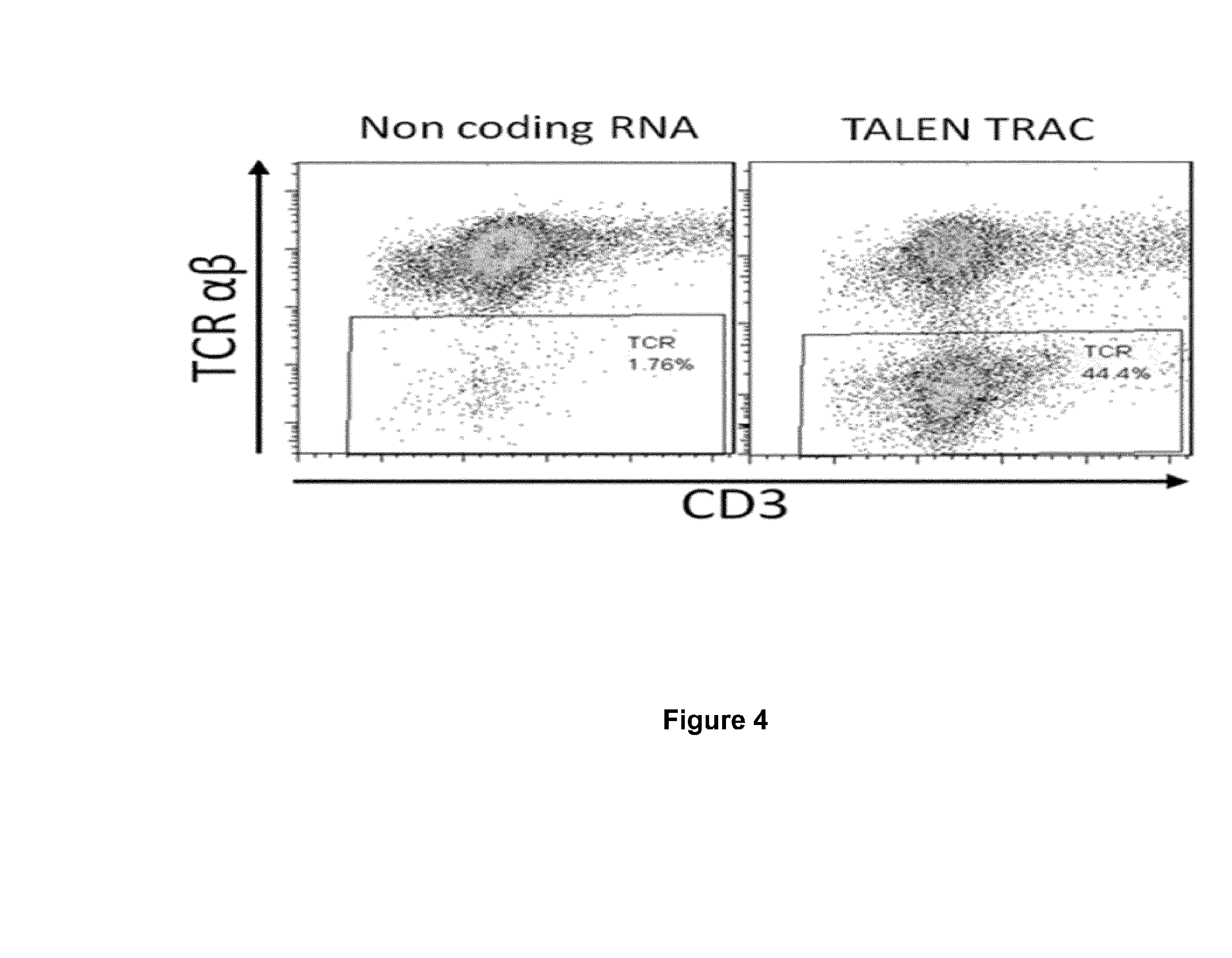
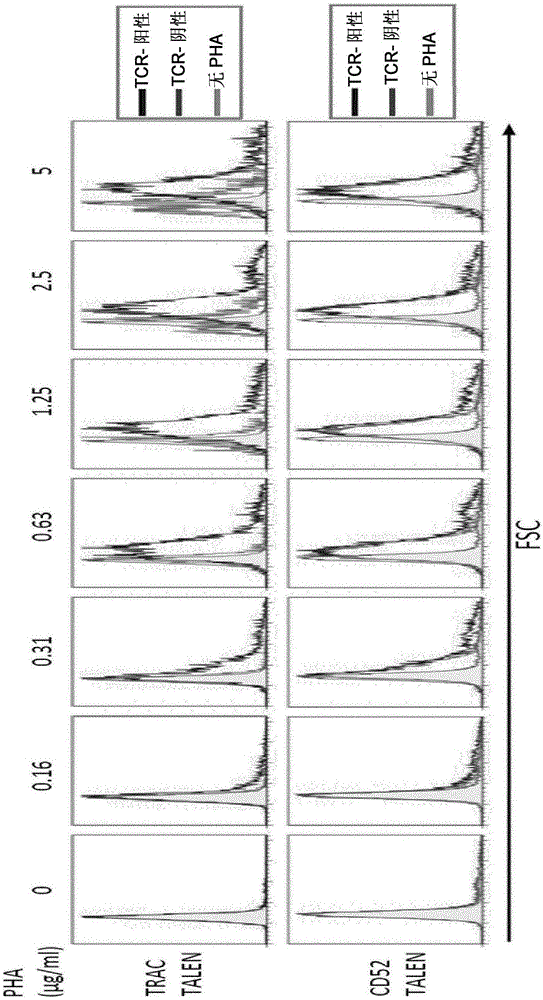
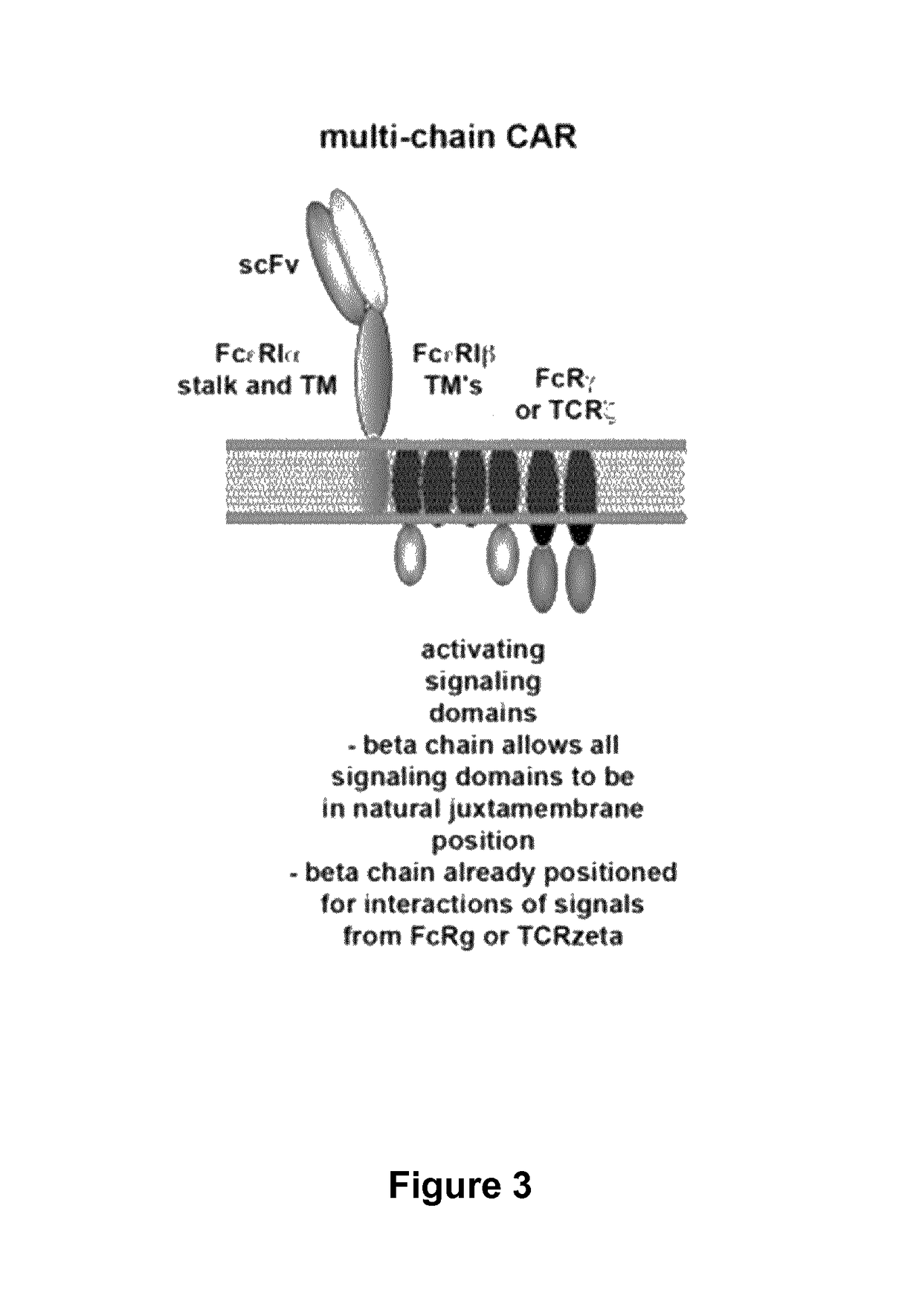
Methods for developing engineered T-cells for immunotherapy that are both non-alloreactive and resistant to immunosuppressive drugs. The present invention relates to methods for modifying T-cells by inactivating both genes encoding target for an immunosuppressive agent and T-cell receptor, in particular genes encoding CD52 and TCR. This method involves the use of specific rare cutting endonucleases, in particular TALE-nucleases (TAL effector endonuclease) and polynucleotides encoding such polypeptides, to precisely target a selection of key genes in T-cells, which are available from donors or from culture of primary cells. The invention opens the way to standard and affordable adoptive immunotherapy strategies for treating cancer and viral infections.
Owner:CELLECTIS SA
Compositions and methods for treatment of neoplastic disease
InactiveUS20050112141A1High productFacilitates their targetingFusions for specific cell targetingReceptors for cytokines/lymphoines/interferonsAbnormal tissue growthDisease
The present invention comprises compositions and methods for treating a tumor or neoplastic disease in a host, The methods employ conjugates comprising superantigen polypeptides or nucleic acids with other structures that preferentially bind to tumor cells and are capable of inducing apoptosis. Also provided are superantigen-glycolipid conjugates and vesicles that are loaded onto antigen presenting cells to activate both T cells and NKT cells. Cell-based vaccines comprise tumor cells engineered to express a superantigen along with glycolipids products which, when expressed, render the cells capable of eliciting an effective anti-tumor immune response in a mammal into which these cells are introduced. Included among these compositions are tumor cells, hybrid cells of tumor cells and accessory cells, preferably dendritic cells. Also provided are T cells and NKT cells activated by the above compositions that can be administered for adoptive immunotherapy.
Owner:TERMAN DAVID
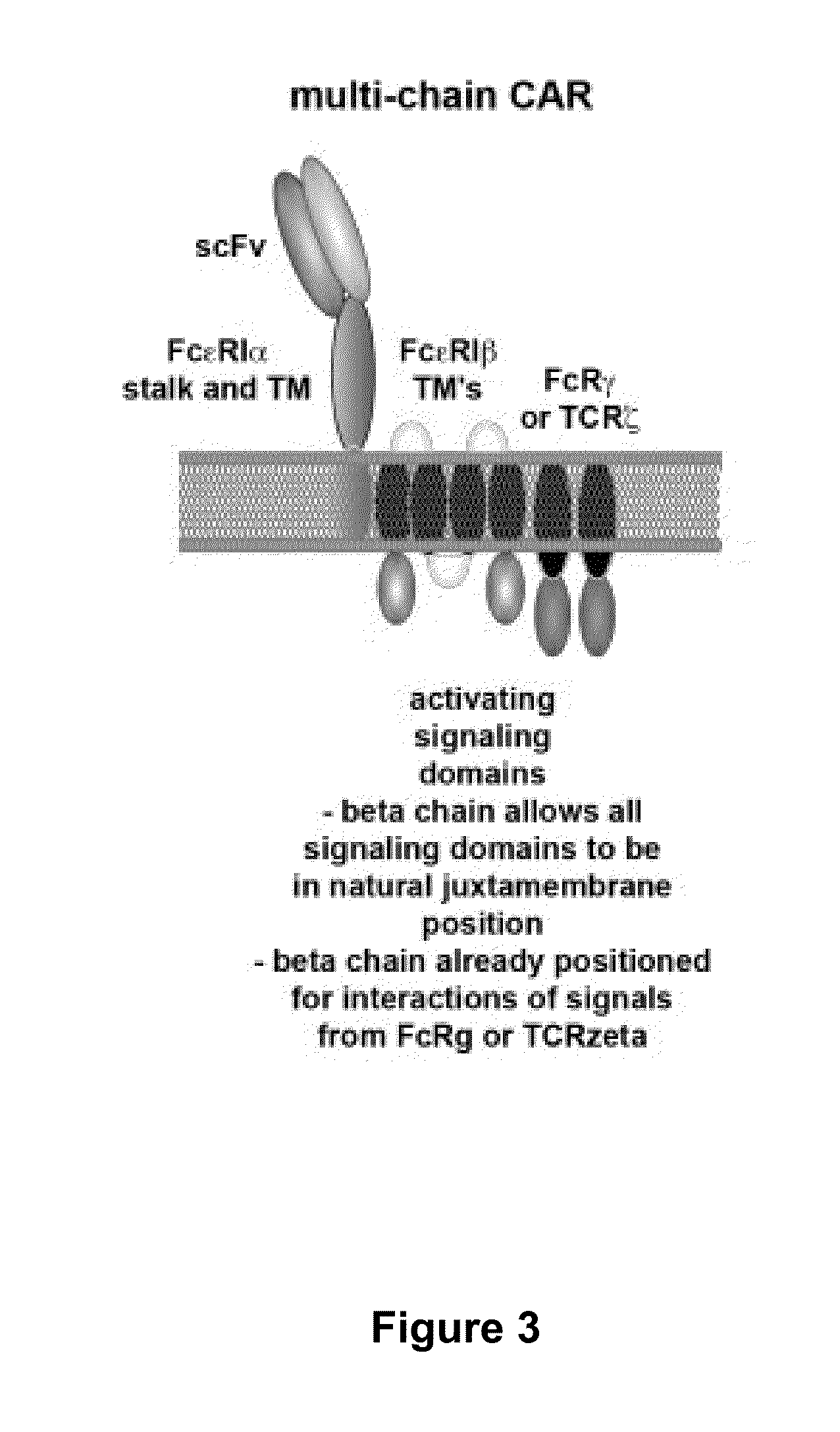
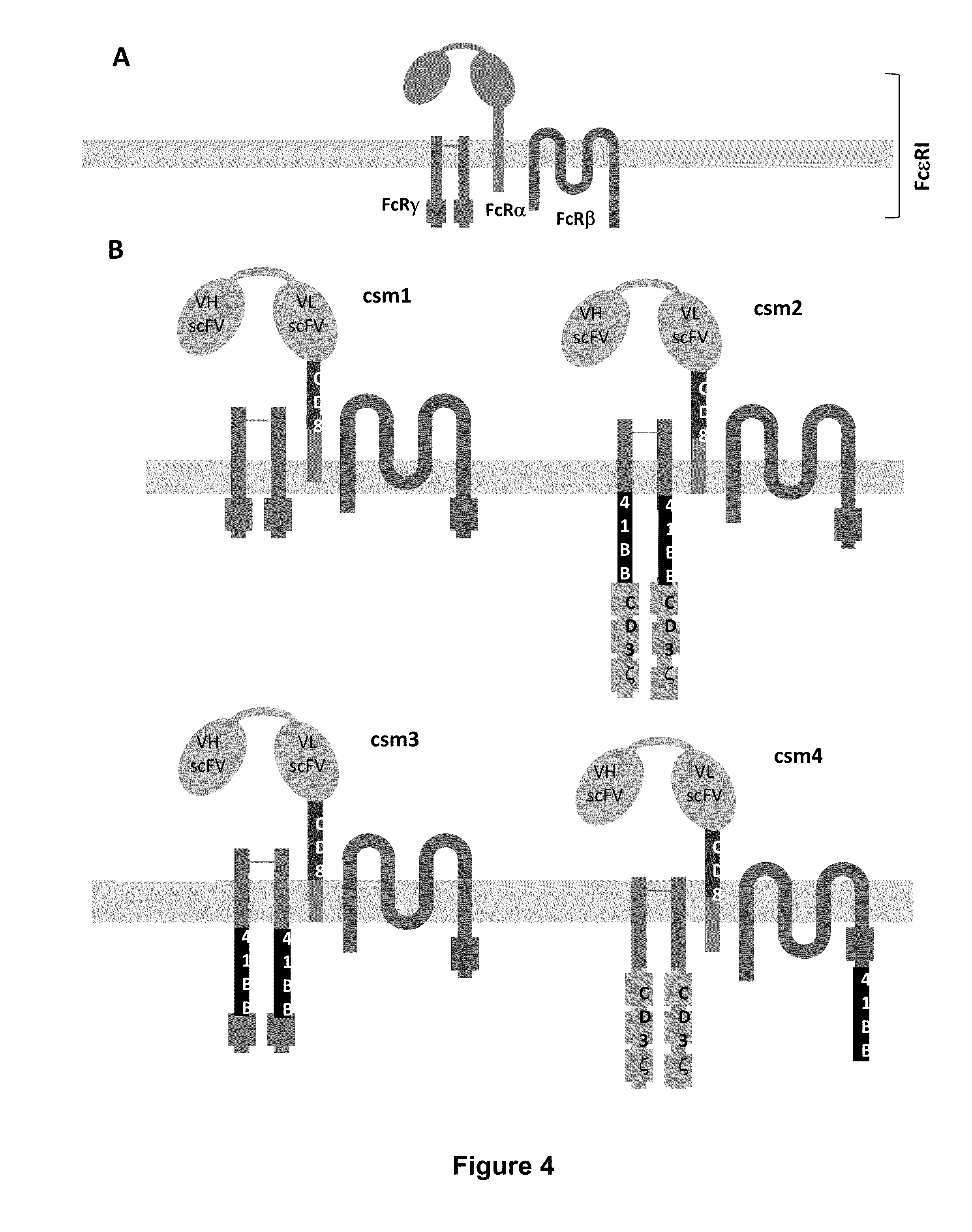
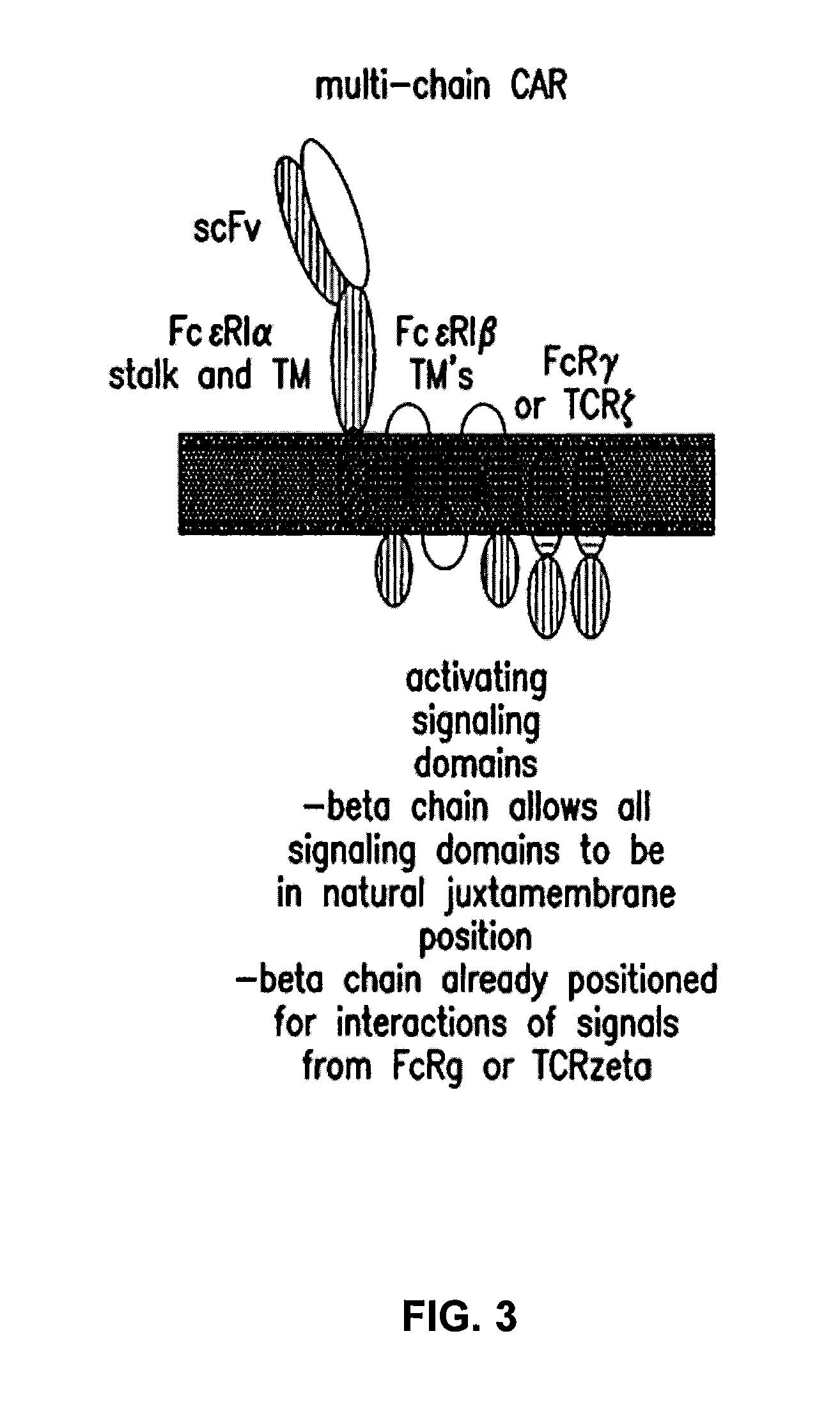
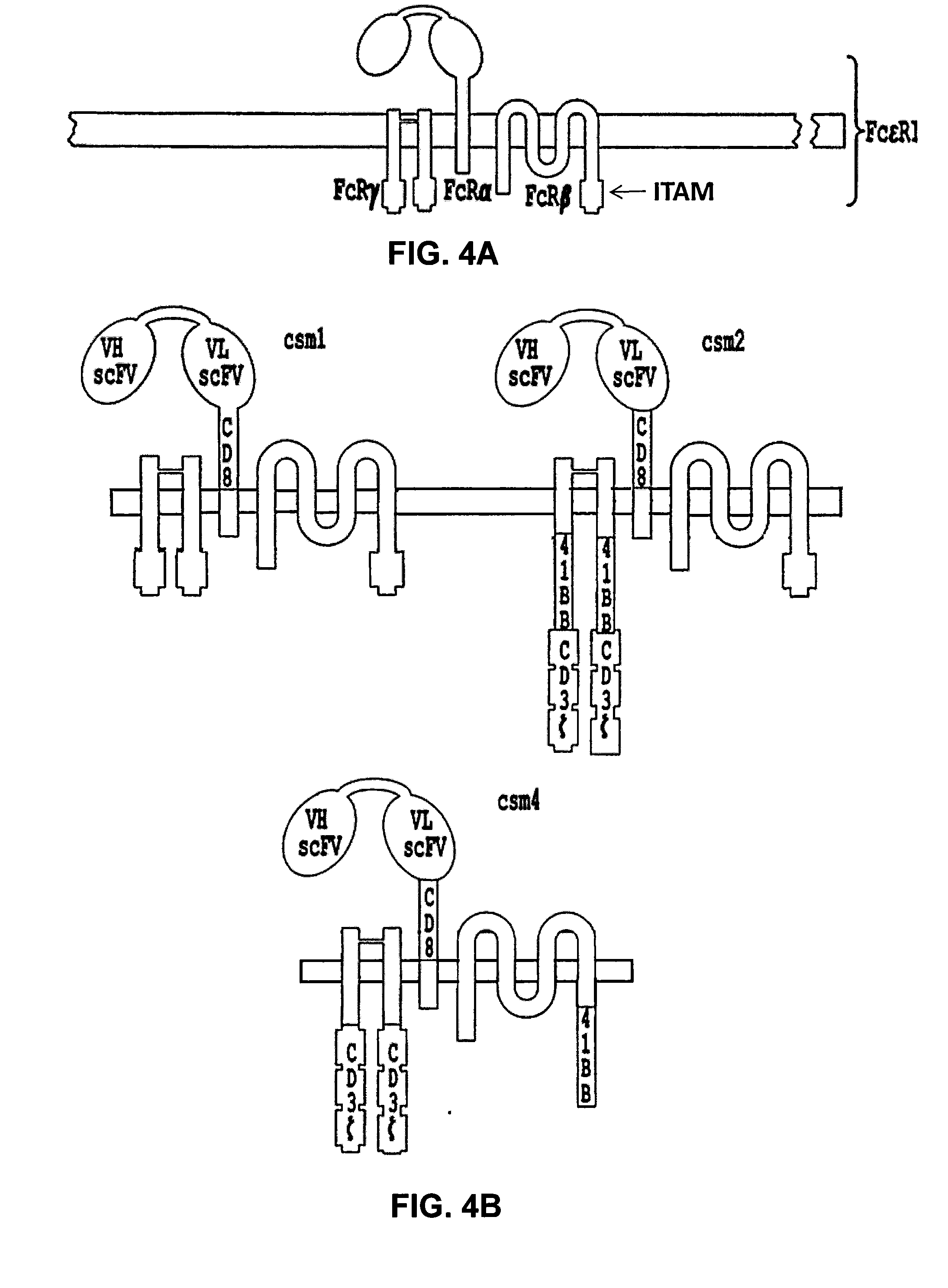
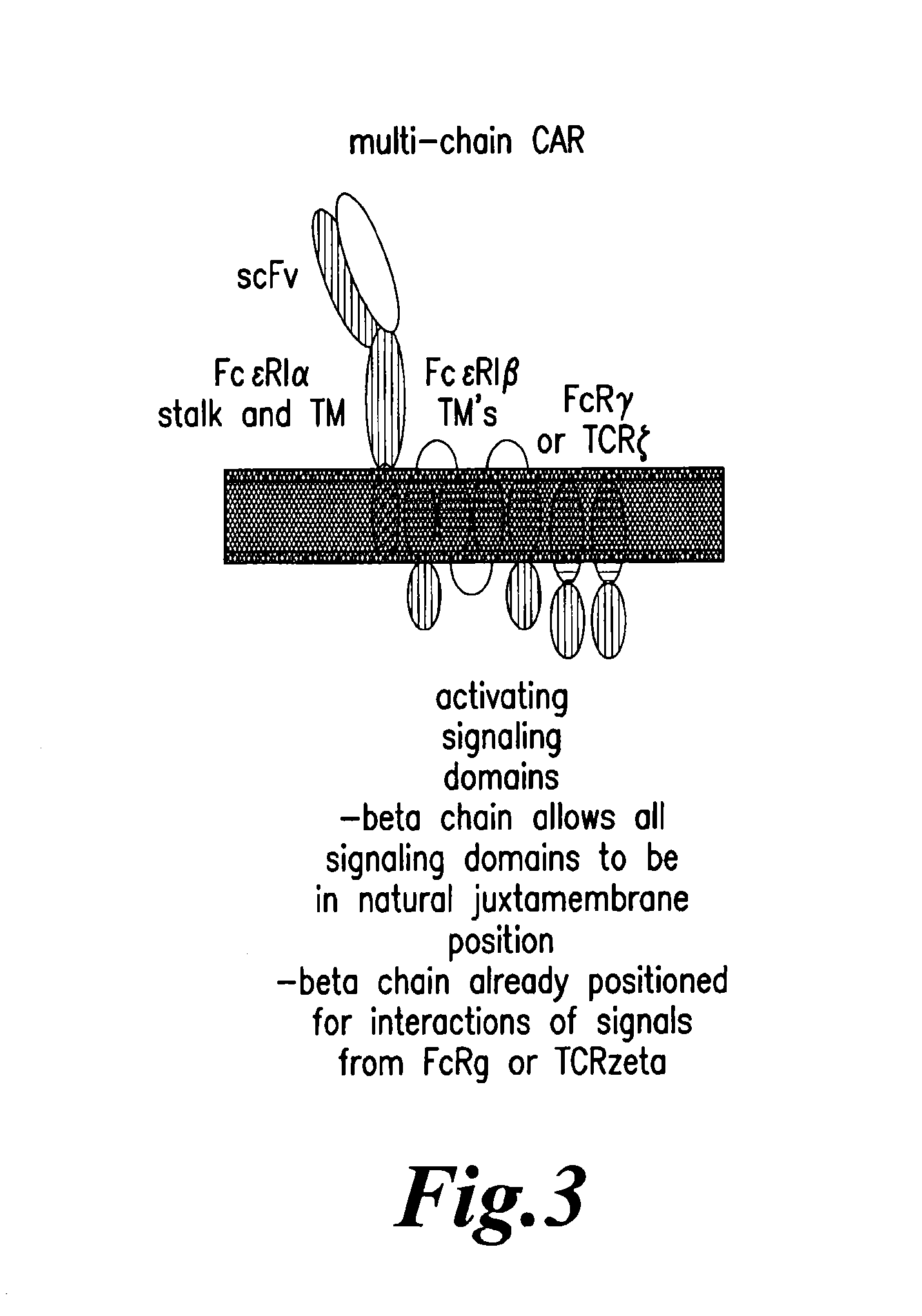
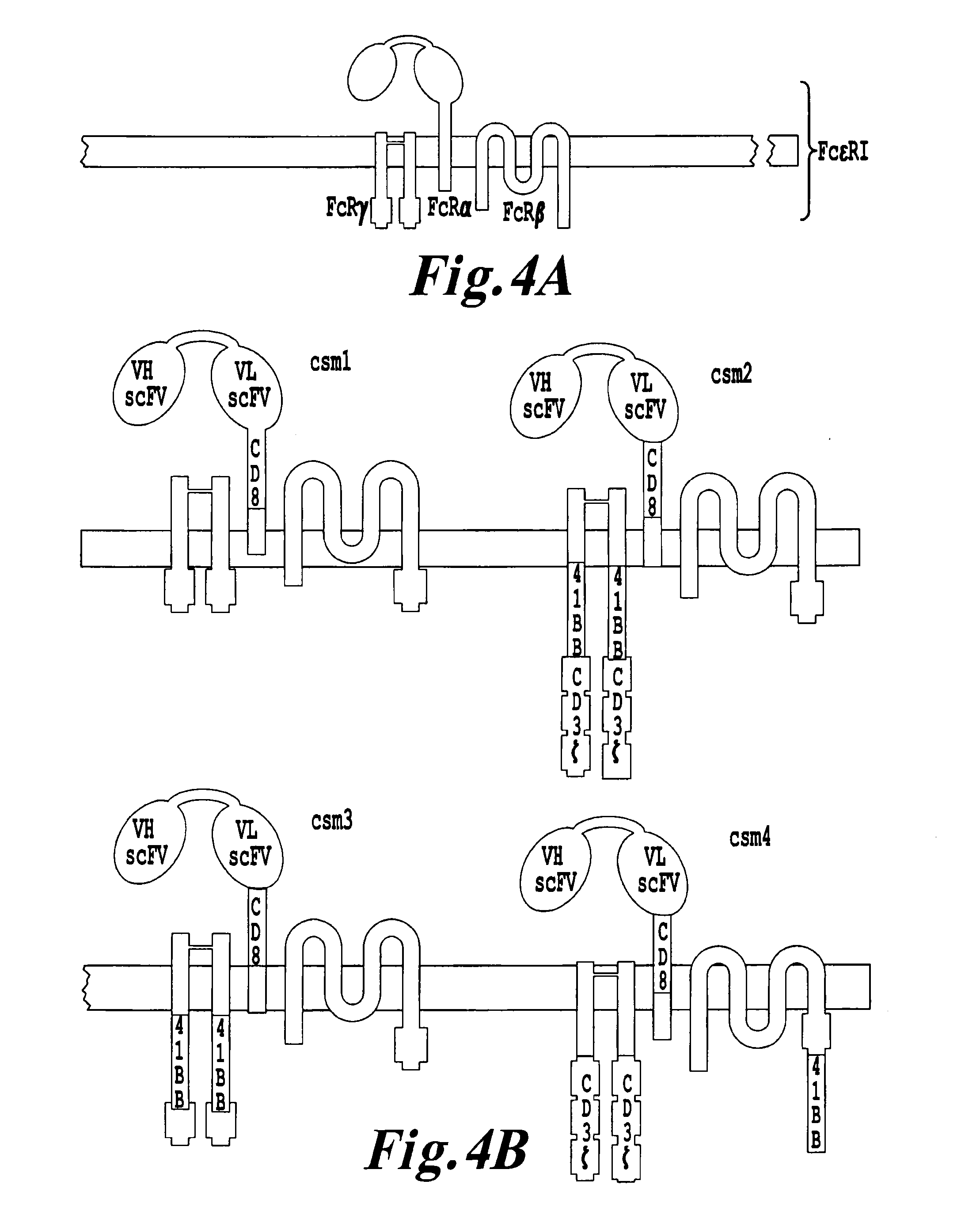
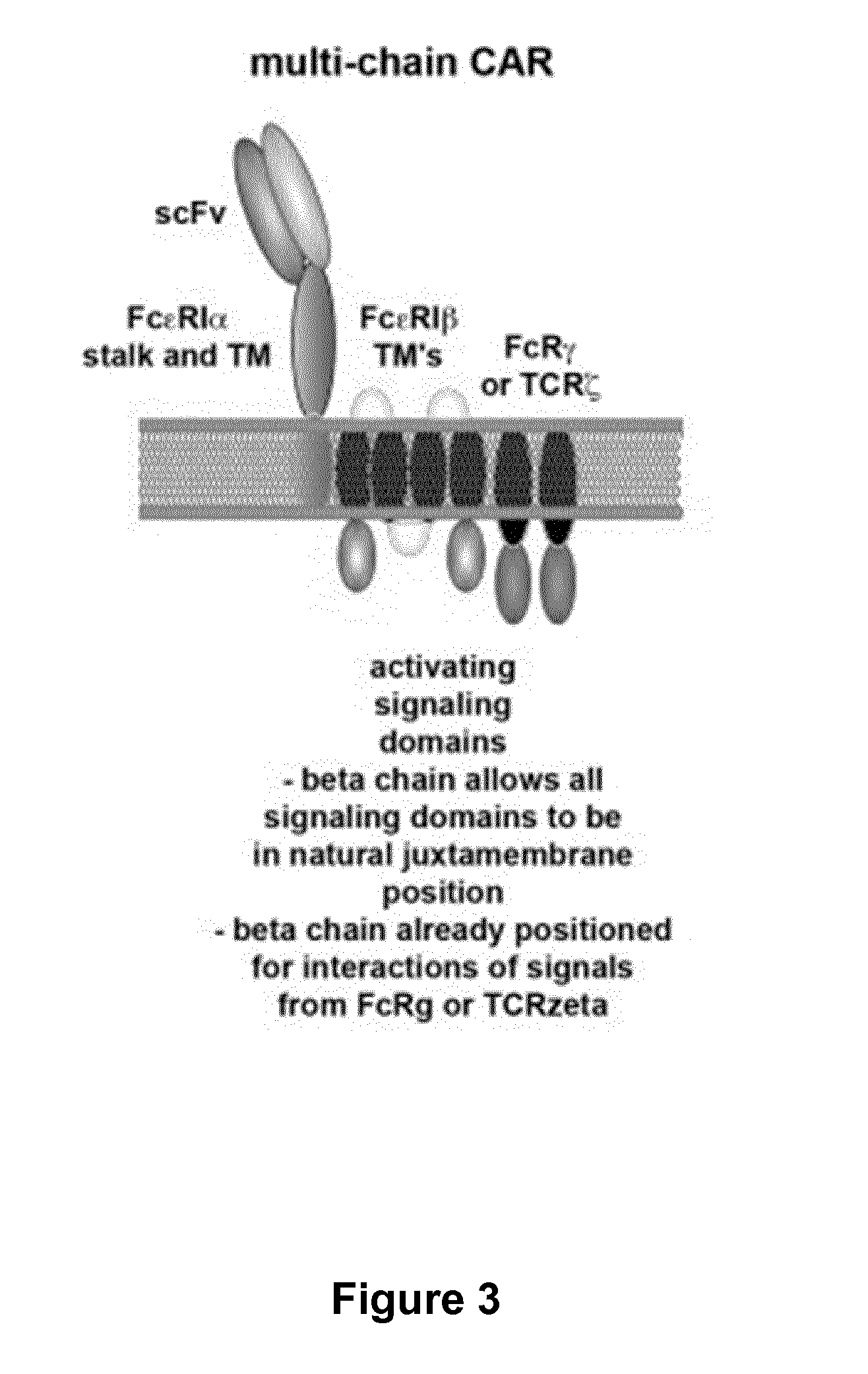
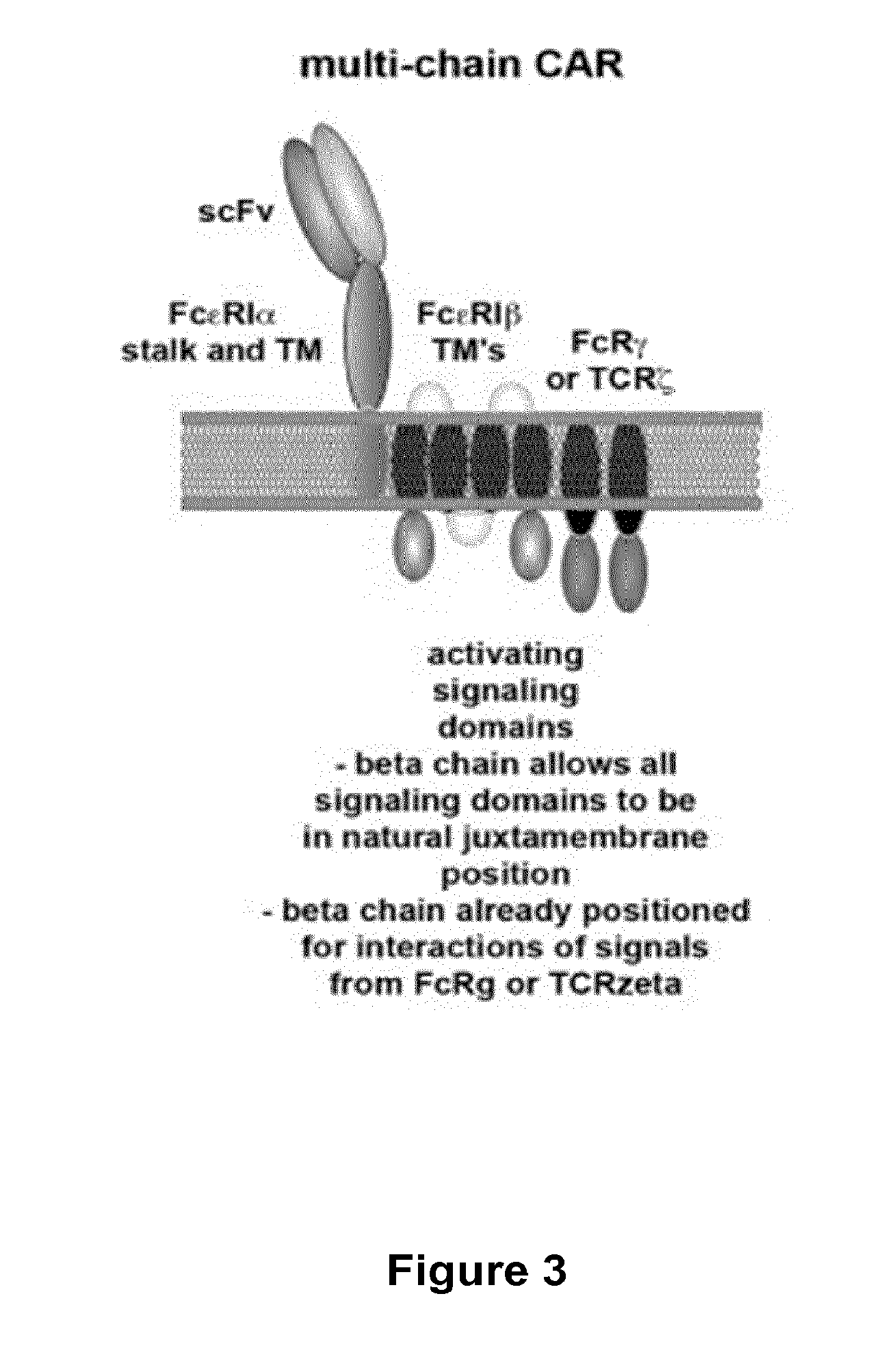
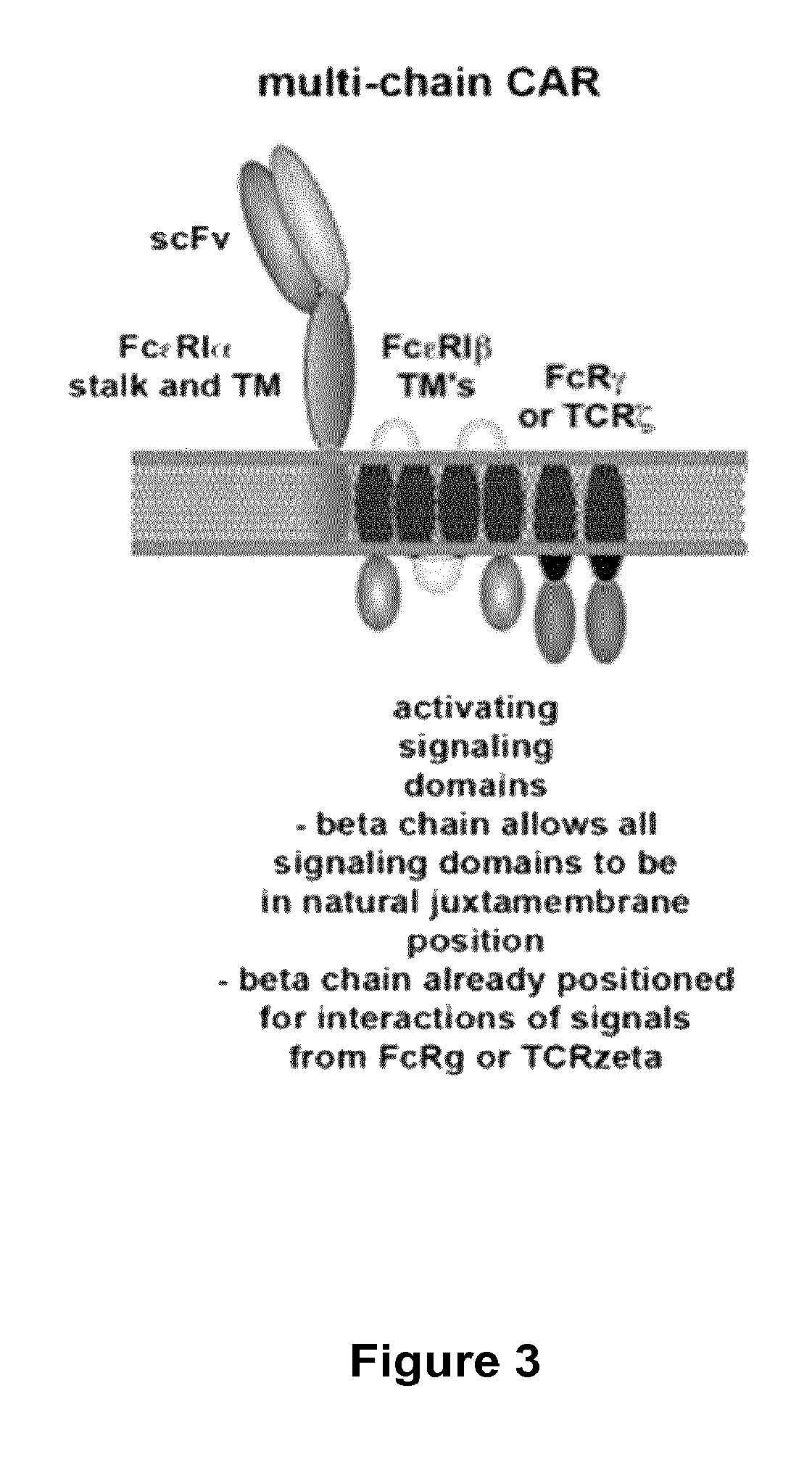
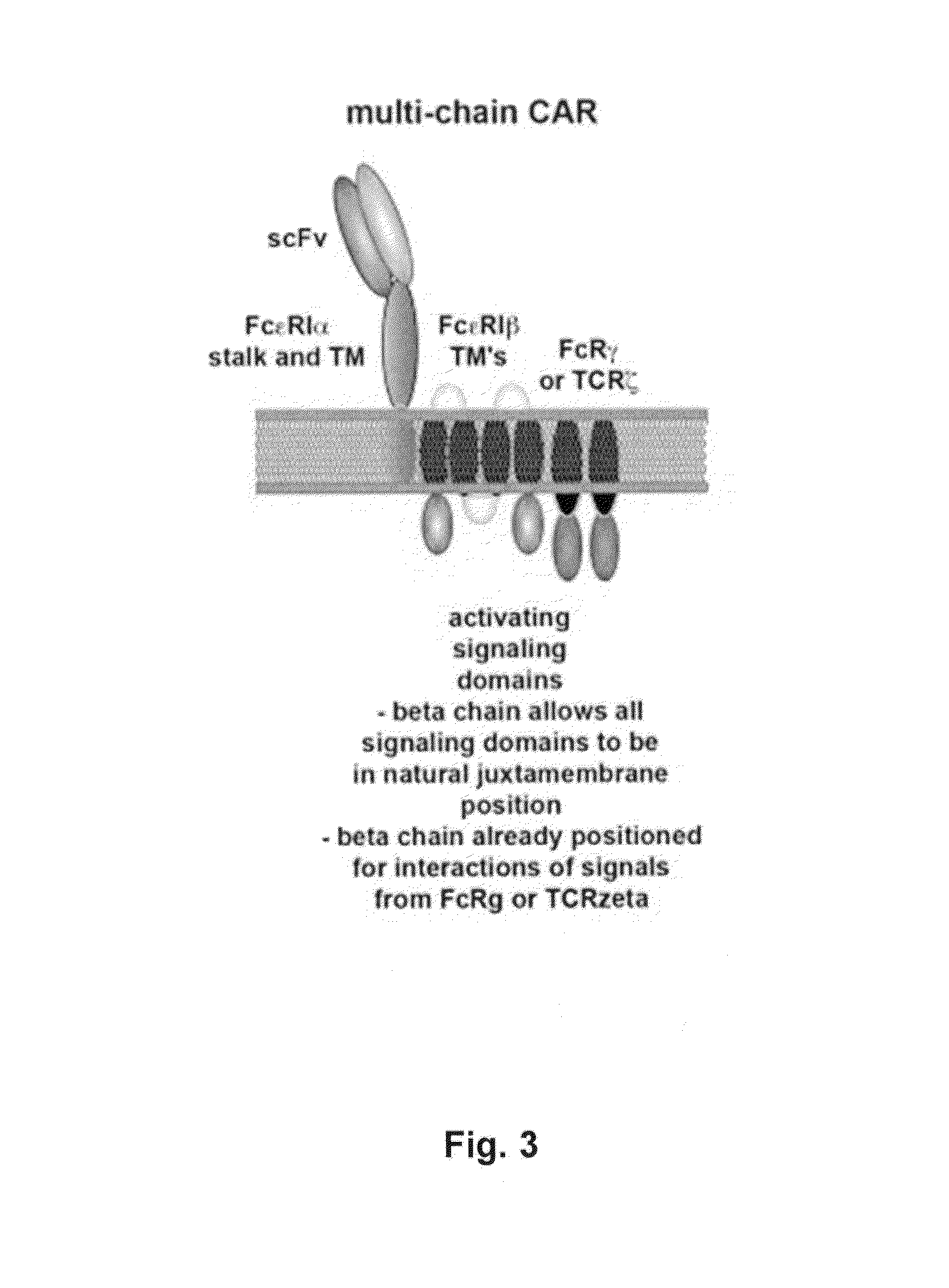
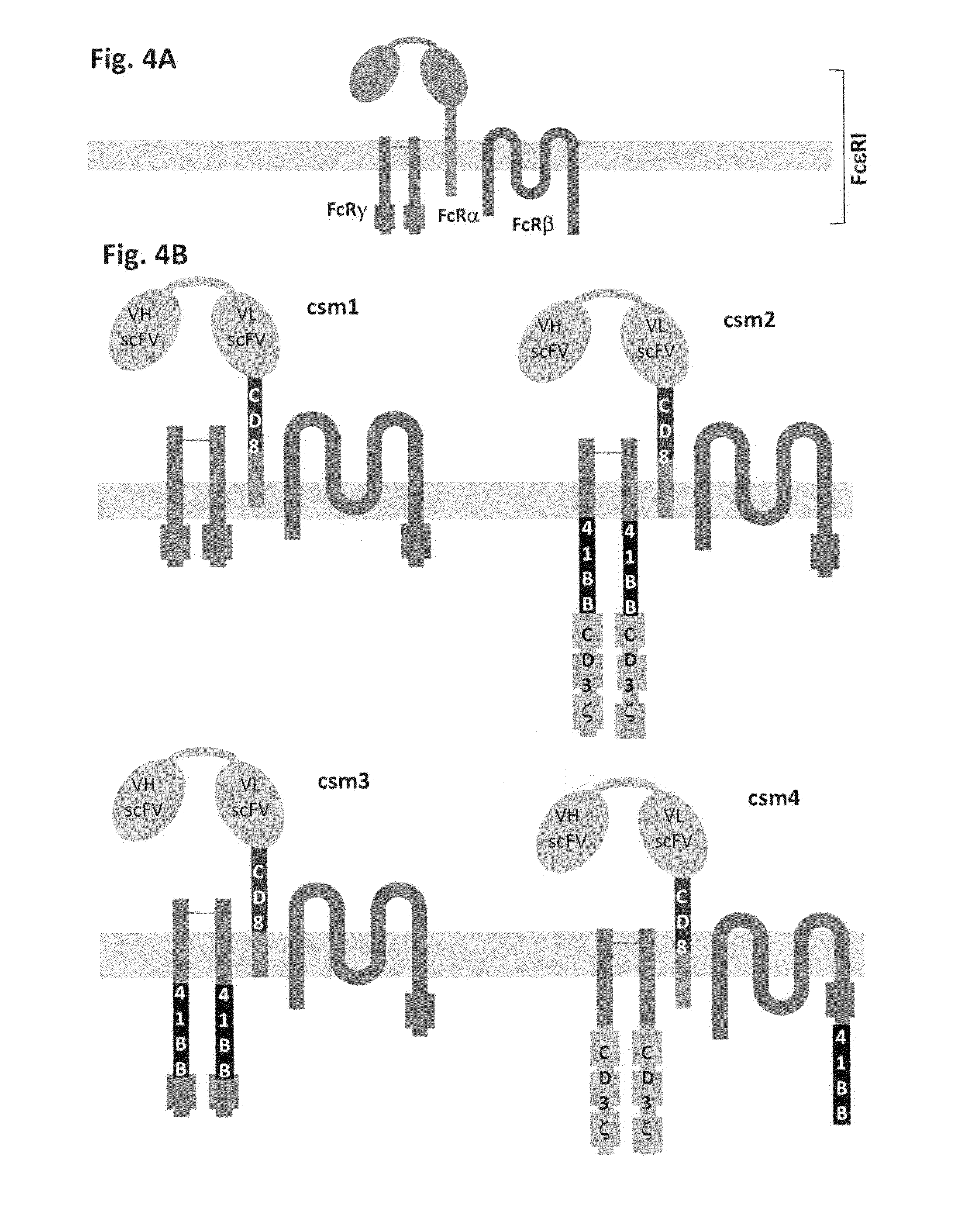
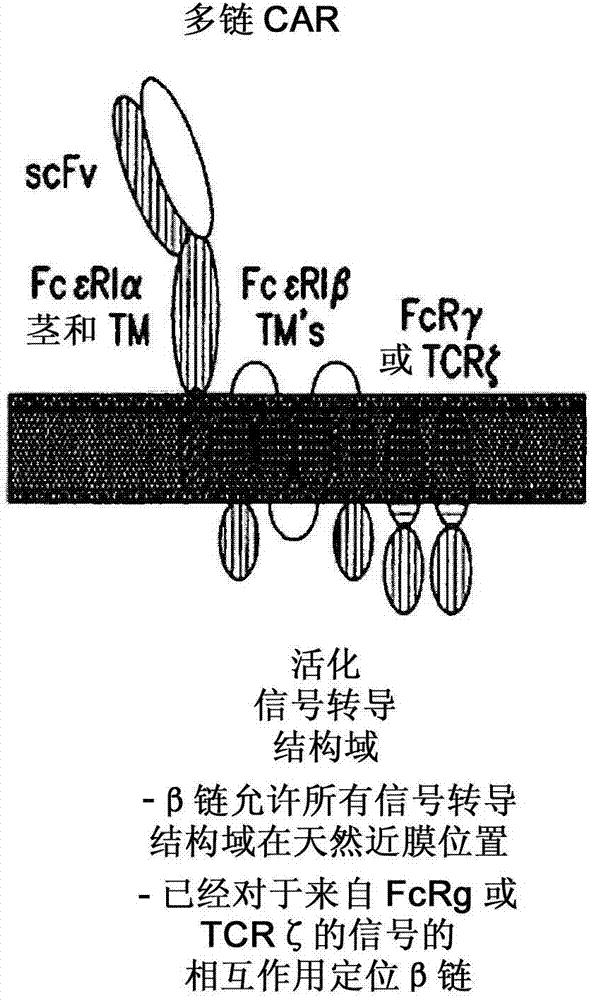
Multi-Chain Chimeric Antigen Receptor and Uses Thereof
ActiveUS20140134142A1Modulate activityStrong specificityPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigen receptorReceptor
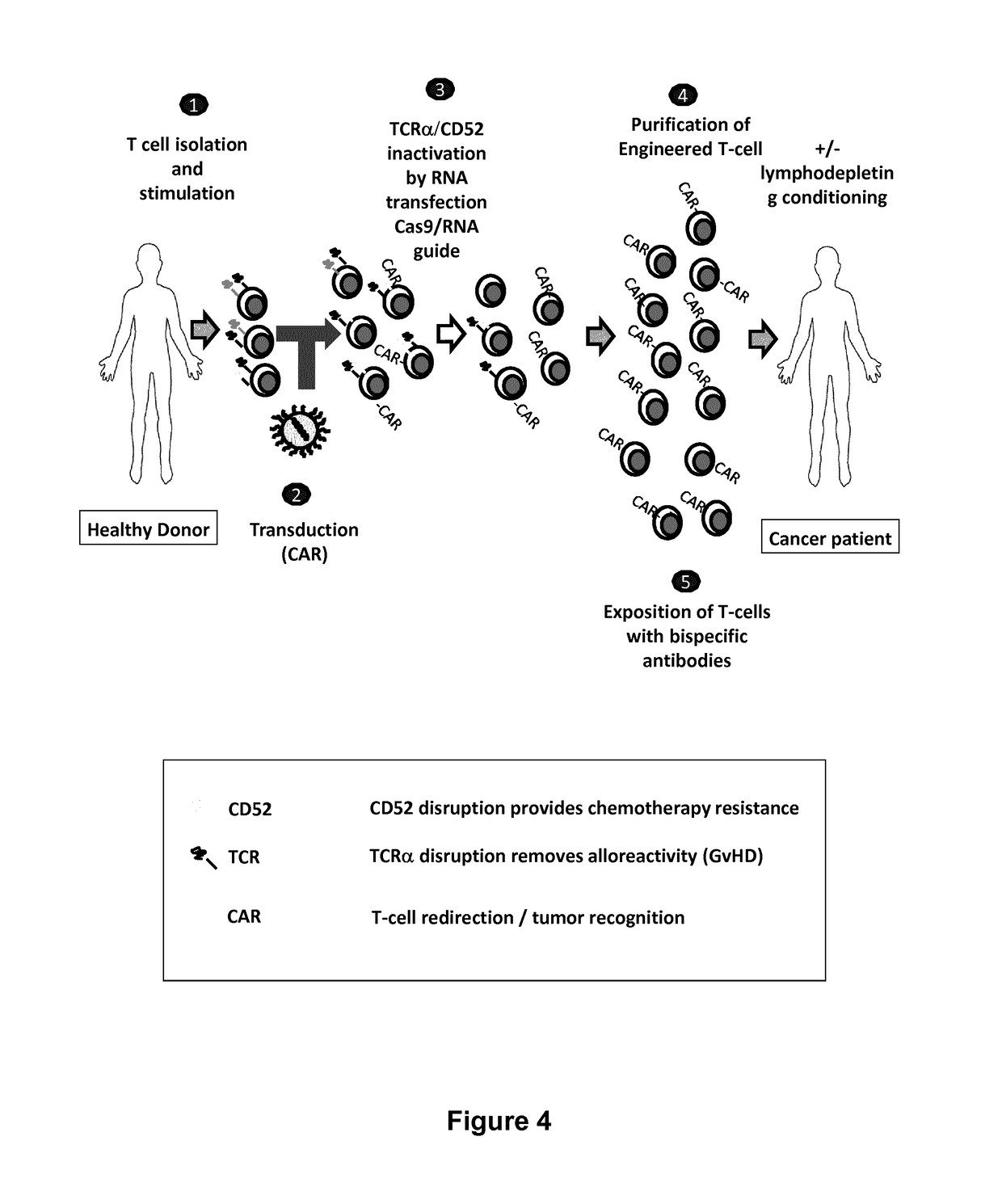
The present invention relates to a new generation of chimeric antigen receptors (CAR) referred to as multi-chain CARs. Such CARs, which aim to redirect immune cell specificity and reactivity toward a selected target exploiting the ligand-binding domain properties, comprise separate extracellular ligand binding and signaling domains in different transmembrane polypeptides. The signaling domains are designed to assemble in juxtamembrane position, which forms flexible architecture closer to natural receptors, that confers optimal signal transduction. The invention encompasses the polynucleotides, vectors encoding said multi-chain CAR and the isolated cells expressing them at their surface, in particularly for their use in immunotherapy. The invention opens the way to efficient adoptive immunotherapy strategies for treating cancer and viral infections.
Owner:CELLECTIS SA
Methods for engineering highly active t cell for immunotheraphy
The present invention relates to methods for developing engineered T-cells for immunotherapy and more specifically to methods for modifying T-cells by inactivating at immune checkpoint genes, preferably at least two selected from different pathways, to increase T-cell immune activity. This method involves the use of specific rare cutting endonucleases, in particular TALE-nucleases (TAL effector endonuclease) and polynucleotides encoding such polypeptides, to precisely target a selection of key genes in T-cells, which are available from donors or from culture of primary cells. The invention opens the way to highly efficient adoptive immunotherapy strategies for treating cancer and viral infections.
Owner:CELLECTIS SA
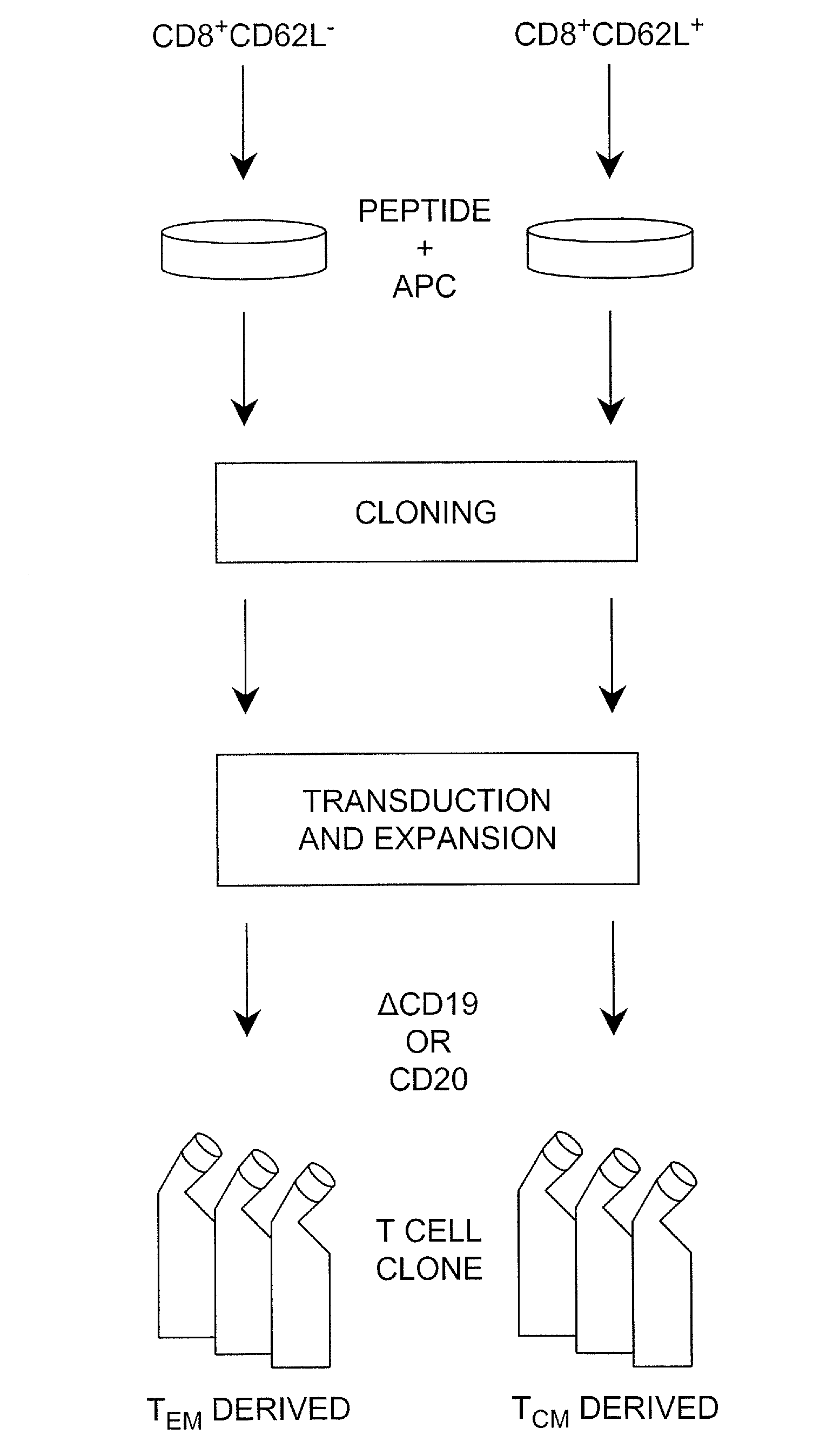
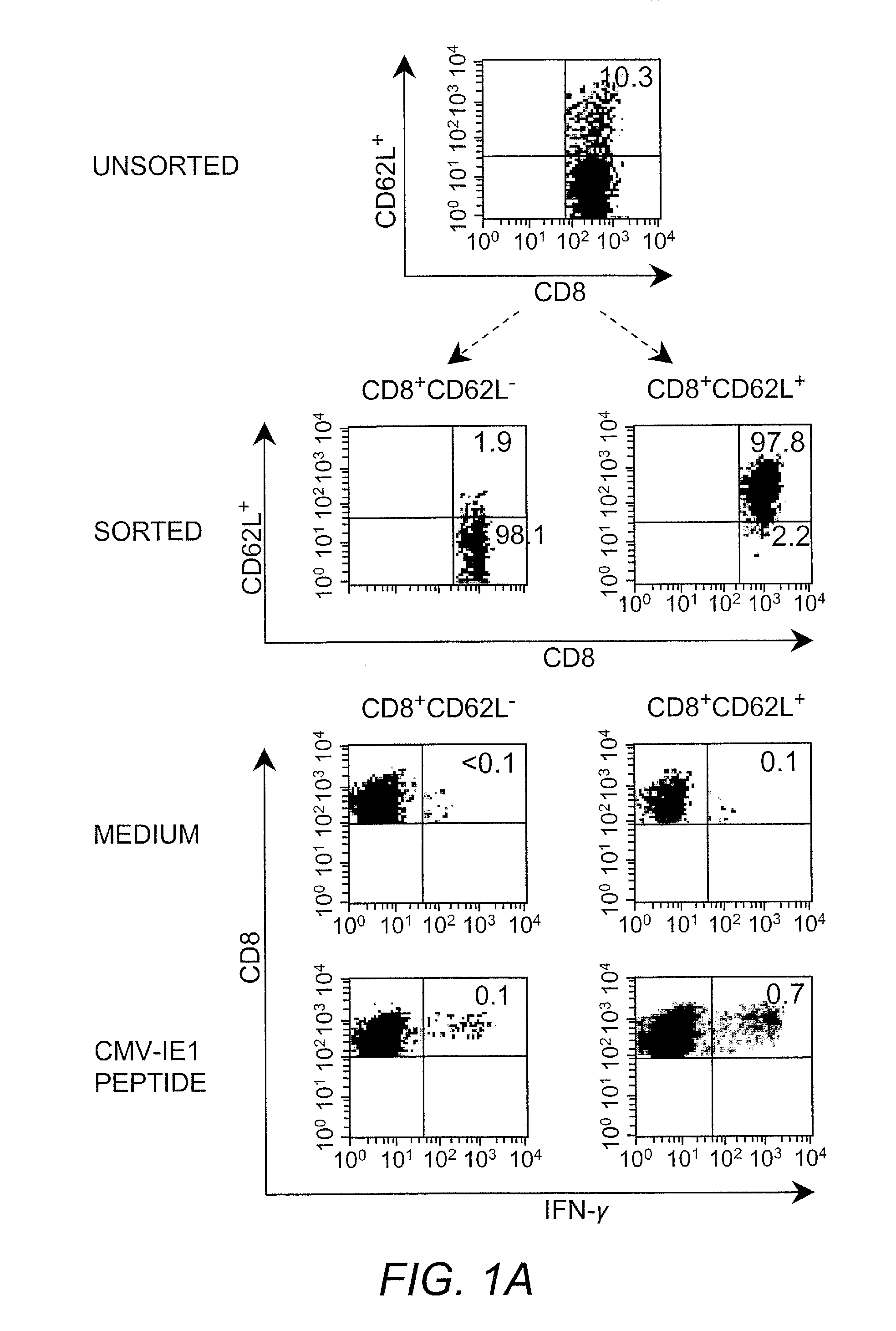
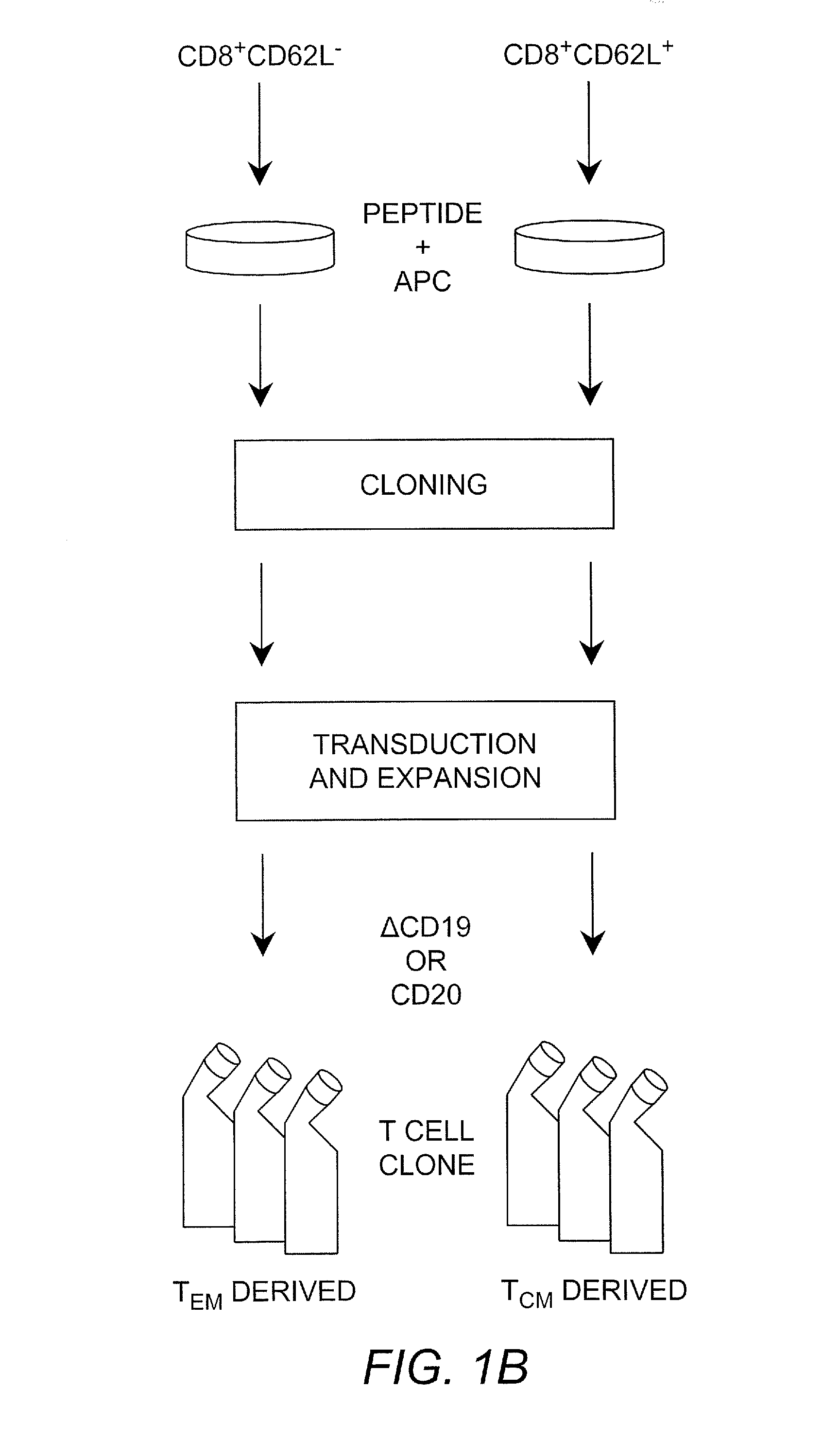
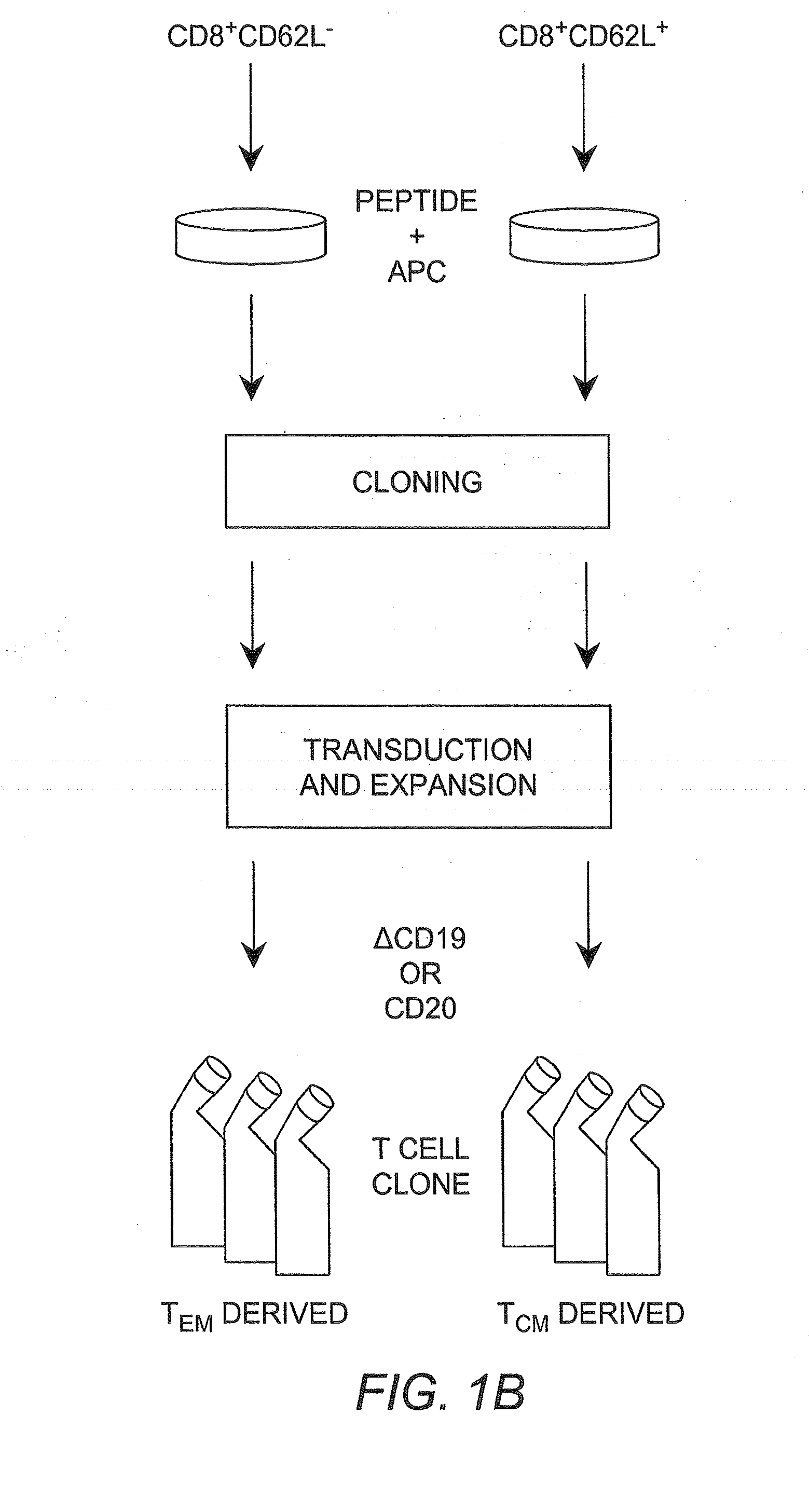
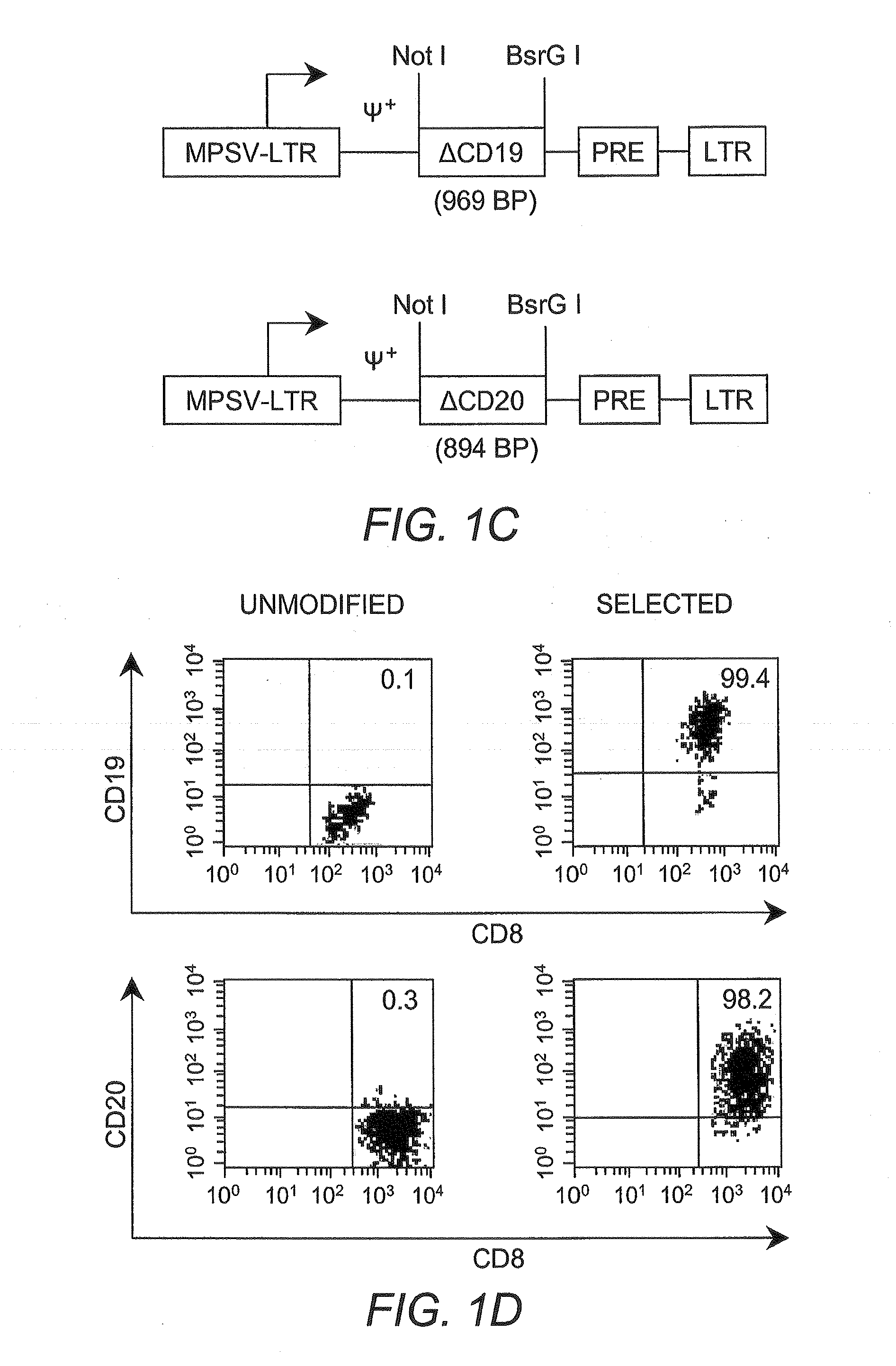
Adoptive transfer of cd8 + t cell clones derived from central memory cells
InactiveUS20080131415A1Increased proliferationBiocideArtificial cell constructsWhite blood cellPharmaceutical formulation
The present invention provides a method of carrying out adoptive immunotherapy in a primate subject in need thereof by administering the subject a cytotoxic T lymphocytes (CTL) preparation in a treatment-effective amount. The method comprises administering as the CTL preparation a preparation consisting essentially of an in vitro expanded primate CTL population, the CTL population enriched prior to expansion for central memory T lymphocytes, and depleted prior to expansion of effector memory T lymphocytes. In some embodiments, the method may further comprise concurrently administering Interleukin-15 to the subject in an amount effective to increase the proliferation of the central memory T cells in the subject. Pharmaceutical formulations produced by the method, and methods of using the same, are also described.
Owner:CITY OF HOPE +1
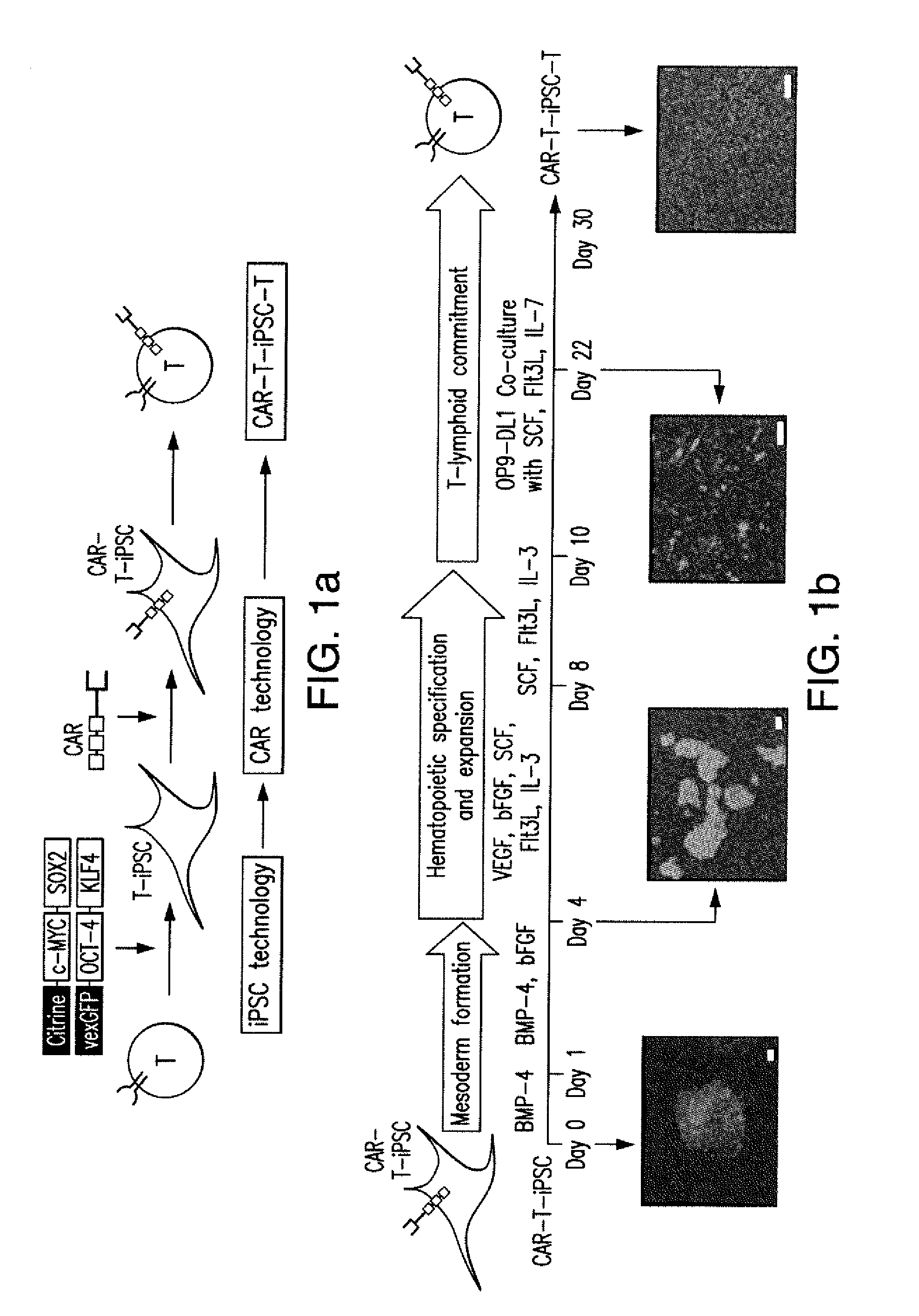
Effective generation of tumor-targeted t cells derived from pluripotent stem cells
ActiveUS20160009813A1Improve survivalGood antitumor activityAntibody mimetics/scaffoldsMammal material medical ingredientsAntigenPluripotential stem cell
The present invention relates to the field of adoptive immunotherapy. The invention provides methods for generating phenotypically defined, functional, and / or expandable T cells from pluripotent stem cells engineered through safe genetic modifications. The engineered cells may provide one or more of: 1) targeting a specific predetermined antigen expressed on the cell surface of a target cell in an HLA independent manner, 2) enhanced survival and functional potential 3) “off-the-shelf” T cells for administration to multiple recipients, eventually across immunogenic barriers, and / or 4) cytotoxic potential and anti-tumor activity.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
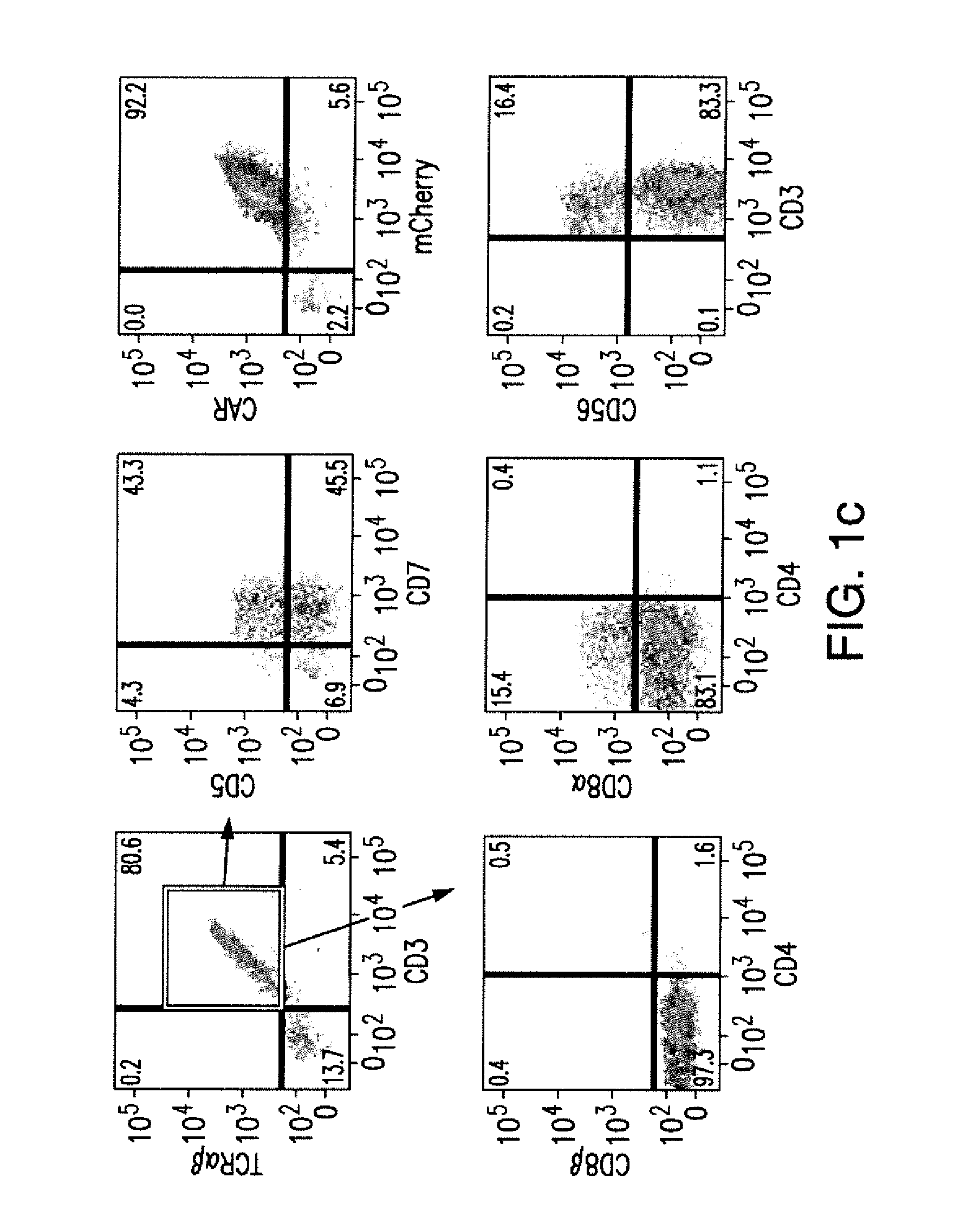
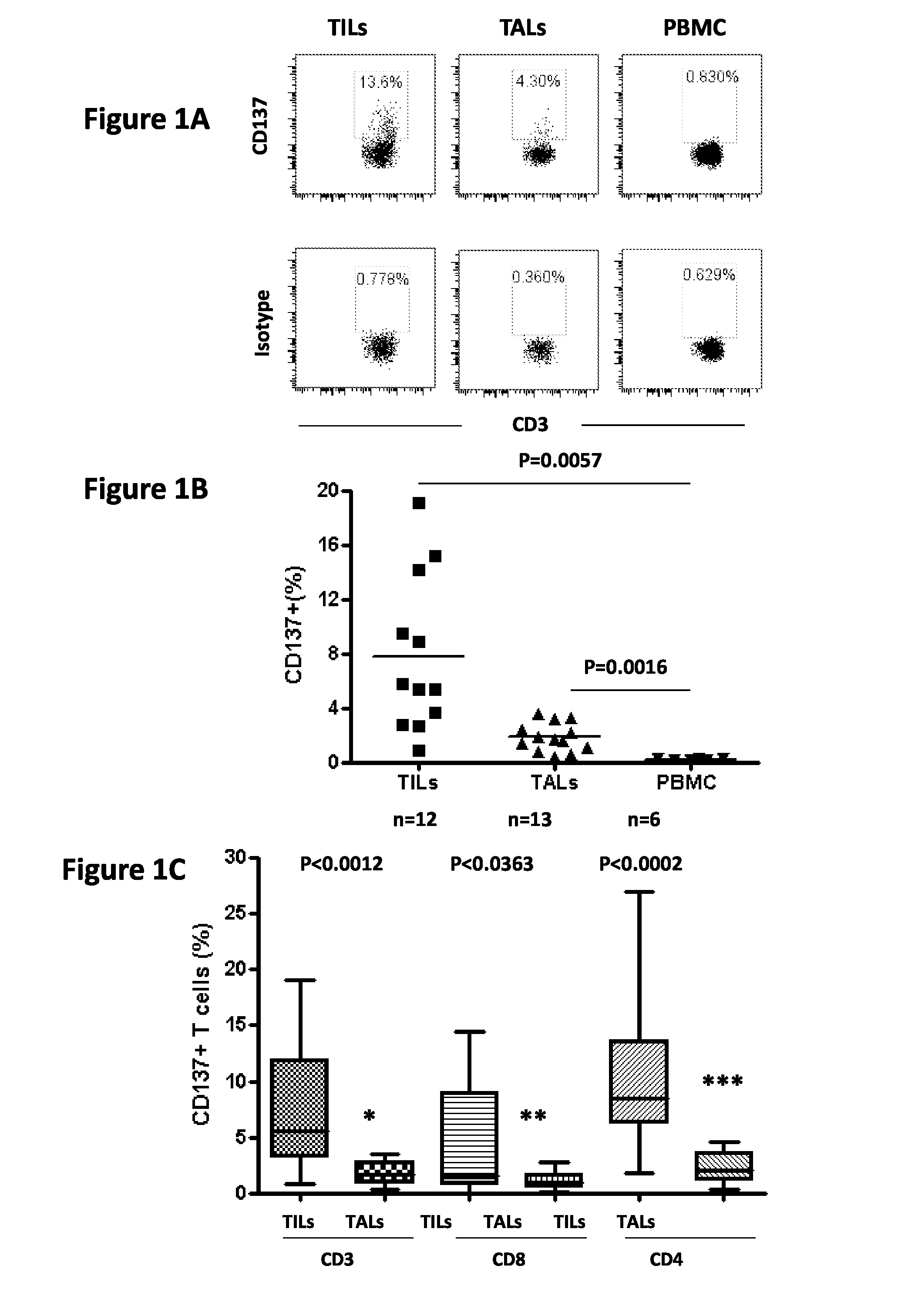
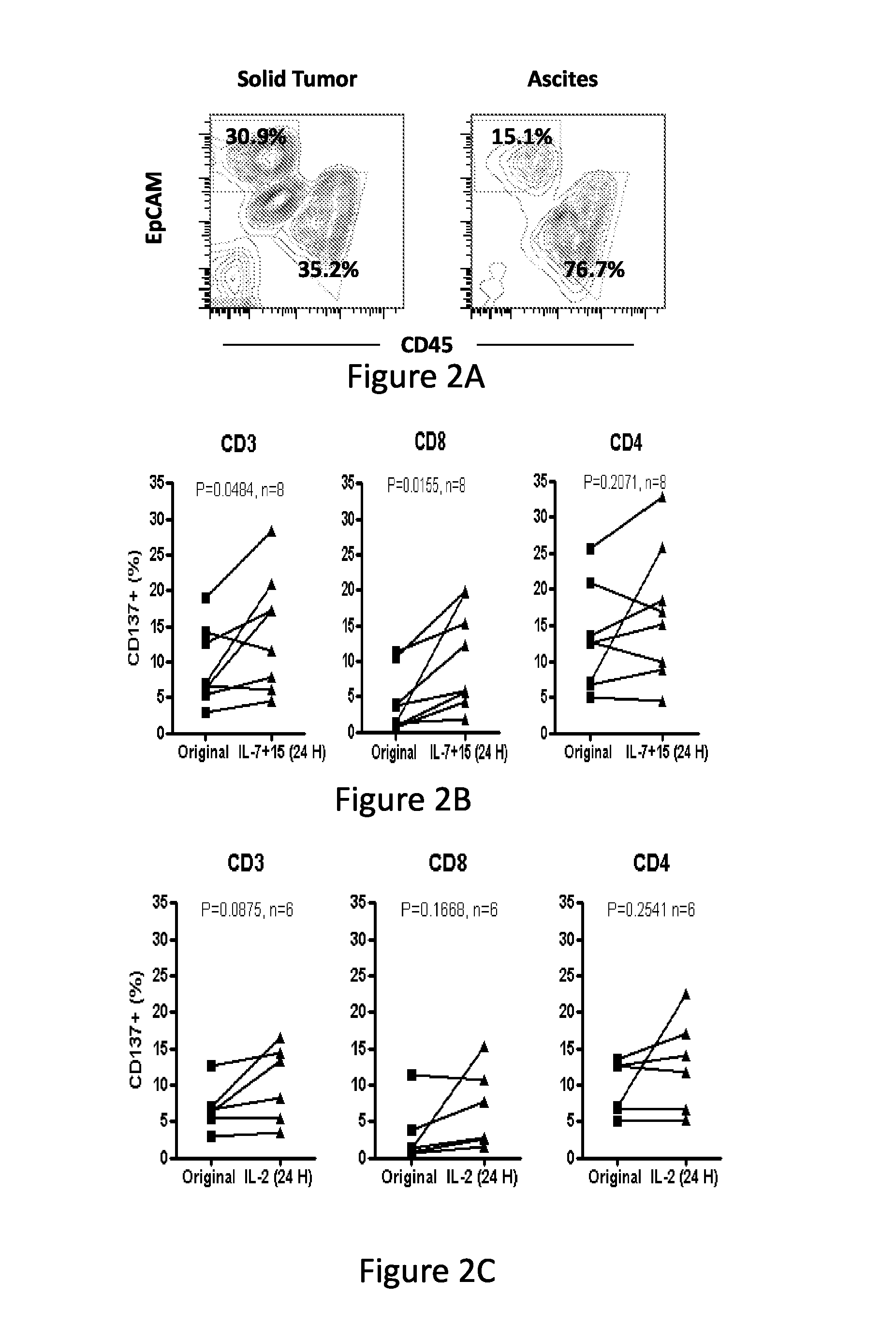
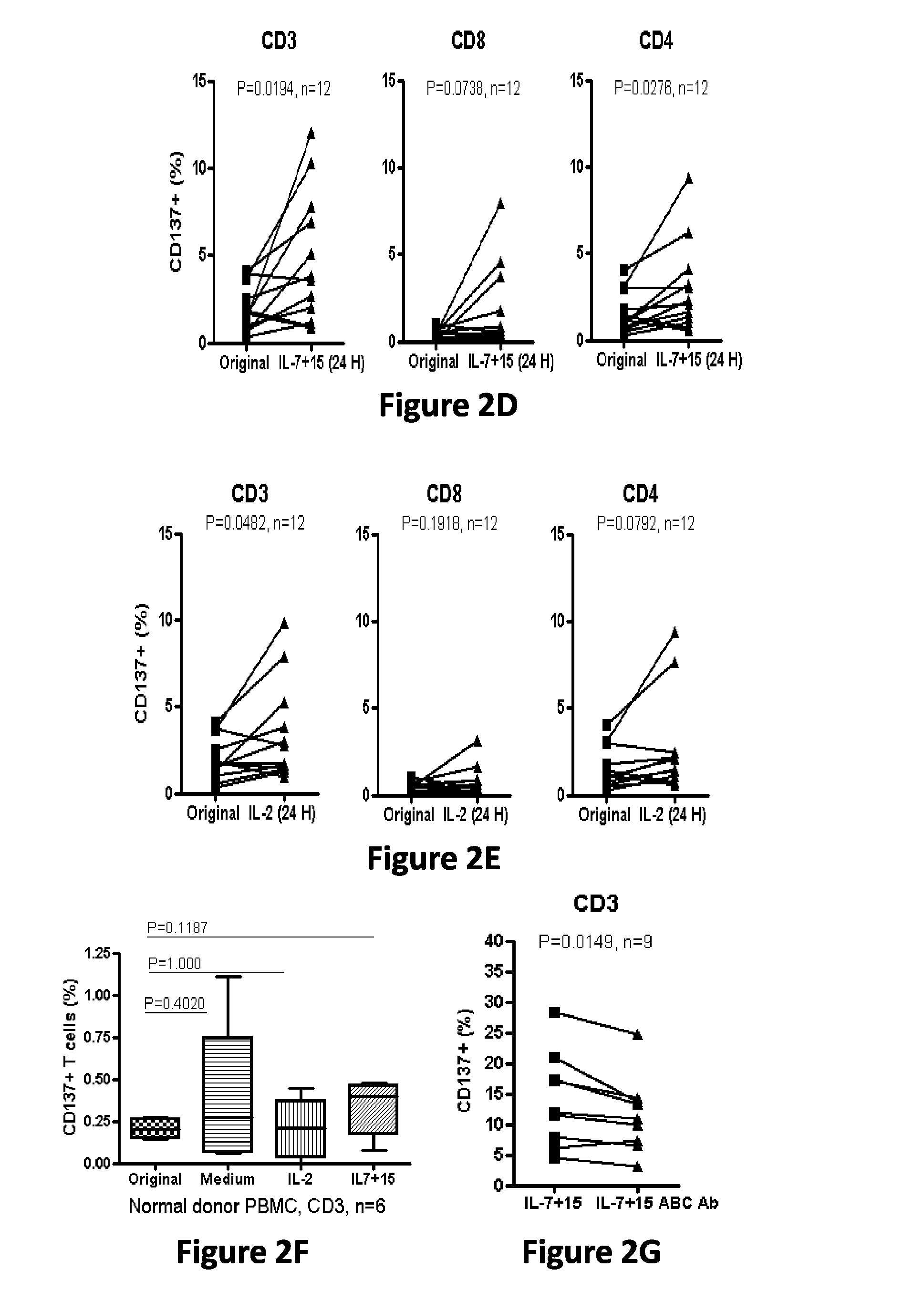
Cd137 enrichment for efficient tumor infiltrating lymphocyte selection
ActiveUS20160215262A1Effective anti-cancer T cell productMammal material medical ingredientsBlood/immune system cellsAbnormal tissue growthCD137
The invention includes compositions and methods to rapidly isolate and culture cells that are potent for use in adoptive immunotherapy. In one embodiment, the isolated cells of the invention are tumor infiltrating lymphocytes (TIL) that express CD137 (also known as 4-1BB and TNFSFR9).
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Methods for engineering t cells for immunotherapy by using rna-guided cas nuclease system
ActiveUS20160272999A1Accurate modificationHydrolasesGenetically modified cellsInfected cellAntigen receptors
The present invention relates to methods of developing genetically engineered, preferably non-alloreactive T-cells for immunotherapy. This method involves the use of RNA-guided endonucleases, in particular Cas9 / CRISPR system, to specifically target a selection of key genes in T-cells. The engineered T-cells are also intended to express chimeric antigen receptors (CAR) to redirect their immune activity towards malignant or infected cells. The invention opens the way to standard and affordable adoptive immunotherapy strategies using T-Cells for treating cancer and viral infections.
Owner:CELLECTIS SA
Methods for engineering t cells for immunotherapy by using rna-guided cas nuclease system
ActiveUS20160184362A1Accurate modificationHydrolasesGenetically modified cellsInfected cellAntigen receptors
The present invention relates to methods of developing genetically engineered, preferably non-alloreactive T-cells for immunotherapy. This method involves the use of RNA-guided endonucleases, in particular Cas9 / CRISPR system, to specifically target a selection of key genes in T-cells. The engineered T-cells are also intended to express chimeric antigen receptors (CAR) to redirect their immune activity towards malignant or infected cells. The invention opens the way to standard and affordable adoptive immunotherapy strategies using T-Cells for treating cancer and viral infections.
Owner:CELLECTIS SA
Adoptive immunotherapy using macrophages sensitized with heat shock protein-epitope complexes
InactiveUS6156302AEnhancing host 's immunocompetenceHigh activityBiocideOrganic active ingredientsDiseaseInterleukin 6
The present invention relates to methods and compositions for enhancing immunological responses and for the prevention and treatment of infectious diseases or primary and metastatic neoplastic diseases based on the administration of macrophages and / or other antigen presenting cells (APC) sensitized with heat shock proteins non-covalently bound to peptide complexes and / or antigenic components. APC are incubated in the presence of hsp-peptide complexes and / or antigenic components in vitro. The sensitized cells are reinfused into the patient with or without treatment with cytokines including but not limited to interferon- alpha , interferon- alpha , interleukin-2, interleukin-4, interleukin-6 and tumor neurosis factor.
Owner:FORDHAM UNIVERSITY
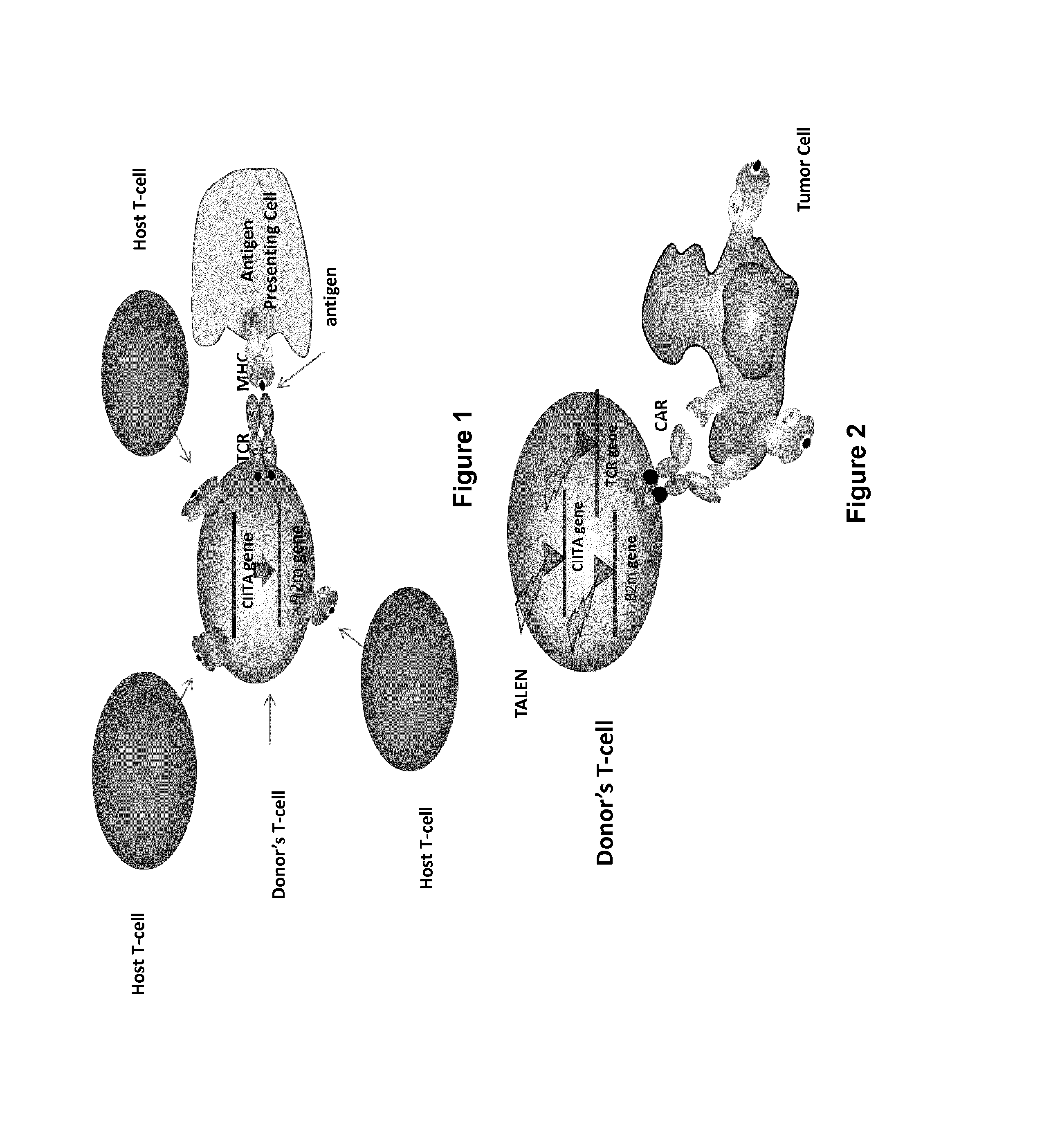
Method for generating t-cells compatible for allogenic transplantation
InactiveUS20170016025A1Polypeptide with localisation/targeting motifImmunoglobulin superfamilyCIITAAuto immune disease
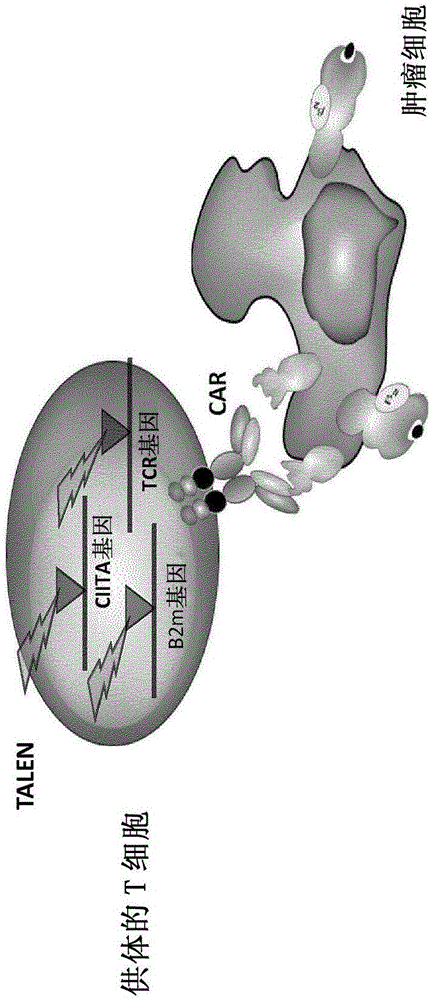
The present invention pertains to engineered T-cells, method for their preparation and their use as medicament, particularly for immunotherapy. The engineered T-cells of the invention are characterized in that the expression of beta 2-microglobulin (B2M) and / or class II major histocompatibility complex transactivator (CIITA) is inhibited, e.g., by using rare-cutting endonucleases able to selectively inactivating by DNA cleavage the gene encoding B2M and / or CIITA, or by using nucleic acid molecules which inhibit the expression of B2M and / or CIITA. In order to further render the T-cell non-alloreactive, at least one gene encoding a component of the T-cell receptor is inactivated, e.g., by using a rare-cutting endonucleases able to selectively inactivating by DNA cleavage the gene encoding said TCR component. In addition, expression of immunosuppressive polypeptide can be performed on those modified T-cells in order to prolong the survival of these modified T cells in host organism. Such modified T-cell is particularly suitable for allogeneic transplantations, especially because it reduces both the risk of rejection by the host's immune system and the risk of developing graft versus host disease. The invention opens the way to standard and affordable adoptive immunotherapy strategies using T-Cells for treating cancer, infections and auto-immune diseases.
Owner:CELLECTIS SA
Method for generating T-cells compatible for allogenic transplantation
ActiveCN106103475APolypeptide with localisation/targeting motifImmunoglobulin superfamilyCIITAAutoimmune disease
The present invention pertains to engineered T-cells, method for their preparation and their use as medicament, particularly for immunotherapy. The engineered T-cells of the invention are characterized in that the expression of beta 2-microglobulin (B2M) and / or class I I major histocompatibility complex transactivator (CIITA) is inhibited, e.g., by using rare-cutting endonucleases able to selectively inactivating by DNA cleavage the gene encoding B2M and / or CIITA, or by using nucleic acid molecules which inhibit the expression of B2M and / or CIITA. In order to further render the T-cell non-alloreactive, at least one gene encoding a component of the T-cell receptor is inactivated, e.g., by using a rare-cutting endonucleases able to selectively inactivating by DNA cleavage the gene encoding said TCR component. In addition, expression of immunosuppressive polypeptide can be performed on those modified T-cells in order to prolong the survival of these modified T cells in host organism. Such modified T-cell is particularly suitable for allogeneic transplantations, especially because it reduces both the risk of rejection by the host's immune system and the risk of developing graft versus host disease. The invention opens the way to standard and affordable adoptive immunotherapy strategies using T-Cells for treating cancer, infections and auto-immune diseases.
Owner:CELLECTIS SA
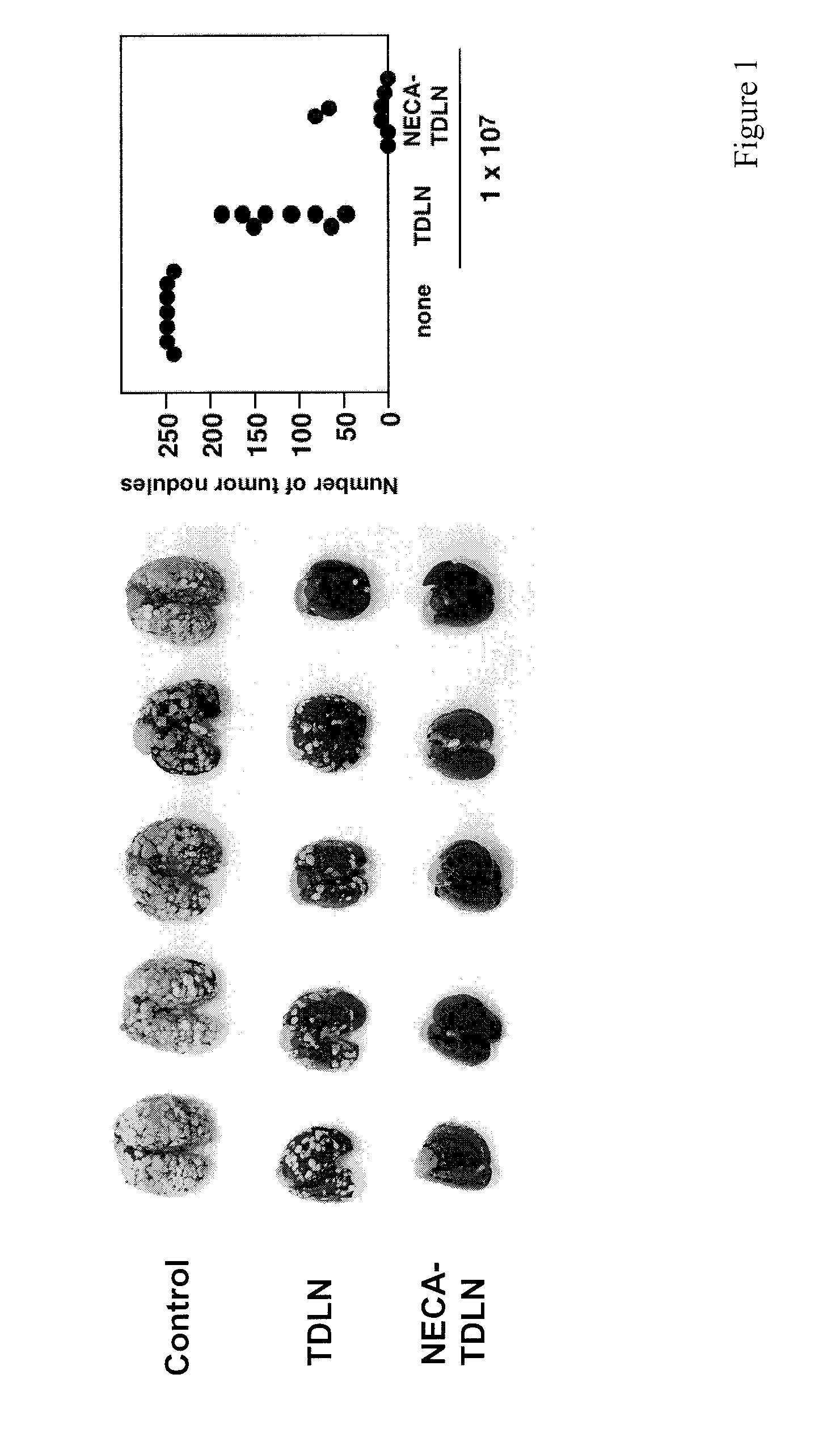
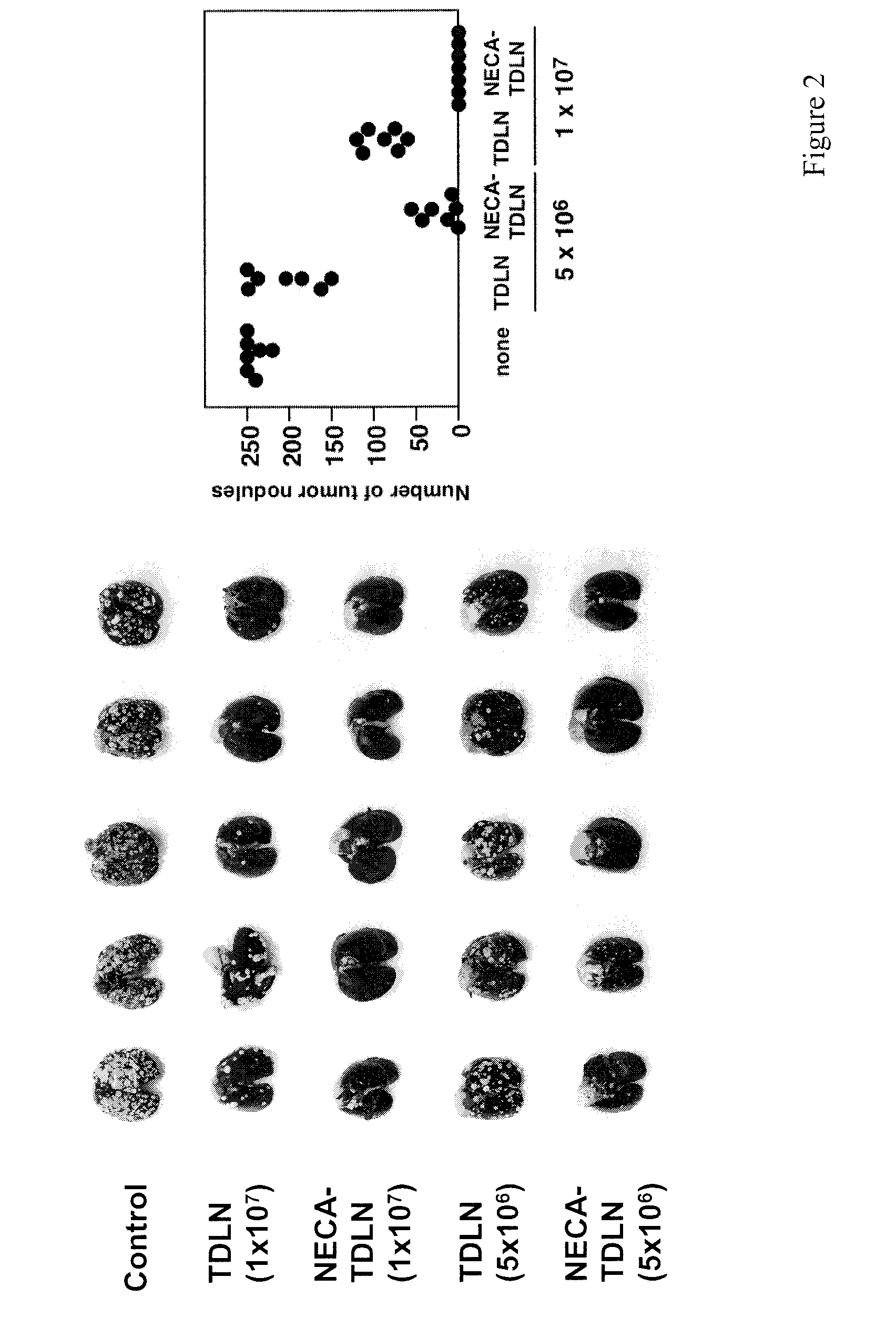
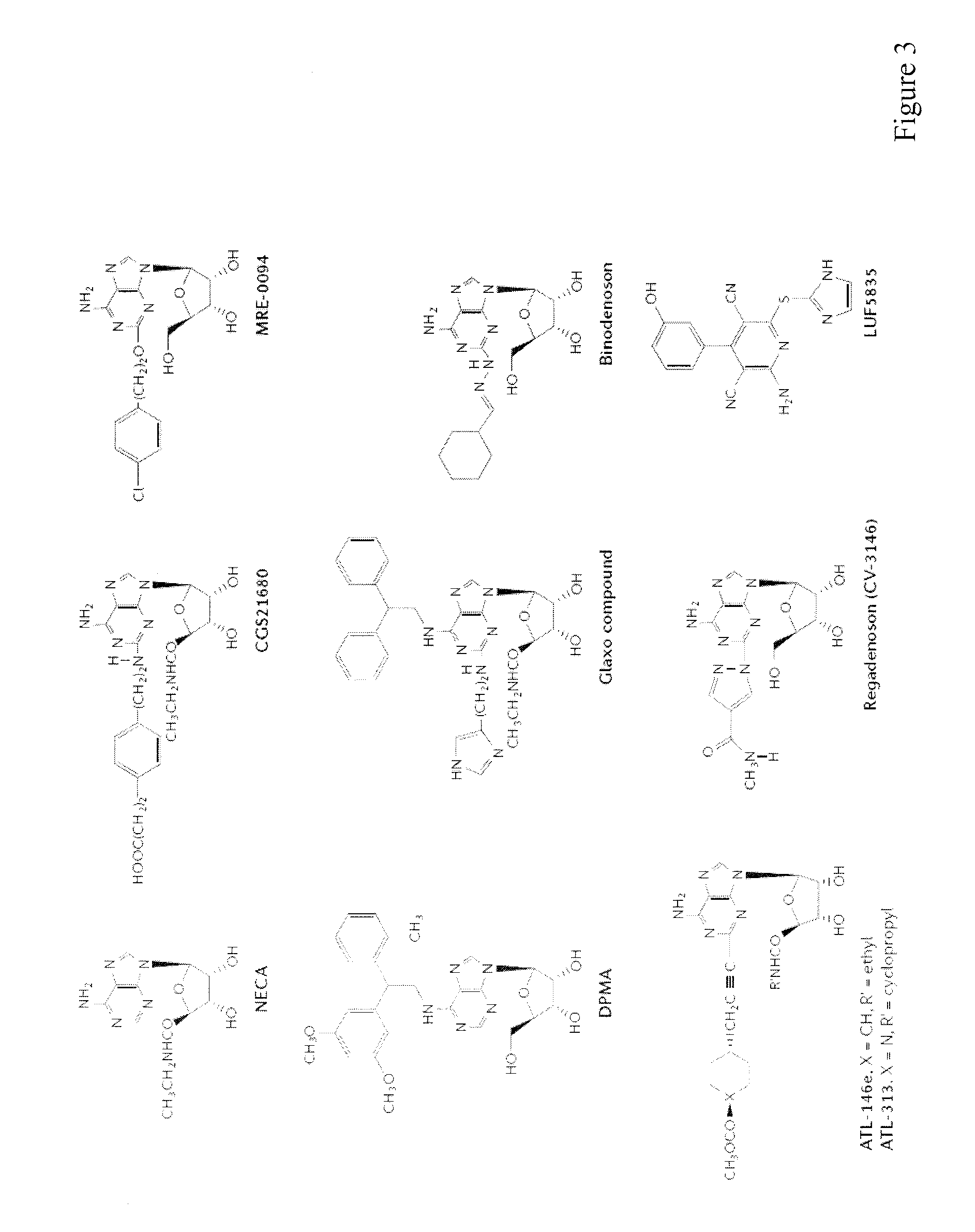
Method of preparing adenosine-resistant anti-tumor T lymphocytes for adoptive immunotherapy
In the past, adoptive immunotherapy often failed because the transferred immune cells were inactive in vivo. This disclosure provides a method of producing immune cells that are highly active in vivo. The immune cells may be expanded in vitro in the presence of an adenosine receptor agonist or an antisense nucleic acid that downregulates expression of an adenosine receptor, for example. The immune cells may be tumor-infiltrating lymphocytes (TIL), cytotoxic T lymphocytes (CTL), natural killer (NK) cells, or lymphokine-activated killer (LAK) cells, for example. The methods described herein may be used to treat a number of diseases including cancer, infectious diseases, and immunodeficiencies.
Owner:NORTHEASTERN UNIV
Methods for engineering T cells for immunotherapy by using RNA-guided CAS nuclease system
ActiveUS9855297B2Accurate modificationHydrolasesGenetically modified cellsInfected cellAntigen receptors
The present invention relates to methods of developing genetically engineered, preferably non-alloreactive T-cells for immunotherapy. This method involves the use of RNA-guided endonucleases, in particular Cas9 / CRISPR system, to specifically target a selection of key genes in T-cells. The engineered T-cells are also intended to express chimeric antigen receptors (CAR) to redirect their immune activity towards malignant or infected cells. The invention opens the way to standard and affordable adoptive immunotherapy strategies using T-Cells for treating cancer and viral infections.
Owner:CELLECTIS SA
Use of cell adhesion inhibitor for the mobilization of antigen presenting cells and immune cells in a cell mixture (AIM) from the peripheral blood and methods of use
Disclosed is a method to recover an antigen presenting cells (APCs) and immune cells rich mixture (AIM) from peripheral blood mononuclear cells (PBMC) mobilized with one or more cell adhesion inhibitors for the preparation of an AIM vaccine or an AIM adoptive immunotherapy preparation. In addition, AIM mobilization can be enhanced by priming, simultaneously or in sequence, one or more of a combination of different chemical compounds, cytokines, hormones, growth factors, etc. The interaction of chemokines and chemokine receptors enable tumor cells attachment or in close proximity to antigen presenting cells and immune cells which possess similar receptors in a micro niche environment. Severing the chemokine / chemokine receptor linkage by a cell adhesion inhibitor will release these specifically primed cell mixtures into the peripheral blood. The collection of these cells from the peripheral blood has never been described and is the basis of this invention. AIM cells can either be used alone or better still, be induced into more target specific preparations with additions, modifications and incubation, pre or post cell adhesion inhibitors mobilization, with vaccines, different target specific antigens, peptides, chemotherapeutic agents, oncolytic viral therapeutic agents, cytokines, co-stimulatory molecules, anti-regulatory T cell therapeutic agents, anti-CTLA4, anti-PD1 molecules and other methodologies of immunological enhancement known to the art. The AIM vaccine or AIM adoptive immunotherapy preparation can then be used, but not limited to, the treatment of cancer and other diseases.
Owner:YEUNG ALEX WAH HIN +1
Methods for engineering allogeneic and highly active t cell for immunotherapy
InactiveUS20150017136A1Precise positioningPeptide/protein ingredientsAntibody mimetics/scaffoldsPrimary cellNuclease
The present invention relates to methods for developing engineered T-cells for immunotherapy that are non-alloreactive. The present invention relates to methods for modifying T-cells by inactivating both genes encoding T-cell receptor and an immune checkpoint gene to unleash the potential of the immune response. This method involves the use of specific rare cutting endonucleases, in particular TALE-nucleases (TAL effector endonuclease) and polynucleotides encoding such polypeptides, to precisely target a selection of key genes in T-cells, which are available from donors or from culture of primary cells. The invention opens the way to standard and affordable adoptive immunotherapy strategies for treating cancer and viral infections.
Owner:CELLECTIS SA
Generation of human regulatory T cells by bacterial toxins for the treatment of inflammatory disorders
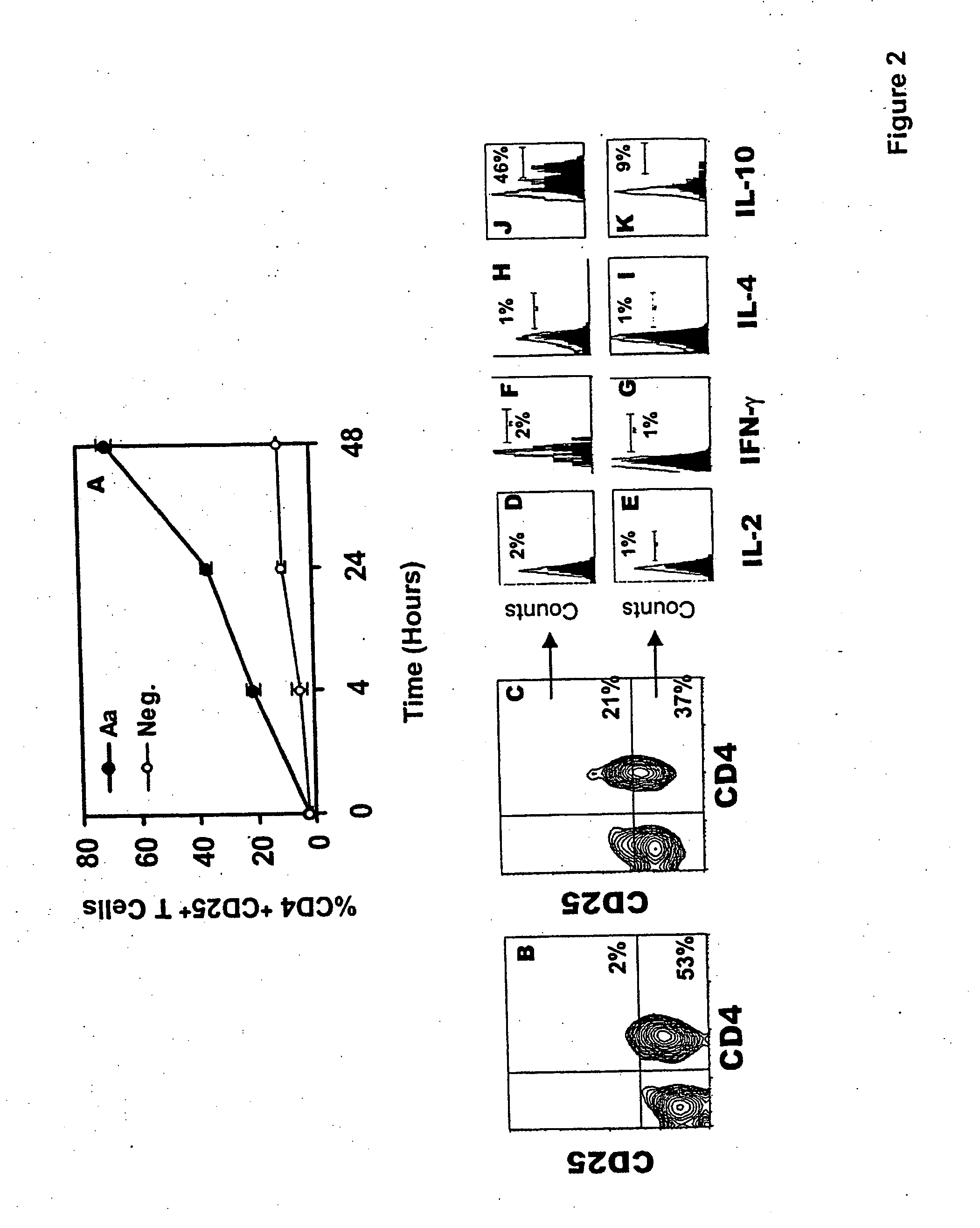
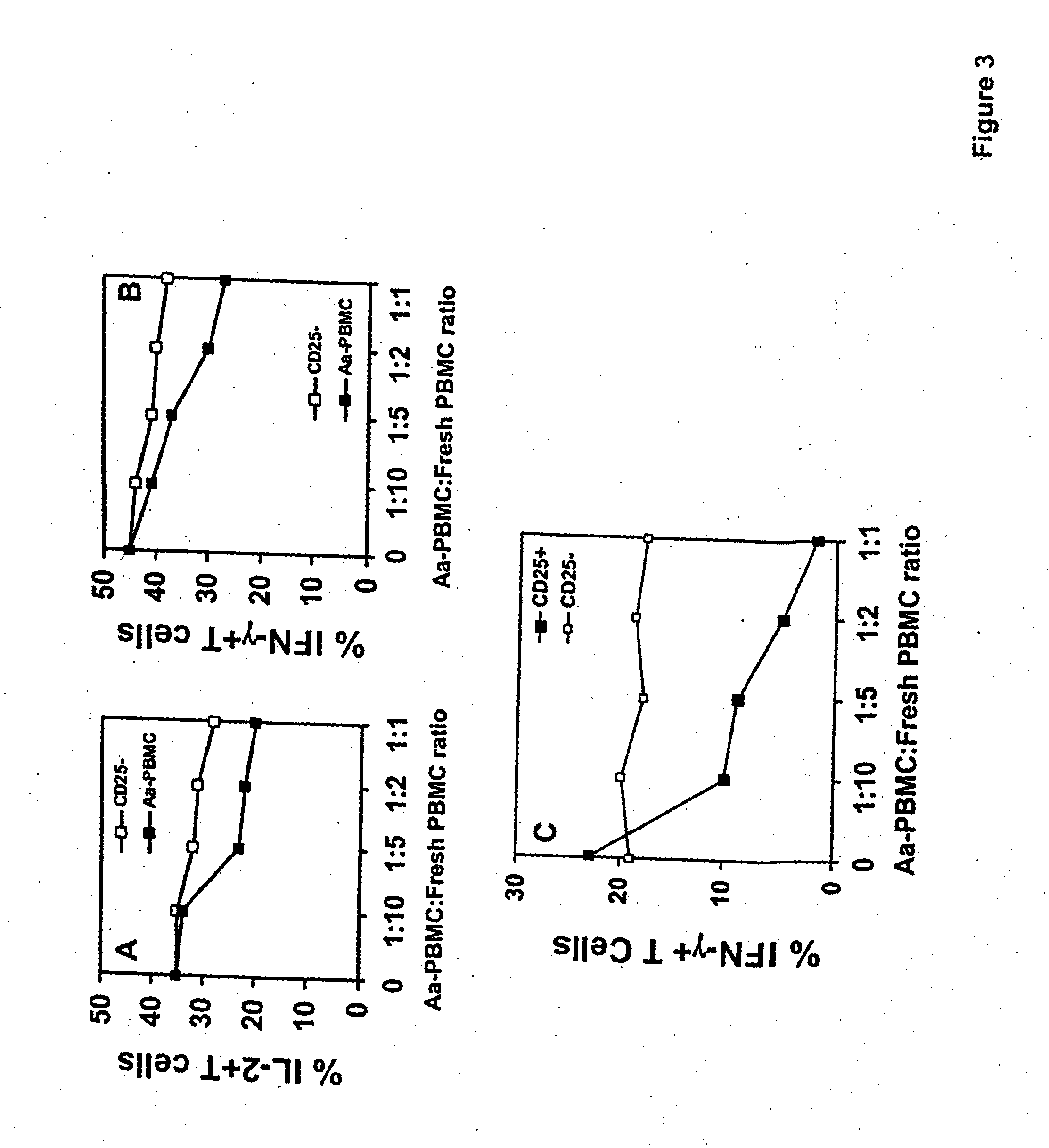
An adoptive immunotherapy using ex vivo-generated regulatory T cells may be used for the suppression of undesireable immune response. T cells are to be obtained from the patient's blood, and upon exposure to a set of toxins from the pathogen A. actinomycetemcomitans, the population of regulatory T cells will be enriched ex vivo and adoptively transferred back to the patient. The novel aspect of the present invention is that it generates large numbers of type 1 regulatory T cells, which secrete Interleukin-10.
Owner:UNIV OF SOUTHERN CALIFORNIA
Method for in situ inhibition of regulatory t cells
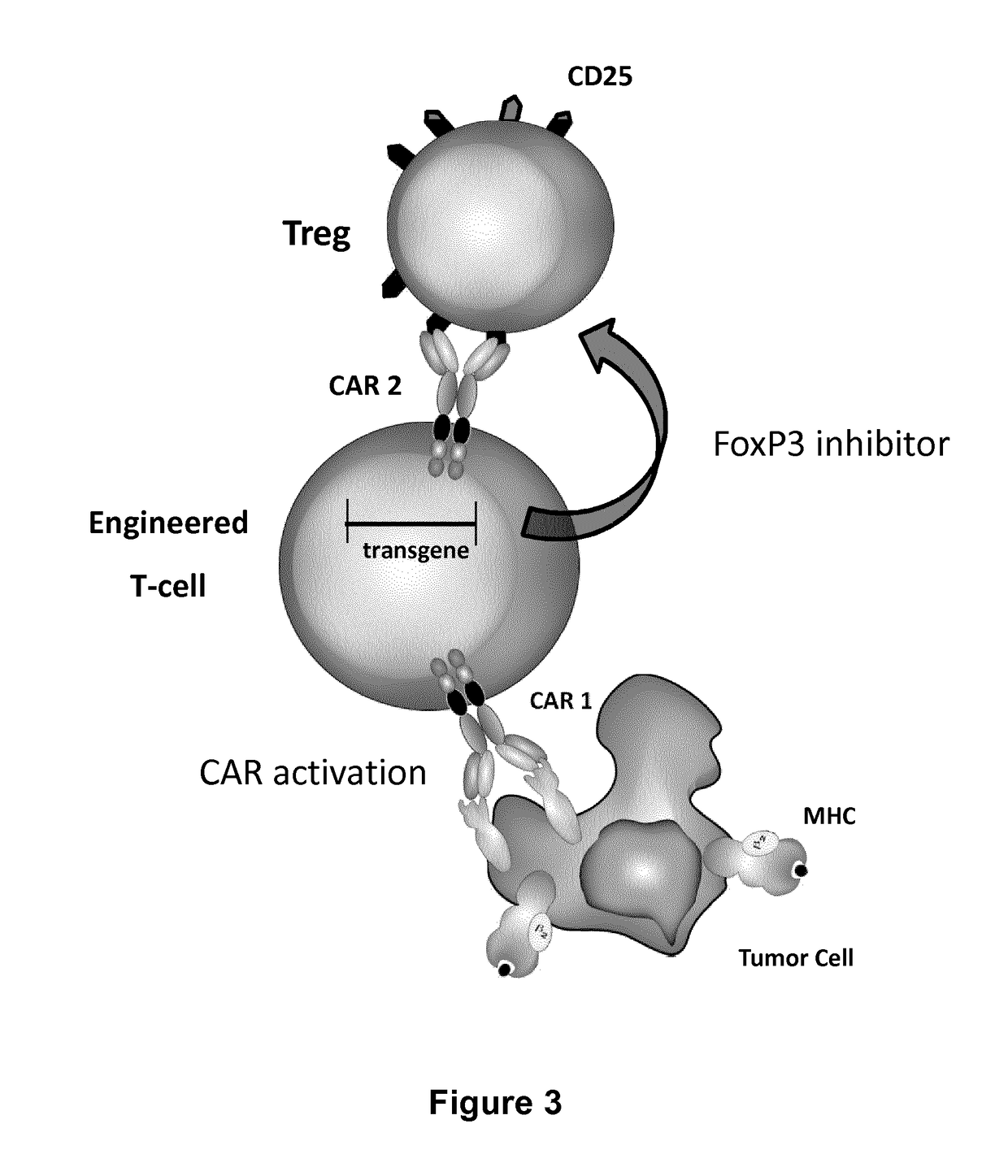
ActiveUS20170067022A1Reducing FoxP transcriptional activityHydrolasesAntibody mimetics/scaffoldsAntigenInfected cell
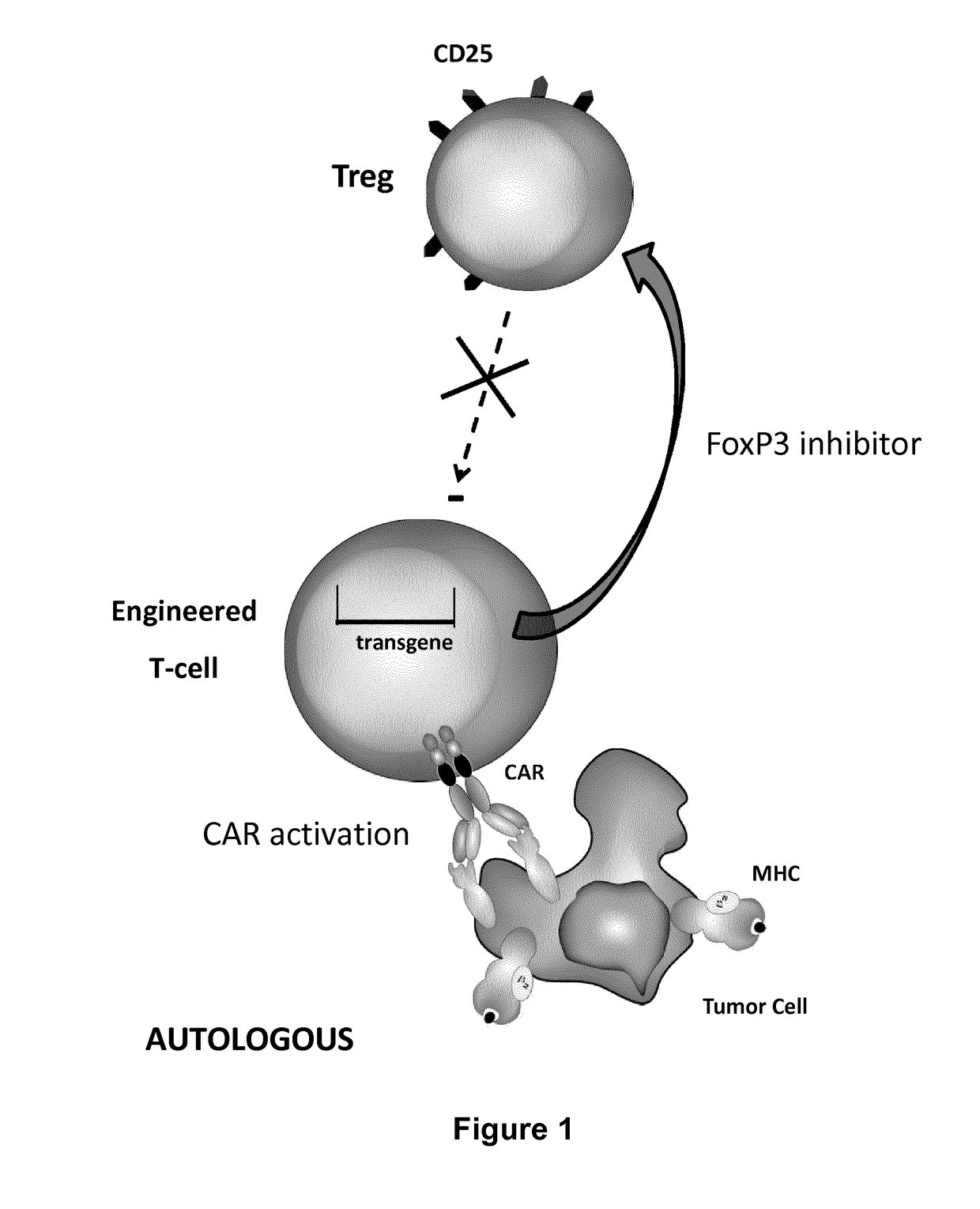
The present invention pertains to engineered T-cells, method for their preparation and their use as medicament, particularly for immunotherapy. The engineered T-cells of the invention are designed to express both a Chimeric Antigen Receptor (CAR) directed against at least one antigen expressed at the surface of a malignant or infected cell, and a secreted inhibitor of regulatory T-cells (Treg). Preferably, such secreted inhibitor is a peptide inhibitor of forkhead / winged helix transcription factor 3 (FoxP3), a specific factor involved into the differentiation of T-cells into regulatory T-cells. The engineered T-cells of the invention direct their immune activity towards specific malignant or infected cells, while at the same time will prevent neighbouring regulatory T-cells from modulating the immune response. The invention opens the way to standard and affordable adoptive immunotherapy strategies, especially for treating or preventing cancer, and bacterial or viral infections.
Owner:CELLECTIS SA
Expansion method of various lymphocyte subpopulations and application of expansion method
ActiveCN105624107ASuppress deathImprove developmentGenetic material ingredientsMammal material medical ingredientsWhite blood cellNatural Killer Cell Inhibitory Receptors
The invention discloses an expansion method of various lymphocyte subpopulations and application of the expansion method to preparation of an adoptive immunotherapy medicine for cancers. The expansion method comprises the following steps: S1, adding IFN gamma and zoledronic acid into a culture medium of PBMC collected in the blood of a tumor patient, and re-adding an OKT3 antibody and recombinant human interleukin-2 after culture to stimulate PBMC so as to finish preliminary expansion; S2, after finish of preliminary expansion, mixing immune lymphocytes with feeder layer cells, and after adding zoledronic acid, an OKT3 antibody and recombinant human interleukin-2, continually performing expansion. By adoption of the expansion method, after twice expansion, the sum of the immune cells can be 10,000 to 80,000 times by expansion, the expanded immune cells include alpha beta T cells, gamma delta T cells, NKT cells and NK cells, and tumor cells can be directly killed, or a cellular immunotherapy drug is prepared to kill the tumor cells.
Owner:杭州朔溪生物医药有限公司
Methods for engineering T cells for immunotherapy by using RNA-guided CAS nuclease system
ActiveUS9890393B2Accurate modificationHydrolasesGenetically modified cellsInfected cellAntigen receptors
The present invention relates to methods of developing genetically engineered, preferably non-alloreactive T-cells for immunotherapy. This method involves the use of RNA-guided endonucleases, in particular Cas9 / CRISPR system, to specifically target a selection of key genes in T-cells. The engineered T-cells are also intended to express chimeric antigen receptors (CAR) to redirect their immune activity towards malignant or infected cells. The invention opens the way to standard and affordable adoptive immunotherapy strategies using T-Cells for treating cancer and viral infections.
Owner:CELLECTIS SA
Adoptive transfer of cd8+ t cell clones derived from central memory cells
ActiveUS20140356398A1Mammal material medical ingredientsArtificial cell constructsWhite blood cellPharmaceutical formulation
The present invention provides a method of carrying out adoptive immunotherapy in a primate subject in need thereof by administering the subject a cytotoxic T lymphocytes (CTL) preparation in a treatment-effective amount. The method comprises administering as the CTL preparation a preparation consisting essentially of an in vitro expanded primate CTL population, the CTL population enriched prior to expansion for central memory T lymphocytes, and depleted prior to expansion of effector memory T lymphocytes. In some embodiments, the method may further comprise concurrently administering Interleukin-15 to the subject in an amount effective to increase the proliferation of the central memory T cells in the subject. Pharmaceutical formulations produced by the method, and methods of using the same, are also described.
Owner:FRED HUTCHINSON CANCER CENT
Method for generating immune cells resistant to arginine and/or tryptophan depleted microenvironment
ActiveUS20170035866A1Enhance immune responseImmunoglobulin superfamilyTransferasesArginineTryptophan
The present invention pertains to engineered immune cells, method for their preparation and their use as medicament, particularly for immunotherapy. The engineered immune cells of the present invention are characterized in that at least one gene selected from a gene encoding GCN2 and a gene encoding PRDM1 is inactivated or repressed. Such modified Immune cells are resistant to an arginine and / or tryptophan depleted microenvironment caused by, e.g., tumor cells, which makes the immune cells of the invention particularly suitable for immunotherapy. The invention opens the way to standard and affordable adoptive immunotherapy strategies using immune cells for treating different types of malignancies.
Owner:CELLECTIS SA
Methods for manufacturing t cells
ActiveUS20190247433A1Shorten the resting timeGenetically modified cellsGenetic material ingredientsVirus typeCD28
The disclosure relates to methods of manufacturing T cells for adoptive immunotherapy. The disclosure further provides for methods of genetically transducing T cells, methods of using T cells, and T cell populations thereof. In an aspect, the disclosure provides for methods of thawing frozen peripheral blood mononuclear cells (PBMC), resting the thawed PBMC, activating the T cell in the cultured PBMC with an anti-CD3 antibody and an anti-CD28 antibody immobilized on a solid phase, transducing the activated T cell with a viral vector, expanding the transduced T cell, and obtaining expanded T cells.
Owner:IMMATICS US INC
Composition capable of stimulating expansion of T cells
InactiveCN105543170AIncrease the number ofRaise the ratioBlood/immune system cellsCell culture active agentsCentral Memory T-CellHalf-life
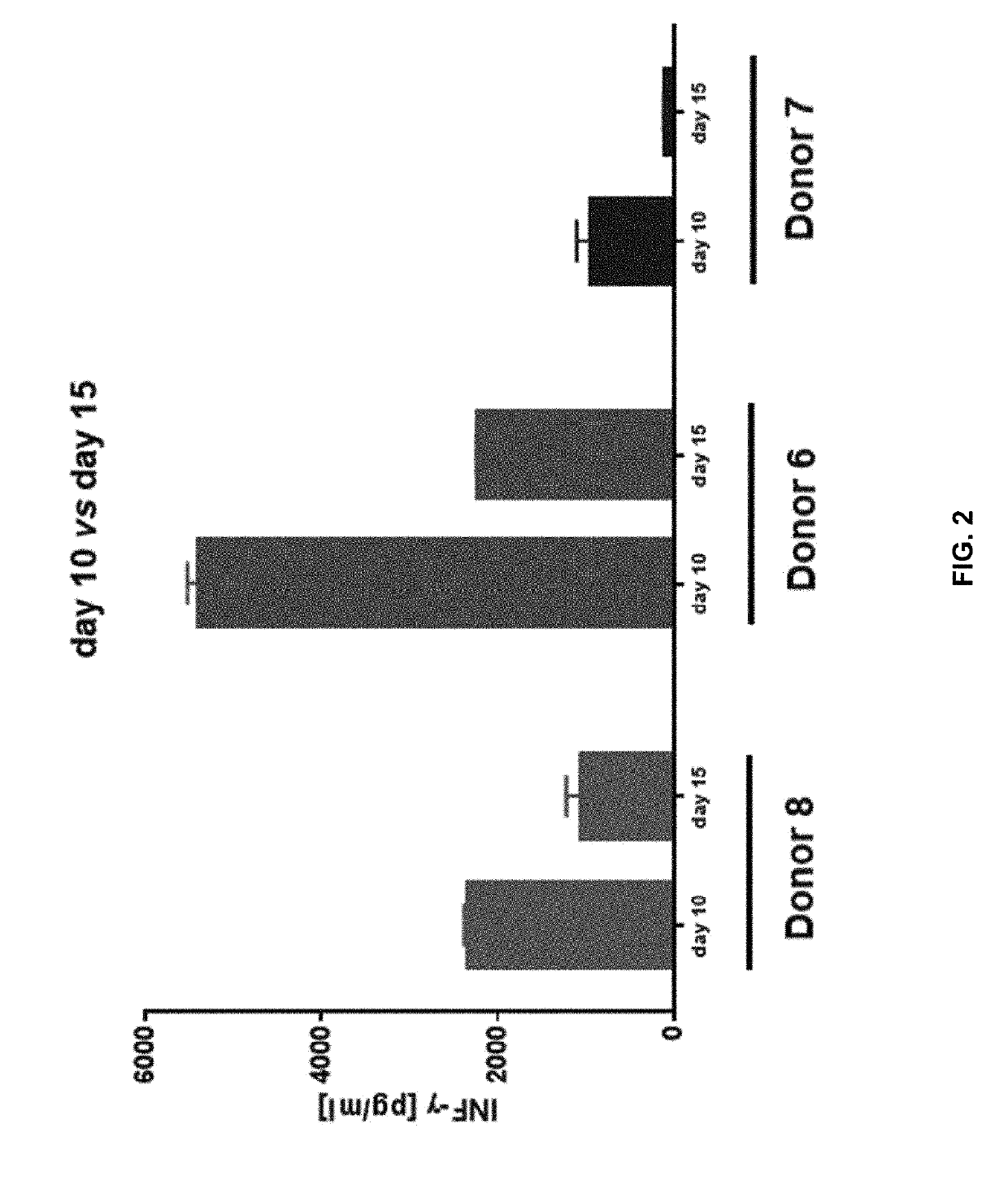
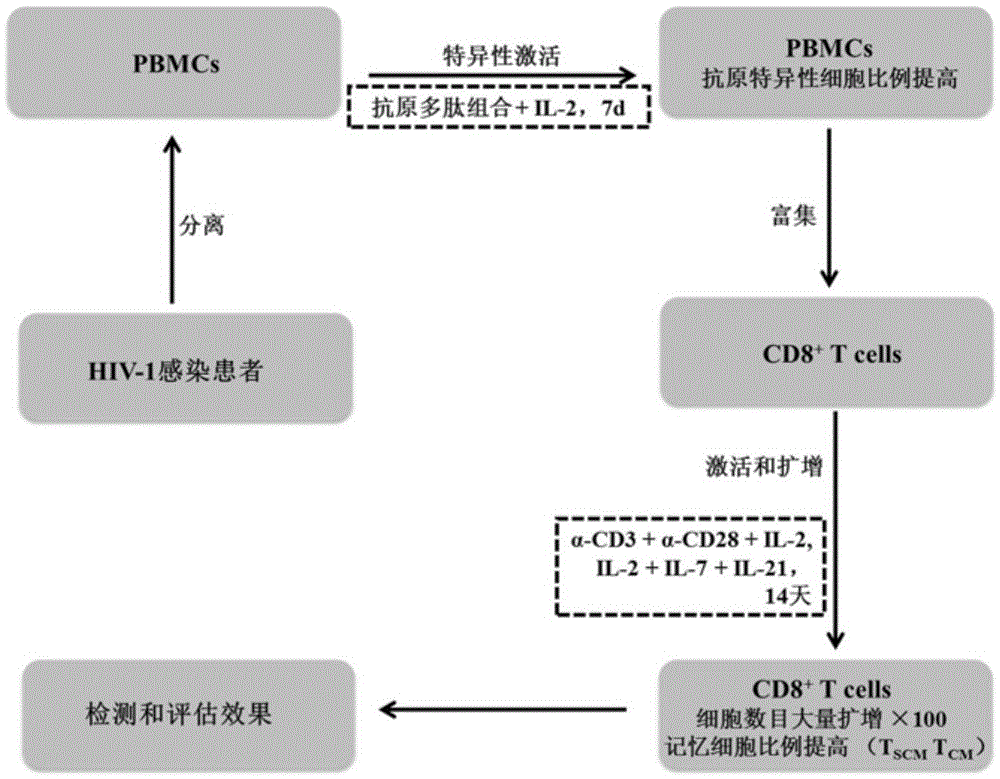
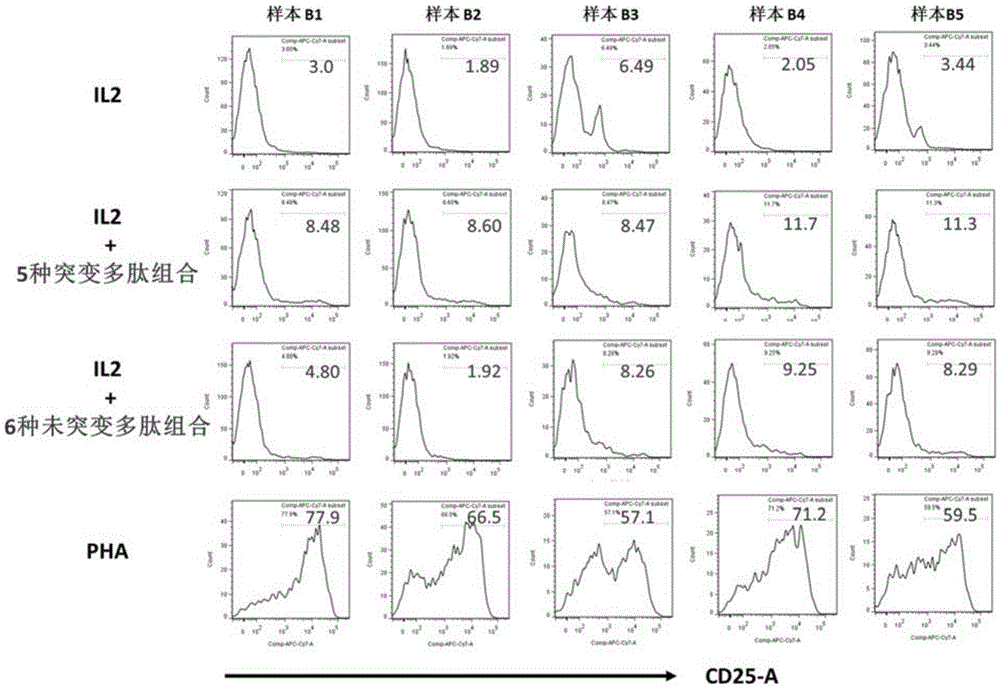
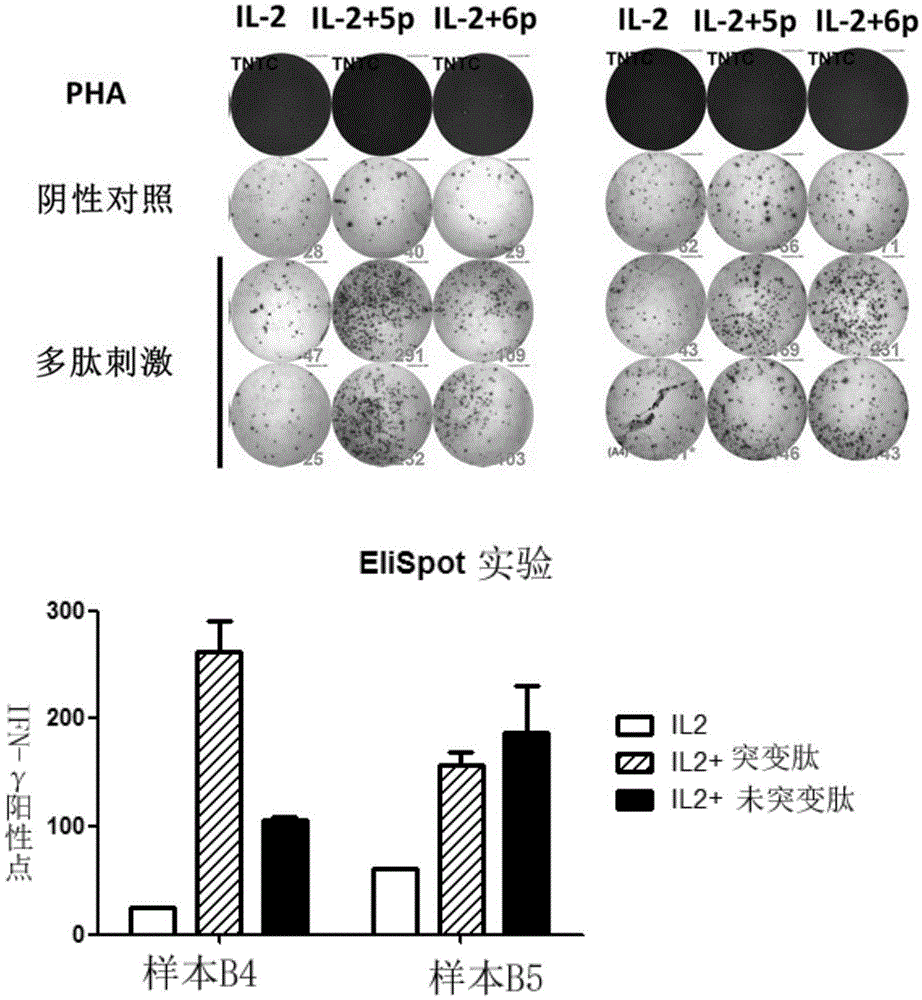
The invention discloses a composition capable of stimulating expansion of T cells. Active factors of the composition comprise an Anti-CD3 antibody, an Anti-CD28 antibody, IL-2, IL-7 and IL-21. Through an HIV-1 specificity polypeptide combination and a multiple cell factor combination, in-vitro expansion of the antigen-specific memory T cells (TSCM and TCM) of an HIV-1 infected person can be effectively and continuously stimulated, and meanwhile the proportion of the expanded CD8<+> memory T cells, especially the CD8<+> TSCM is increased. The half-life period of adoptive immunotherapy reinjection cells can be prolonged, therefore, the immune surveillance achieving ageing of the adoptive immunotherapy reinjection cells is prolonged, and the potential of controlling HIV-1 latent infection for a long term is achieved. According to the method, the advantages that operation is simple, and materials are easy to obtain are achieved, and good technical support is supplied to wide development of anti-infection adoptive immunotherapy.
Owner:SUN YAT SEN UNIV
Adoptive immunotherapy for treating cancer
ActiveUS20170349880A1Cancer antigen ingredientsBlood/immune system cellsTumor-infiltrating lymphocytesCancer therapy
The present invention provides methods for producing and / or expanding tumor-infiltrating lymphocytes (TILs) that can be used in adoptive immunotherapy in cancer treatment.
Owner:UNIVERSITY OF LAUSANNE
Immortalized car-t cells genetically modified to elminate t-cell receptor and beta 2-microglobulin expression
InactiveUS20190175651A1None is suitable for purposeTumor rejection antigen precursorsGenetic material ingredientsT cellAuto immune disease
The present invention pertains to engineered immortalized T-cell lines, method for their preparation and their use as medicament, particularly for immunotherapy. The engineered immortalized T-cell lines of the invention are characterized in that the expression of endogenous T-cell receptors (TCRs) and beta 2-microglobulin (B2M) is inhibited, e.g., by using an endonuclease able to selectively inactivate the TCR and B2M genes in order to render the immortalized T-cells non-alloreactive. In addition, expression of immunosuppressive polypeptide can be performed on those engineered immortalized T-cells in order to prolong the survival of these T-cells in host organisms. Such engineered immortalized T-cells are particularly suitable for allogeneic transplantations, especially because it reduces both the risk of rejection by the host's immune system and the risk of developing graft versus host disease. The invention opens the way to standard and affordable adoptive immunotherapy strategies using immortalized T-cells for treating cancer, infections and auto-immune diseases.
Owner:JANSSEN BIOTECH INC
Multi-chain chimeric antigen receptor and uses thereof
The present invention relates to the generation of chimeric antigen receptors (CAR) referred to as multi-chain CARs. Such CARs, which aim to redirect immune cell specificity and reactivity toward a selected target exploiting the ligand-binding domain properties, comprise separate extracellular ligand binding and signaling domains in different transmembrane polypeptides. The signaling domains are designed to assemble in juxtamembrane position, which forms flexible architecture closer to natural receptors, that confers optimal signal transduction. The invention encompasses the polynucleotides, vectors encoding said multi- chain CAR and the isolated cells expressing them at their surface, in particularly for their use in immunotherapy. The invention opens the way to efficient adoptive immunotherapy strategies for treating cancer and viral infections.
Owner:CELLECTIS SA
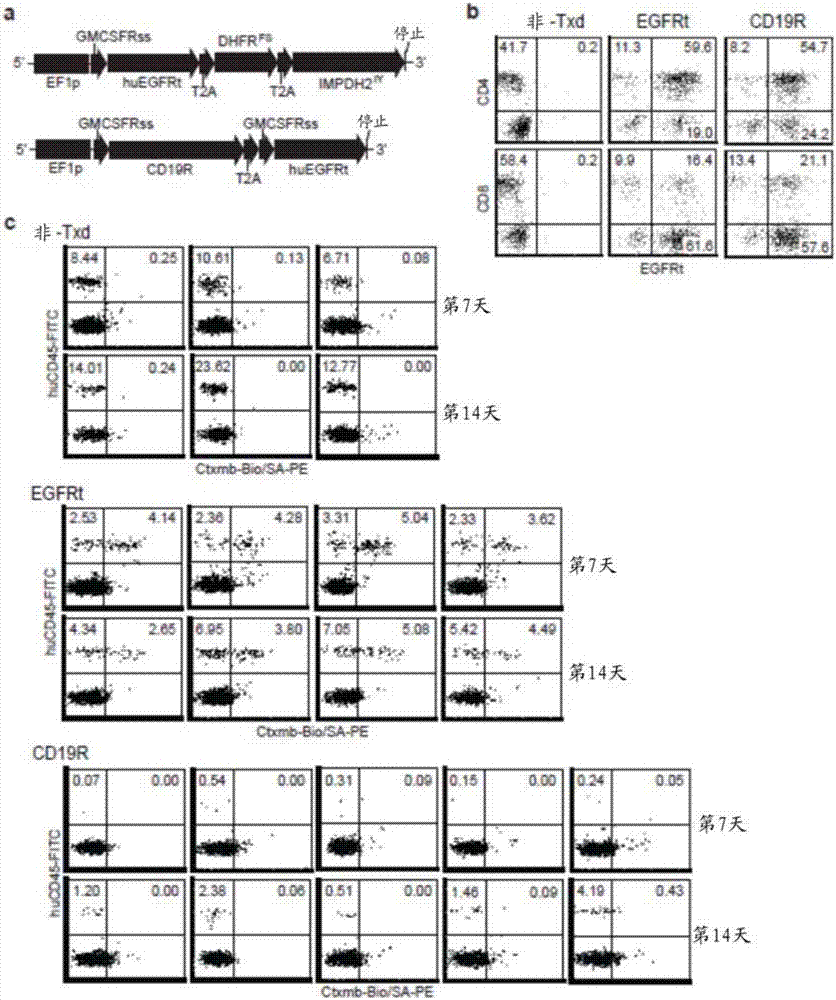
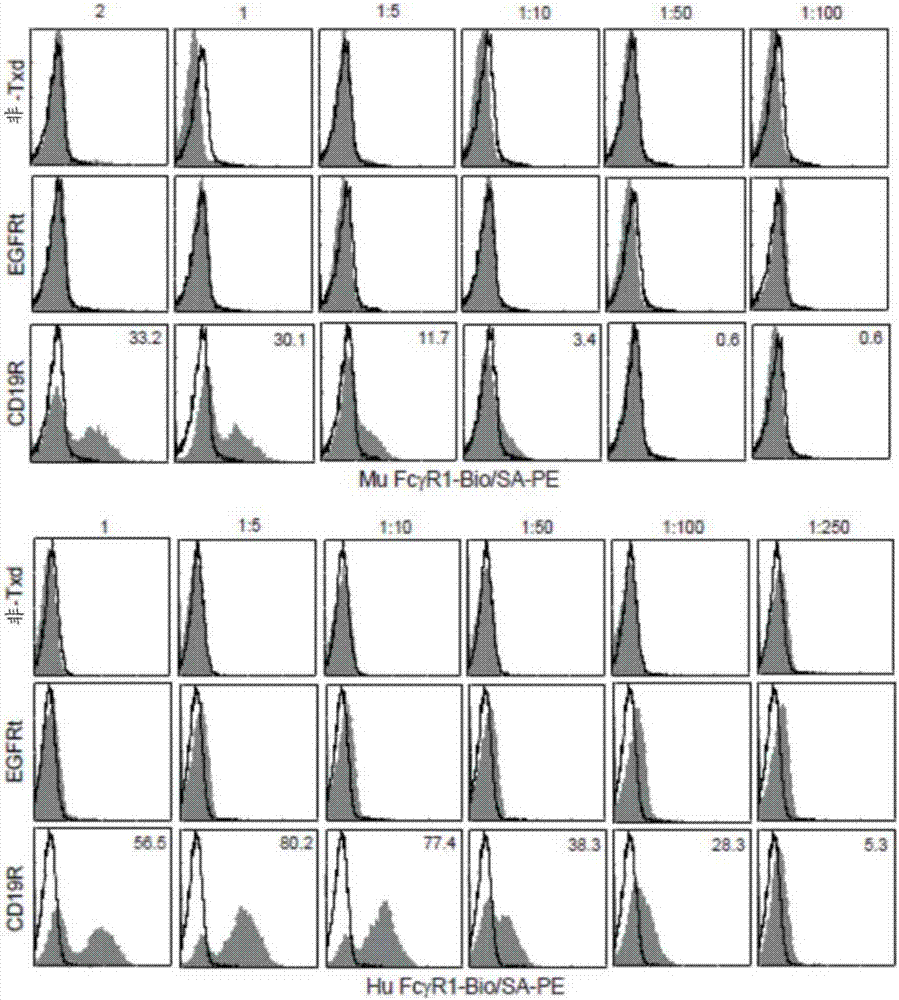
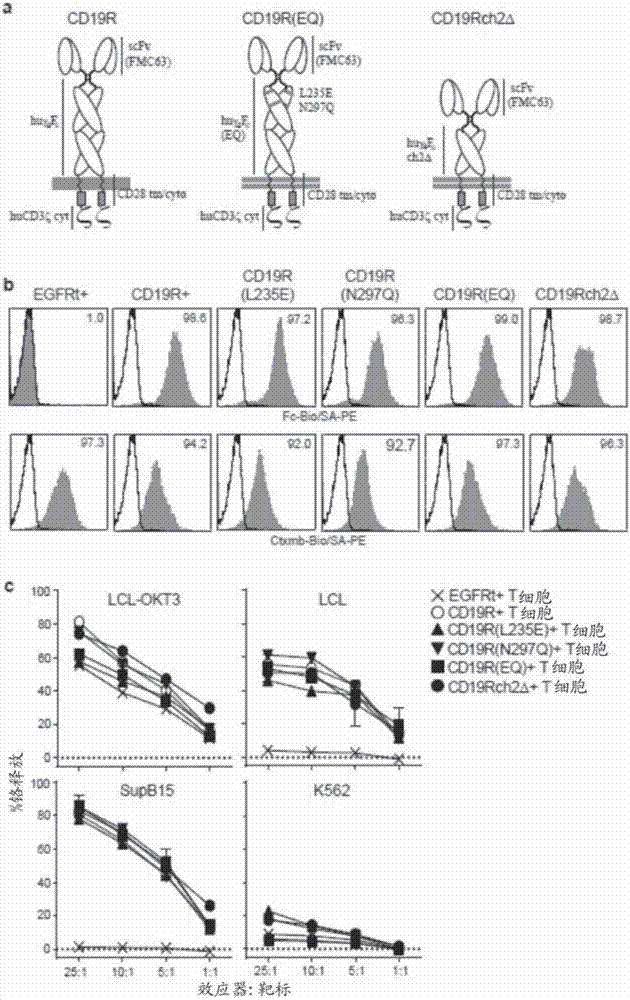
Chimeric antigen receptors (CARs) having mutations in the Fc spacer region and methods for their use
ActiveCN107074957APrevent identification and damageAvoid clearingPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigen receptorsChimeric antigen receptor
Adoptive immunotherapy using T cells genetically redirected via expression of chimeric antigen receptors (CARs) is a promising approach for cancer treatment. However, this immunotherapy is dependent in part on the optimal molecular design of the CAR, which involves an extracellular ligand-binding domain connected to an intracellular signaling domain by spacer and / or transmembrane sequences.
Owner:CITY OF HOPE
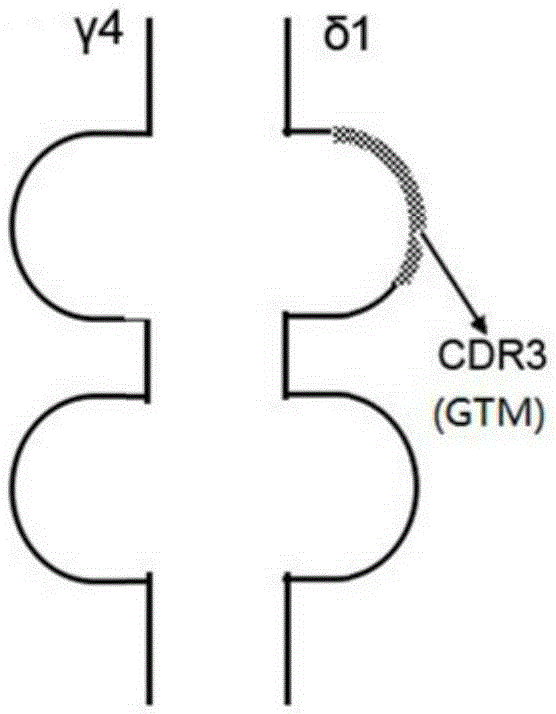
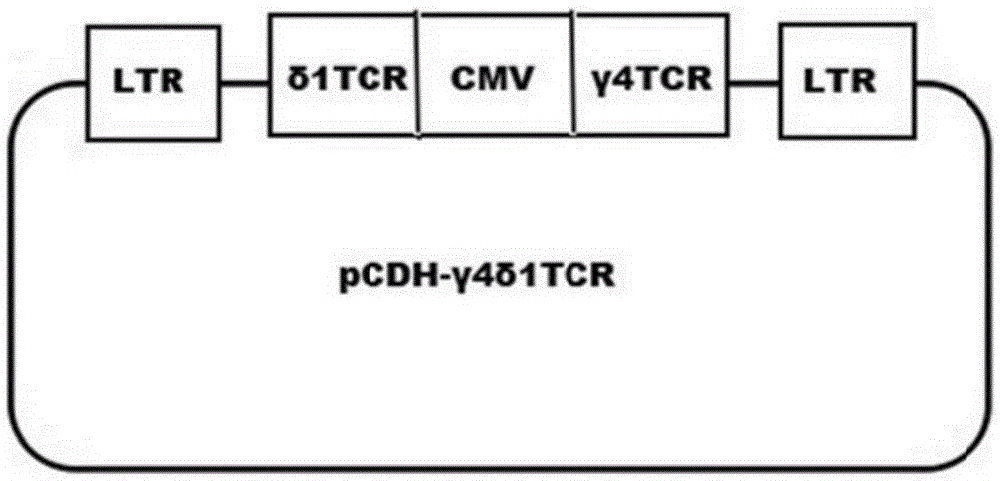
Tumor binding specific gamma delta TCR gene modified alpha beta T cell and cancer suppression application thereof
ActiveCN105296431ABroad anti-tumor effectNo cytotoxic activityMammal material medical ingredientsGenetic engineeringCytotoxicityT cell
The invention relates to a tumor binding specific gamma delta TCR gene modified alpha beta T cell and cancer suppression application thereof. The alpha beta T cell expresses the tumor binding specific gamma delta TCR; the tumor binding specific gamma delta TCR comprises a gamma 4 chain and a delta 1 chain; the amino acid sequence of the delta 1 chain CDR3 is shown as SEQ ID NO: 1. Experiment results prove that the cell has cytotoxicity for a plurality of tumor cells, and a novel method and a novel strategy are provided for adoptive immunotherapy of tumors.
Owner:北京佳德和细胞治疗技术有限公司
Adoptive immunotherapy with enhanced T lymphocyte survival
Owner:UNITED STATES OF AMERICA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com