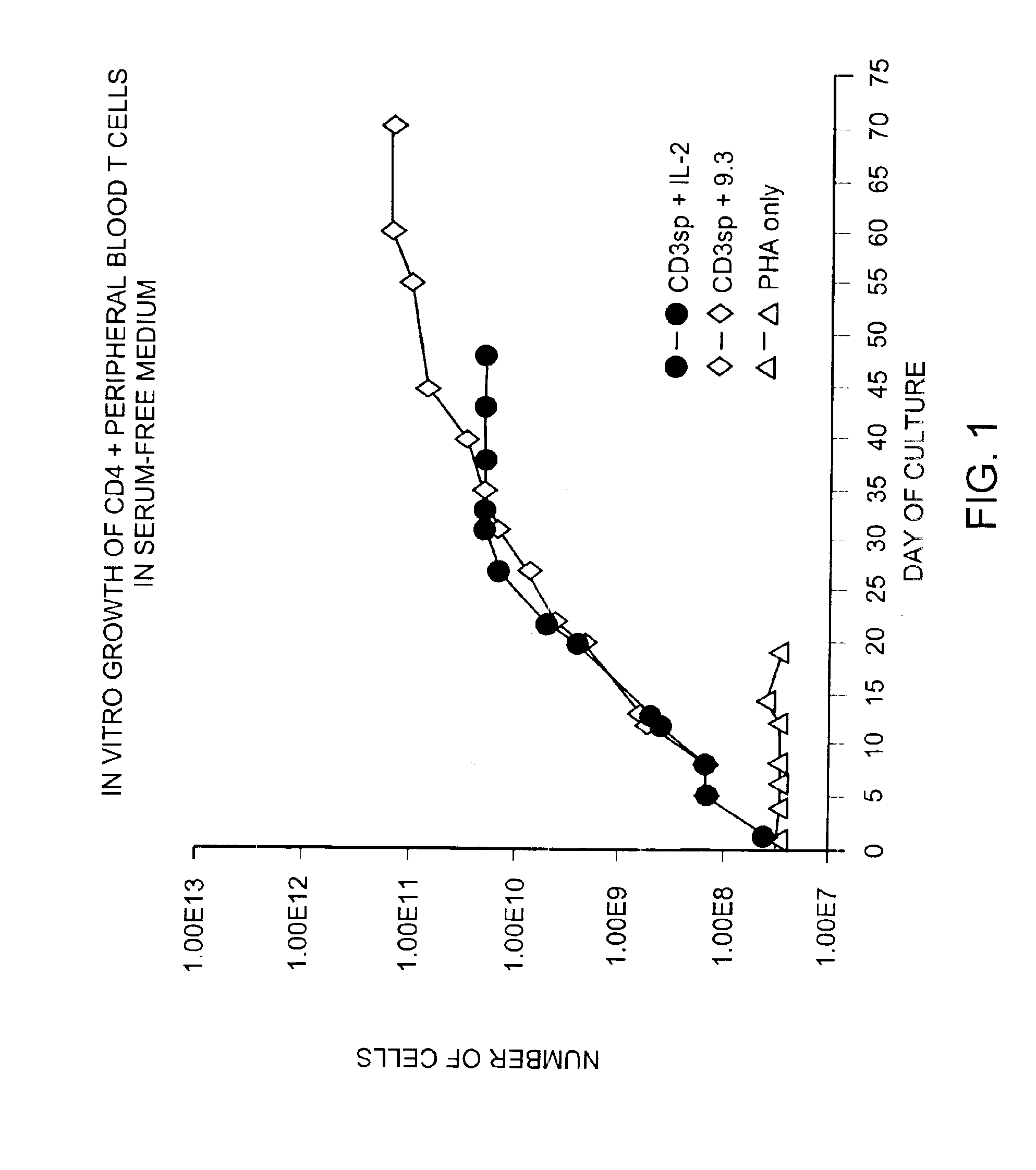
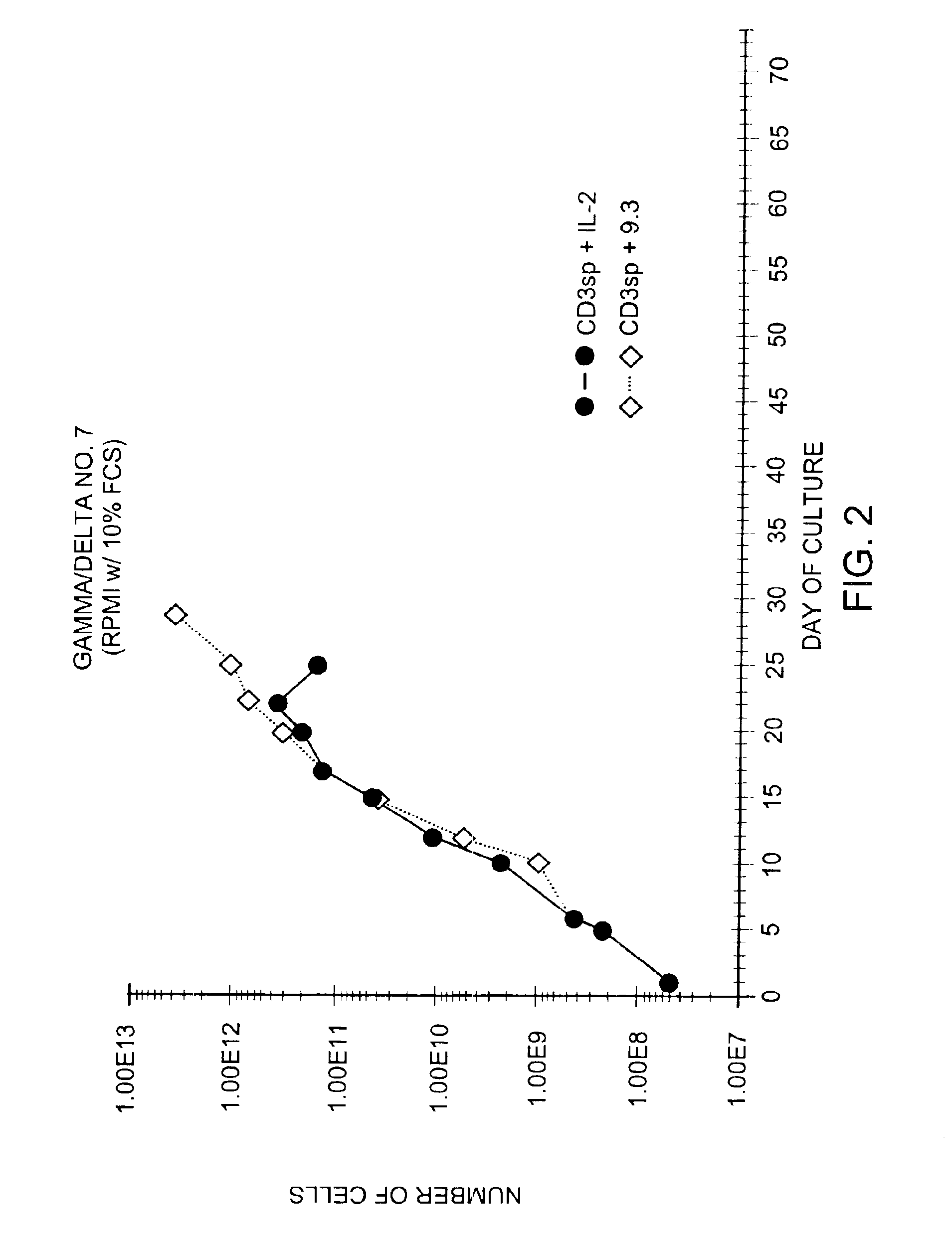
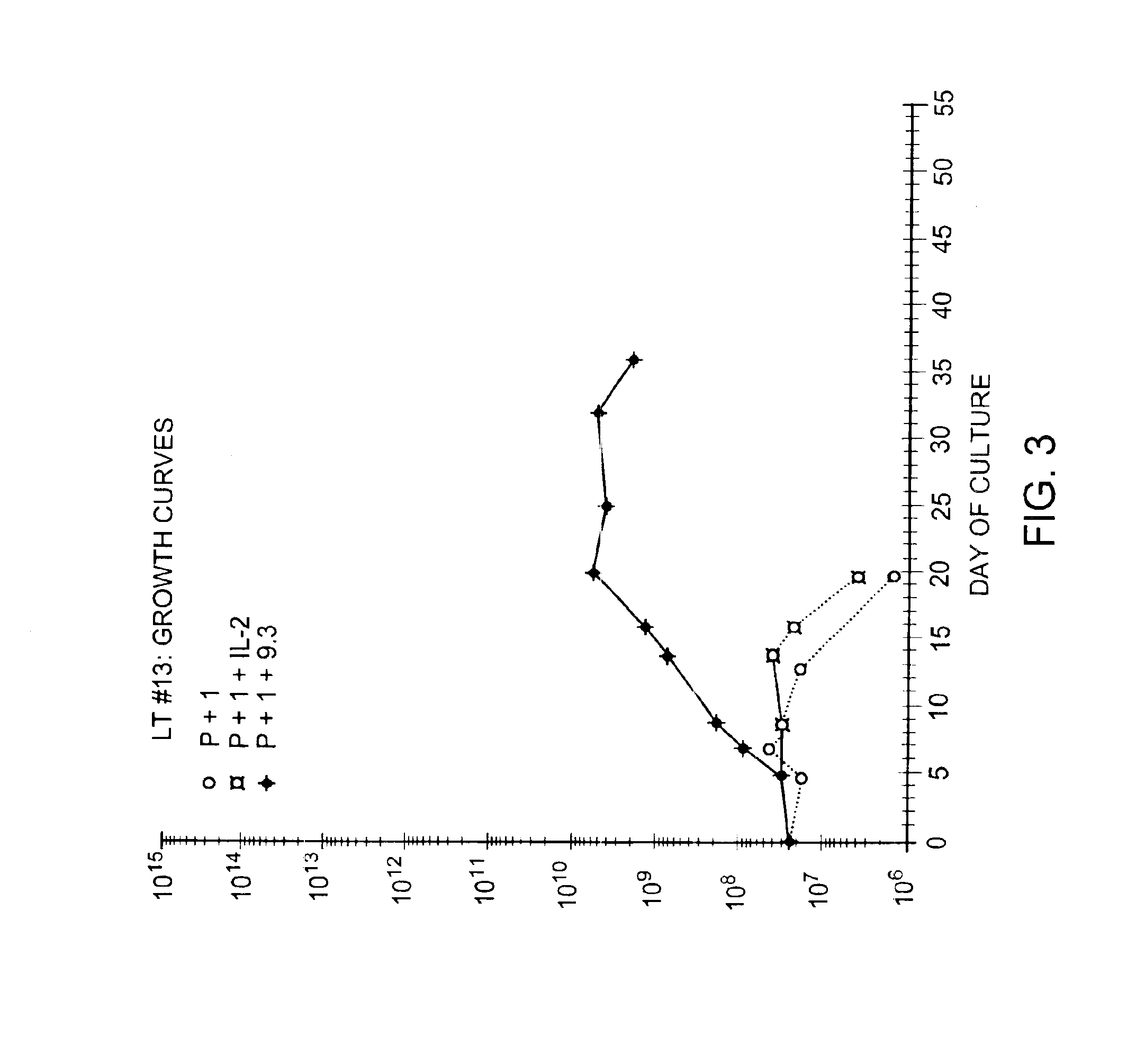
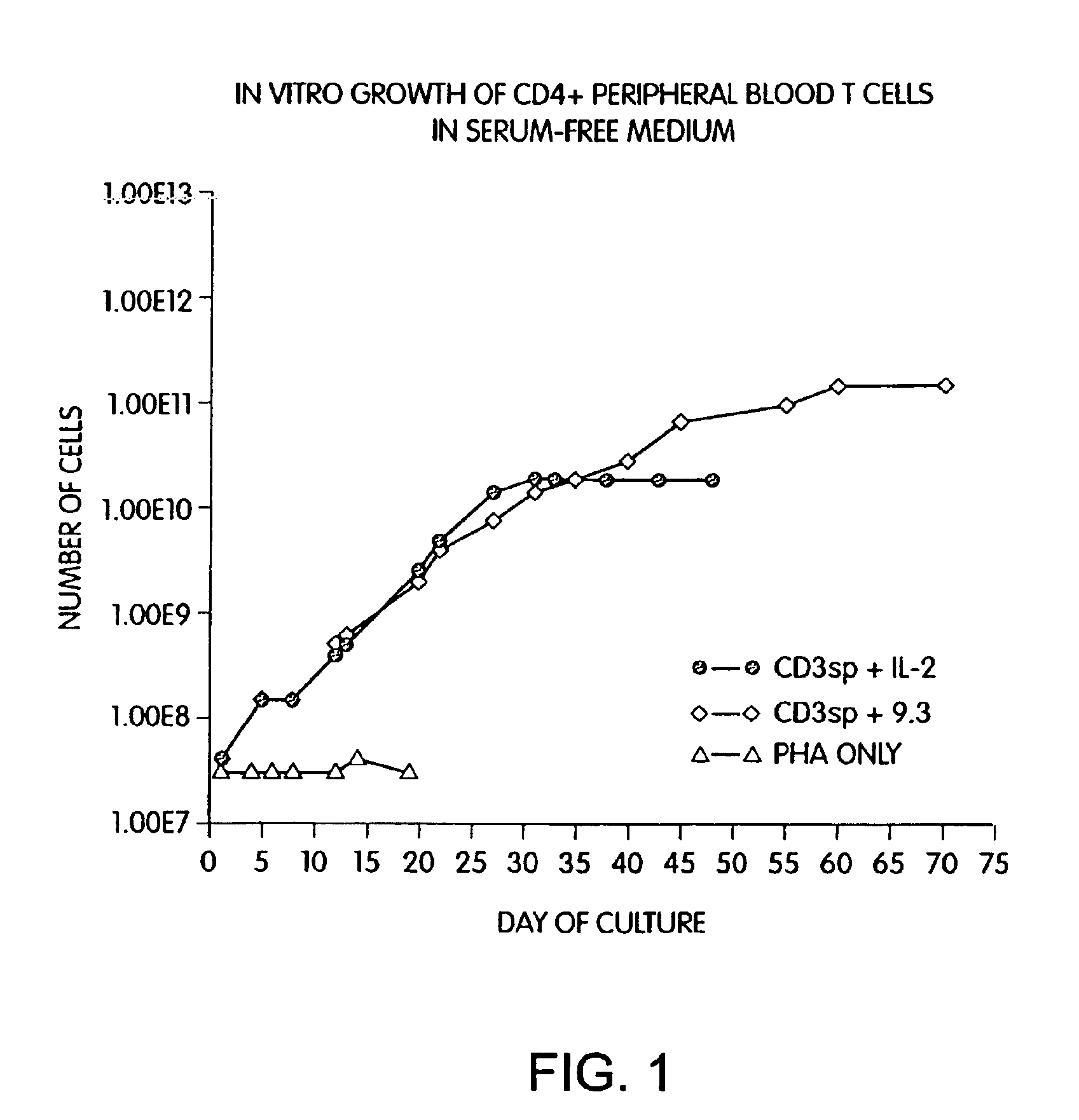
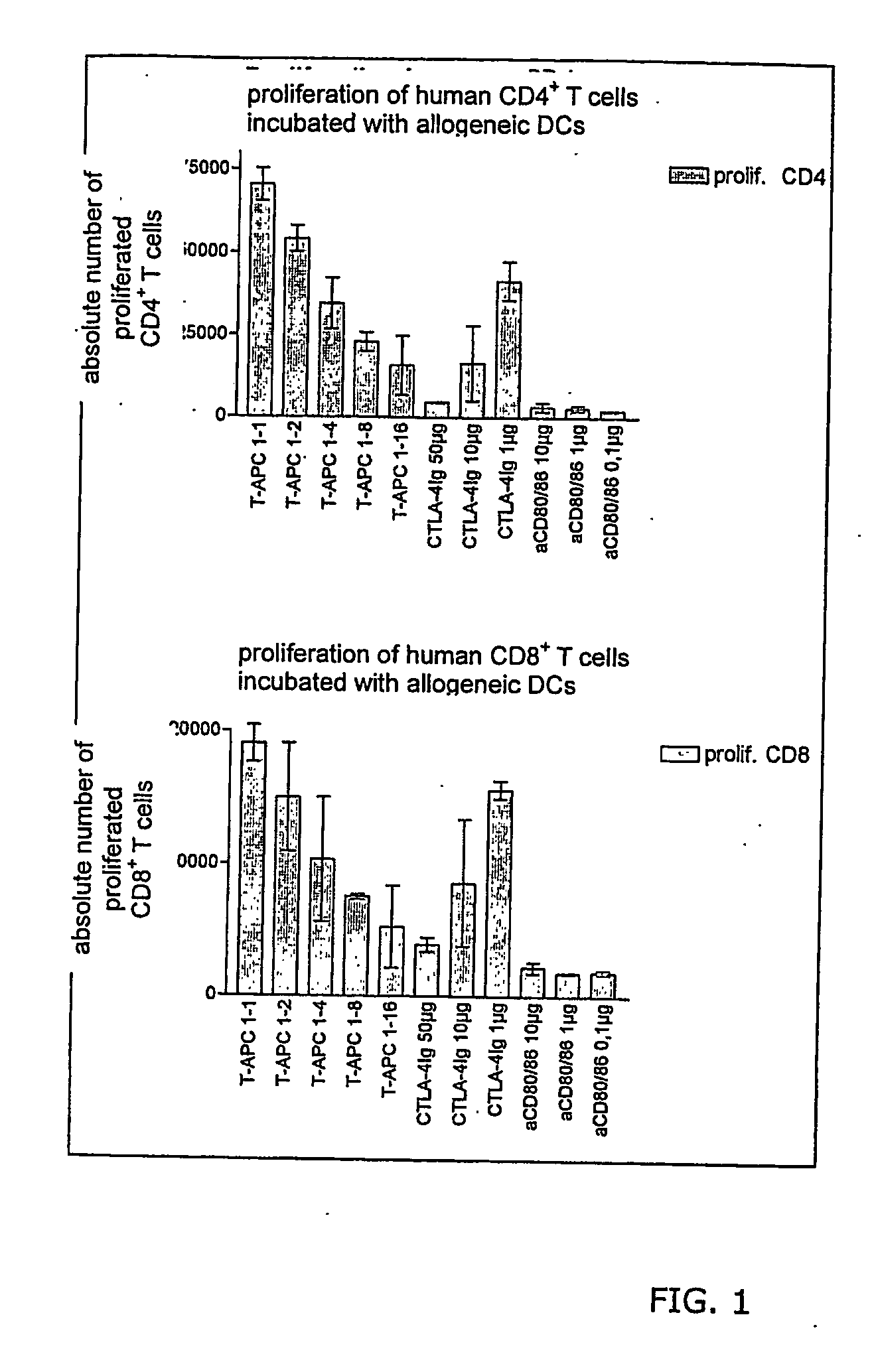
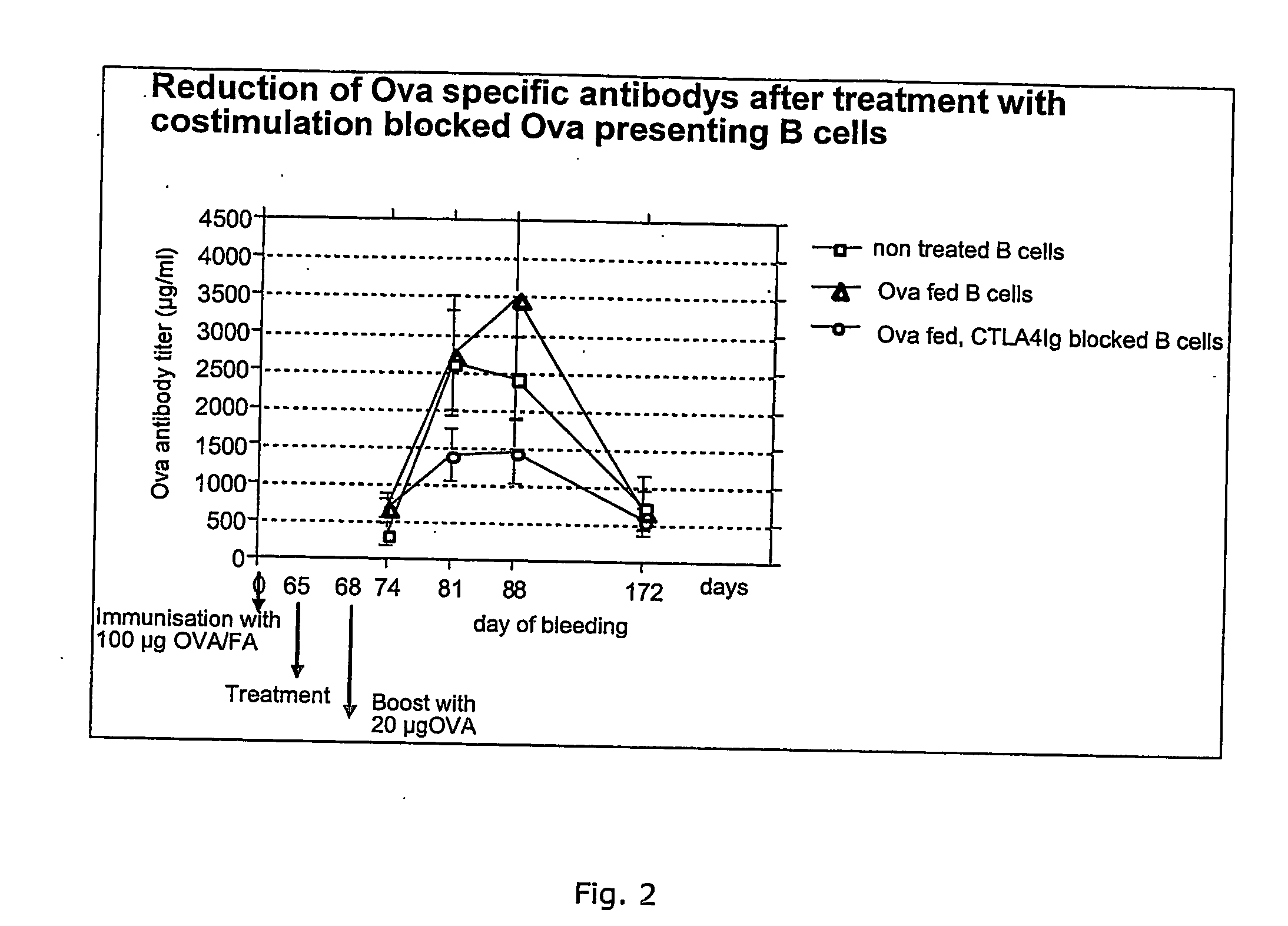
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
411 results about "CD28" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
CD28 (Cluster of Differentiation 28) is one of the proteins expressed on T cells that provide co-stimulatory signals required for T cell activation and survival. T cell stimulation through CD28 in addition to the T-cell receptor (TCR) can provide a potent signal for the production of various interleukins (IL-6 in particular).
Methods for selectively stimulating proliferation of T cells
InactiveUS6905681B1Increase the number ofVirusesPeptide/protein ingredientsAccessory moleculeExogenous growth
Owner:GENETICS INST INC +2
Methods for selectively stimulating proliferation of T cells
InactiveUS6887466B2Expanding population of cellIncrease the number ofVirusesPeptide/protein ingredientsAccessory moleculeExogenous growth
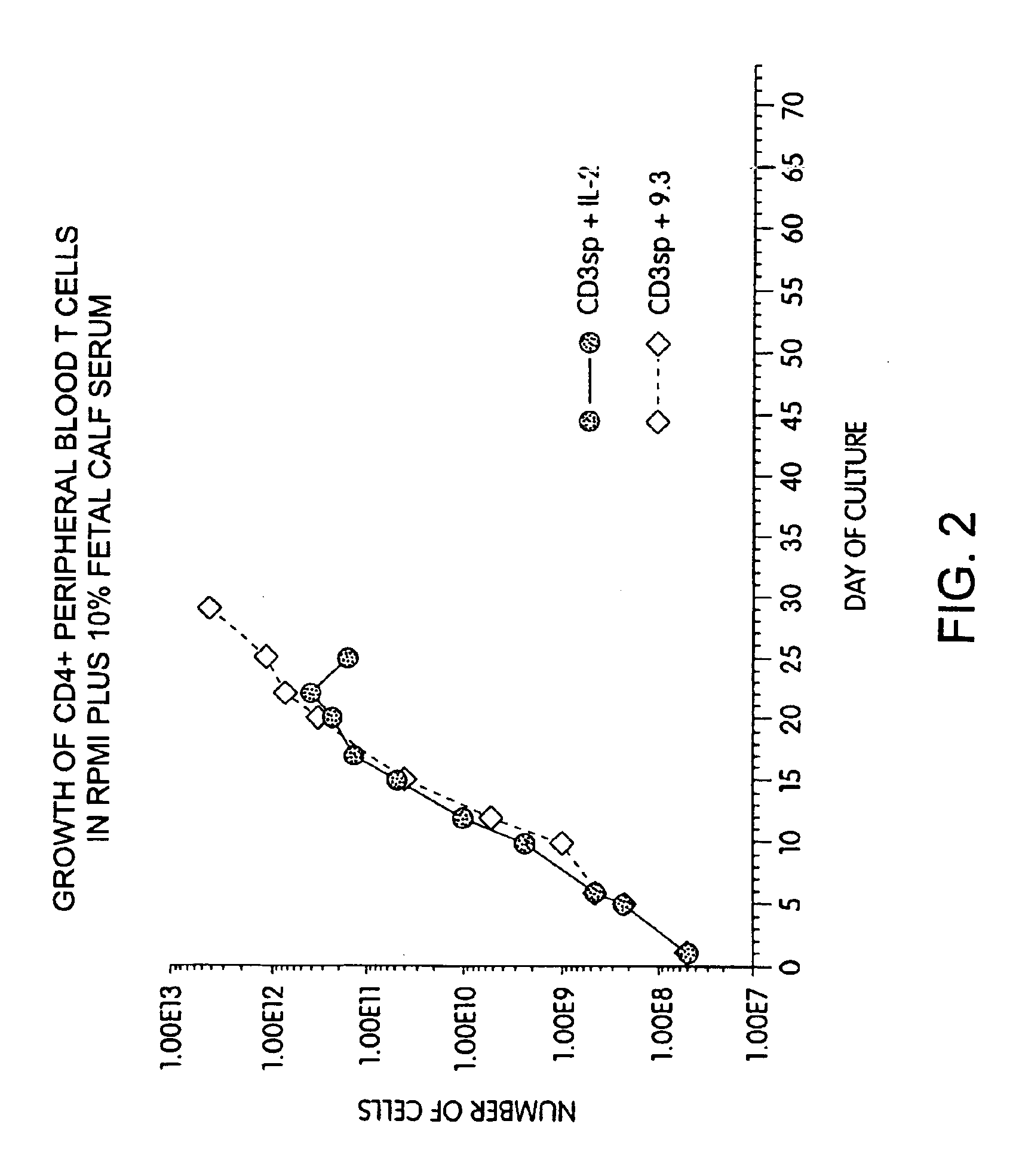
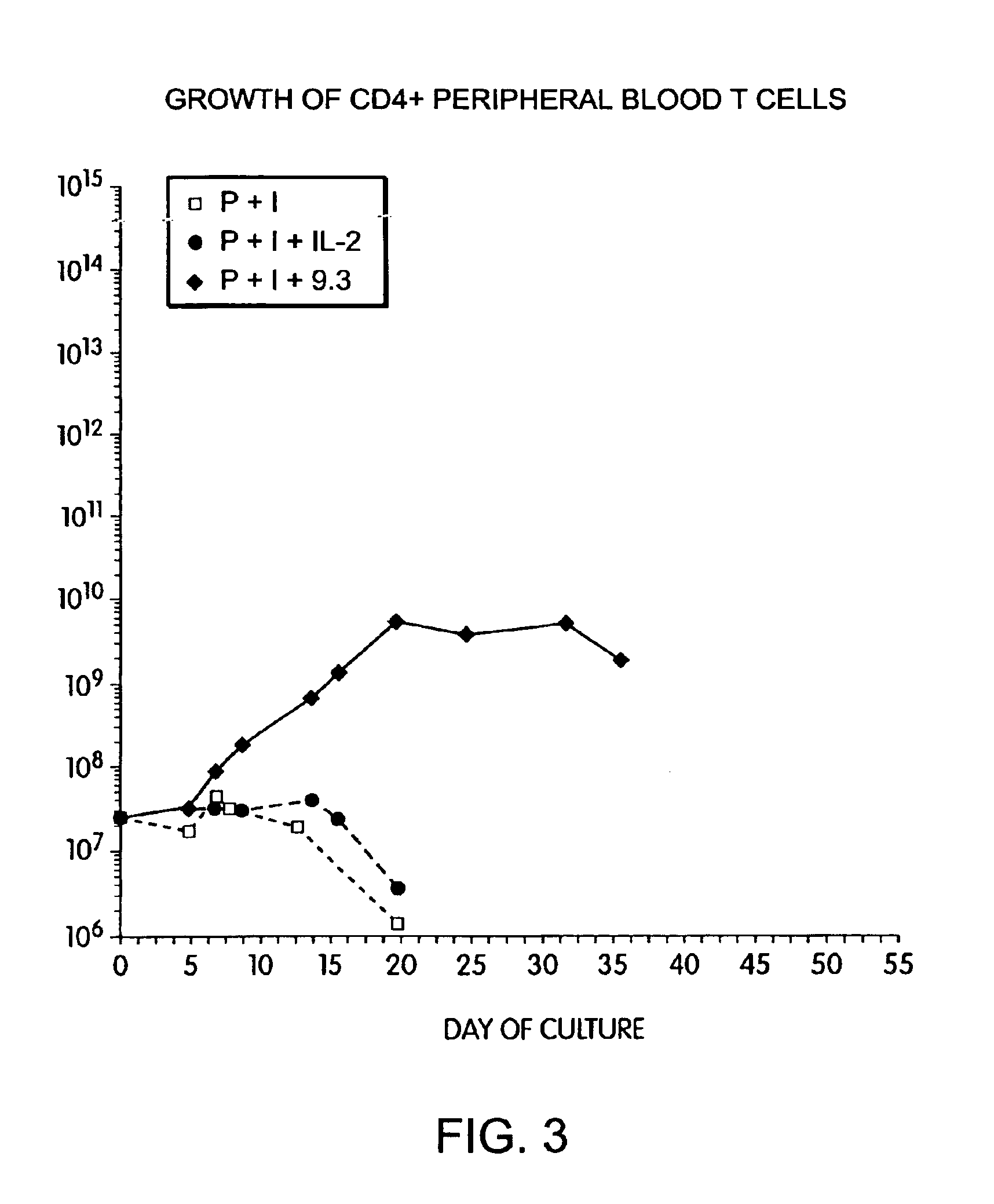
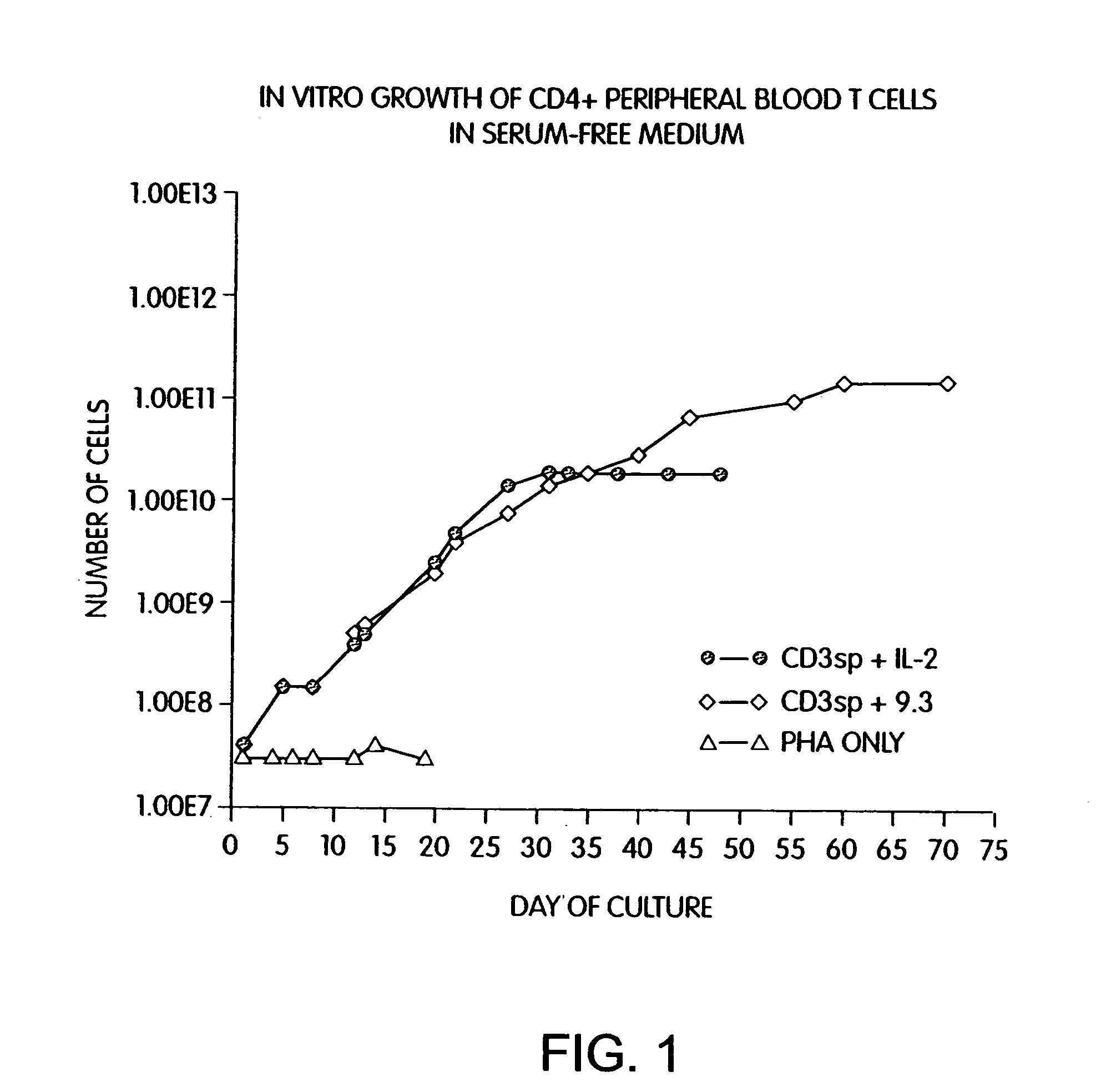
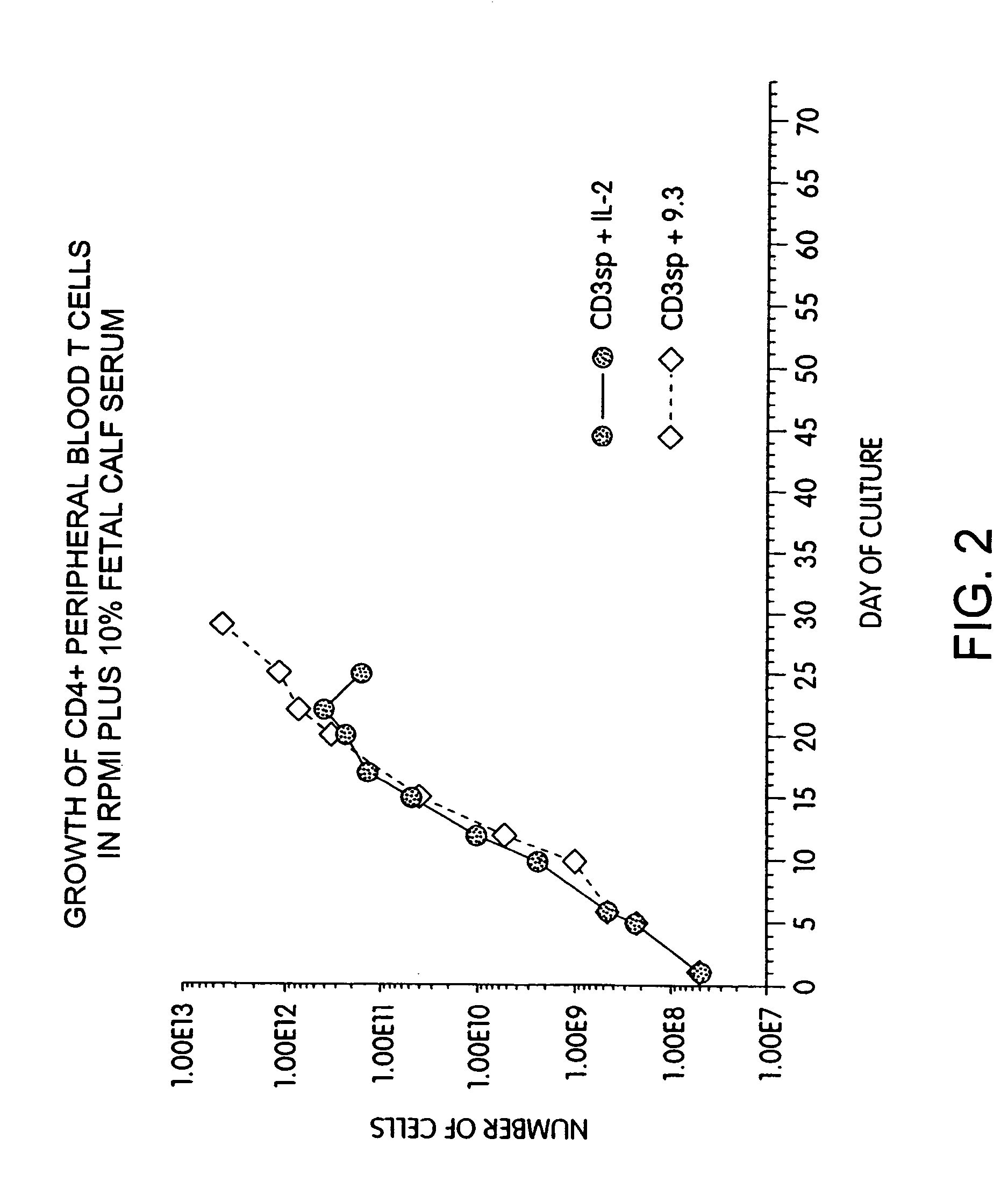
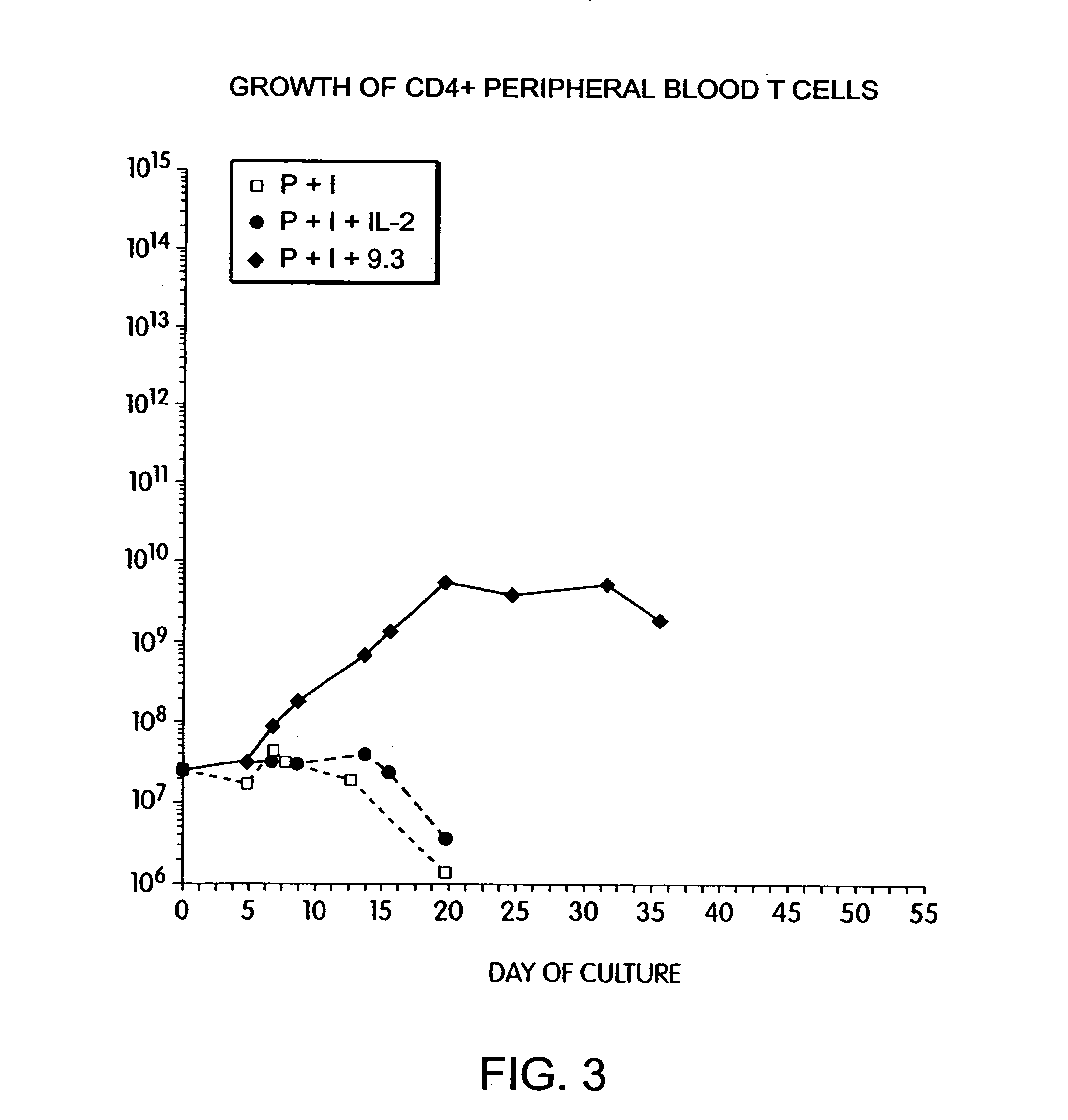
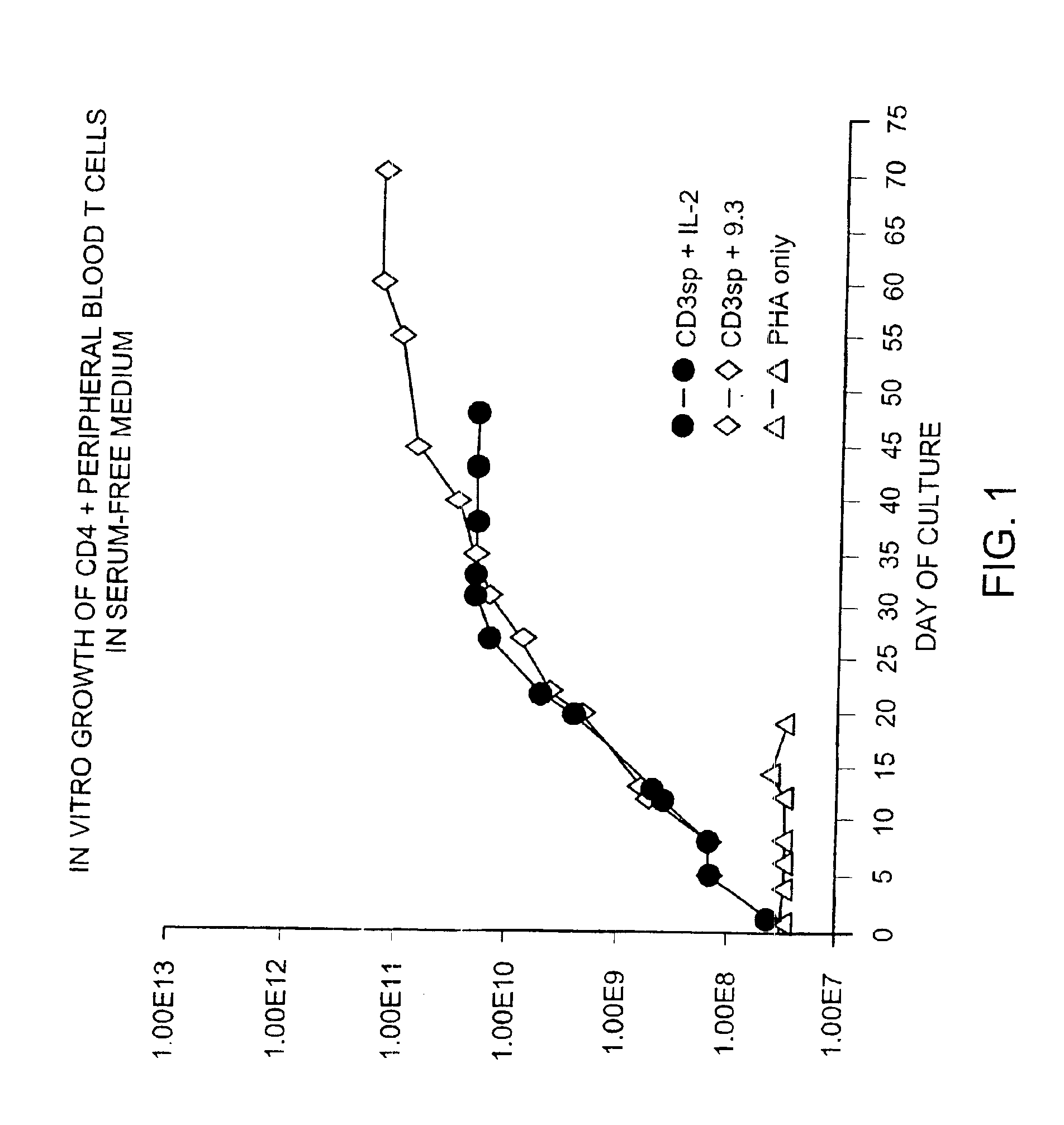
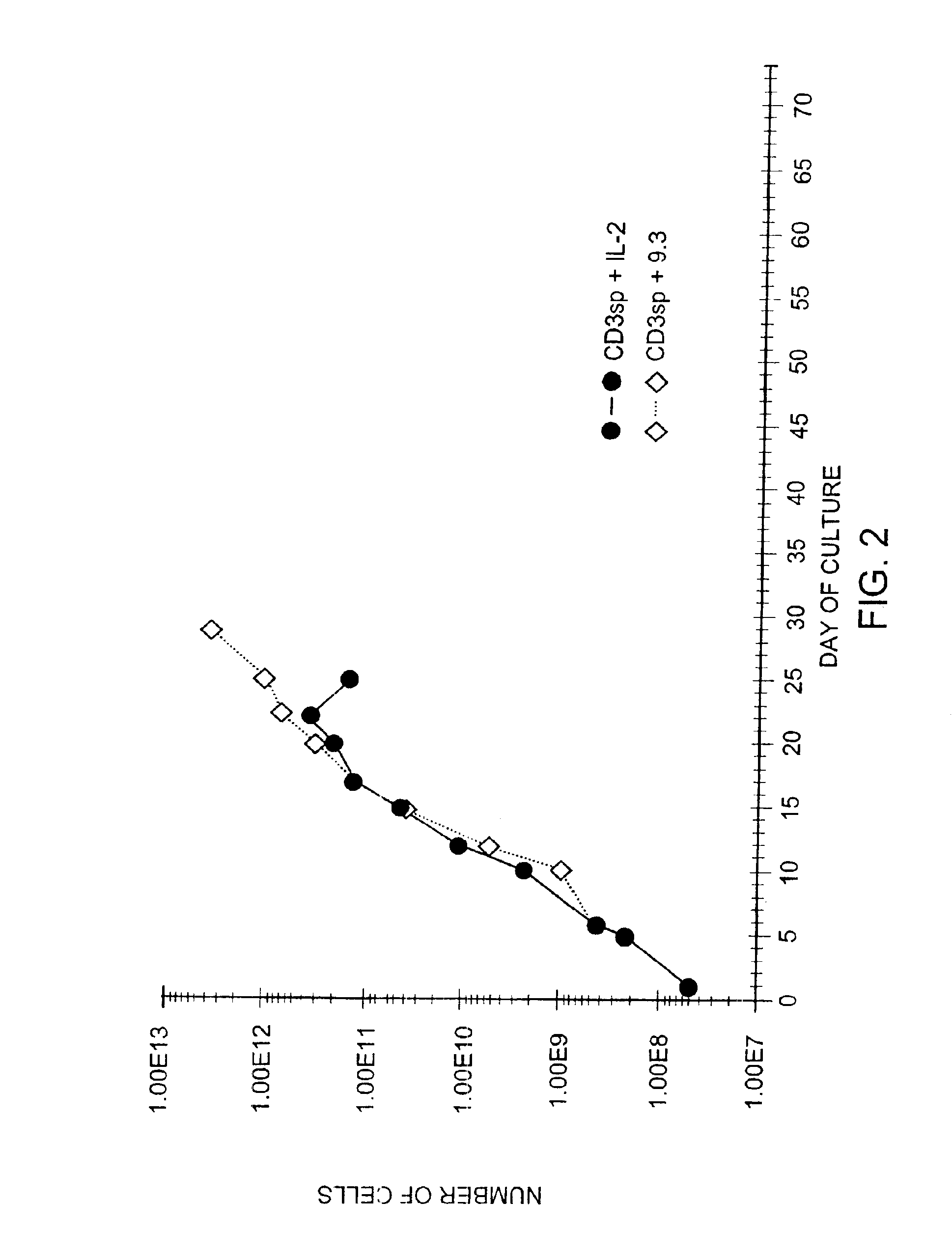
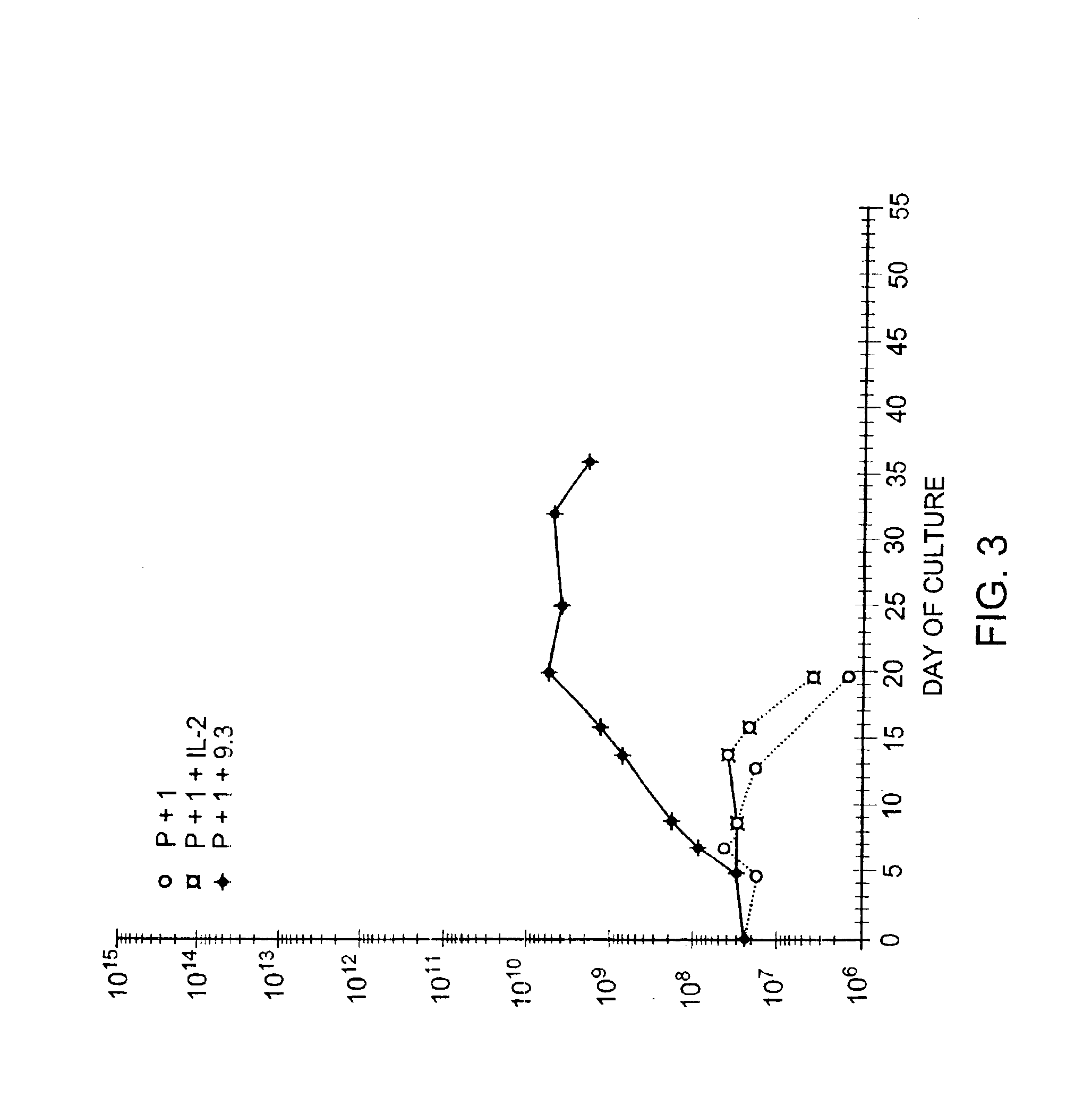
Methods for inducing a population of T cells to proliferate by activating the population of T cells and stimulating an accessory molecule on the surface of the T cells with a ligand which binds the accessory molecule are described. T cell proliferation occurs in the absence of exogenous growth factors or accessory cells. T cell activation is accomplished by stimulating the T cell receptor (TCR) / CD3 complex or the CD2 surface protein. To induce proliferation of an activated population T cells, an accessory molecule on the surface of the T cells, such as CD28, is stimulated with a ligand which binds the accessory molecule. The T cell population expanded by the method of the invention can be genetically transduced and used for immunotherapy or can be used in methods of diagnosis.
Owner:GENETICS INST INC +2
Methods for selectively stimulating proliferation of T cells
InactiveUS7175843B2Increase the number ofBiocideCell receptors/surface-antigens/surface-determinantsAccessory moleculeExogenous growth
Owner:GENETICS INST LLC +2
Methods of treating HIV infected subjects
InactiveUS6905680B2Expanding population of cellIncrease the number ofVirusesPeptide/protein ingredientsAccessory moleculeExogenous growth
Methods for inducing a population of T cells to proliferate by activating the population of T cells and stimulating an accessory molecule on the surface of the T cells with a ligand which binds the accessory molecule are described. T cell proliferation occurs in the absence of exogenous growth factors or accessory cells. T cell activation is accomplished by stimulating the T cell receptor (TCR) / CD3 complex or the CD2 surface protein. To induce proliferation of an activated population T cells, an accessory molecule on the surface of the T cells, such as CD28, is stimulated with a ligand which binds the accessory molecule. The T cell population expanded by the method of the invention can be genetically transduced and used for immunotherapy or can be used in methods of diagnosis.
Owner:GENETICS INST INC +2
Methods for selectively stimulating proliferation of T cells
InactiveUS7144575B2Increase the number ofBiocidePeptide/protein ingredientsAccessory moleculeExogenous growth
Methods for inducing a population of T cells to proliferate by activating the population of T cells and stimulating an accessory molecule on the surface of the T cells with a ligand which binds the accessory molecule are described. T cell proliferation occurs in the absence of exogenous growth factors or accessory cells. T cell activation is accomplished by stimulating the T cell receptor (TCR) / CD3 complex or the CD2 surface protein. To induce proliferation of an activated population T cells, an accessory molecule on the surface of the T cells, such as CD28, is stimulated with a ligand which binds the accessory molecule. The T cell population expanded by the method of the invention can be genetically transduced and used for immunotherapy or can be used in methods of diagnosis.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY +2
Cyclic single-chain trispecific antibody
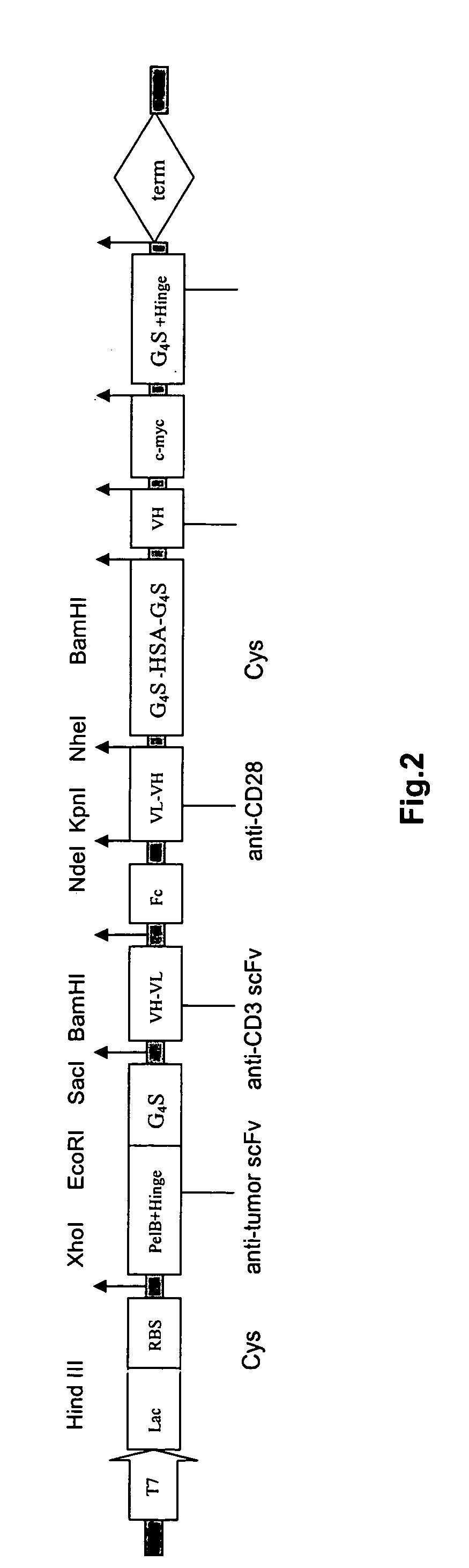
The invention provides a cyclic single-chain trispecific antibody against human tumor. It comprises three parts. The first part is an anti-tumor Fab antibody, an anti-tumor single-domain antibody or an scFv. The second part is a reshaped Fab antibody against human CD3, a reshaped single-domain antibody against human CD3 or a reshaped scFv against human CD3. The third part is a reshaped Fab antibody against human CD28, a reshaped single-domain antibody against human CD28 or a reshaped scFv against human CD28. The present invention also offers the DNA sequence coding for this trispecific antibody, expression vectors containing this DNA sequence and host cells (E. coli) containing the vectors.
Owner:DONGGUAN HAOFA BIOTECH DEVAL +2
Human B7.1-specific primatized antibodies and transfectomas expressing said antibodies
InactiveUS6113898AShrink tumorInhibit tumor growthPeptide/protein ingredientsAntipyreticDiseaseOrgan transplant rejection
The present invention relates to the identification of macaque antibodies to human B7.1 and B7.2 by screening of phage display libraries or monkey heterohybridomas obtained using B lymphocytes from B7.1 and / or B7.2 immunized monkeys. More specifically, the invention provides four monkey monoclonal antibodies 7B6, 16C10, 7C10 and 20C9 which inhibit the B7:CD28 pathway and thereby function as effective immunosuppressants. The invention further provides the complete DNA and amino acid sequences of the light and heavy chain of three primatized antibodies derived from those monkey monoclonal antibodies which bind B7.1 and possibly B7.2, primatized 7C10, primatized 7B6 and primatized 16C10. These primatized and monkey antibodies may be used as specific immunosuppressants, e.g., for the treatment of autoimmune diseases and to prevent organ transplant rejection.
Owner:BIOGEN INC
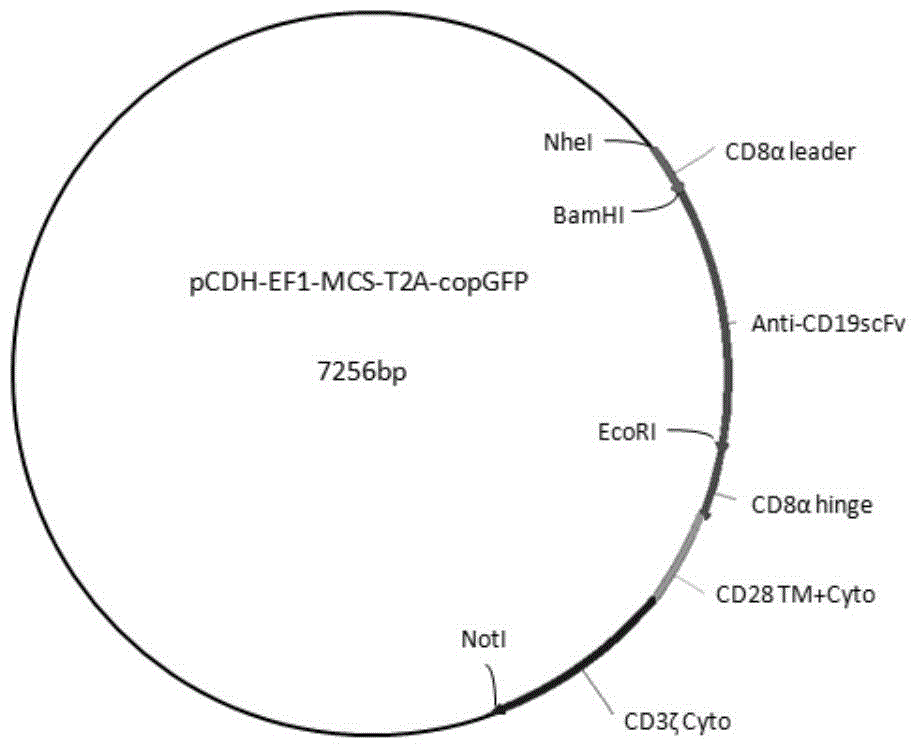
Chimeric antigen receptor hCD19scFv-CD8a-CD-28-CD3zata and application thereof
ActiveCN104788573AConfirmed specific killing effectMammal material medical ingredientsHybrid peptidesAntigen receptorHeavy chain
The invention discloses a chimeric antigen receptor hCD19scFv-CD8a-CD-28-CD3zata and application thereof. The chimeric antigen receptor is serially connected by an anti-human CD19 monoclonal antibody H119a light chain and heavy chain variable region (hCD19scFv), human CD8a hinge region, a human CD28 transmembrane region, an intracellular region and a human CD3zata intracellular region. The chimeric antigen receptor is used for modifying T lymphocyte, and the modified T lymphocyte (CAR-T cell) can be used for treating the tumor with positive surface CD19.
Owner:JUVENTAS CELL THERAPY LTD
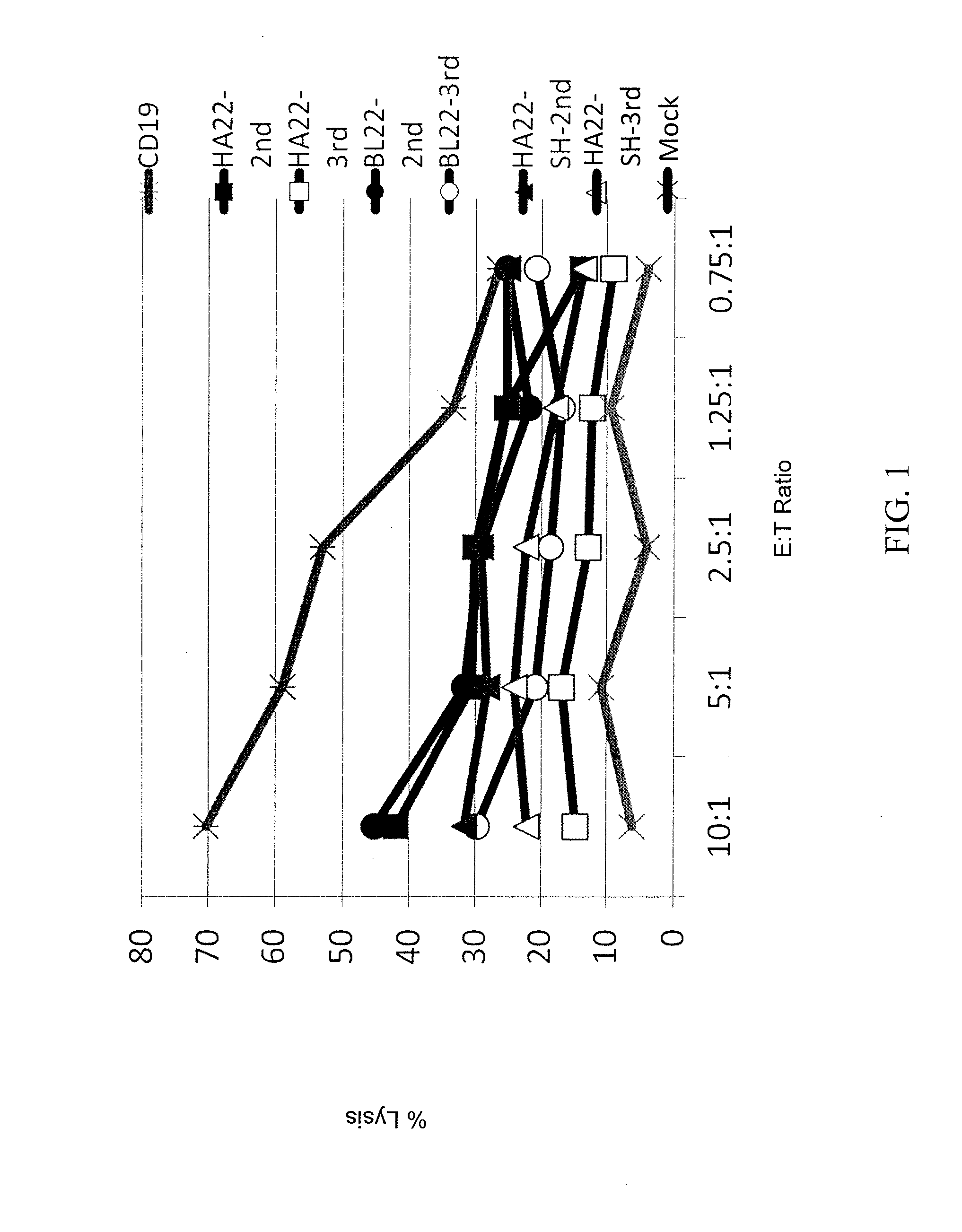
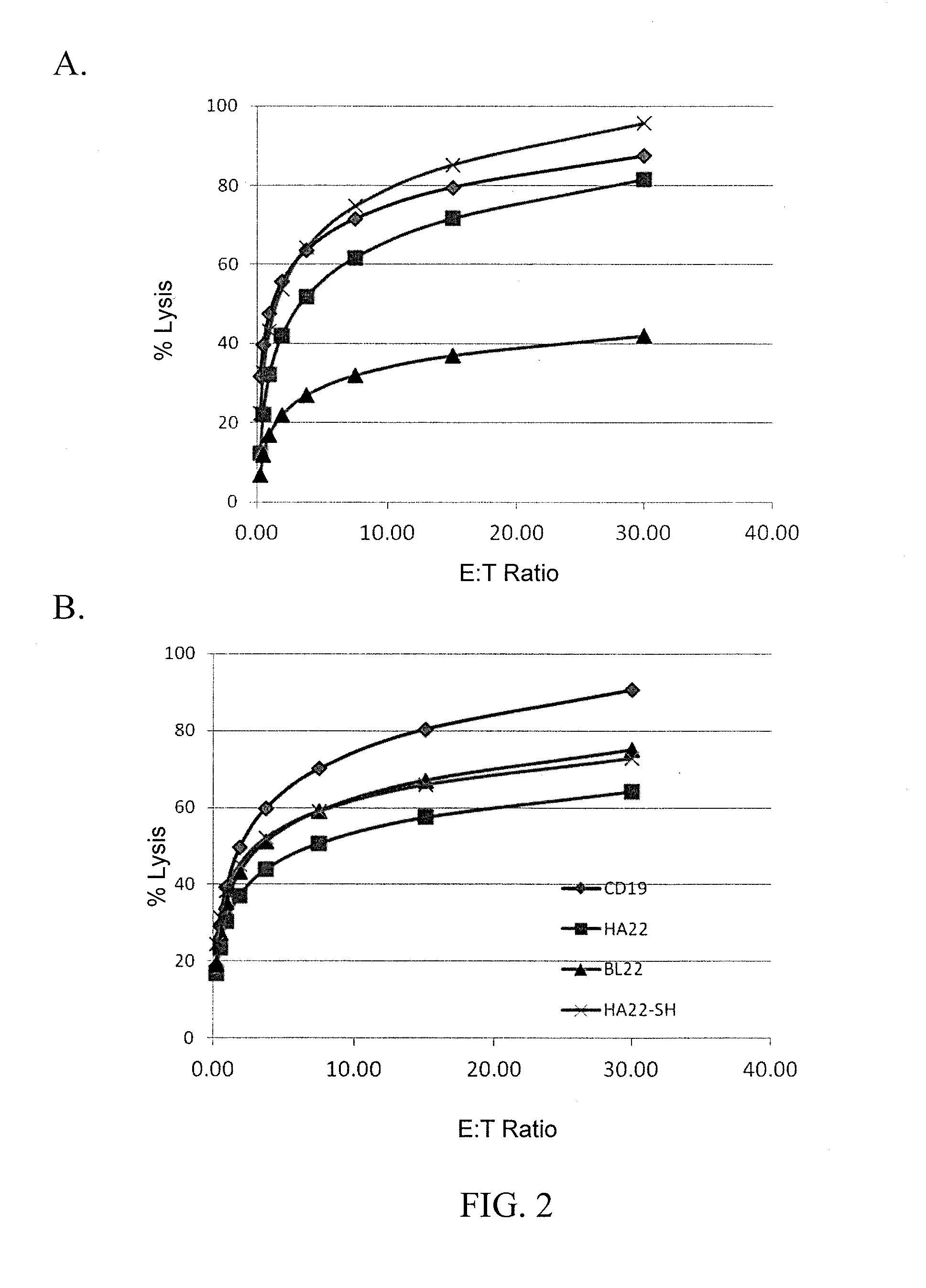
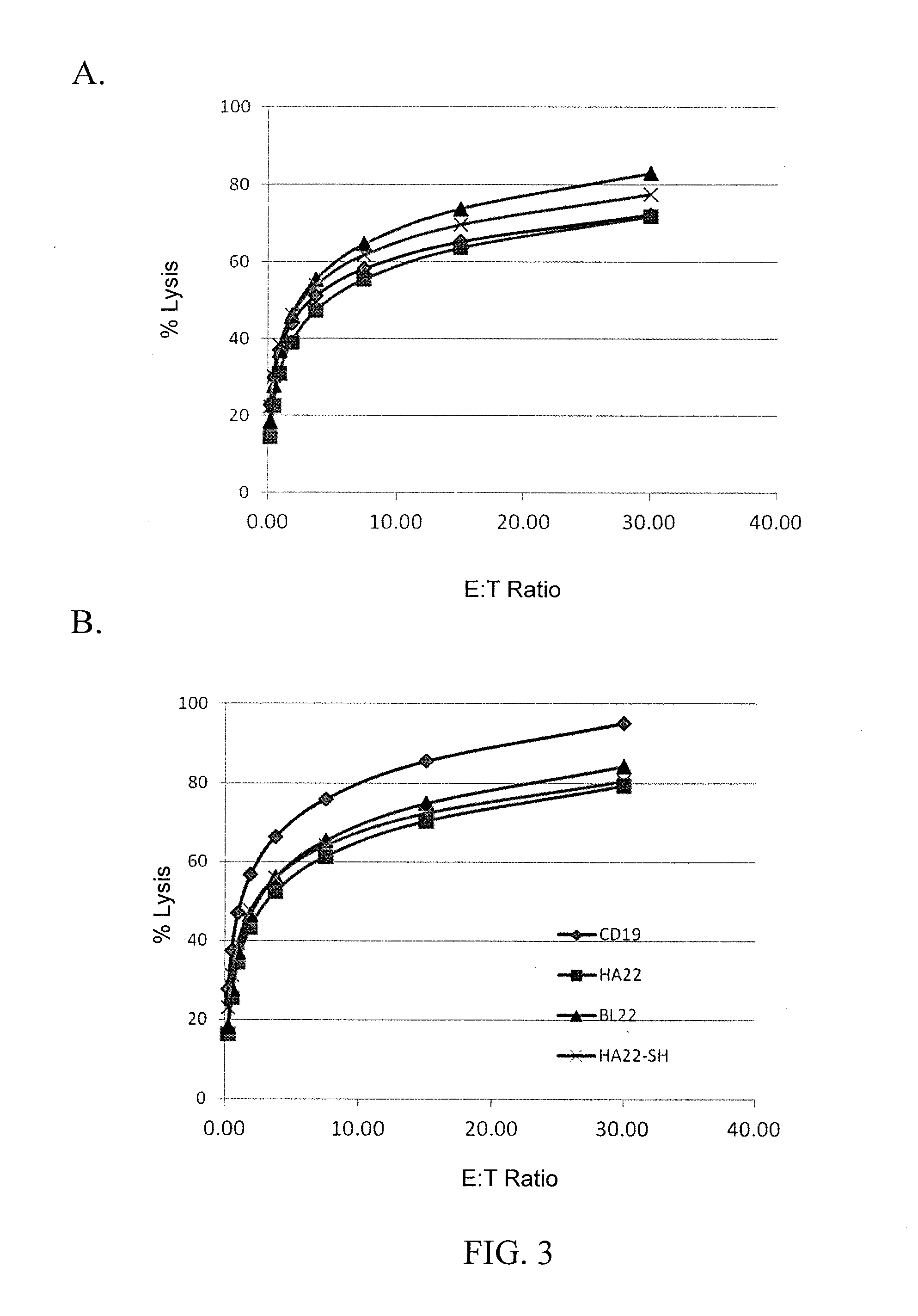
Anti-cd22 chimeric antigen receptors
The disclosure provides a chimeric antigen receptor (CAR) comprising a) an antigen binding domain of HA22, a transmembrane domain, and an intracellular T cell signaling domain; or b) an antigen binding domain of BL22, a transmembrane domain, and an intracellular T cell signaling domain comprising CD28 and / or CD137. Nucleic acids, recombinant expression vectors, host cells, populations of cells, antibodies, or antigen binding portions thereof, and pharmaceutical compositions relating to the CARs are disclosed. Methods of detecting the presence of cancer in a mammal and methods of treating or preventing cancer in a mammal are also disclosed.
Owner:UNITED STATES OF AMERICA
Modified polynucleotides encoding cd28 molecule
Owner:MODERNA THERAPEUTICS INC
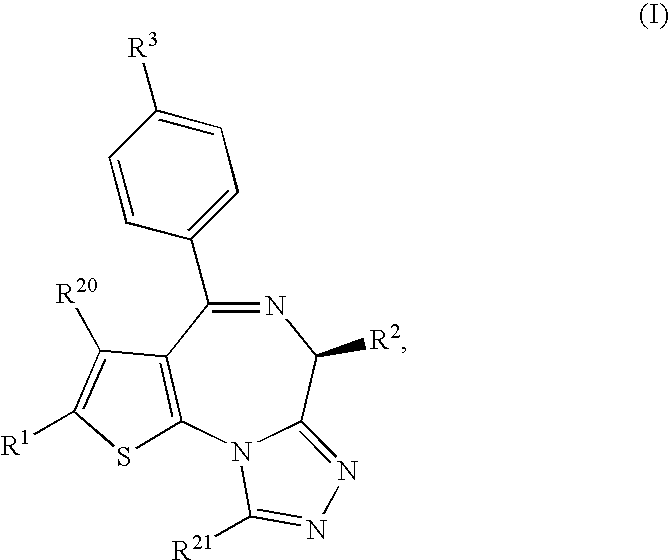
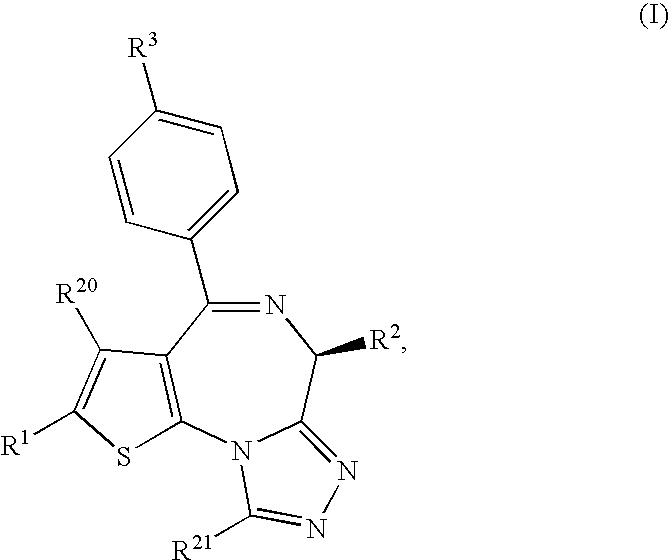
Thienotriazolodiazepine compound and medicinal use thereof
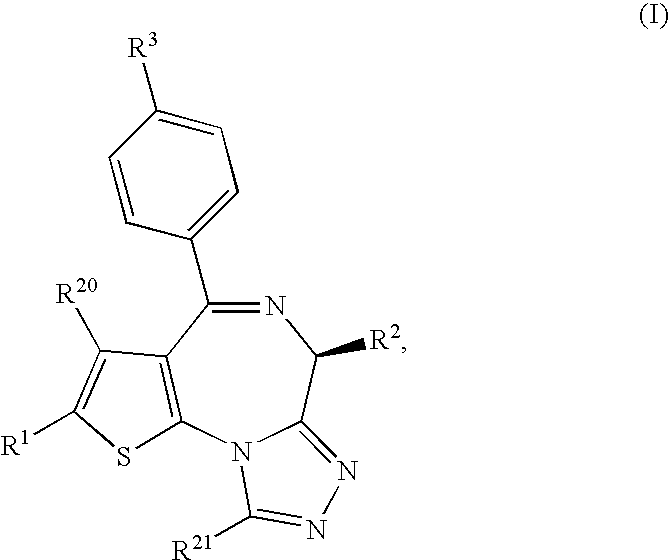
ActiveUS8044042B2Induce antigen specific immunological toleranceProphylaxis or treatmentBiocideSenses disorderDiseaseAutoimmune disease
A thienotriazolodiazepine compound of the following formula (I)a pharmaceutical agent containing the compound as an active ingredient, and a production intermediate and a production method of the thienotriazolodiazepine compound.Since this compound has an inhibitory action on costimulatory signal from CD28 on T cell, it is useful for the prophylaxis or suppression of rejection reaction in transplantation of organ or bone marrow and the like, and the prophylaxis or treatment of autoimmune diseases or allergic diseases.
Owner:MITSUBISHI TANABE PHARMA CORP
Chimeric antigen receptor and its use
InactiveCN103145849ASpeed up entryGood treatment effectGenetic material ingredientsAntiviralsLatent Membrane Protein-1Single-Chain Antibodies
The invention belongs to the biotechnical field of tumors, and discloses a preparation method of a chimeric antigen receptor and an application of the chimeric antigen receptor. The chimeric antigen receptor is formed through the structural series connection of a single chain antibody of a human anti-EB virus latent membrane protein 1, CH2CH3 of a human antibody IgG1, and intracellular signals of immune co-stimulating signal molecules CD28, CD134 and CD3zeta. The chimeric antigen receptor is used for modifying T lymphocytes, and the modified lymphocytes can be used for treating EB virus related tumors and preparing EB virus related tumor resisting medicines.
Owner:SINOBIOWAY CELL THERAPY CO LTD
Preparation method and application of autologous CAR (chimeric antigen receptor)-T cell
InactiveCN106755088ALittle side effectsNo MHC restrictionGenetically modified cellsMammal material medical ingredientsSide effectAntigen binding
The invention relates to a preparation method and application of an autologous CAR (chimeric antigen receptor)-T cell. An established CD28-CD137-CD19-CD3 full-length gene is guided into a T-cell of a patient by a CRISPR / Cas9 technology to prepare the CAR-T cell, and the CAR-T cell is subjected to expansion in vitro and then returns in the body of the patient to perform anti-tumor treatment. Compared with the traditional tumor treatment method, the method has the advantages that the method is cell targeted therapy and small in side effect; the gene modified T cell can stably express an antigen binding domain on the surface and identify a target antigen, and does not have MHC limit; and the tumor treatment effect is improved.
Owner:GUANGDONG PANGUARD CELL BIOLOGICAL TECH CO LTD
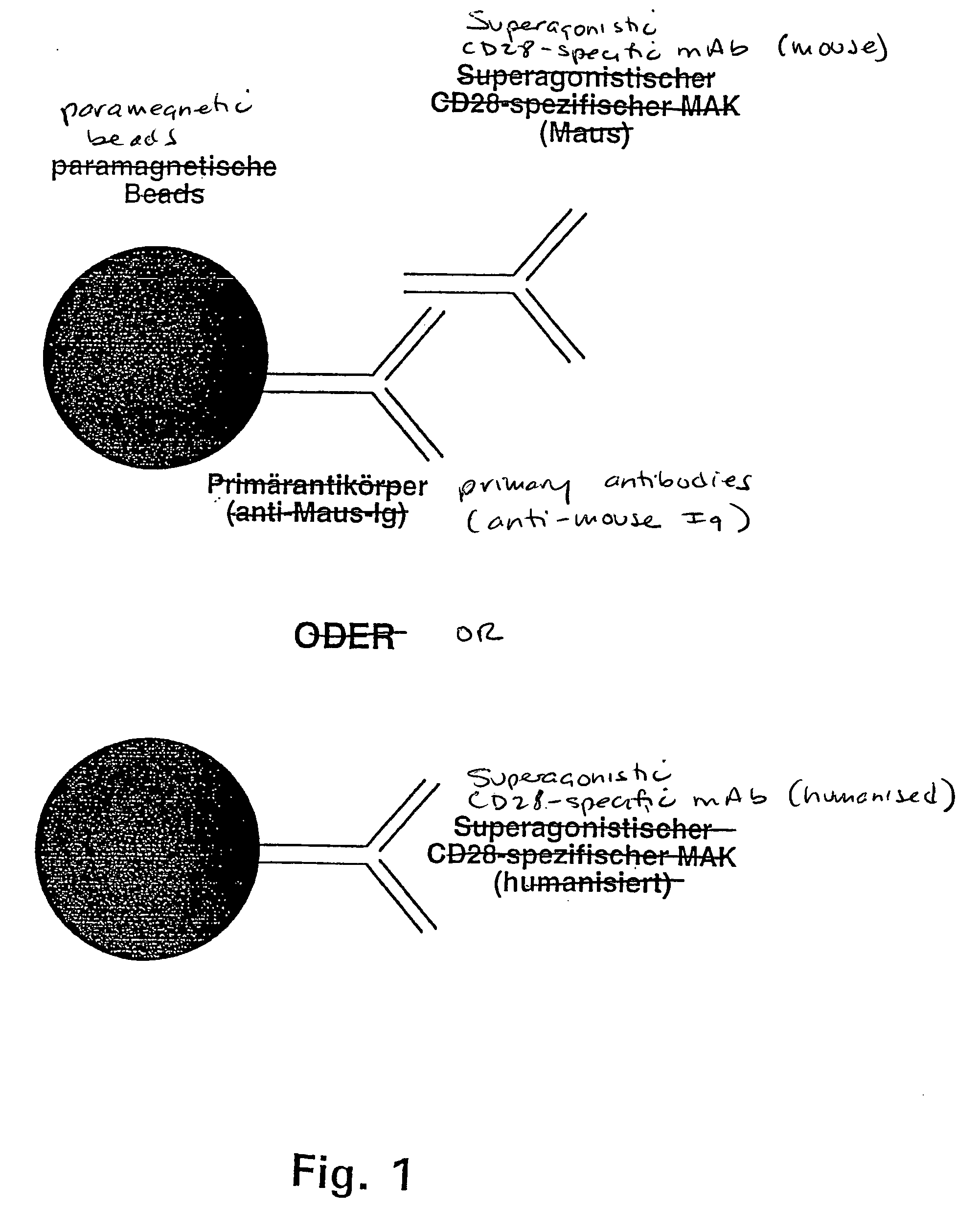
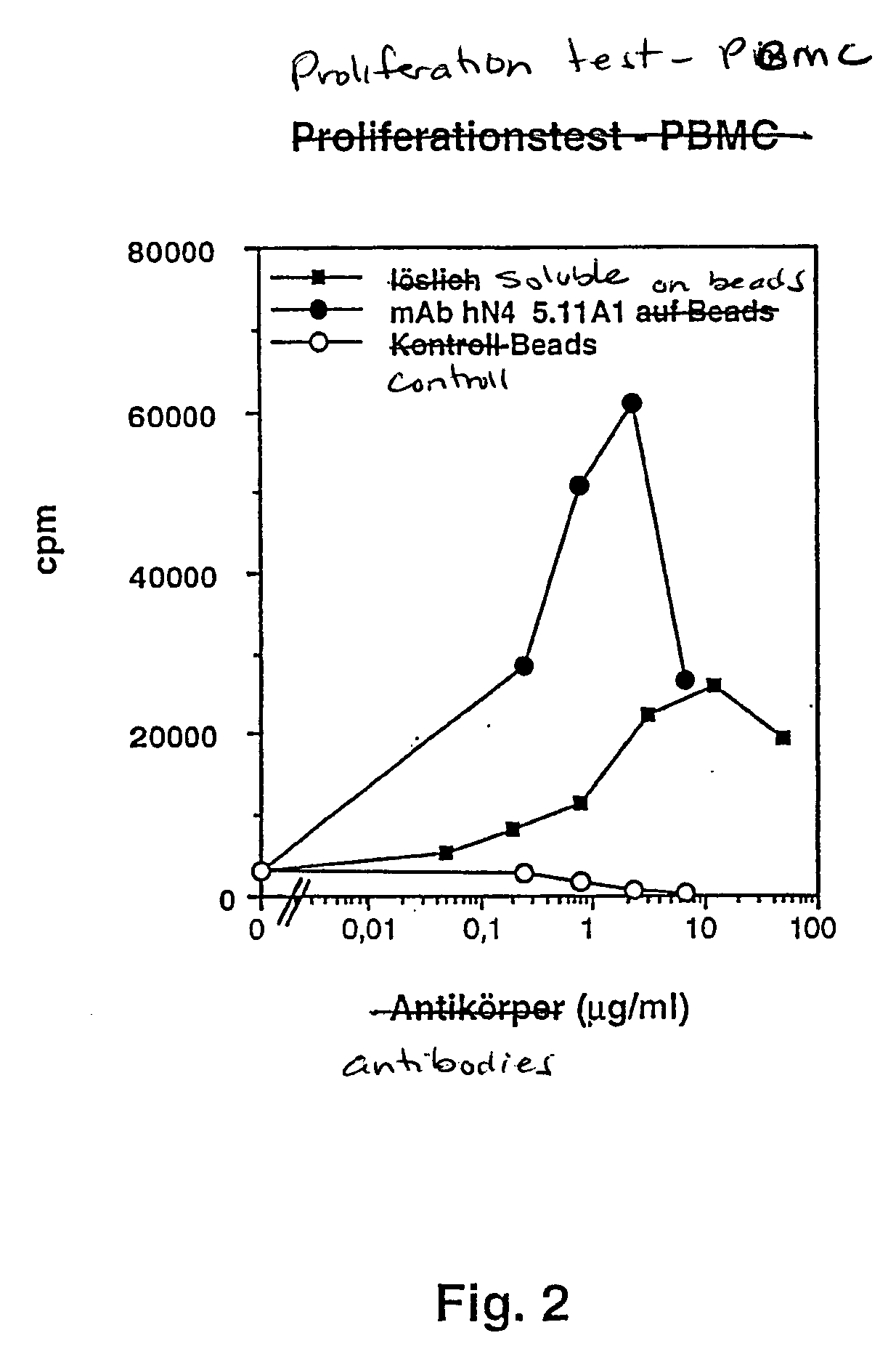
Microparticle with cd28-specific monoclonal antibodies
InactiveUS20060121021A1Reduce the amount requiredPowder deliveryImmunoglobulins against cell receptors/antigens/surface-determinantsMonoclonal antibodyMicroparticle
The invention relates to microparticles with a support structure and CD28-specific superagonistic monoclonal antibodies (mAbs) bonded to the support structure or a compound mimicking the above.
Owner:THERAMAB
Antibodies against PD-1 and uses therefor
This disclosure provides antibodies and antigen-binding fragments that can act as agonists and / or antagonists of PD-1 (Programmed Death 1), thereby modulating immune responses in general, and those mediated by TcR and CD28, in particular. The disclosed compositions and methods may be used for example, in treating autoimmune diseases, inflammatory disorders, allergies, transplant rejection, cancer, and other immune system disorders.
Owner:WYETH LLC +1
Molecule which binds cd80 and cd86
InactiveUS20070148162A1Prevent rejectionAvoid immune responseAnimal cellsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenT cell mediated immunity
The present invention relates to the identification of molecules, which are specific to CD80 and CD86 antigens. Also preferably, such antibodies are capable of inhibiting the binding of CD28 and CTLA4 to those receptors. Those molecules are able to inhibit T cell mediated immune reactions.
Owner:THERAVISION
Recombining single chained three specific antibodies of anti CCA, anti CD 3, anti CD 28 through genetic engineering
InactiveCN1563092AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntiendomysial antibodiesSingle-Chain Antibodies
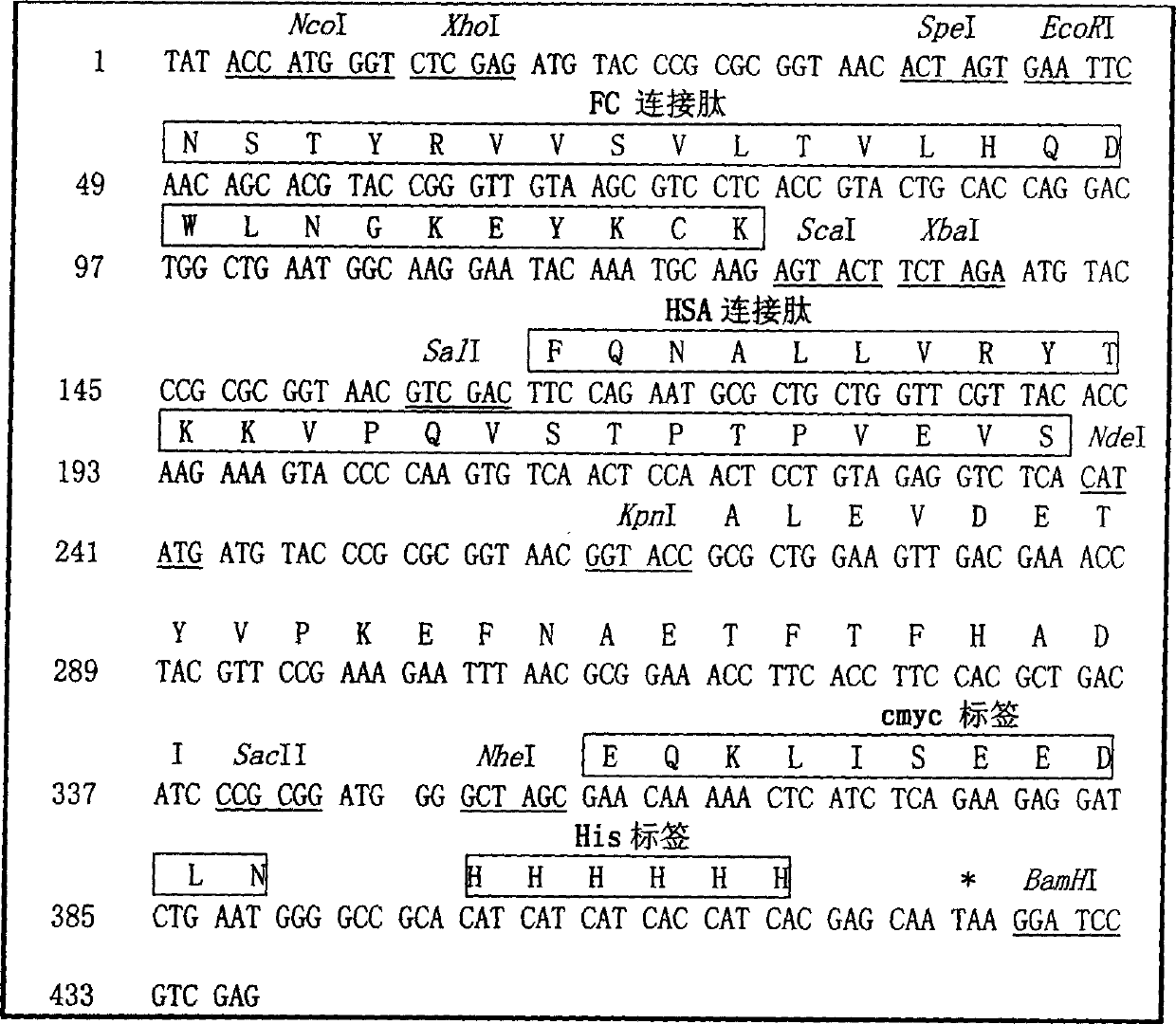
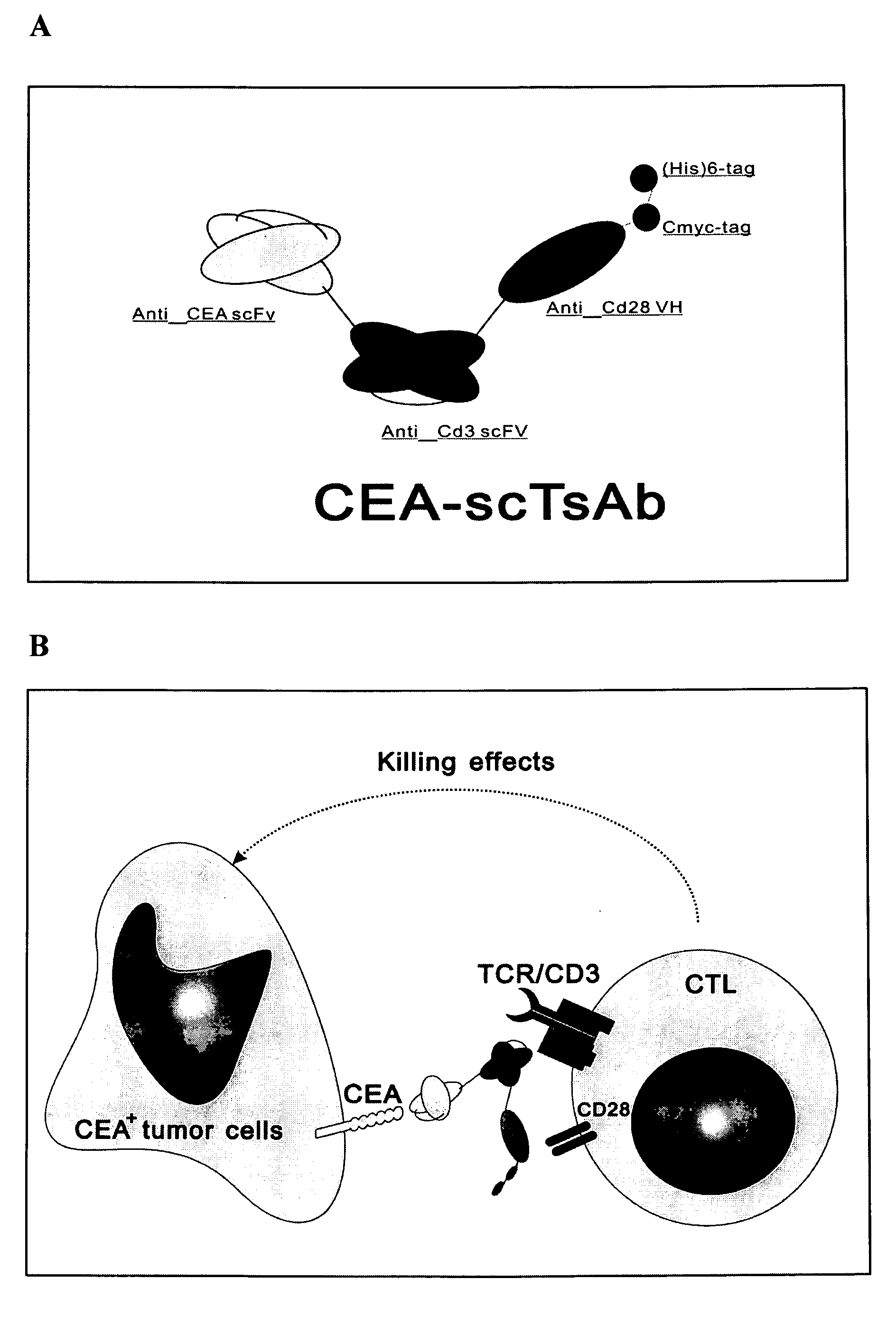
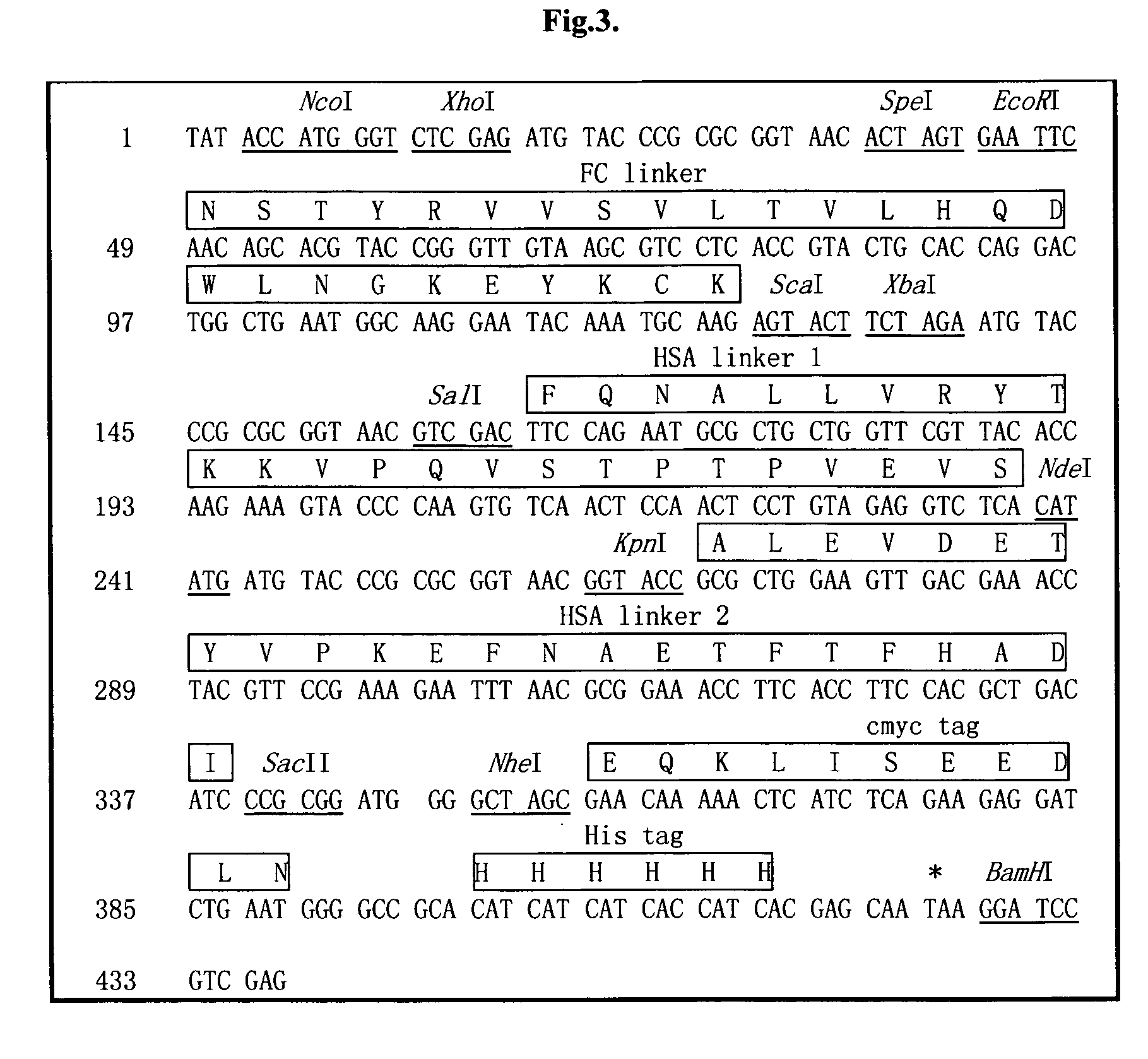
The invention relates to a recombinant single chain tri-specific antibody, and it is characterized by that it is made by successively connecting antibody of antineoplastic related antigen, FC onnecting peptide, antihuman CD3 antibody, HSA connecting peptide and antihuman CD28 antibody. More specifically, said invention relates to a recombinant single chain tri-specific antibody; CEA-TsAb of anti-CEA, anti-CD3 and anti-CD28. Said antibody is made up by using three antibody fragments (unti-CEA single chain antibody, anti-CD3 single chain antibody and anti-CD28 single chain antibody), utilizing two connecting peptides (FC connecting peptide and HSA connecting peptide) and series-connecting them together, and on its C end C-myc tag and (His)6-tag are added. Said invention also relates to a method for constructing expressing and purifying said antibody, including exonic DNA sequence of said antibody, expression vector and host cell containing said vector.
Owner:弘业新创抗体技术股份有限公司
Gene Engineering Recombinant Anti-CEA, Anti-CD3, And Anti-CD28 Single-Chain Tri-Specific Antibody
InactiveUS20090117108A1Increase production costHigh expressionBacteriaSugar derivativesAntibody fragmentsSingle-domain antibody
The invention is related to a recombinant single-chain tri-specific antibody made from anti-Tumor Associated Antigen (TAA) antibody, FC interlinker, anti-CD3 antibody, HSA interlinker and anti-CD28 antibody in turn. Particularly, the invention relates to an anti-CEA, anti-CD3, anti-CD28 recombinant single-chain tri-specific antibody, CEA-scTsAb, which was constructed with three tandem antibody fragments (anti-CEA scFv, anti-CD3 scFv and anti-CD28 single-domain antibody) linked by two interlinkers (FC interlinker, HSA interlinker), and could be appended by C myc tag or histidine tag ((His)6-tag) at the C terminal. It also concerns a method for construction, expression and purification of the antibody. It also offers the encoded DNA sequence of the antibody, expression vectors and host cells for the vectors.
Owner:WANG XIANGBIN +5
Thienotriazolodiazepine compound and medicinal use thereof
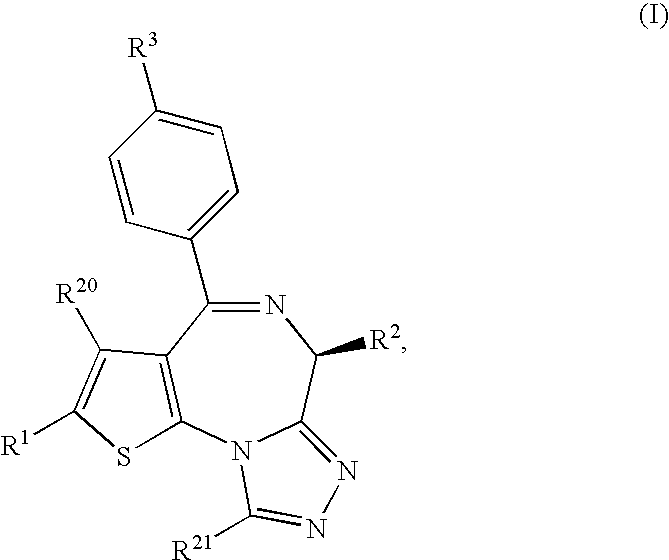
ActiveUS20100041643A1Induce antigen specific immunological toleranceAvoid signalingBiocideSenses disorderImmunologic disordersAutoimmune disease
[Solving means] A thienotriazolodiazepine compound of the following formula (I)a pharmaceutical agent containing the compound as an active ingredient, and a production intermediate and a production method of the thienotriazolodiazepine compound.[Effect]Since this compound has an inhibitory action on costimulatory signal from CD28 on T cell, it is useful for the prophylaxis or suppression of rejection reaction in transplantation of organ or bone marrow and the like, and the prophylaxis or treatment of autoimmune diseases or allergic diseases.
Owner:MITSUBISHI TANABE PHARMA CORP
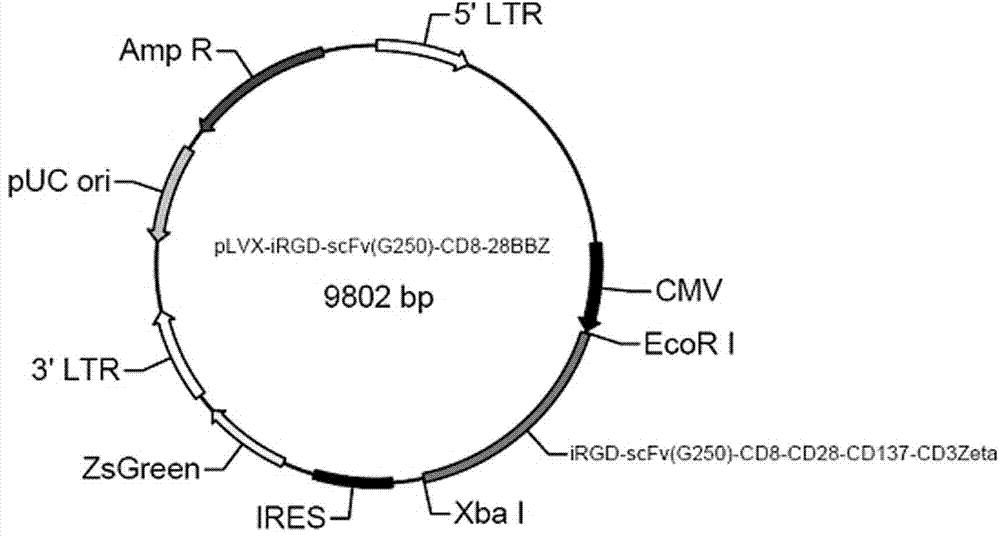
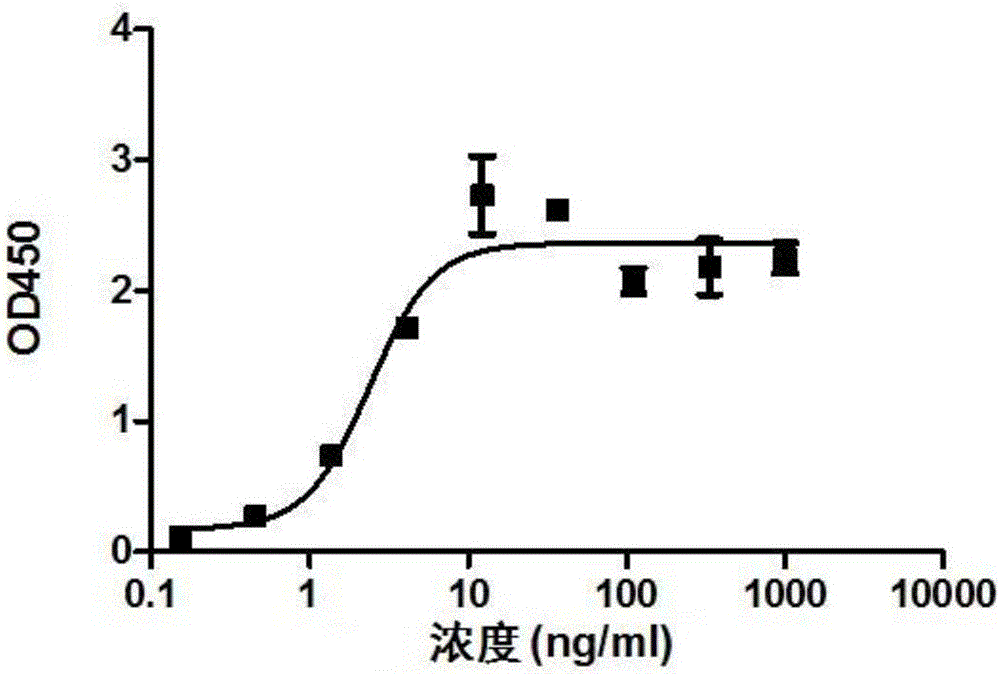
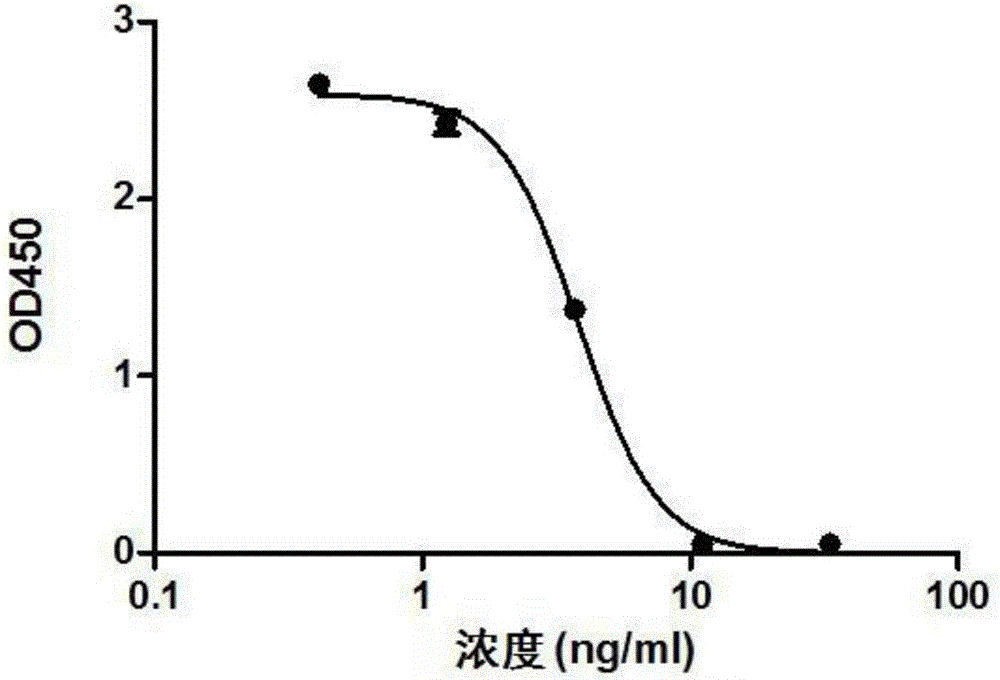
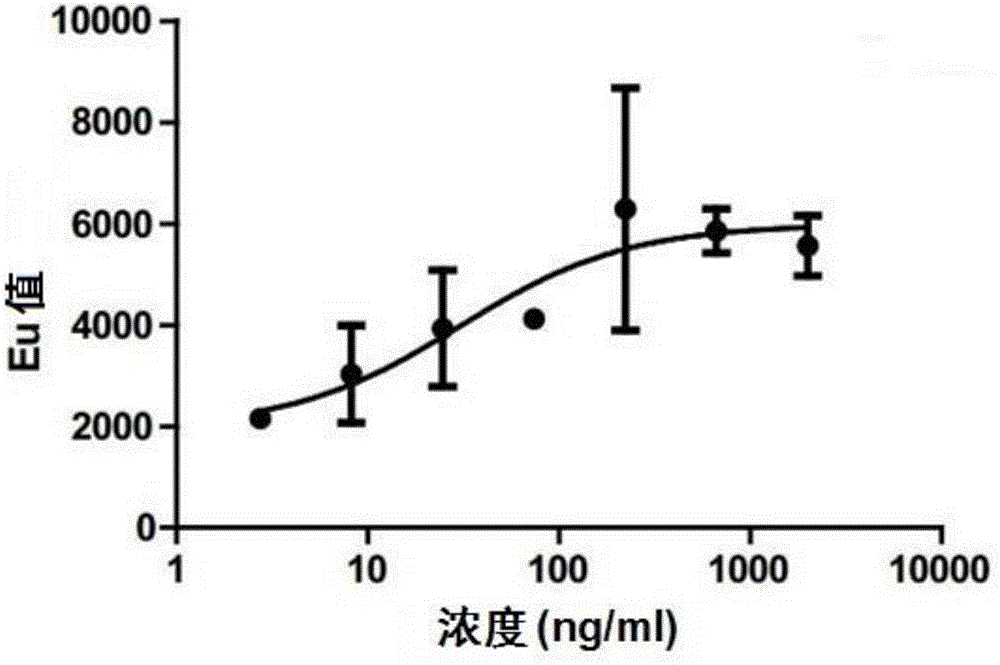
Chimeric antigen receptor iRGD-scFv (G250)-CD8-CD28-CD137-CD3zeta and application thereof
InactiveCN102775500APeptide/protein ingredientsPharmaceutical non-active ingredientsAntigen receptorSingle-Chain Antibodies
The invention belongs to the technical field of biology and new medicines and discloses a chimeric antigen receptor (CAR) iRGD-scFv (G250)-CD8-CD28-CD137-CD3zeta and application thereof. The chimeric antigen receptor is formed by series connection of tumor penetrating peptide iRGD, a single-chain antibody of a humanized anti-human renal carcinoma antigen G250, a hinge region and a transmembrane region of CD8, and intracellular signal structural domains of CD28, CD137 and CD3zeta. The chimeric antigen receptor can be used for modifying T lymphocytes, and the modified T lymphocytes can be used for renal carcinoma treatment.
Owner:郑骏年
Anti-human PD-1 humanized monoclonal antibody and application thereof
ActiveCN106008714AStrong specificityHigh affinityAntibacterial agentsAntibody mimetics/scaffoldsMonoclonal antibodyPD-L1
The invention relates to the field of biological medicine and particularly relates to an anti-human PD-1 humanized monoclonal antibody and application thereof. By virtue of sieving, the anti-human PD-1 humanized monoclonal antibody with favorable specificity and relatively high compatibility and stability is obtained, can be specifically combined with human PD-1, but is not combined with other members of the CD28 family, is capable of blocking combination of PD-L1 and PD-1 and partially restoring functions of T cells, and has an obvious inhibiting effect on tumor growth.
Owner:REYOUNG SUZHOU BIOLOGY SCI & TECH CO LTD
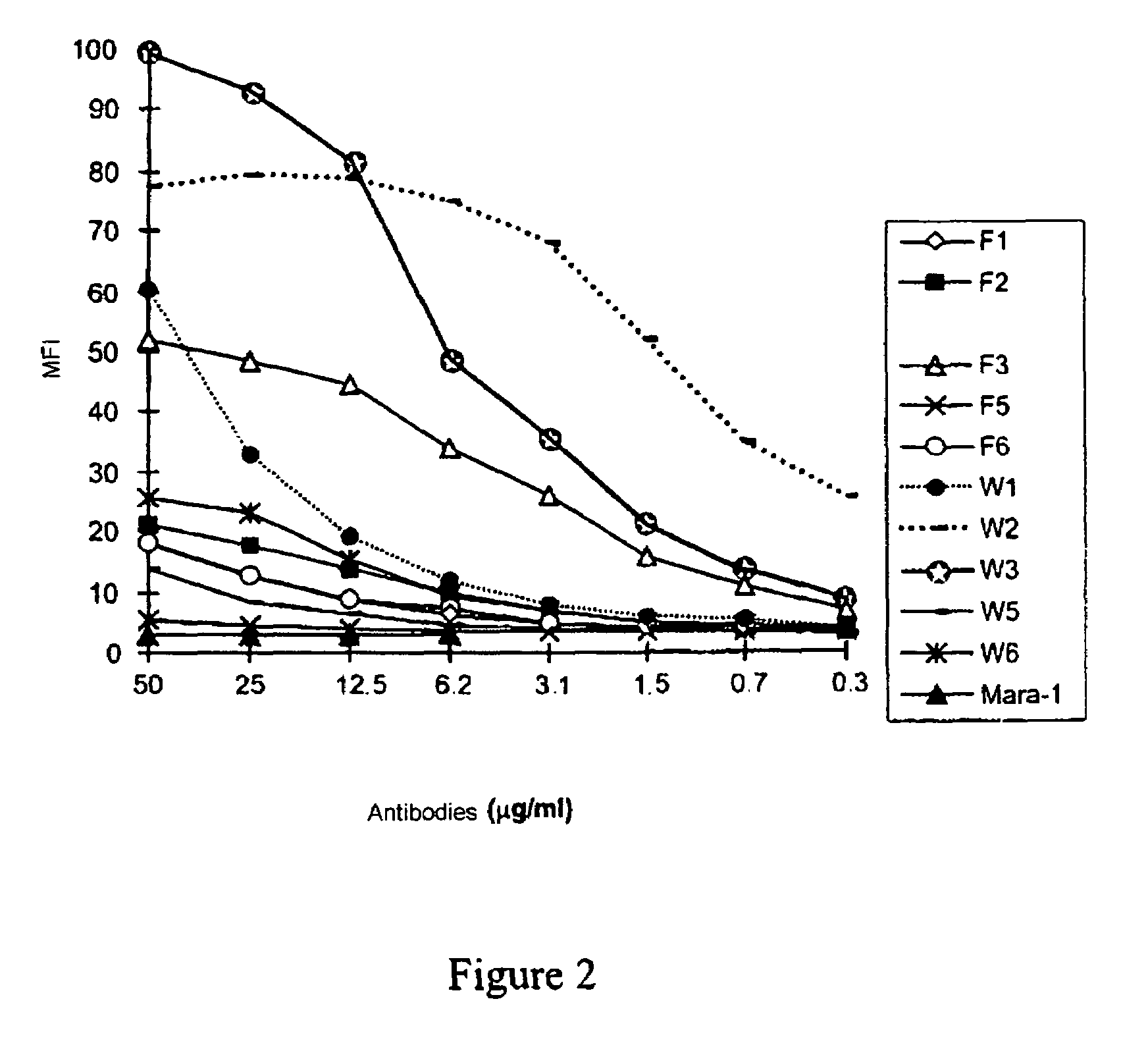
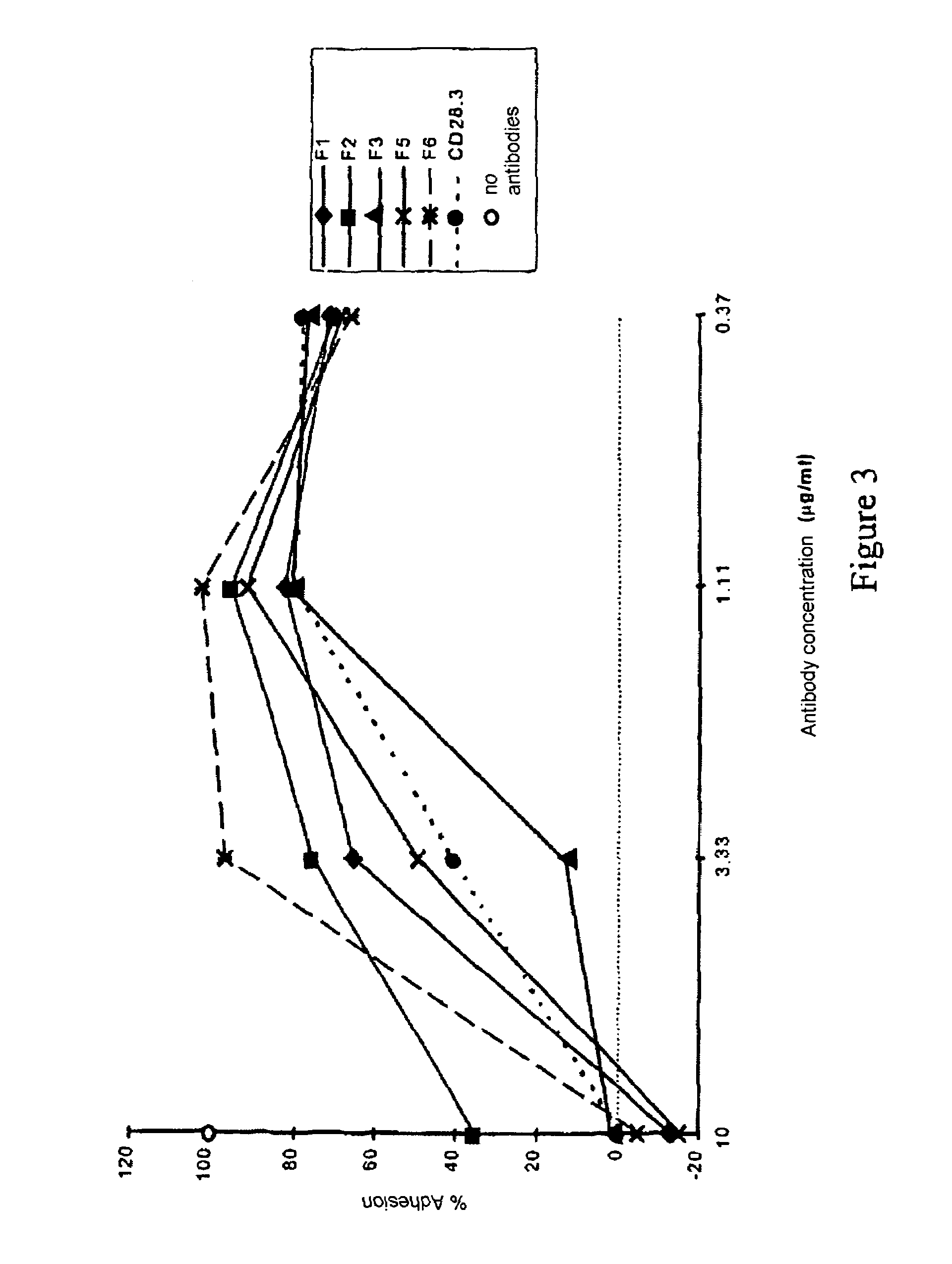
Anti-CD28 antibody
ActiveUS7723482B2Curb clinical symptomPrevent T lymphocyte activationPeptide/protein ingredientsAntipyreticAntibody fragmentsLymphocyte
An antibody or antibody fragment directed against the CD28 receptor which blocks the interaction between B7-1 or B7-2 and CD28. Methods for blocking activation via CD28, including CD28-dependent lymphocyte activation.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM)
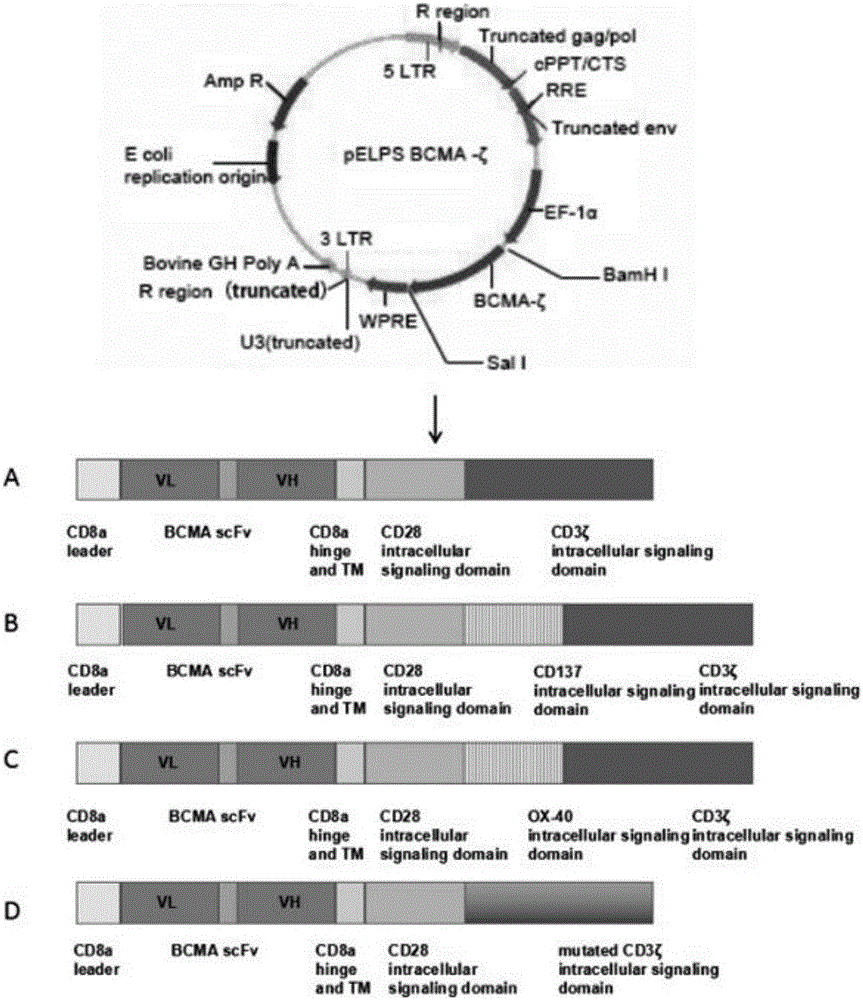
BCMA-based (B cell maturation antigen-based) chimeric antigen receptor and preparation method and application thereof
InactiveCN105837693AImprove anti-apoptotic abilityImprove bindingAntibody mimetics/scaffoldsMammal material medical ingredientsTumor targetAntigen
The invention provides a BCMA-based (B cell maturation antigen-based) chimeric antigen receptor, comprising following cis-form cascade domains: CD8a leader region, scFv fragment BCMA scFv of anti-BCMA antibody, CD8a hinge region and transmembrane region, CD28 intracellular signal domain and CD3 Zeta intracellular signal domain; the CD3 Zeta intracellular signal domain is wild CD3 Zetaintracellular signal domain or mutant CD3 Zeta mut intracellular signal domain; the BCMA-based chimeric antigen receptor can enhance anti-apoptotic capability of T-cells and enhance CAR T-cell and antigen bonding and signal conduction; T-cells with the BCMA-based chimeric antigen receptor show good tumor targeting performance in in-vivo experiments, tumors diminish significantly after two weeks of dosage, and the T-cells have excellent therapeutic effect in vivo.
Owner:李斯文 +1
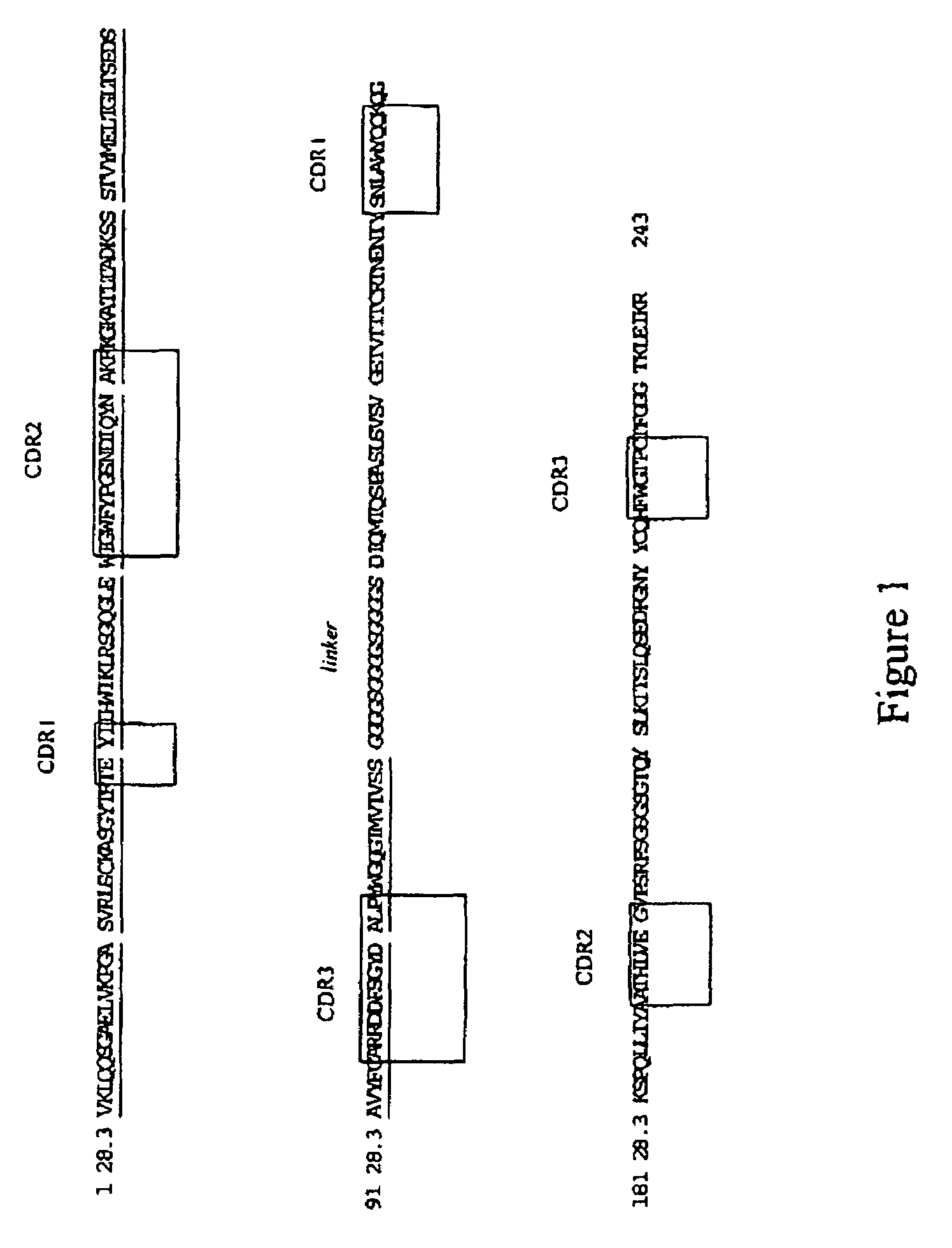
Nucleic acids encoding superagonistic anti-CD28 antibodies
The present invention relates to one or more nucleic acid(s) encoding a binding molecule specifically binding to a human CD28 molecule, comprising(a) a nucleic acid sequence encoding a VH region and a nucleic acid sequence encoding a VL region comprising CDRs in a human immunoglobulin framework, wherein(i) the CDRs of the VH region (CDR-H) comprise the amino acid sequences of SEQ ID NOS: 2 or 18 (CDR-H3), 4 or 20 (CDR-H2) and 6 or 22 (CDR-H1) or are encoded by the nucleic acid sequences of SEQ ID NOS: 1 or 17 (CDR-H3), 3 or 19 (CDR-H2) and 5 or 21 (CDR-H1); and(ii) the CDRs of the VL region (CDR-L) comprise the amino acid sequences of SEQ ID NOS: 8 or 24 (CDR-L3), 10 or 26 (CDR-L2) and 12 or 28 (CDR-L1) or are encoded by the nucleic acid sequences of SEQ ID NOS: 7 or 23 (CDR-L3), 9 or 25 (CDR-L2) and 11 or 27 (CDR-L1); and(b) a nucleic acid sequence encoding the constant region of a human IgG1 or IgG4 antibody.
Owner:THERAMAB
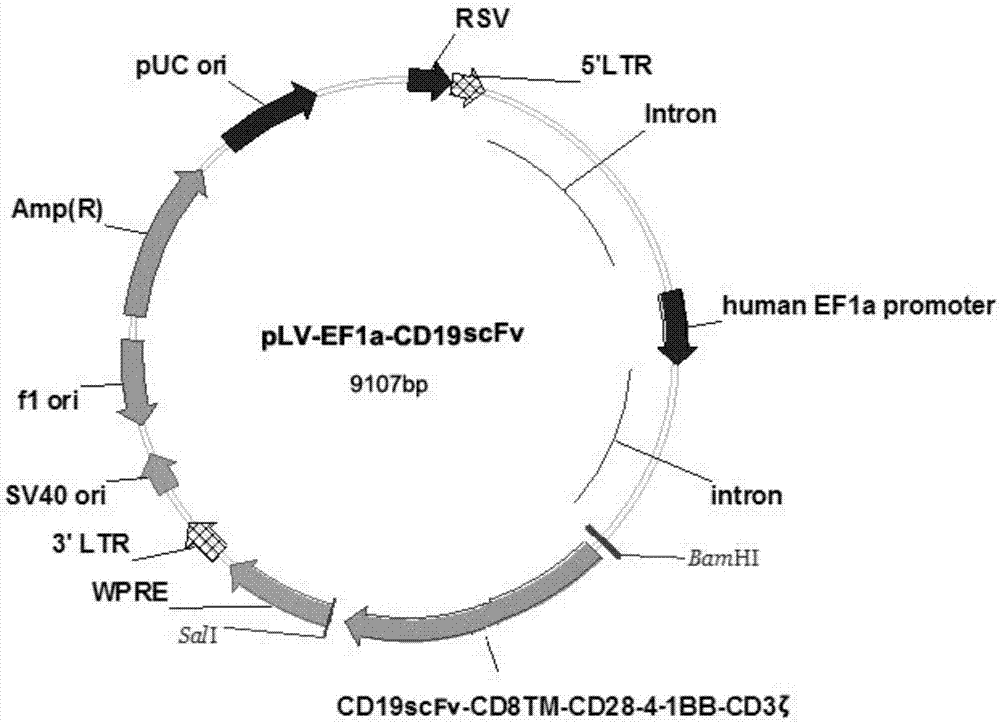
CD19 targeted CAR (chimeric antigen receptor)-T cell, preparation method and application
InactiveCN107287164AIncrease lethalityHigh transduction efficiencyGenetically modified cellsMammal material medical ingredientsCAR T-cell therapySingle-Chain Antibodies
The invention provides a CD19 targeted CAR (chimeric antigen receptor)-T cell, a preparation method and an application and relates to the field of immune cells. The activation capacity of T cells in CAR-T cell therapy can be improved, the problem of insufficient transfection efficiency can be solved, and the CAR-T cell has a high killing capacity for CD19. The CD19 targeted CAR-T cell expresses a CD19 targeted CAR gene on surface, and the CD19 targeted CAR gene is formed by connecting a single-chain antibody CD19ScFv of CD19, a hinge region and a transmembrane region of CD8, an intracellular signal structure of CD28, an intracellular signal structure of 4-1BB and an intracellular signal structural domain of CD3zeta in series, and the base sequence of the CD19 targeted CAR gene is shown as SEQ ID NO:2.
Owner:青岛见康华美医学检验有限公司
Amplifying, freezing and storing and recovering method of activated lymphocyte with CD3+CD8+as major
InactiveCN102839153ASolve the problem of multiple blood collectionHigh purityDead animal preservationBlood/immune system cellsPatient needT lymphocyte
The invention discloses culturing, freezing and recovering methods of an activated lymphocyte with CD3+CD8+as major, which can solve problems that a patient needs carrying out blood sampling for many times caused by continuously utilizing the activated lymphocyte with CD3+CD8+as major. The method comprises the following steps of: (1) contacting the extracted lymphocyte of the peripheral blood with IL-2, IL-15, an anti-CD3 antibody and an anti-CD28 antibody, so as to amplify the activated lymphocyte with CD3+CD8+as major; (2) freezing and storing the activated lymphocyte; and (3) recovering the activated lymphocyte. The activated lymphocyte cultured via the method disclosed by the invention has clear components, and comprises few CD4+CD25+Treg cells and more CD8+T lymphocyte; feedback time and frequency of the activated lymphocyte can be adjusted according to other treatments for a patient, such as a radiotherapy or a chemotherapy, so that diseases, such as tumor, infectious diseases and immunodeficiency can be treated well.
Owner:JINAN TAISHENG BIOLOGICAL TECH CO LTD
Universal donor chimeric antigen receptor cells
InactiveUS20160237407A1Good curative effectImprove localizationPolypeptide with localisation/targeting motifImmunoglobulin superfamilyProgenitorCord blood stem cell
Disclosed are allogeneic cells useful for the treatment of cancer in a universal donor, off the shelf, manner. In one embodiment of the invention cord blood derived T cell progenitors are matured with anti-CD3 and anti-CD28, interleukin-7 and transfected with a construct encoding a chimeric antigen receptor (CAR) targeting a tumor antigen or a tumor endothelial associated antigen on the antigen binding domain. The intracellular domain containing CD3 zeta chain and at least one shRNA domain encoding a transcript which generates at least one siRNA capable of inhibiting expression of HLA I and / or HLA II. In another embodiment mesenchymal stem cells are transfected with CAR to enhance migration into tumors and induce tumor death, reduction of inflammation, or immune sensitization. In another embodiment universal donor CAR-MSC are disclosed.
Owner:BATU BIOLOGICS
Targeting chimeric antigen receptor modified immune cell as well as preparation method and application thereof
InactiveCN105906720ABroaden the range of targetsAccurate targetAntibody mimetics/scaffoldsMammal material medical ingredientsSequence signalHinge region
Owner:WUHAN HAMILTON BIOTECH
Genetically engineered lymphocyte targeting Human EGFR (Epidermal Growth Factor Receptor), preparation method and application of genetically engineered lymphocyte
ActiveCN103113470AGood effectInhibit tumor formationGenetic material ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsSingle-Chain AntibodiesTumor antigen
The invention relates to the field of genetic engineering, and in particular relates to an anti-EGFR ((Epidermal Growth Factor Receptor) chimeric antigen receptor, and a lymphocyte for expressing the antigen receptor. The technical solution of the invention provides a new effective selection for the anti-tumor technical field. The technical scheme is that a single-chain antibody targeting the EGFR is provided. The single-chain antibody is further designed to be constructed into the chimeric antigen receptor by means of a genetic engineering technology, and the structure of the gained chimeric antigen receptor is anti-EGFR (scFv)-IgG2 (Fc)-CD28-CD3 Zeta. According to the genetically engineered lymphocyte targeting the human EGFR, the chimeric antigen receptor is transfected into the lymphocyte by the nucleofaction technology, and thus the nonspecific lymphocyte can be endowed with the capacity of specifically distinguishing the EGFR tumor antigen and targeting and killing the EGFR over-expression tumor cells. According to the technical scheme, the adoptive cell therapy and the genetic therapy are organically combined, so that remarkable effect of killing the tumor is gained, and a novel effective selection is provided for the field.
Owner:WEST VAC BIOPHARMA CO LTD
CTLA4-Cy4 fusion proteins
CTLA4-immunoglobulin fusion proteins having modified immunoglobulin constant region-mediated effector functions, and nucleic acids encoding the fusion proteins, are described. The CTLA4-immunoglobulin fusion proteins comprise two components: a first peptide having a CTLA4 activity and a second peptide comprising an immunoglobulin constant region which is modified to reduce at least one constant region-mediated biological effector function relative to a CTLA4-IgG1 fusion protein. The nucleic acids of the invention can be integrated into various expression vectors, which in turn can direct the synthesis of the corresponding proteins in a variety of hosts, particularly eukaryotic cells. The CTLA4-immunoglobulin fusion proteins described herein can be administered to a subject to inhibit an interaction between a CTLA4 ligand (e.g., B7-1 and / or B7-2) on an antigen presenting cell and a receptor for the CTLA4 ligand (e.g., CD28 and / or CTLA4) on the surface of T cells to thereby suppress an immune response in the subject, for example to inhibit transplantation rejection, graft versus host disease or autoimmune responses.
Owner:REPLIGEN CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com