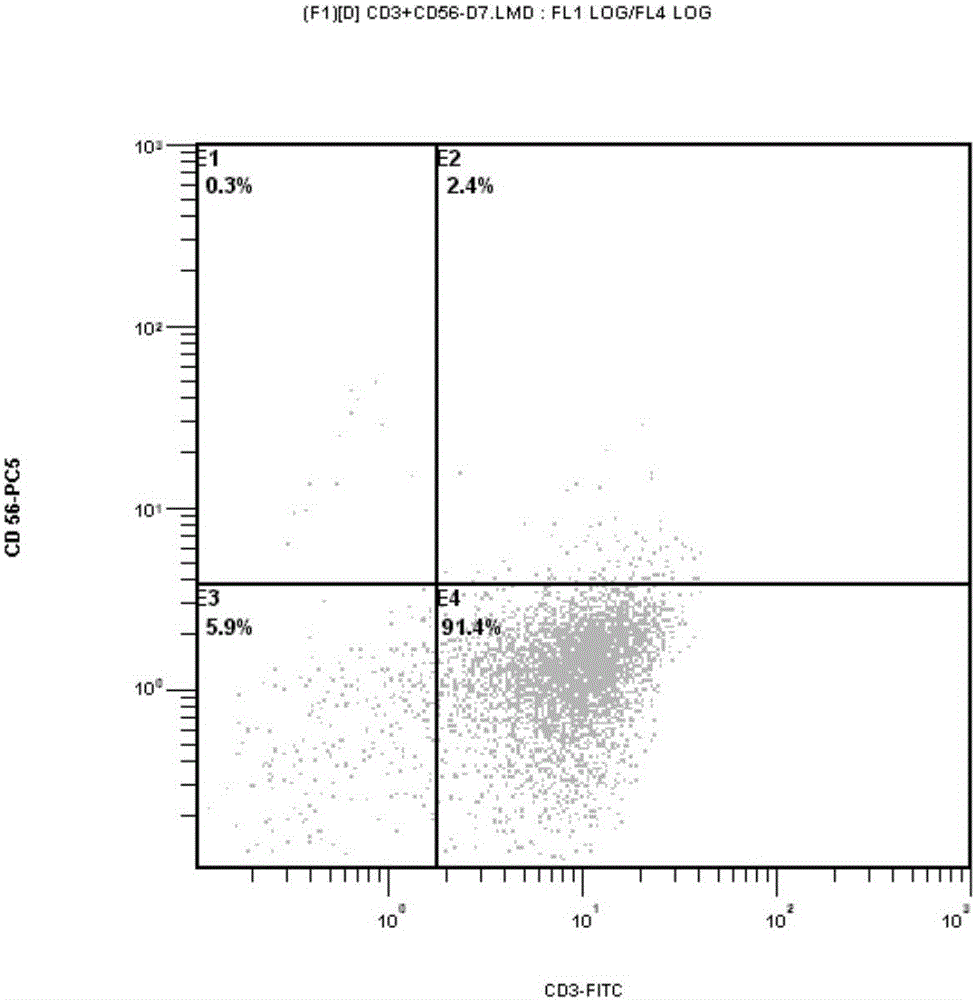
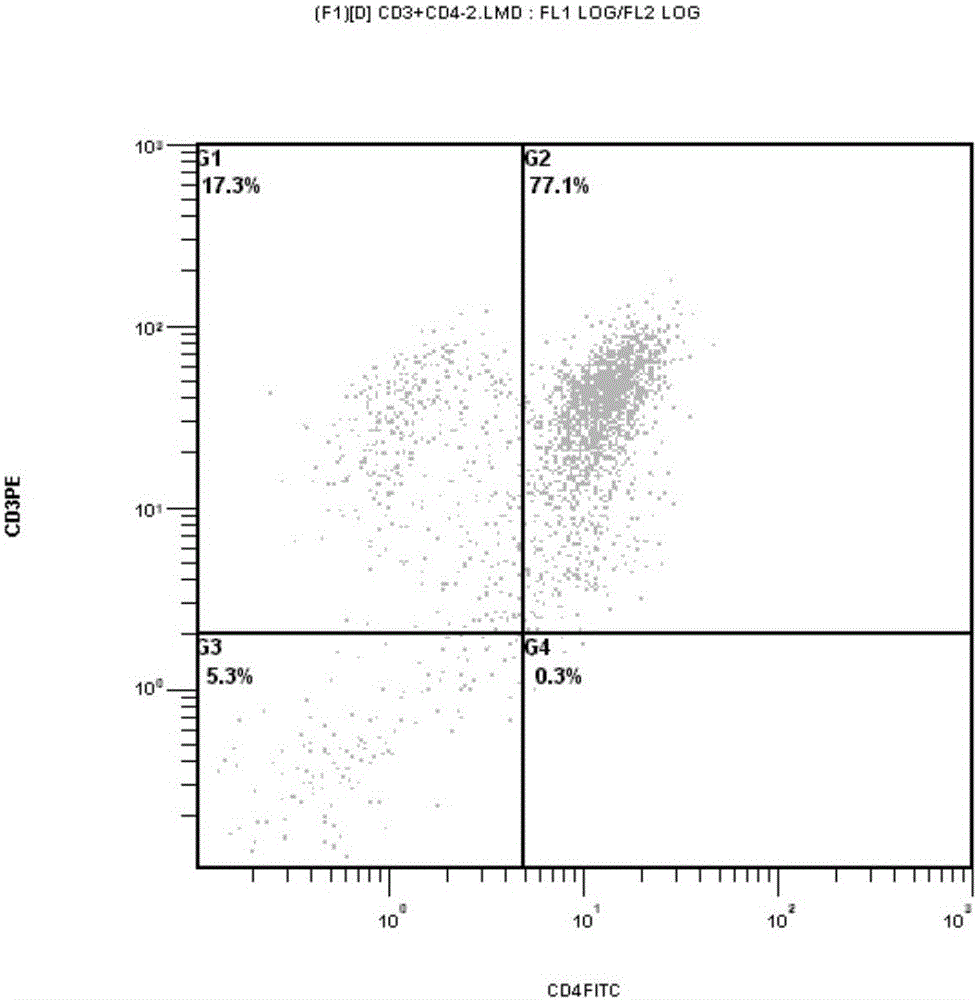
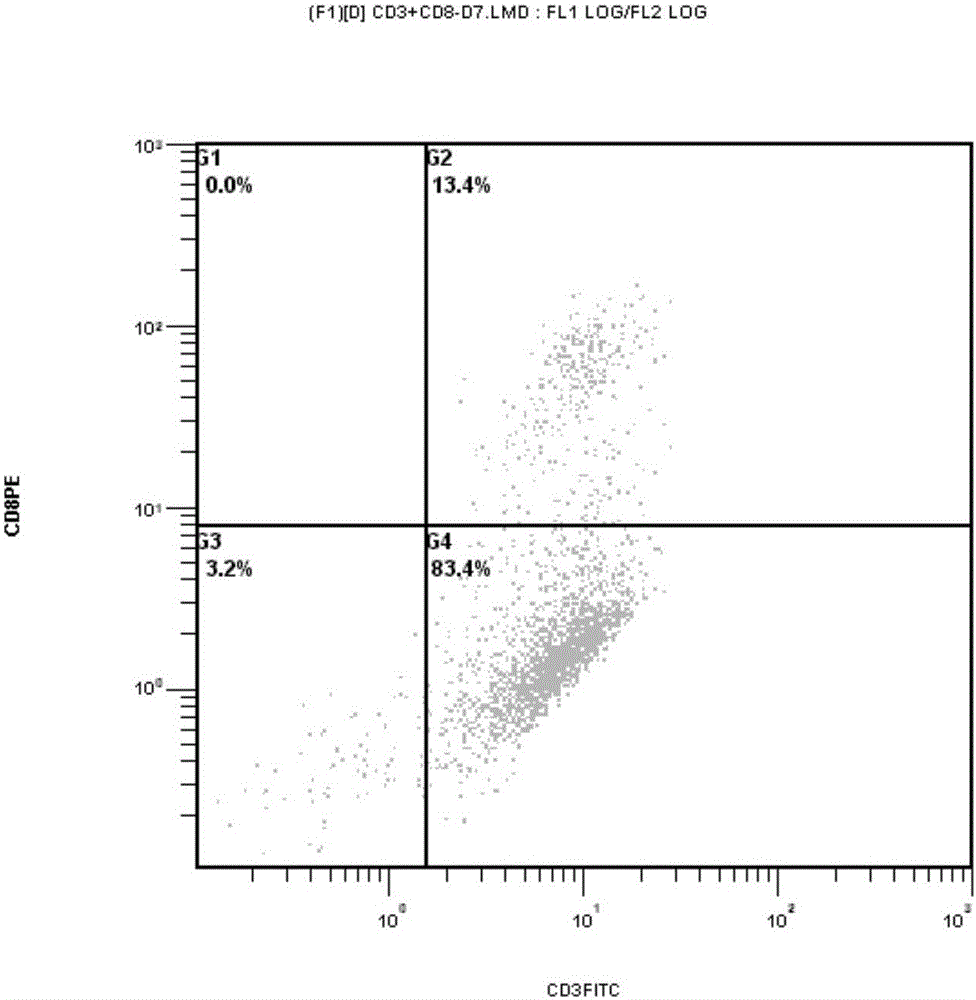
Targeting chimeric antigen receptor modified immune cell as well as preparation method and application thereof
A technology of chimeric antigen receptors and immune cells, which is applied to genetically modified cells, cells modified by introducing foreign genetic material, and targeted specific cell fusion. High cost and other issues, to achieve the effect of expanding the target range, lasting tumor killing effect, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1 Preparation of T cells
[0074] (1) Take human venous blood in a vacuum tube containing heparin. Mononuclear cells (PBMCs) were obtained by using lymphocyte separation medium and density gradient centrifugation.
[0075] (2) PBMCs were washed three times, and the final concentration of cells was adjusted to 2×10 by T cell culture medium (X-VIVO 15 serum-free medium) containing 5% autologous serum. 6 cell / mL; the cells were inoculated in a 75 cm cell culture flask coated with a final concentration of 5 ug / mL LCD3 monoclonal antibody and a final concentration of 10 ug / mL of retronectin (purchased from TAKORA). Add recombinant human protein interferon-γ with a final concentration of 1000U / mL and recombinant human interleukin-2 at a final concentration of 1000U / mL to the culture medium, and store at 37°C and saturated humidity of 5% CO 2 cultured in an incubator.
[0076] (3) On the fourth day, 100 ml of T cell culture medium containing 0.6% autologous serum by...
Embodiment 2
[0077] Example 2: Construction of chimeric antigen receptor (pCDH-mCD19ScFv-CD28-41BB-CD3ζ) lentiviral expression vector
[0078] (1) Human CD8α signal peptide gene, human CD8α hinge region gene, and human CD28 transmembrane gene were searched from the GennBank database of the National Library of Medicine (htp: / / www.ncbi.nlm.nih.gov / entroz) region and intracellular region genes, human 41BB intracellular region and human CD3ζ intracellular region gene sequences. The mouse-derived anti-CD19 single-chain antibody (anti-CD19ScFv) gene sequence comes from a patent (patent number: ZL200610015650.9), and its codons are optimized to ensure that it is more suitable for human cell expression without changing the encoded amino acid sequence. The DNA sequence of CD19scFV is shown in SEQ ID NO.3, and the encoded amino acid sequence is shown in SEQ ID NO.4; the DNA sequence of CD8 is shown in SEQ ID NO.5, and the encoded amino acid sequence is shown in SEQ ID NO.6; the DNA sequence of CD28 ...
Embodiment 3
[0106] Example 3 Preparation of Chimeric Antigen Receptor pCDH-CD19ScFv-CD28-41BB-CD3ζ Modified T Cells
[0107] (1) Packaging and concentration of lentivirus
[0108] The lentiviral expression plasmid pCDH-CD19scFv-CD28-41BB-CD3ζ and the helper plasmids pCMV-G, pRSV-Rev and pMDLG-pRRE were respectively extracted with the plasmid extraction kit to measure the concentration of the plasmids. The four plasmids were divided into 3:1: 293T cells were co-transfected with calcium phosphate transfection reagent at a mass ratio of 2:2. Collect the virus supernatant in 15ml tubes at 72 hours after transfection, centrifuge at 500G for 10min at 4°C, transfer the supernatant to a new 15ml tube, filter the virus supernatant with a 0.45um filter; filter the virus supernatant with 5×PEG6000- Mix NaCI according to the volume ratio of 4:1, shake it at 4°C overnight, then centrifuge at 10,000G for 60 minutes at 4°C, discard the supernatant, and dissolve the precipitate in X-VIVO 15 serum-free m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com