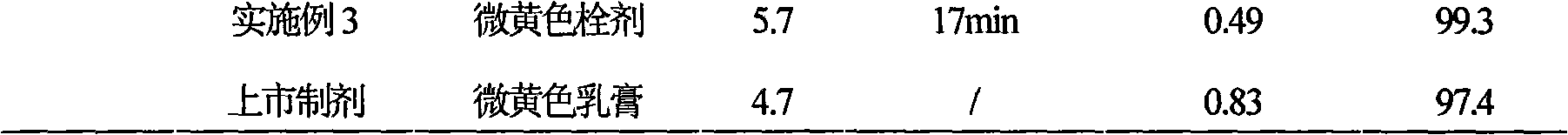Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
89 results about "Pessary" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
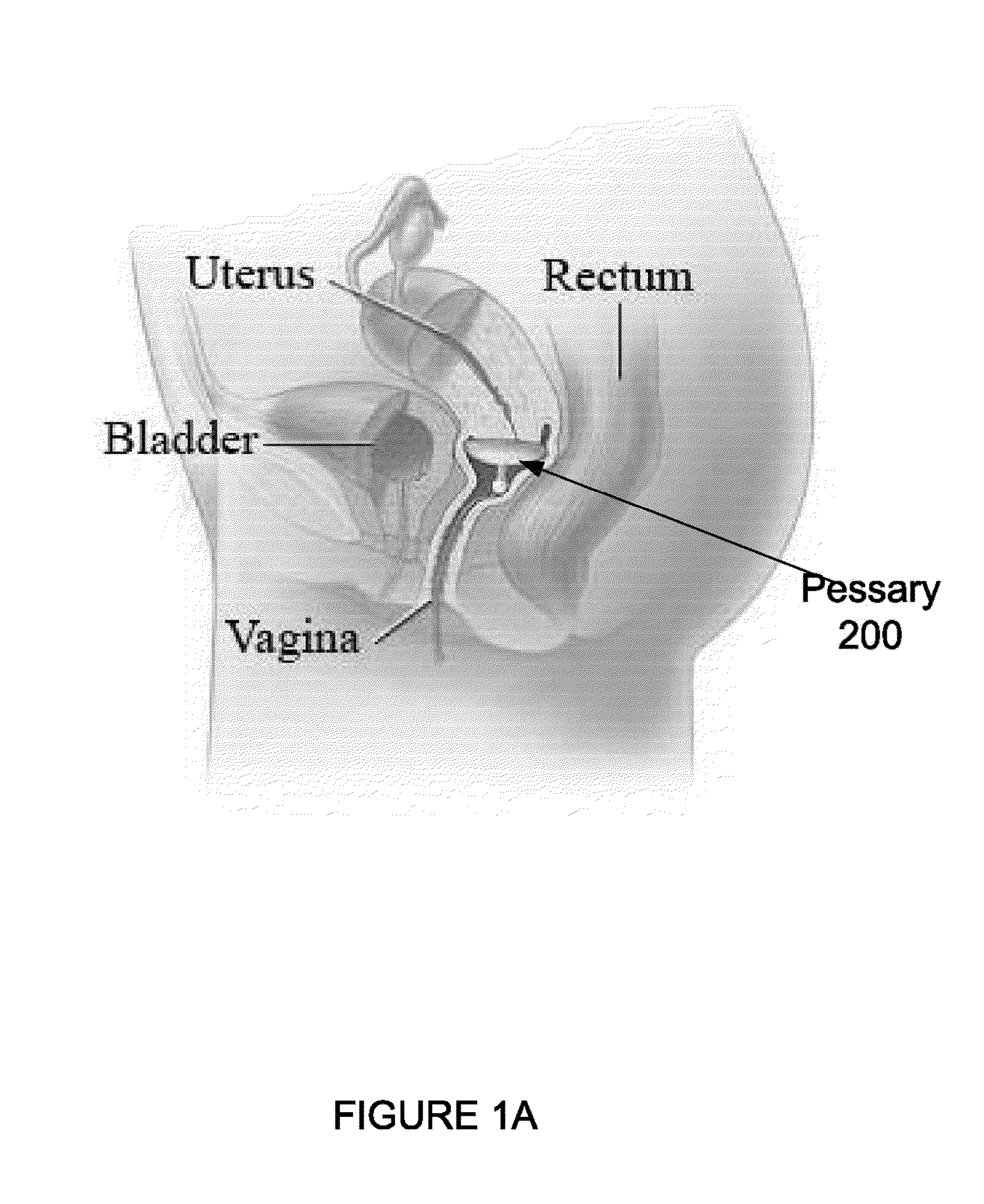
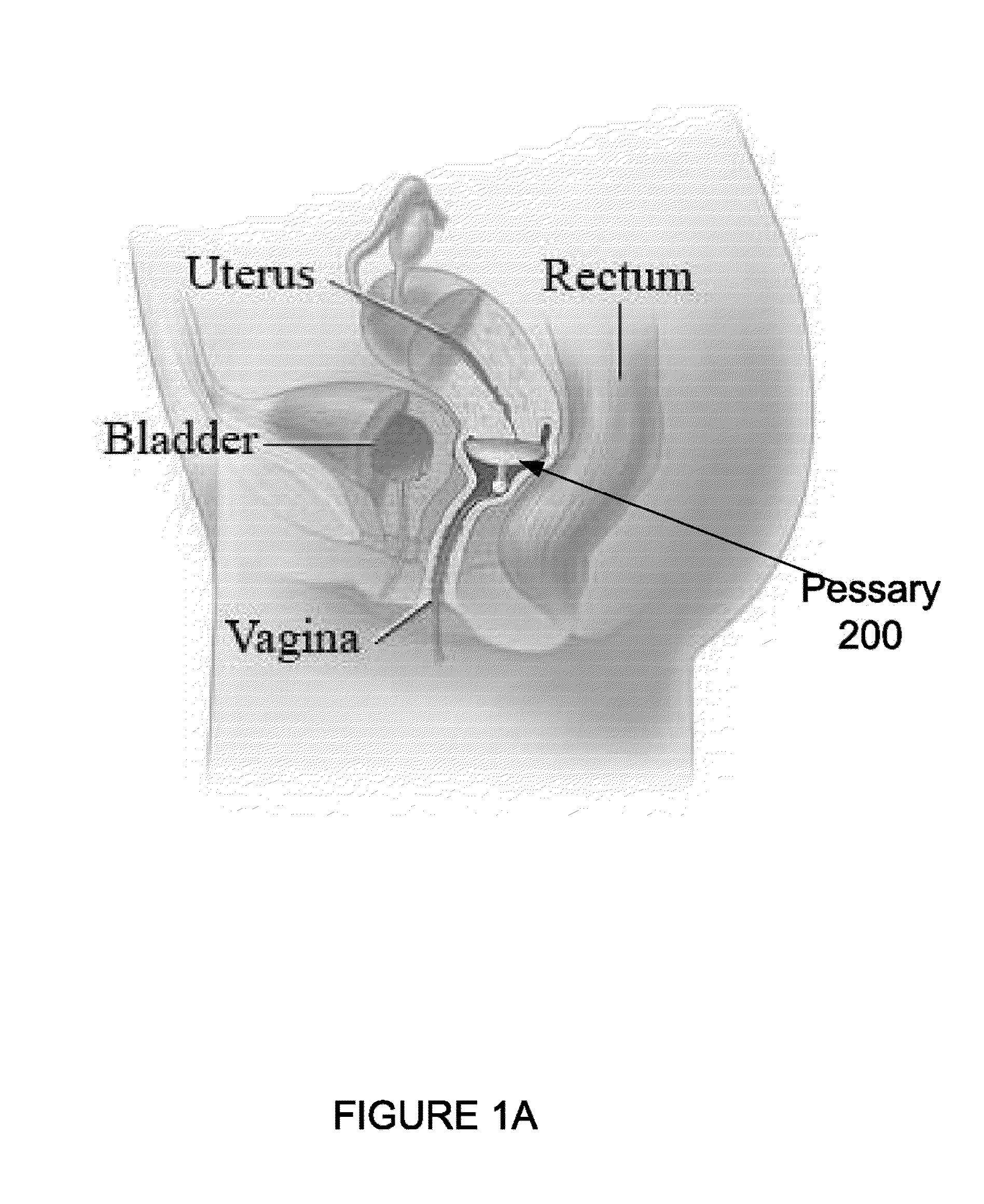
A pessary is a prosthetic device inserted into the vagina to reduce the protrusion of pelvic structures into the vagina. It can be a route of administration of medication and provides a slow and consistent release of the medication. Pessaries are of varying shapes and sizes. They may cause vaginal ulceration if they are not correctly sized and routinely cleansed. Depending on locale, pessaries can be fitted by health care practitioners; in some countries, pessaries may be available over the counter.
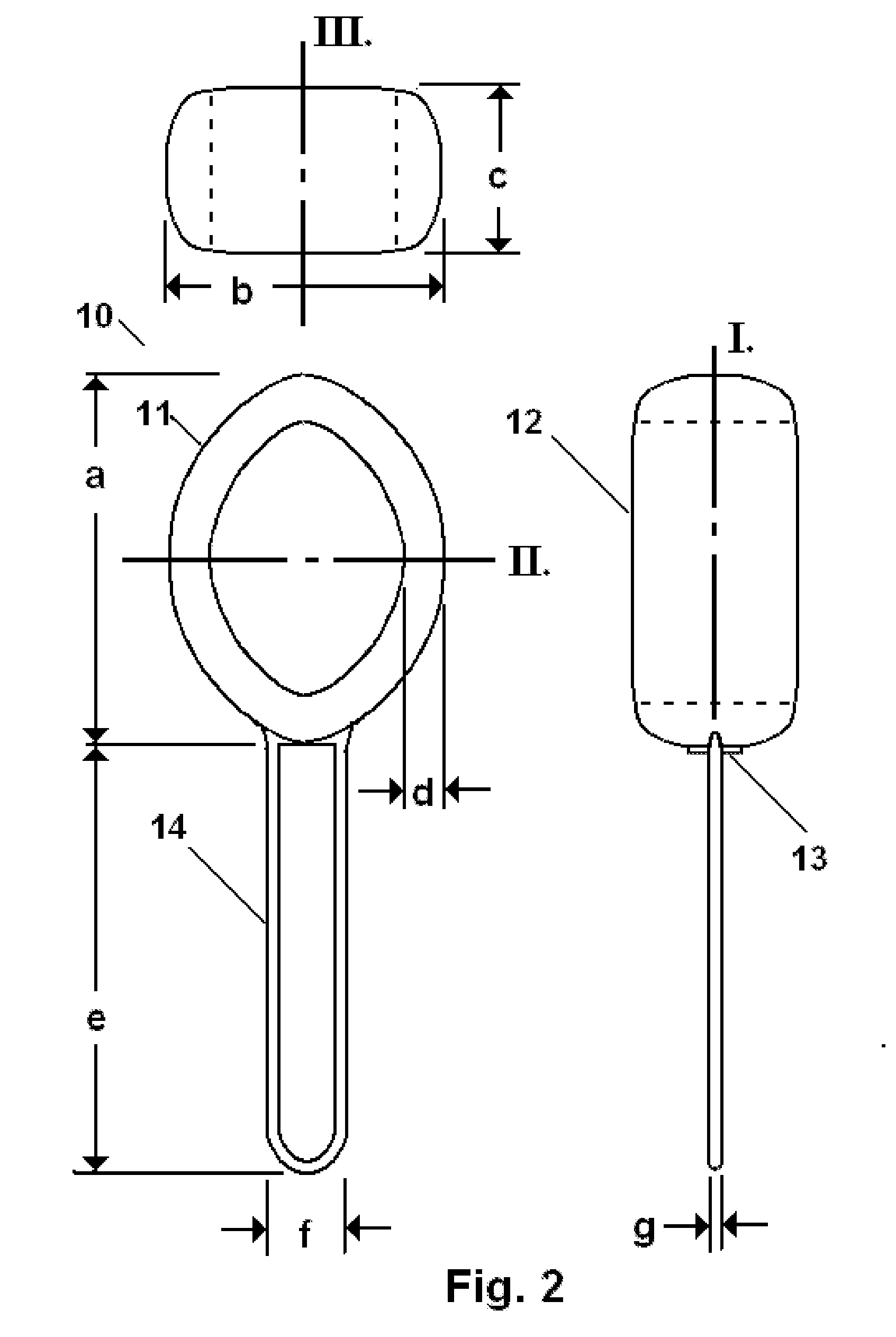
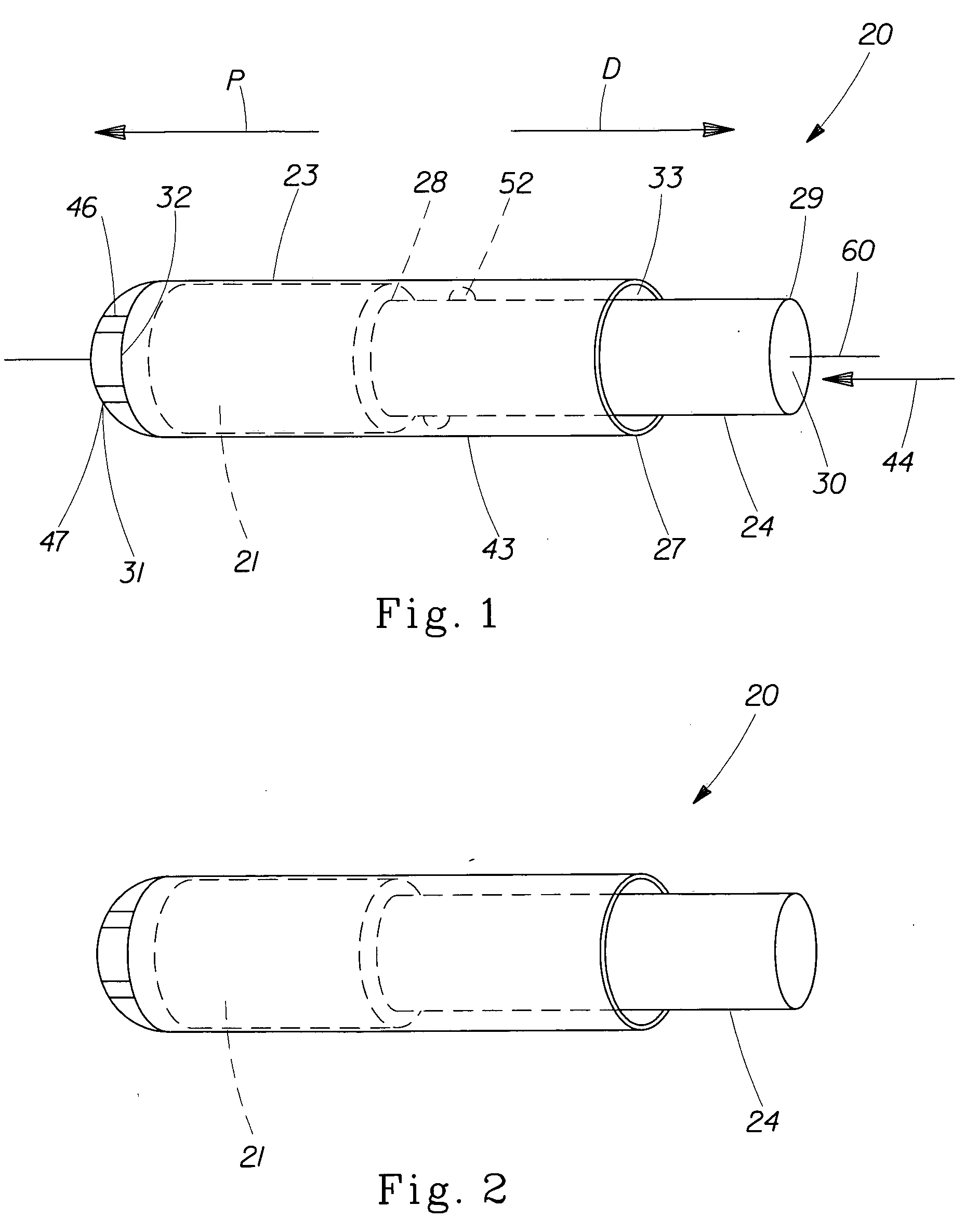
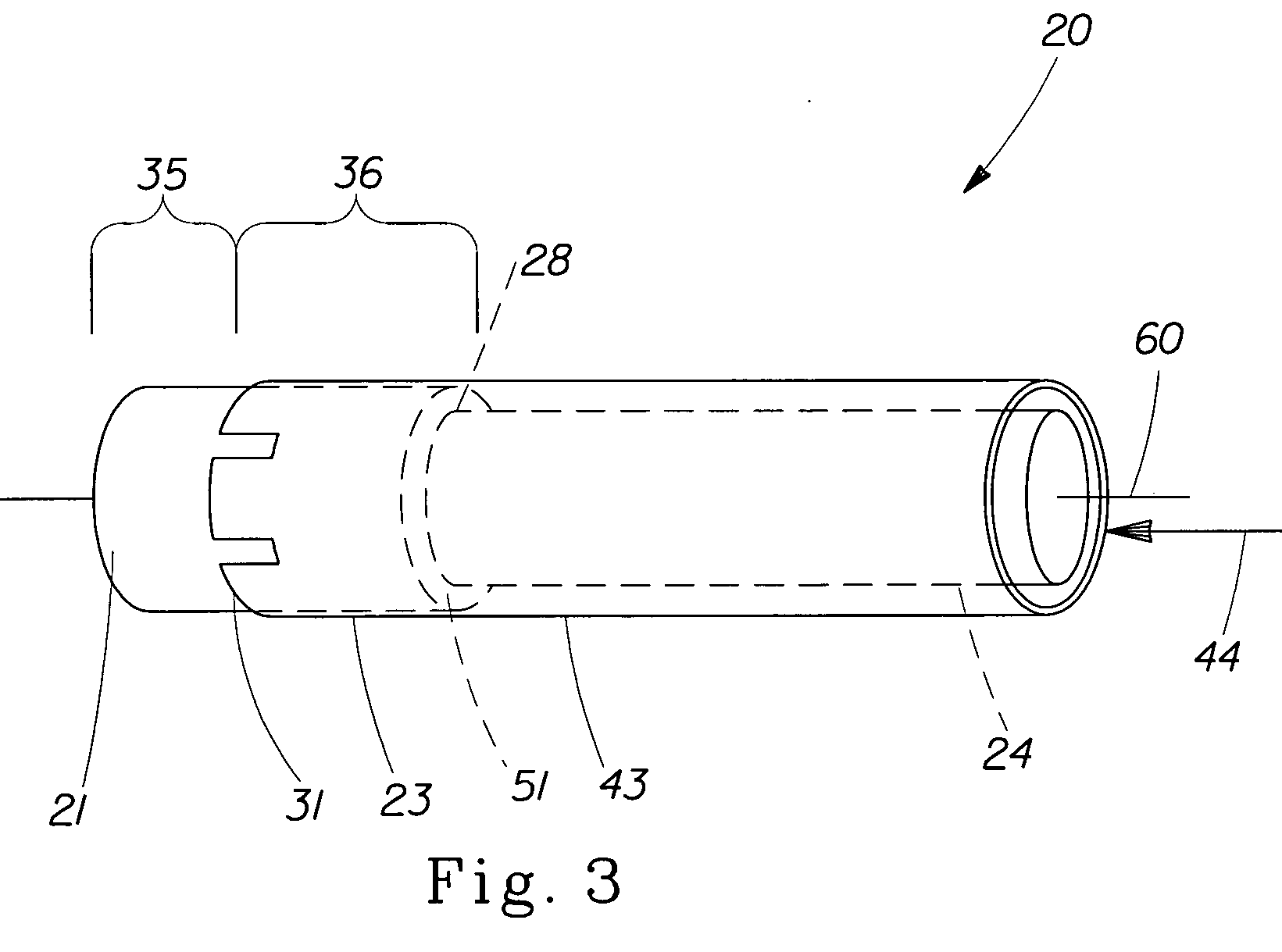
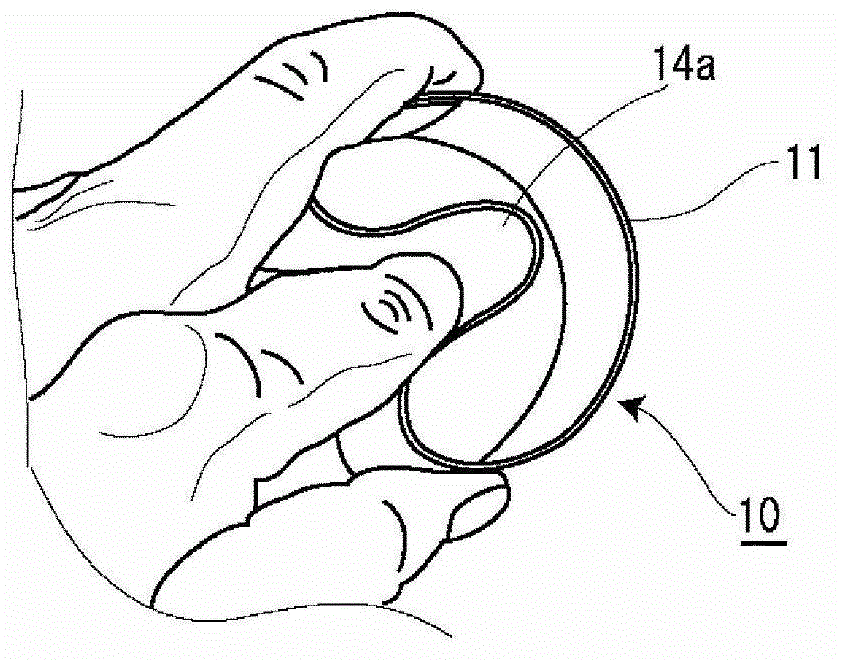
Pessary Device
InactiveUS20090216071A1Easy to inflateEasy to disassembleAnaesthesiaAnti-incontinence devicesUterusPessary
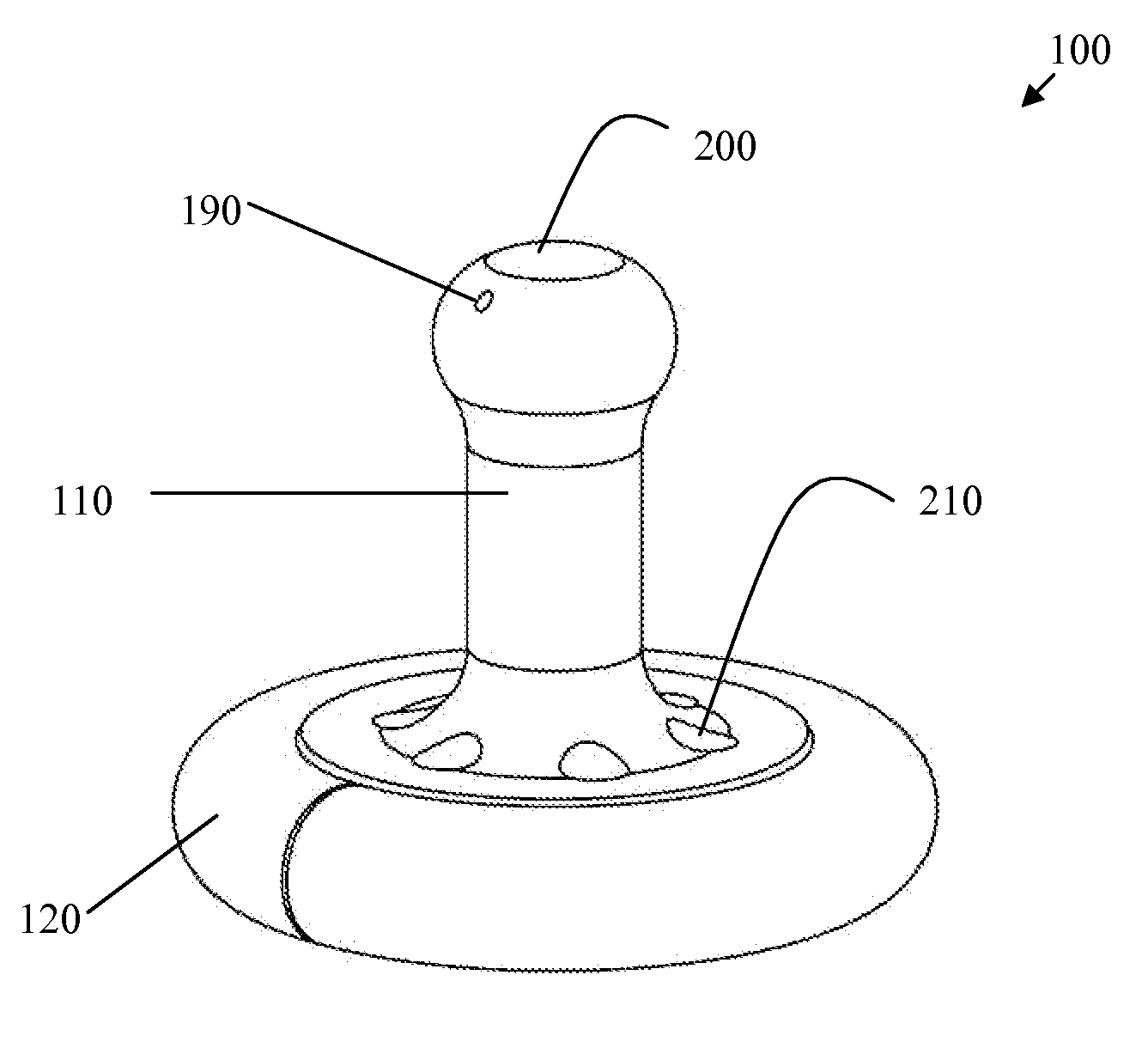
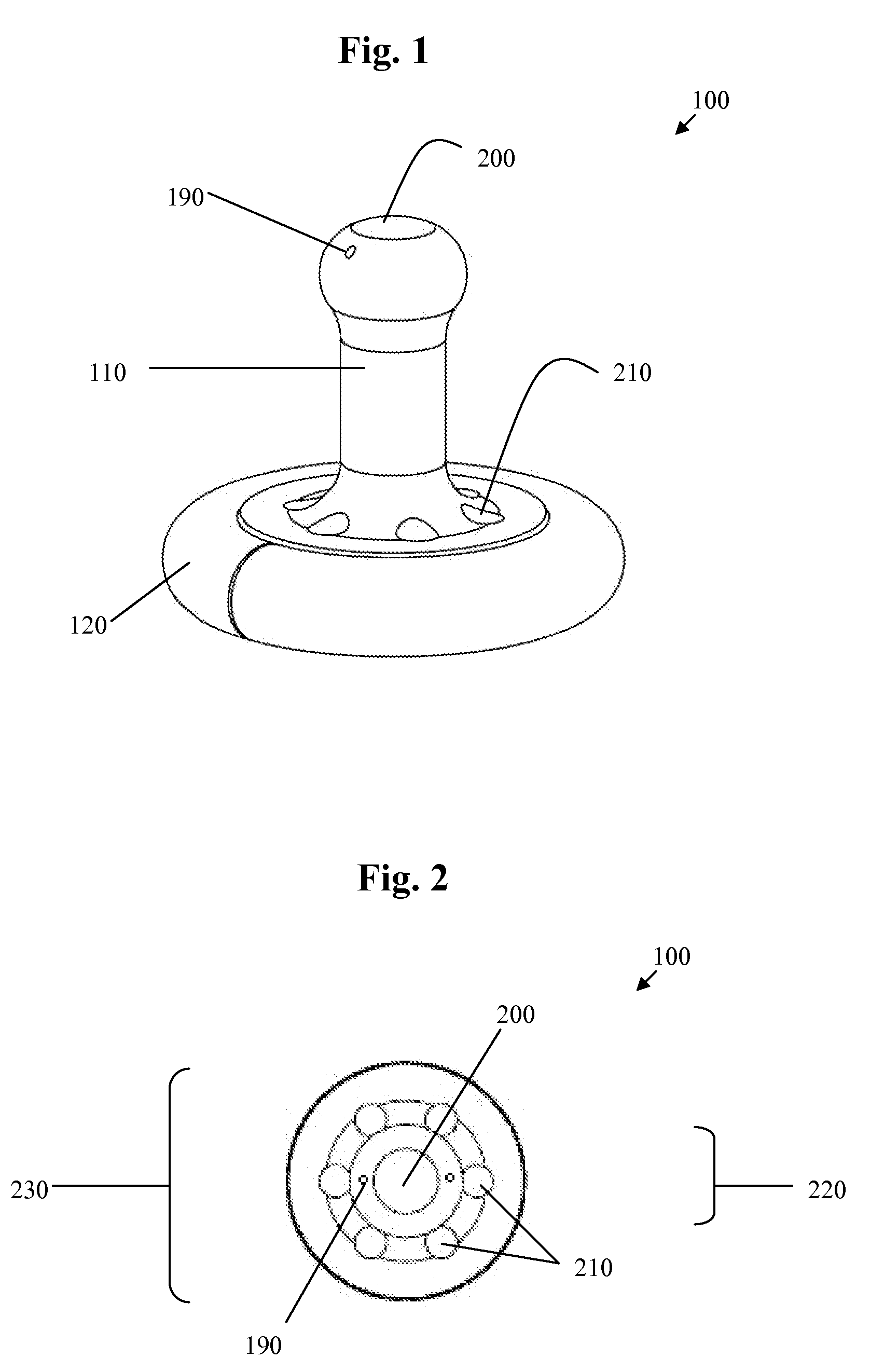
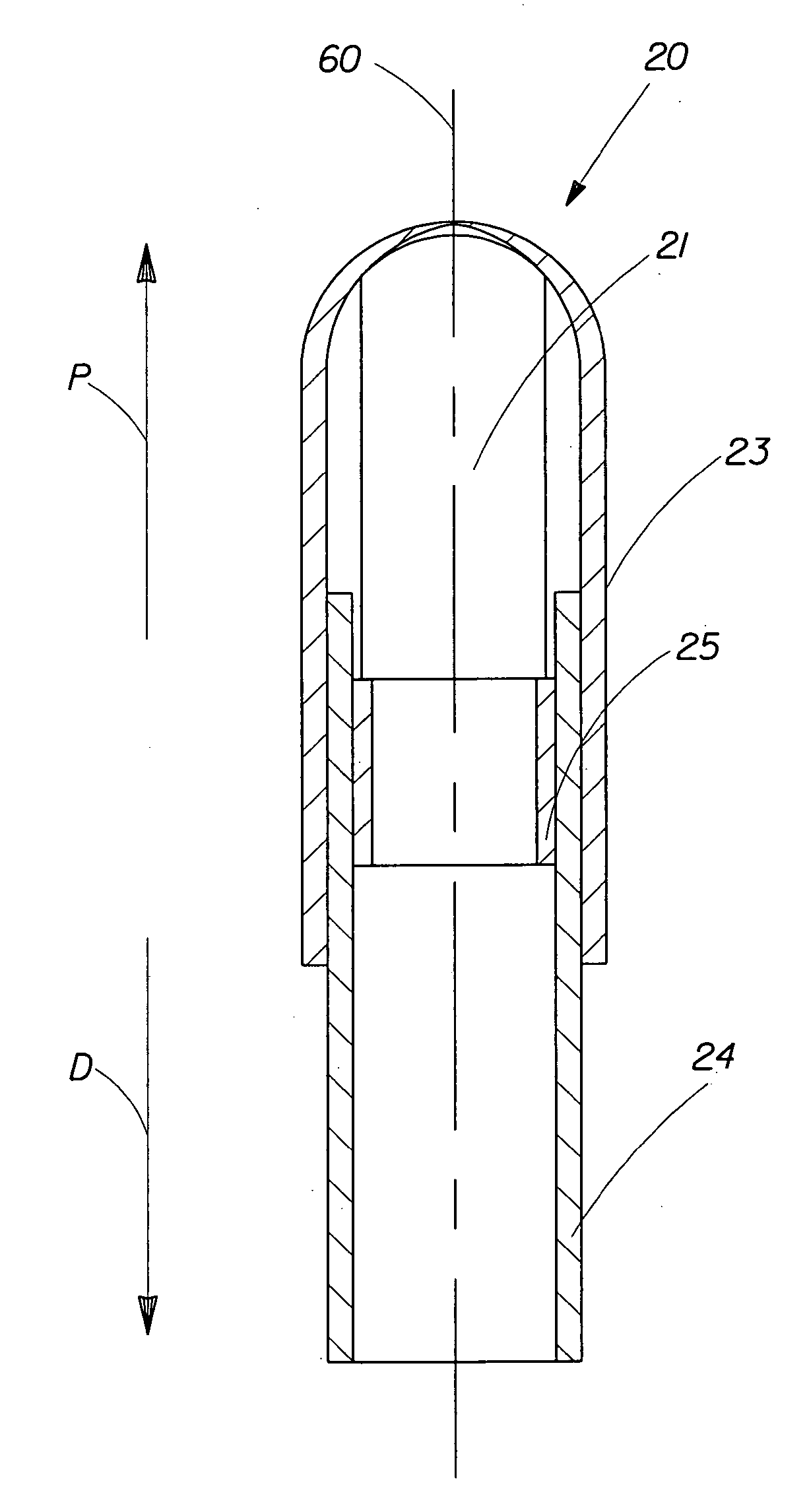
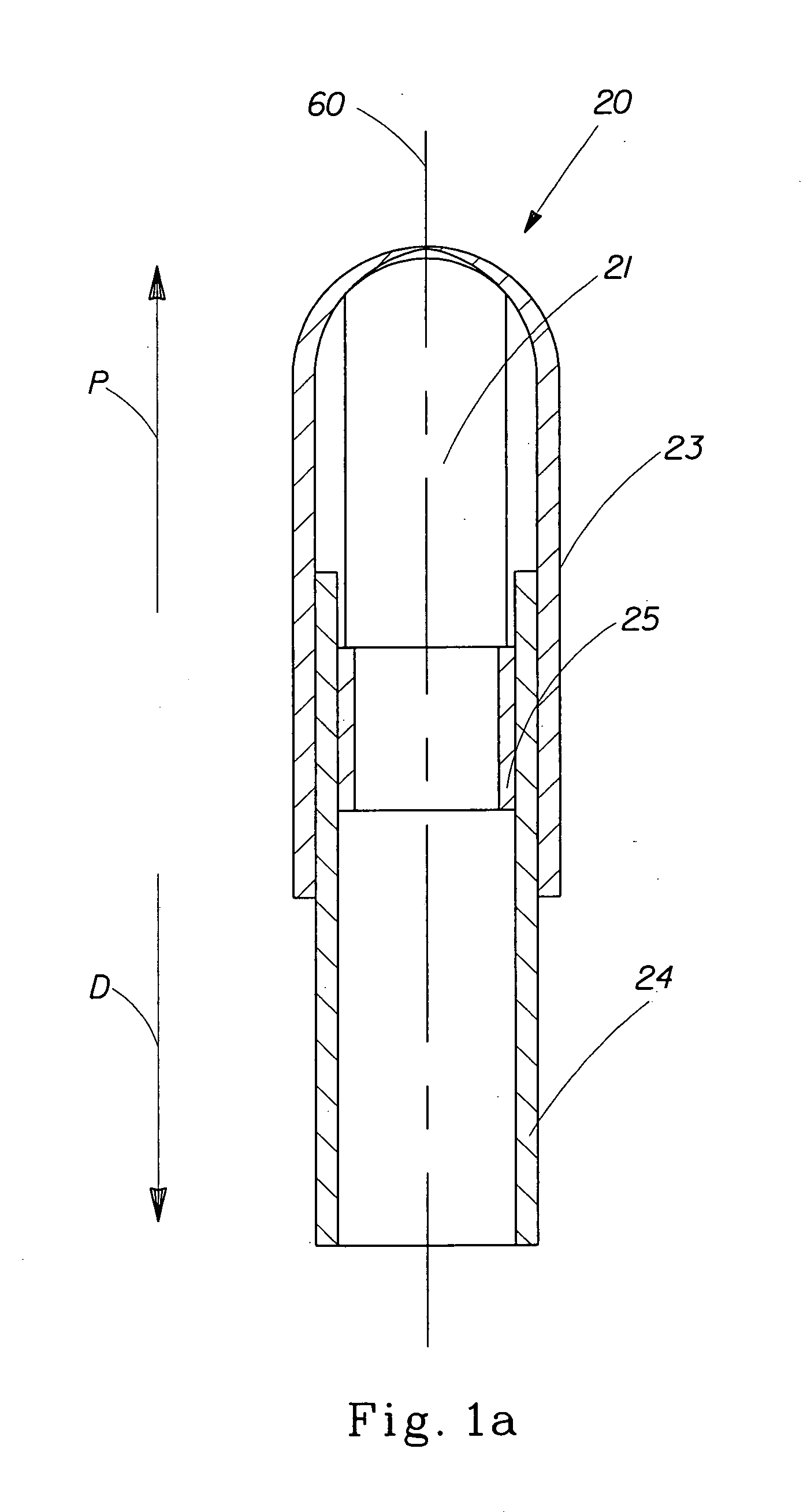
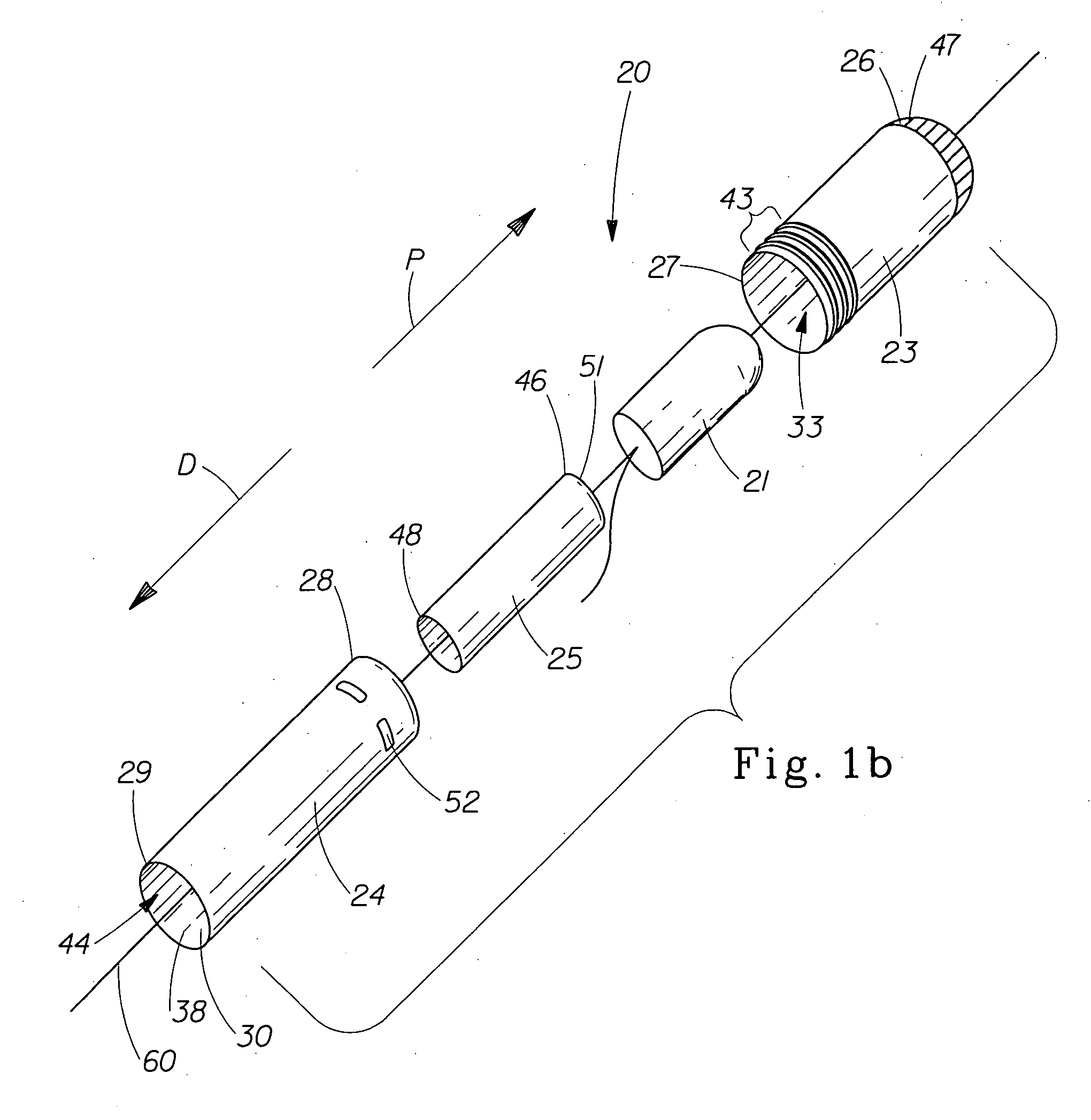
A pessary comprising a main stem and an inflatable bladder disposed about the proximal larger diameter section of the main stem. The main stem further comprises a distal smaller diameter portion having a cap at its distal tip and a check valve disposed there under. The check valve communicates with a central fluid passage that may extend into the proximal larger diameter section of the main stem. Secondary fluid passages connect the central fluid passage to the inflatable bladder volume disposed about the outer circumference of the proximal larger diameter section of the main stem. The main stem provides support to the pelvic structures and the inflatable bladder acts to hold the device in position. At least one void through the proximal larger diameter portion of the main stem allows bodily material to pass from the vagina through the pessary device thereby decreasing the amount of vaginal discharge.
Owner:ZIPPER RALPH
Medical exercise device
InactiveUS20130324380A1Simple designFacilitate ease of insertion and flexibility and resistanceResilient force resistorsRegimenPelvic diaphragm muscle
Disclosed is an intra-vaginal device of a new and innovative design that in combination with kegel exercises or performing ones daily routine strengthens the muscles of the pelvic floor. The device employs a combination of unique shape, materials as well as carefully engineered deflection, frictional, testing and clean-ability characteristics with particular attention to a sexually neutral appearance, comfort during insertion and exercise as well as positive physical feedback to encourage prolonged and frequent use. Benefits of the device include improvement or prevention of urinary leakage and prolapse, increases in sexual intensity, to keep the uterus / bladder / rectum in place as a pessary as well as relief for women suffering from moderate prolapse. As a dilator to aid women who suffer from vaginismus. With its multiple features and benefits, the device is a vital tool in a woman's quest for a complete and balanced wellness regimen.
Owner:HORSLEY CARYN M
Single use pessary devices
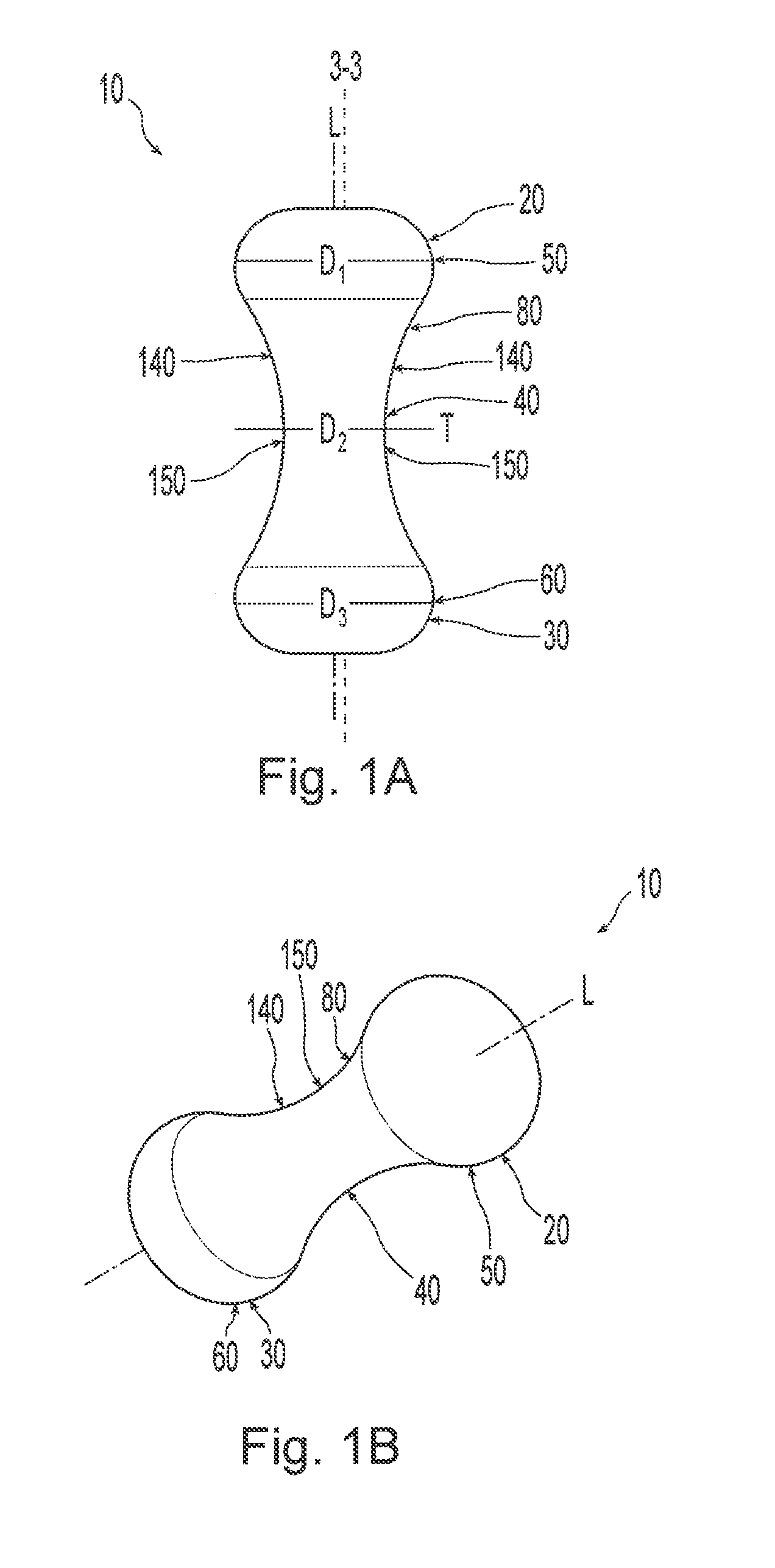
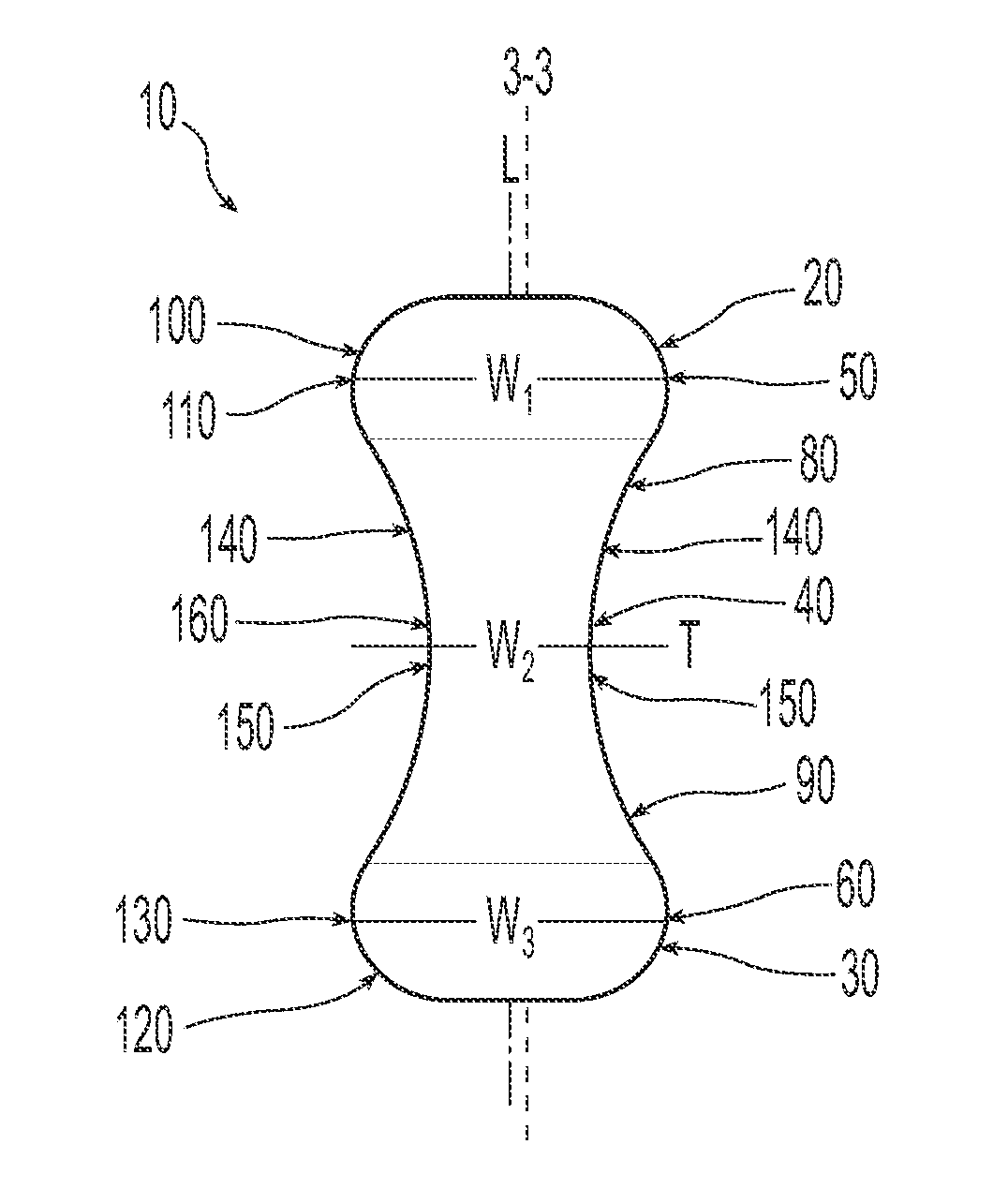
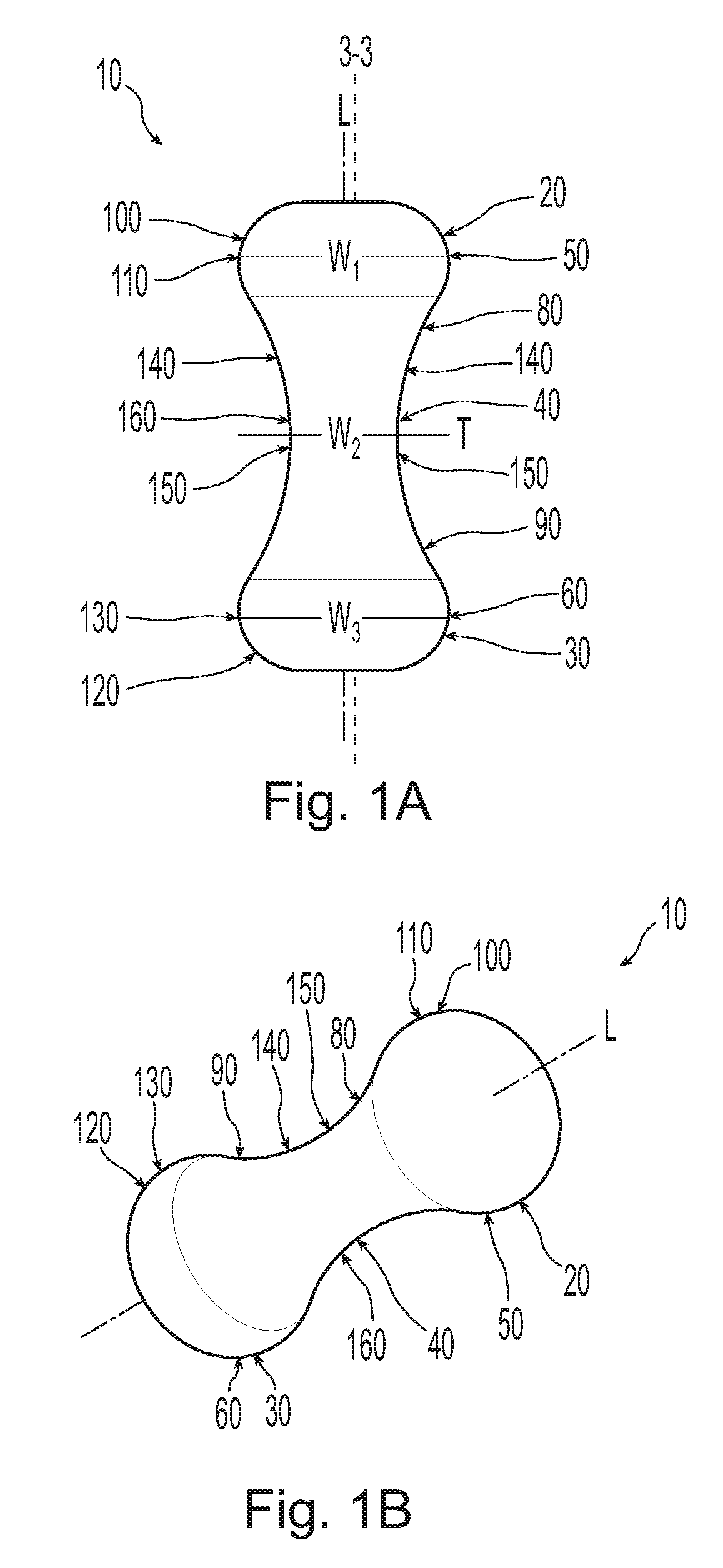
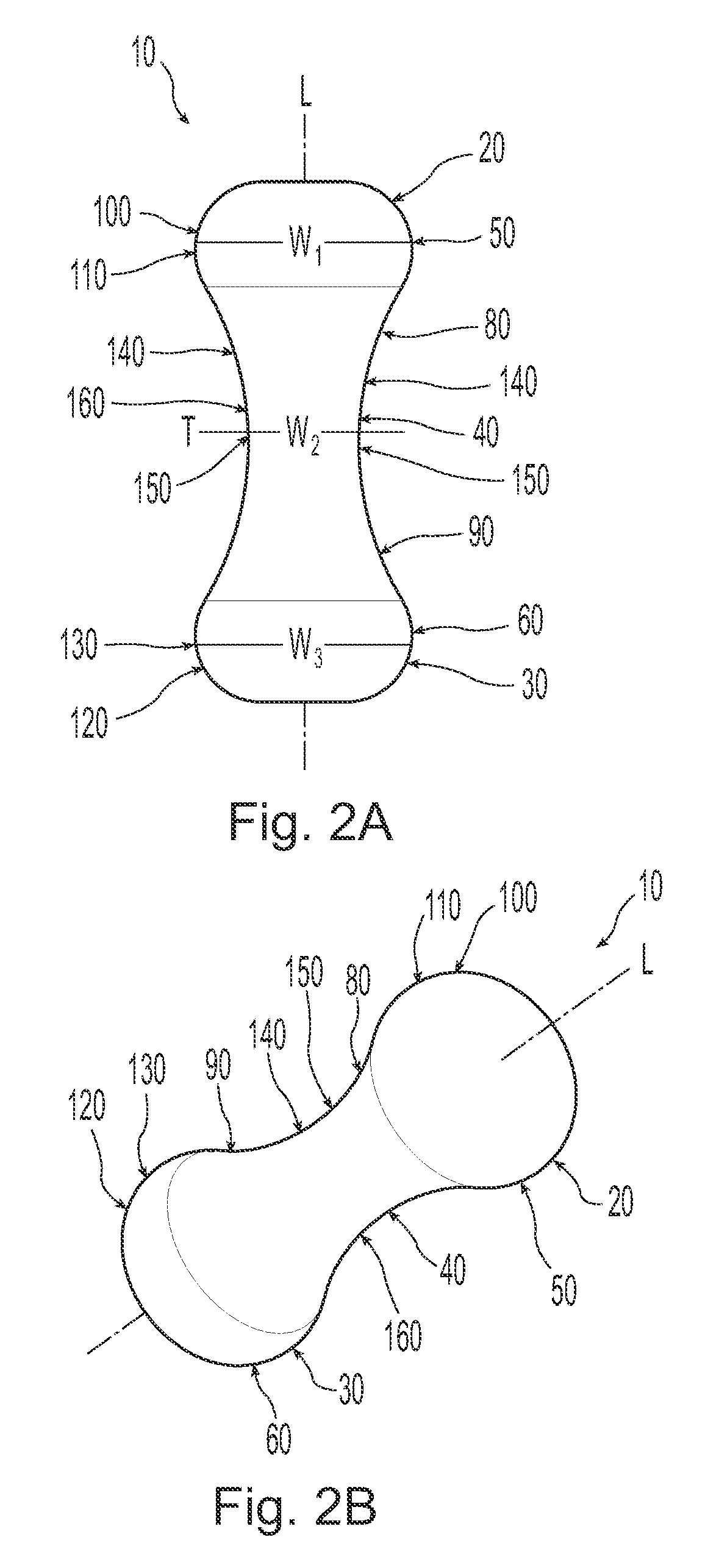
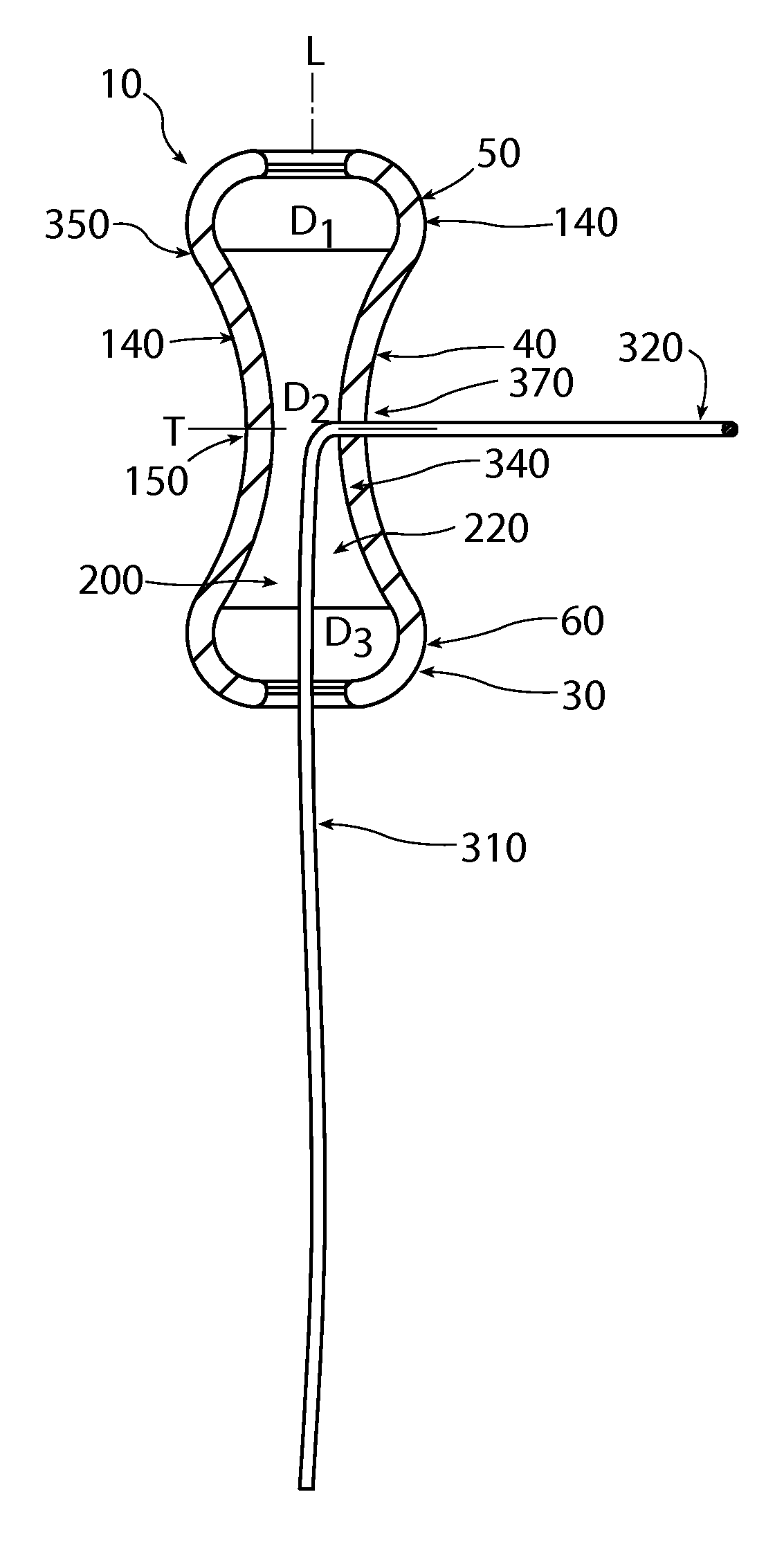
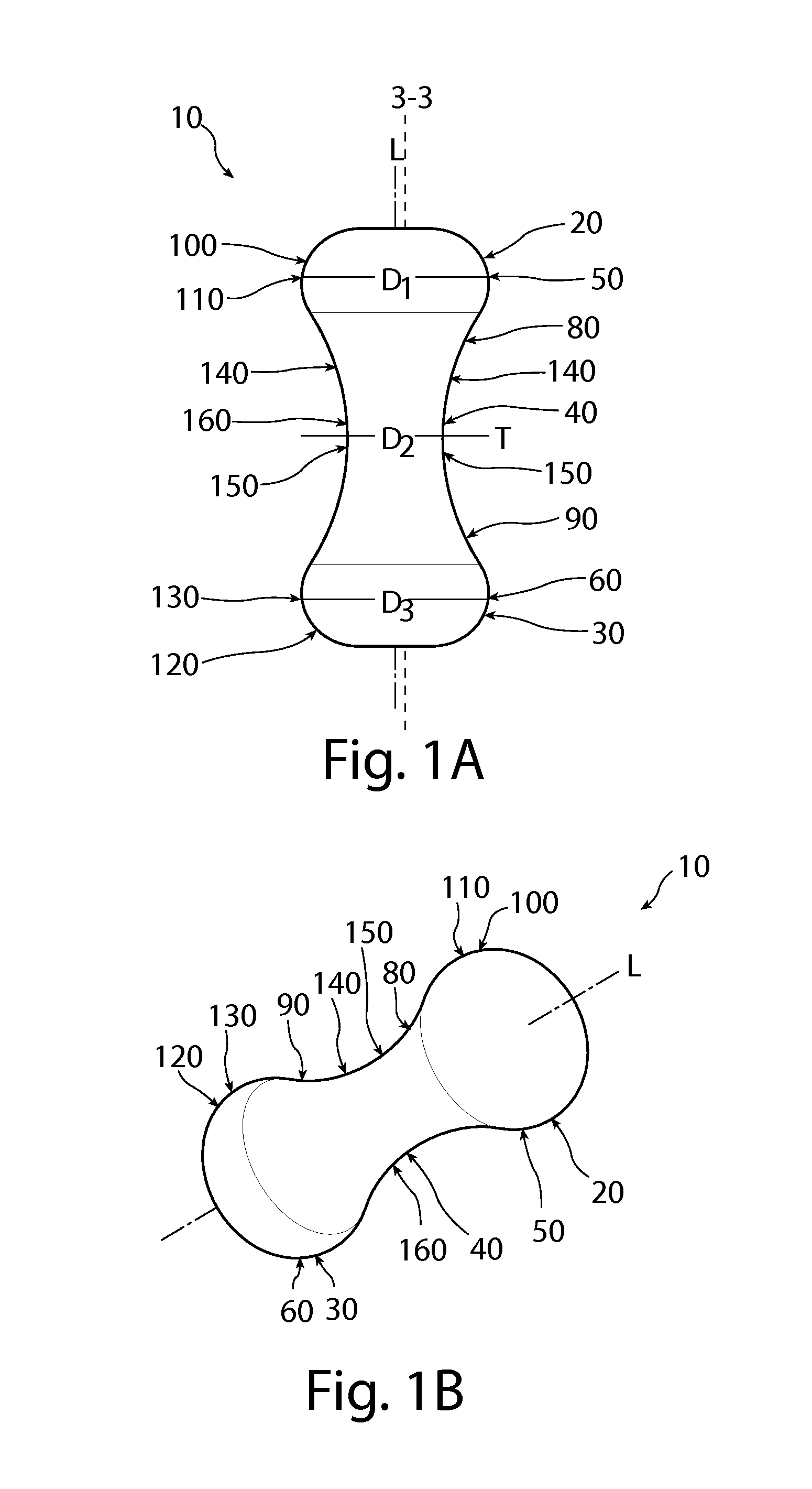
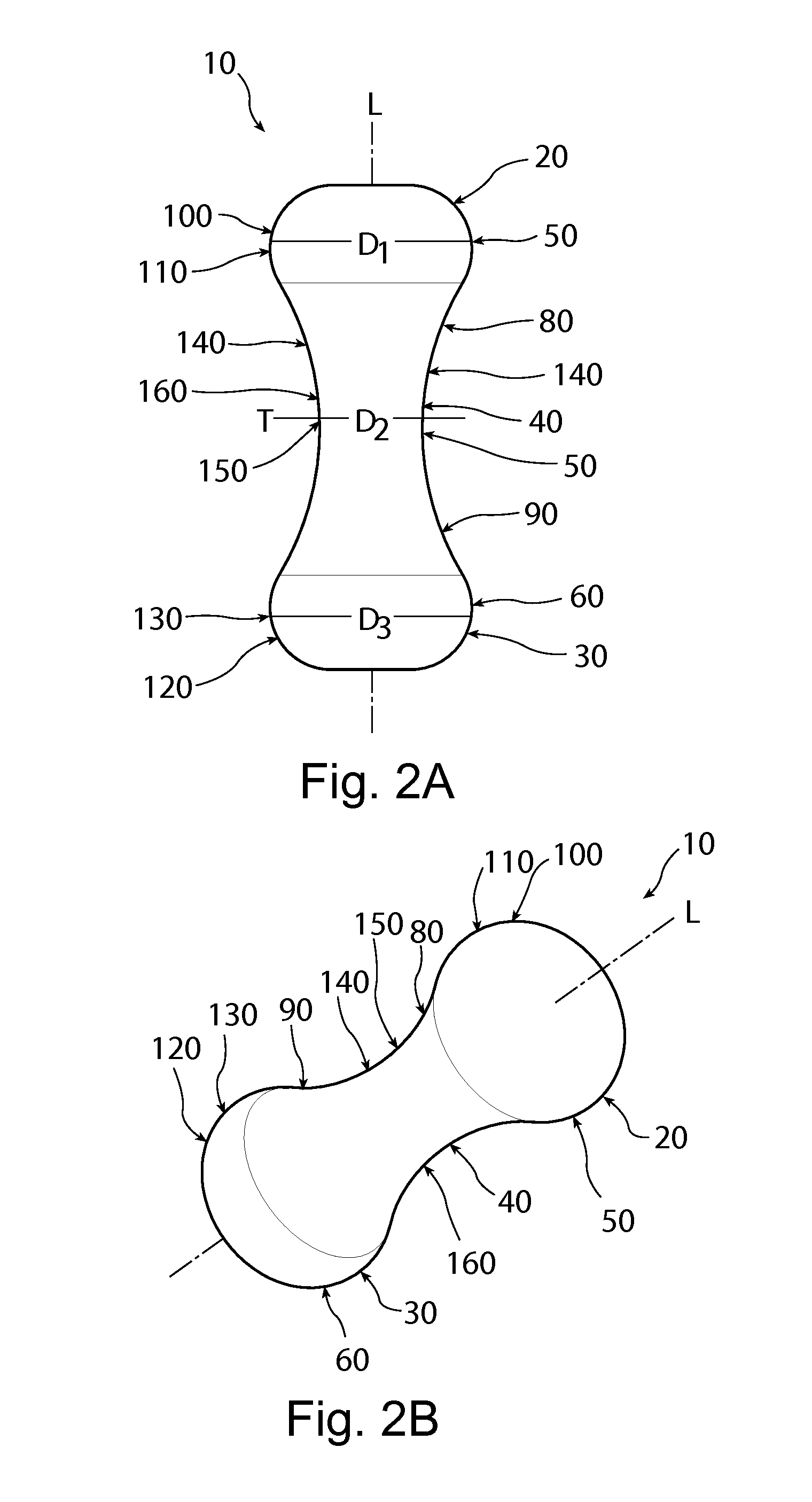
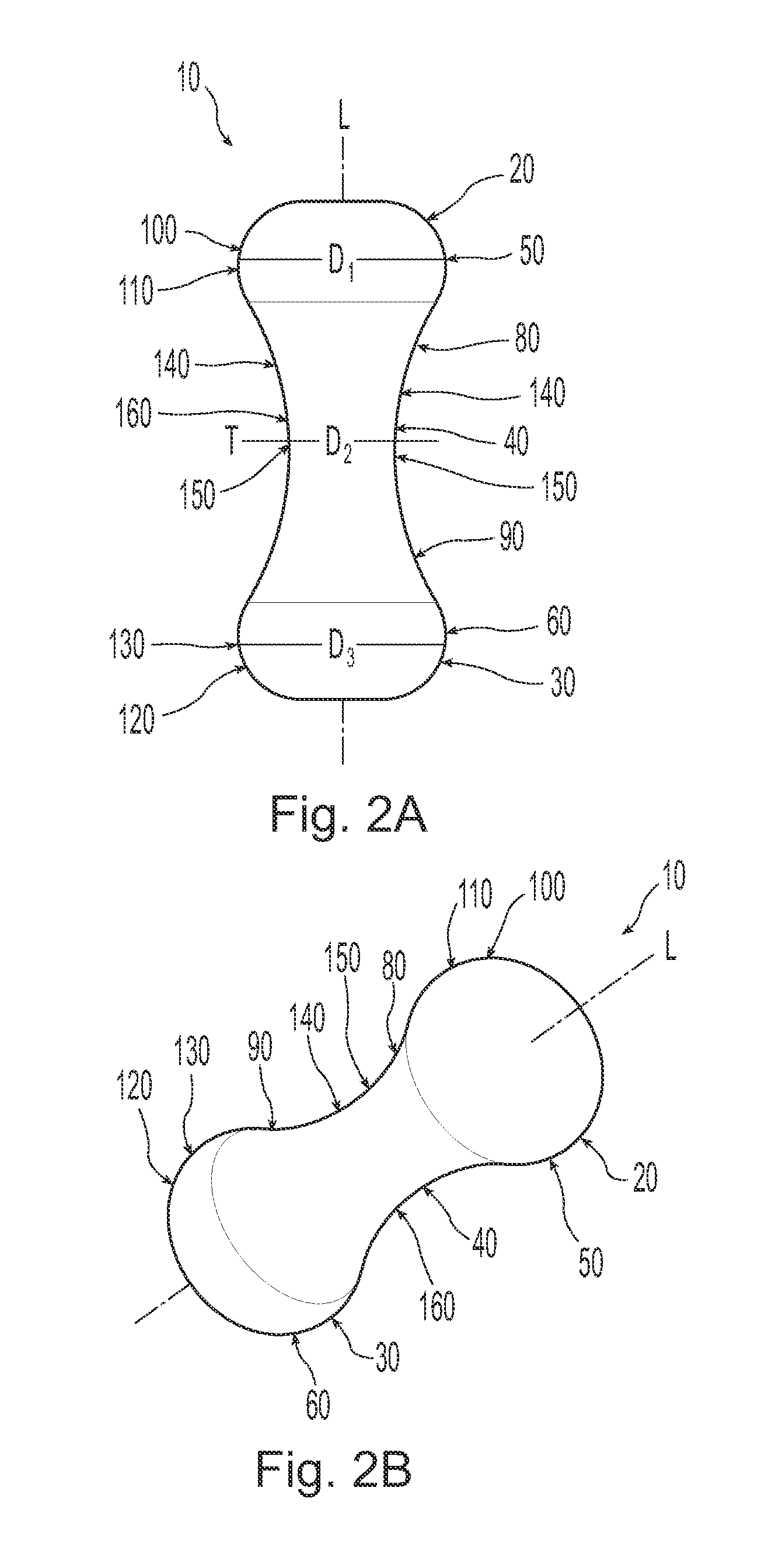
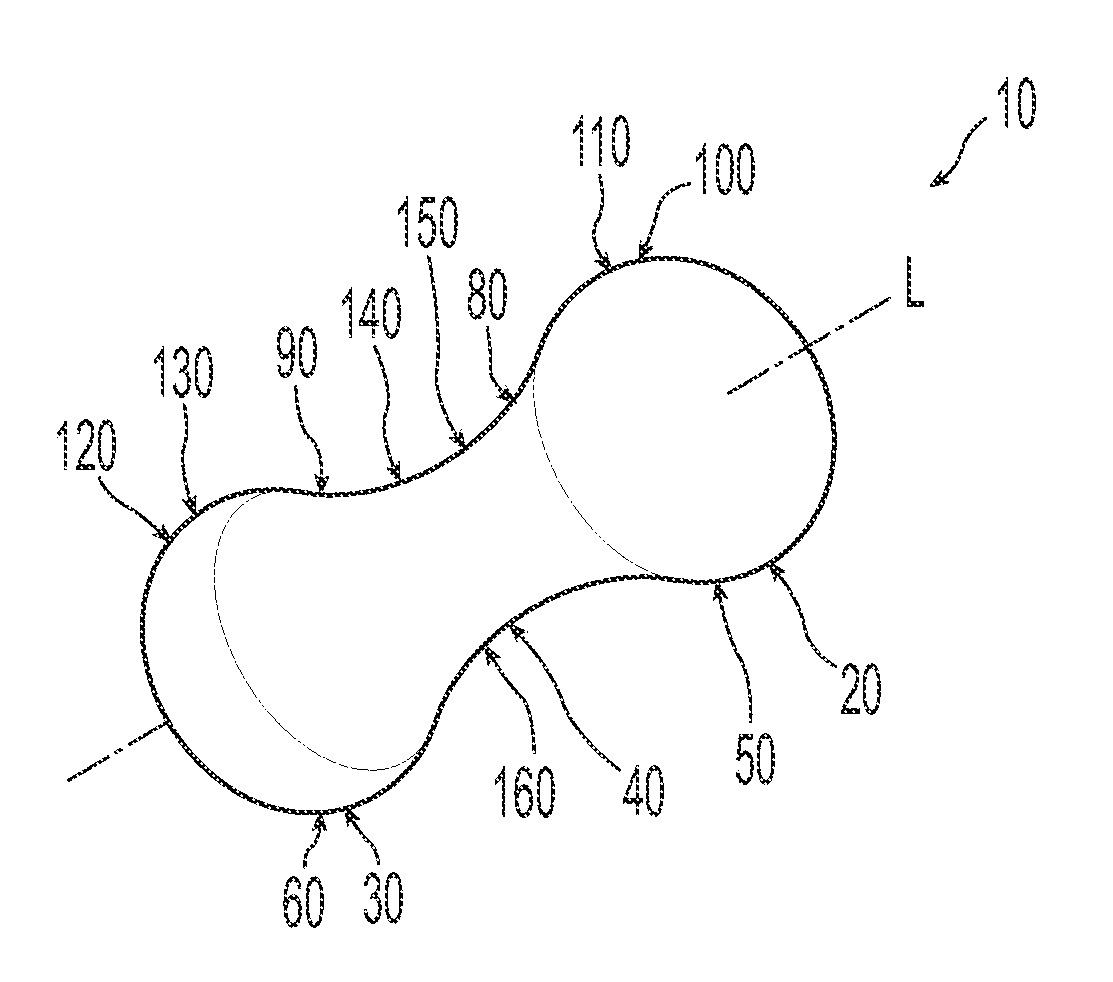
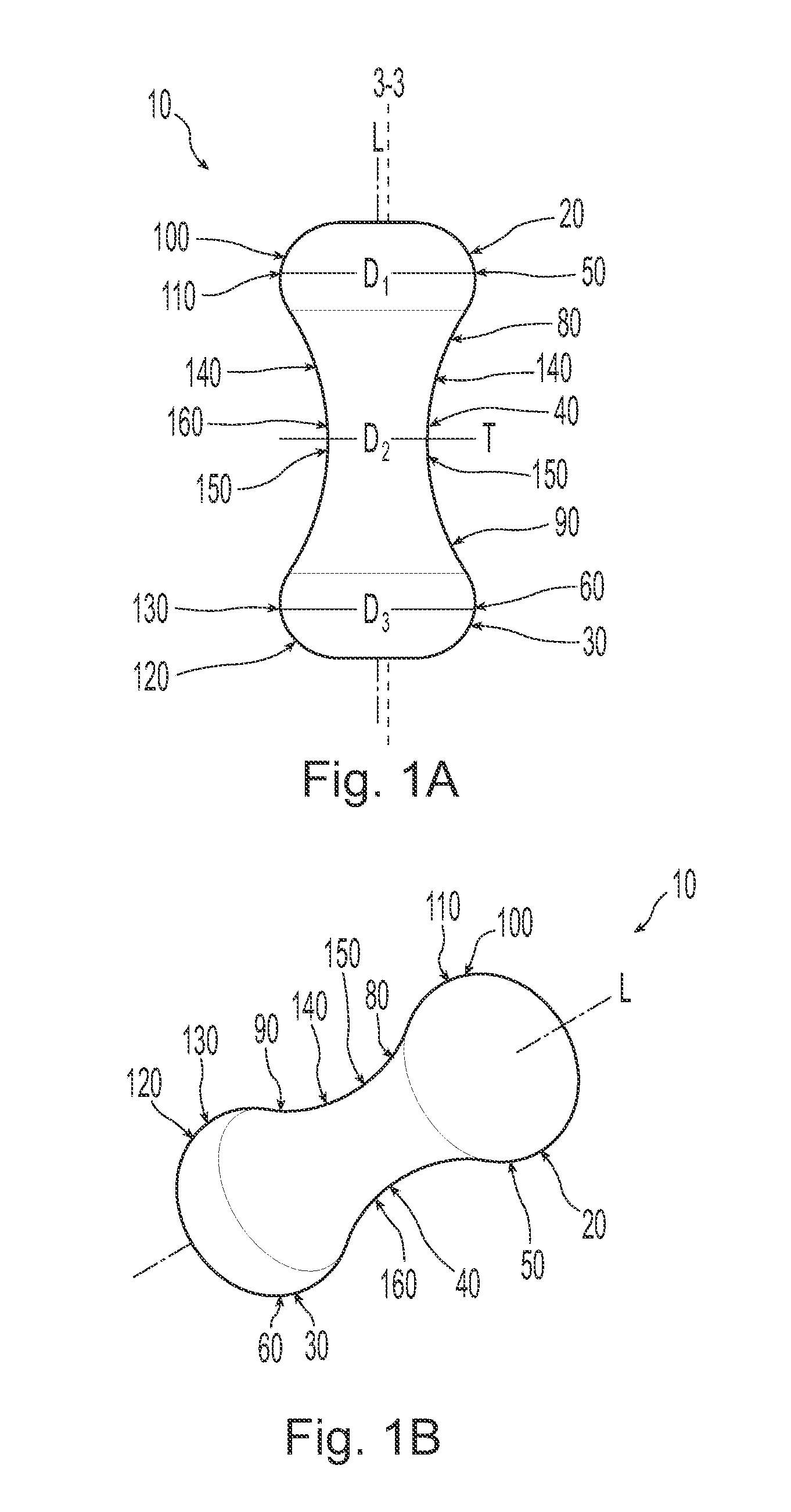
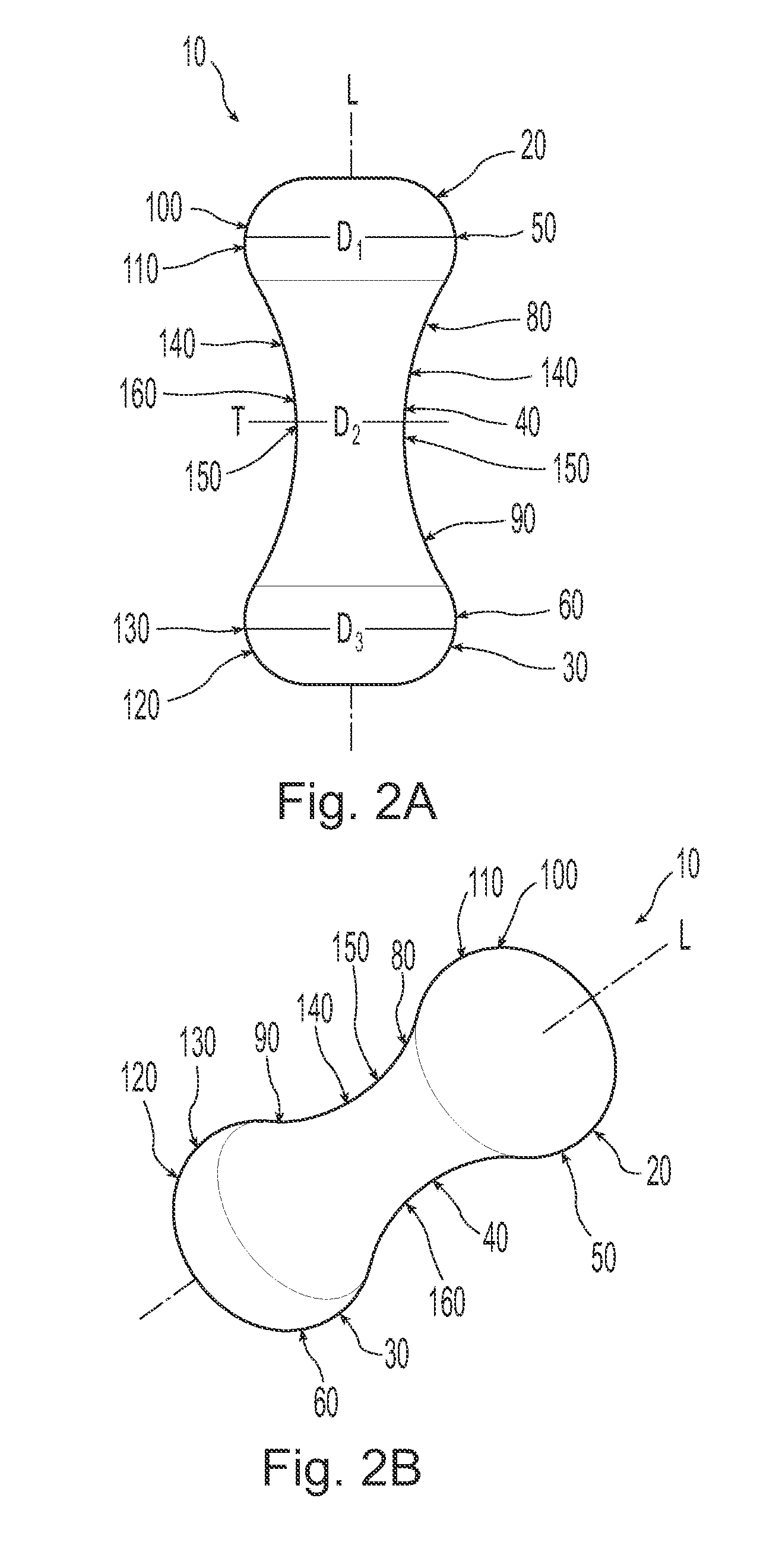
A non-expandable single use pessary device. The pessary device has a top, a base, a length, an outer surface, a longitudinal axis, a maximum diameter, and a minimum diameter that is less than the maximum diameter. The pessary device includes a pressure region adapted to extend between an anterior vaginal wall and a posterior vaginal wall of a user to provide pressure on the user's urethra through the vaginal wall. The pressure region includes the maximum diameter. In addition, the outer surface includes a usage indicator that is visible to a user viewing the outer surface of the device after the device is removed from the user's vagina.
Owner:THE PROCTER & GAMBLE COMPANY
Device and method for fitting a pessary
ActiveUS20100274159A1Easy to cleanAvoid excessive forceAnti-incontinence devicesPerson identificationEngineeringPessary
There is provided a device for measuring an inside shape of a vagina, including a device body, operationally connected to one or more movable cheeks, the cheeks movable in a direction substantially transaxially relative to the device body longitudinal axis, and a width indicator, for indicating a distance between opposite faces of the cheeks. There is provided a method for fitting a pessary including inserting a Pessary Caliber (PC) into a vagina, extending cheeks of the PC to press against sides of the vagina, measuring a distance between the cheeks, measuring how deep the PC is inserted into the vagina, and selecting a pessary size based, at least in part, on the measuring. Related apparatus and methods are also described.
Owner:CONTIPI IP LLC
Applicator having plunger with gripping elements
The present invention provides applicators for inserting feminine hygiene articles, such as and pessaries into a user's body.
Owner:THE PROCTER & GAMBLE COMPANY
Pessary device with improved pressure profile
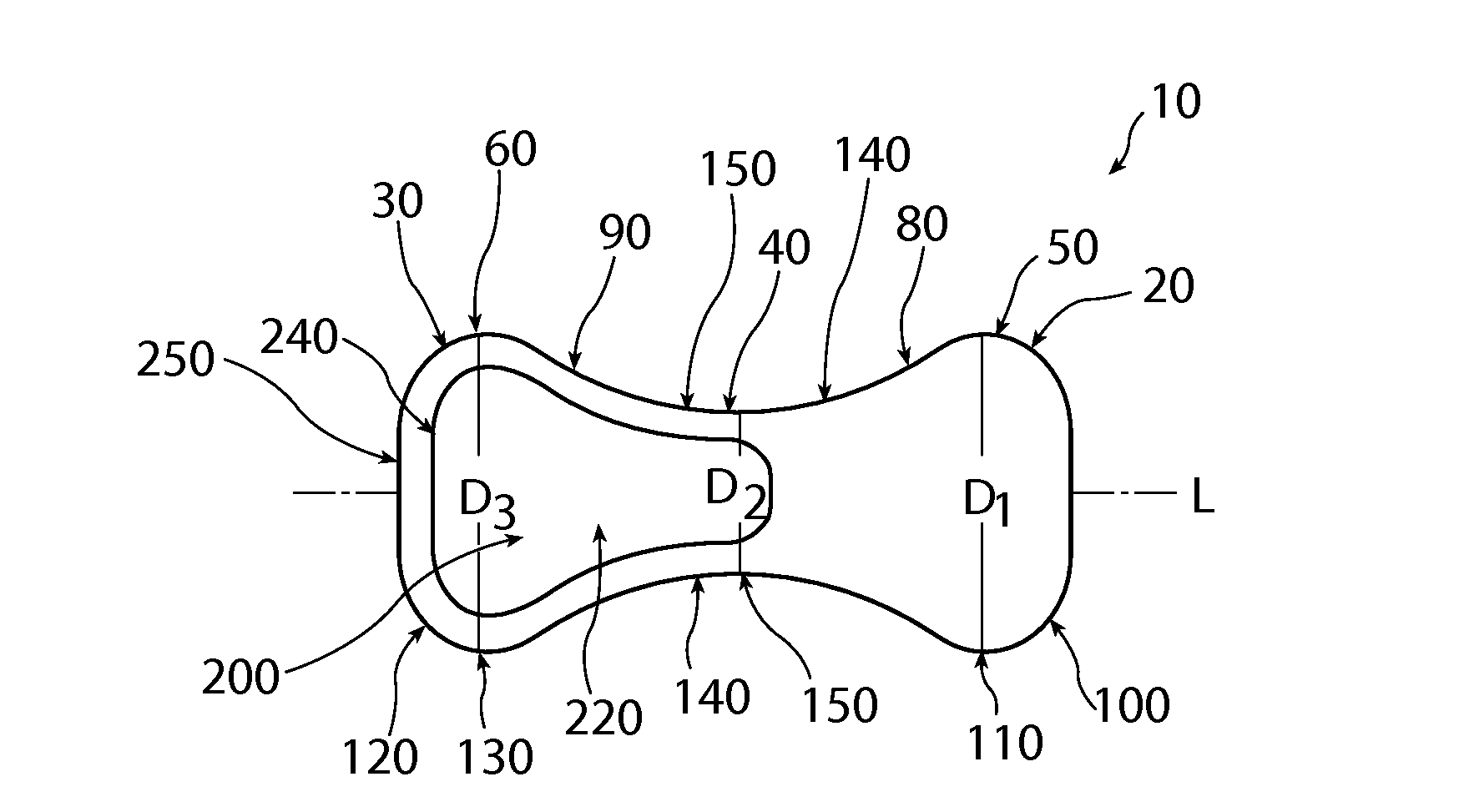
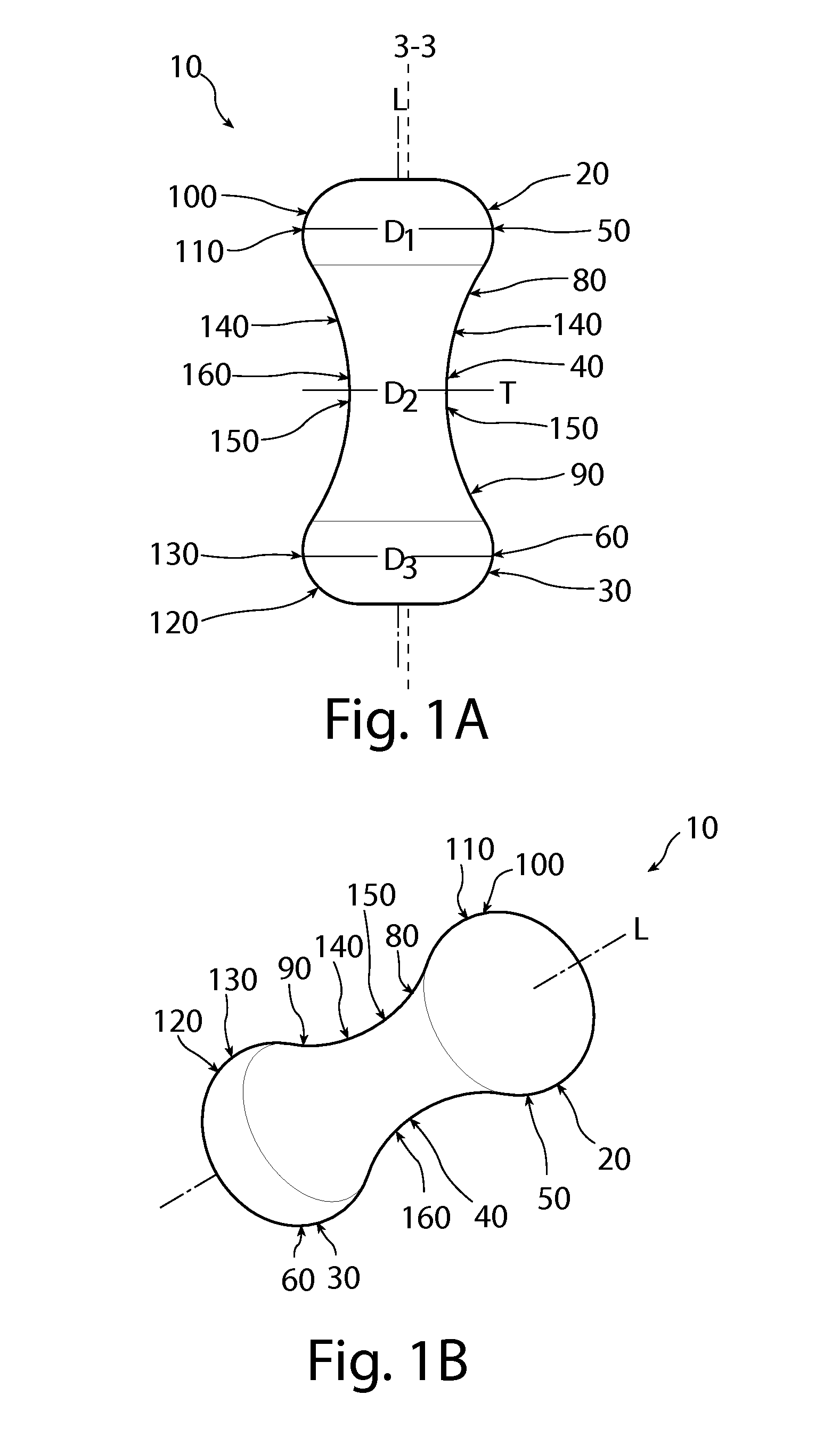
A non-expandable intravaginal pessary device capable of providing varying pressure along the length of a woman's urethra when inserted into the woman's vagina. The device has a top, a bottom, and a sidewall that extends between the top and the bottom, and the sidewall, top and bottom form an enclosed outer periphery defining a total area of the device. The sidewall has a convex bottom portion, a mid-section, and a convex top portion. The convex bottom portion connects the bottom to the mid-section, the convex upper portion connects the top to the mid-section, and the convex bottom portion and the convex upper portion are capable of providing pressure to two distinct locations on a woman's urethra when inserted into a woman's vagina.
Owner:THE PROCTER & GAMBLE COMPANY
Pessary with slow-release support
The invention relates to a pessary with a slow-release support. The slow-release support is installed inside the pessary, medicines are arranged on the support, medicines are separated from the outside through micro-hole films, and diameters of micro holes of micro-hole films are in a range from 0.1 nm to 10 mm in accordance with diameters of passing medicine. Due to own movement, organ oppression and micro hole diameter limitation, medicines slowly act on a vagina and a cervix and directly act on target organs in accordance with preset speed and the clinical effect is achieved. By the aid of the pessary, a physical mode and a chemical mode can be used for curing prolapsus and supplementing hormone.
Owner:江苏奥博金医药科技有限公司
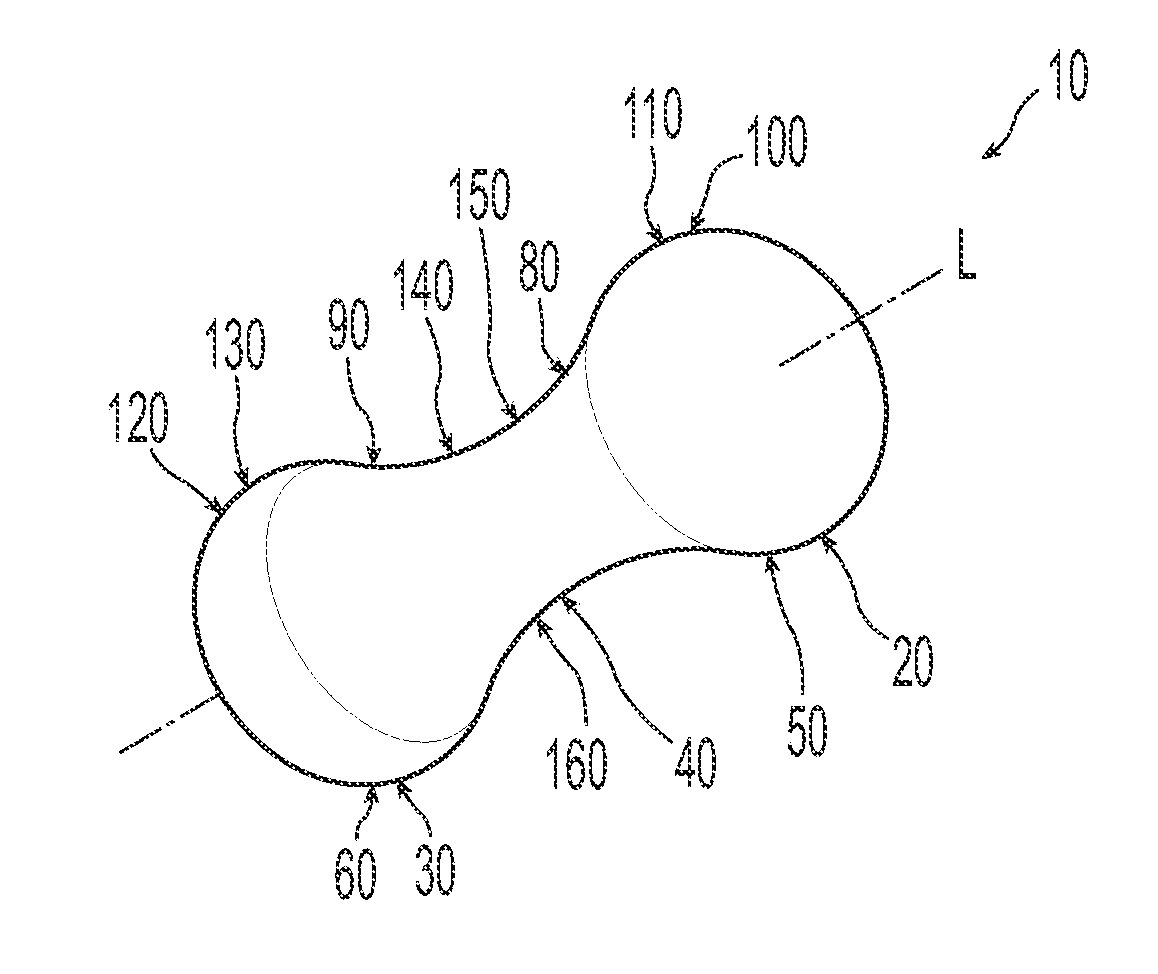
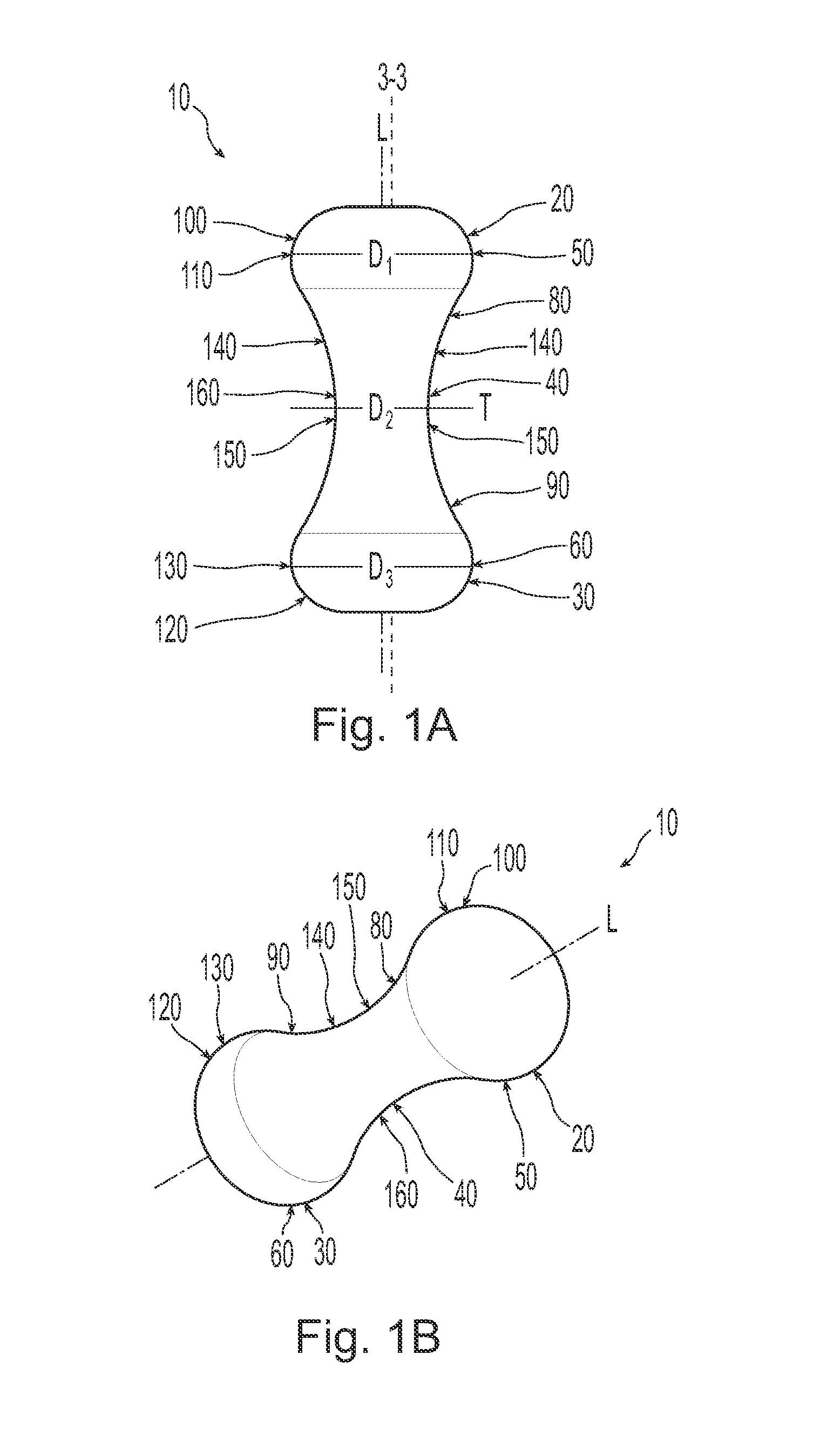
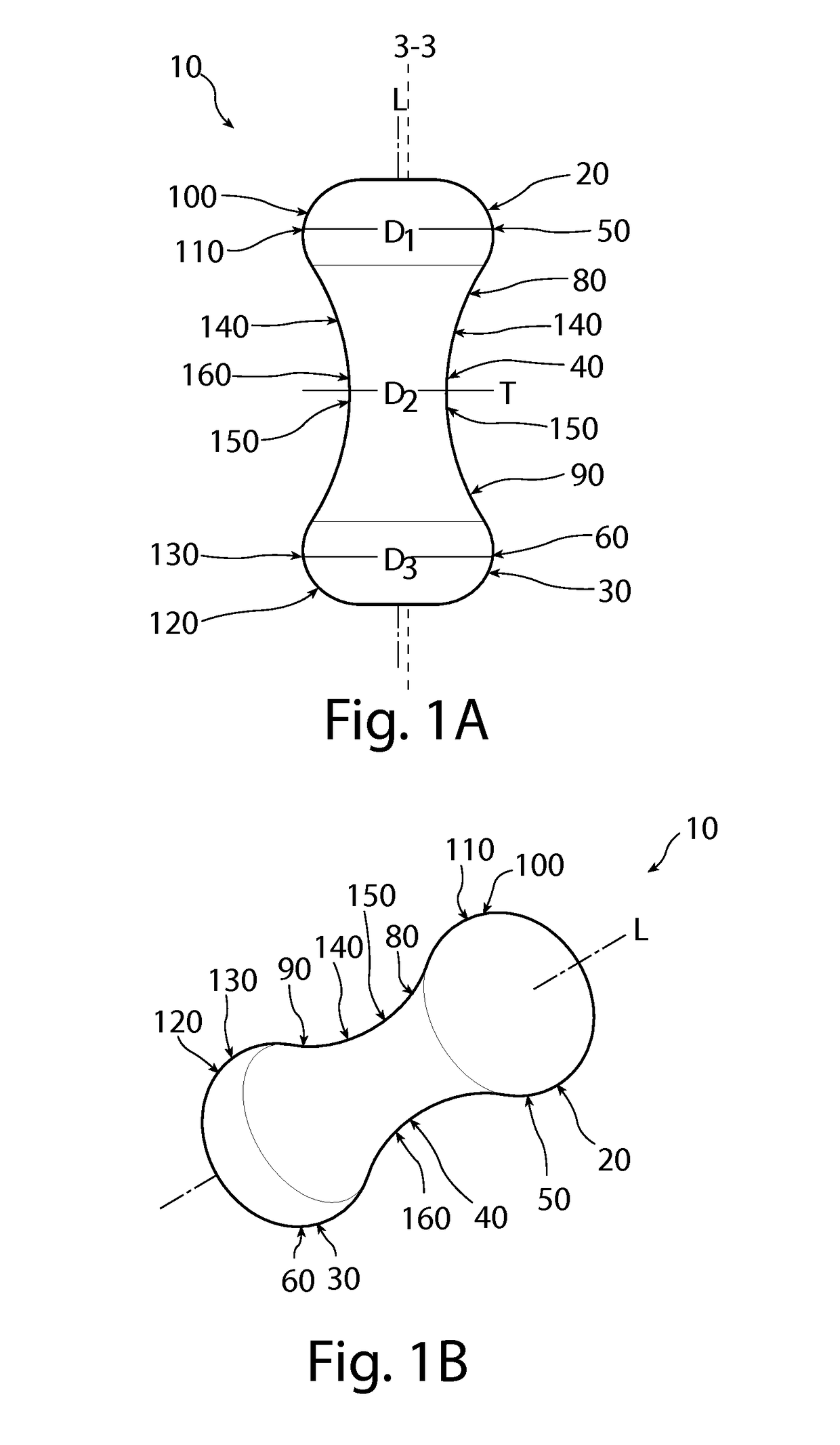
Pessary device with longitudinal flexibility
An intravaginal pessary device. The device has a top, a mid-section, and a bottom. The device has a sidewall that extends between the top and the bottom, and the top, the bottom, and the sidewall form an outer periphery defining a total area of the device. The top has a first width and the mid-section has a second width, and the first width is greater than the second width. In addition, the top has a first flexibility and the mid-section has a second flexibility, and the first flexibility is greater than the second flexibility.
Owner:PROCTER & GAMBLE CO
Pessary applicator providing low placement
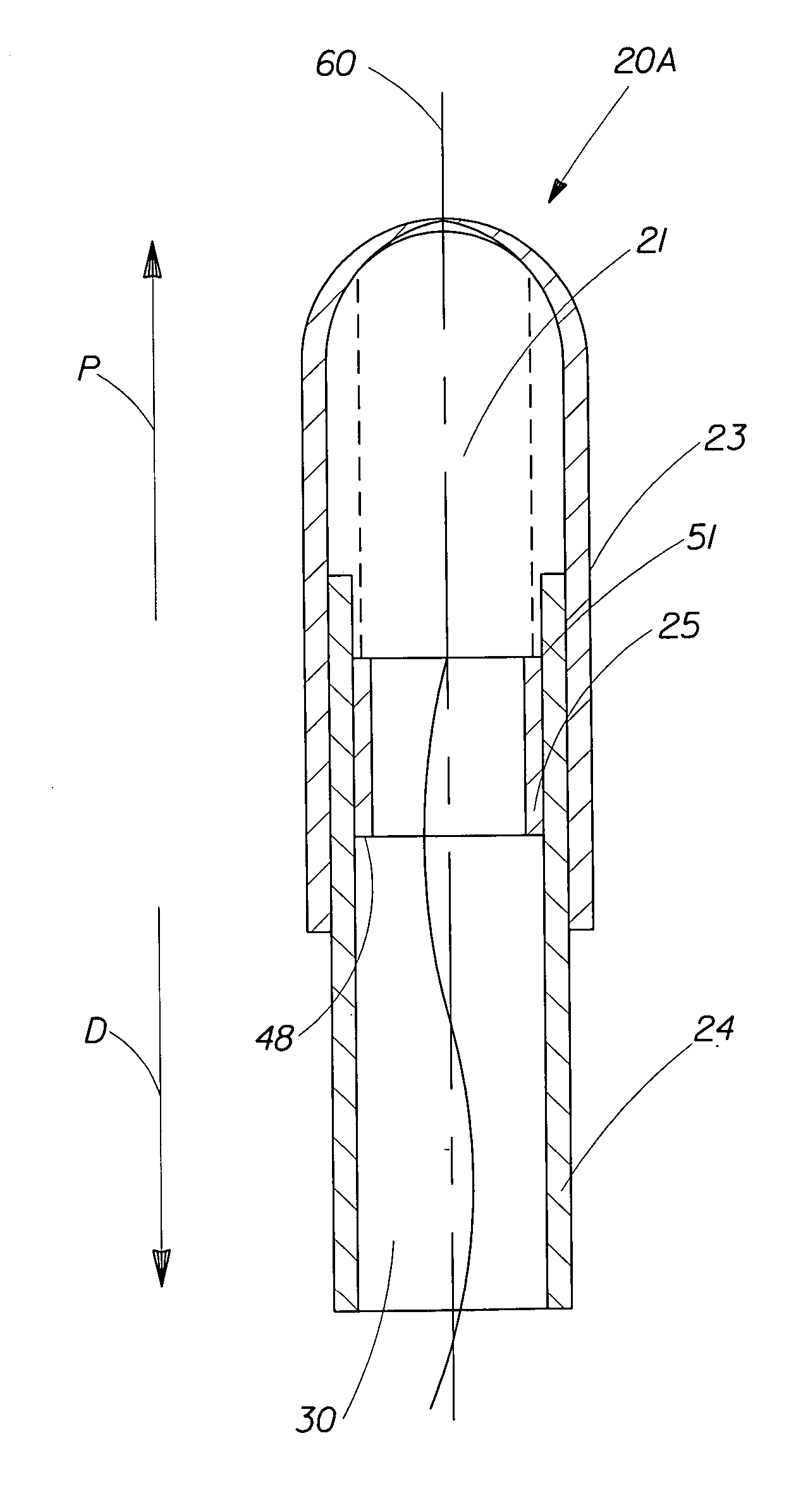
A pessary applicator for positioning a pessary inside a vaginal cavity. The pessary applicator includes an outer member disposed co-axially with an inner member for insertion of the pessary into the vaginal cavity. The inner member is slidable within the outer member wherein at least 15% of a length of the pessary remains in the outer member when the inner member is fully engaged with the outer member to define a remaining portion of the pessary. The remaining portion of the pessary requires a force of less than about 0.30 N to be removed from the pessary applicator. Alternatively, when the inner member is fully engaged with the outer member to define a remaining portion of the pessary, the remaining portion of the pessary requires a force of less than about 0.04 N / mm to be removed from the outer member.
Owner:PROCTER & GAMBLE CO
Device and method for fitting a pessary
There is provided a device for measuring an inside shape of a vagina, including a device body, operationally connected to one or more movable cheeks, the cheeks movable in a direction substantially transaxially relative to the device body longitudinal axis, and a width indicator, for indicating a distance between opposite faces of the cheeks. There is provided a method for fitting a pessary including inserting a Pessary Caliber (PC) into a vagina, extending cheeks of the PC to press against sides of the vagina, measuring a distance between the cheeks, measuring how deep the PC is inserted into the vagina, and selecting a pessary size based, at least in part, on the measuring. Related apparatus and methods are also described.
Owner:CONTIPI IP LLC
Pessary and lubricant system and method
A device comprising a tubular applicator and lotion dispenser in combination. The device includes a tubular applicator and a lotion dispenser for applying lotion to the tubular applicator. The lotion dispenser includes a lotion selected from the group consisting of gels, solutions, compositions, lubricants, and creams.
Owner:THE PROCTER & GAMBLE COMPANY
Pessary
Owner:石井贤治
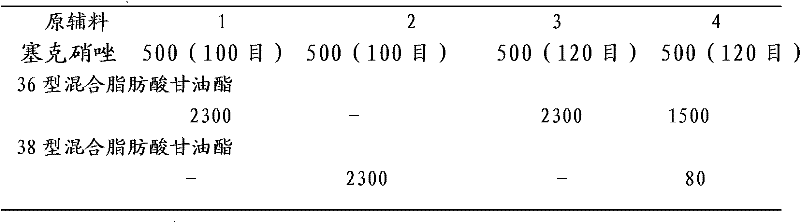
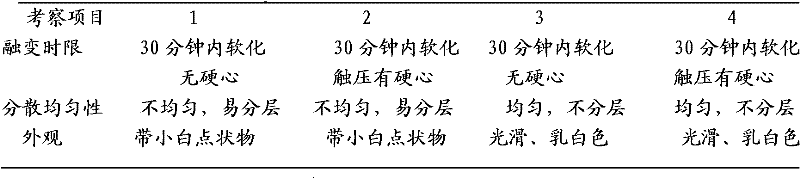
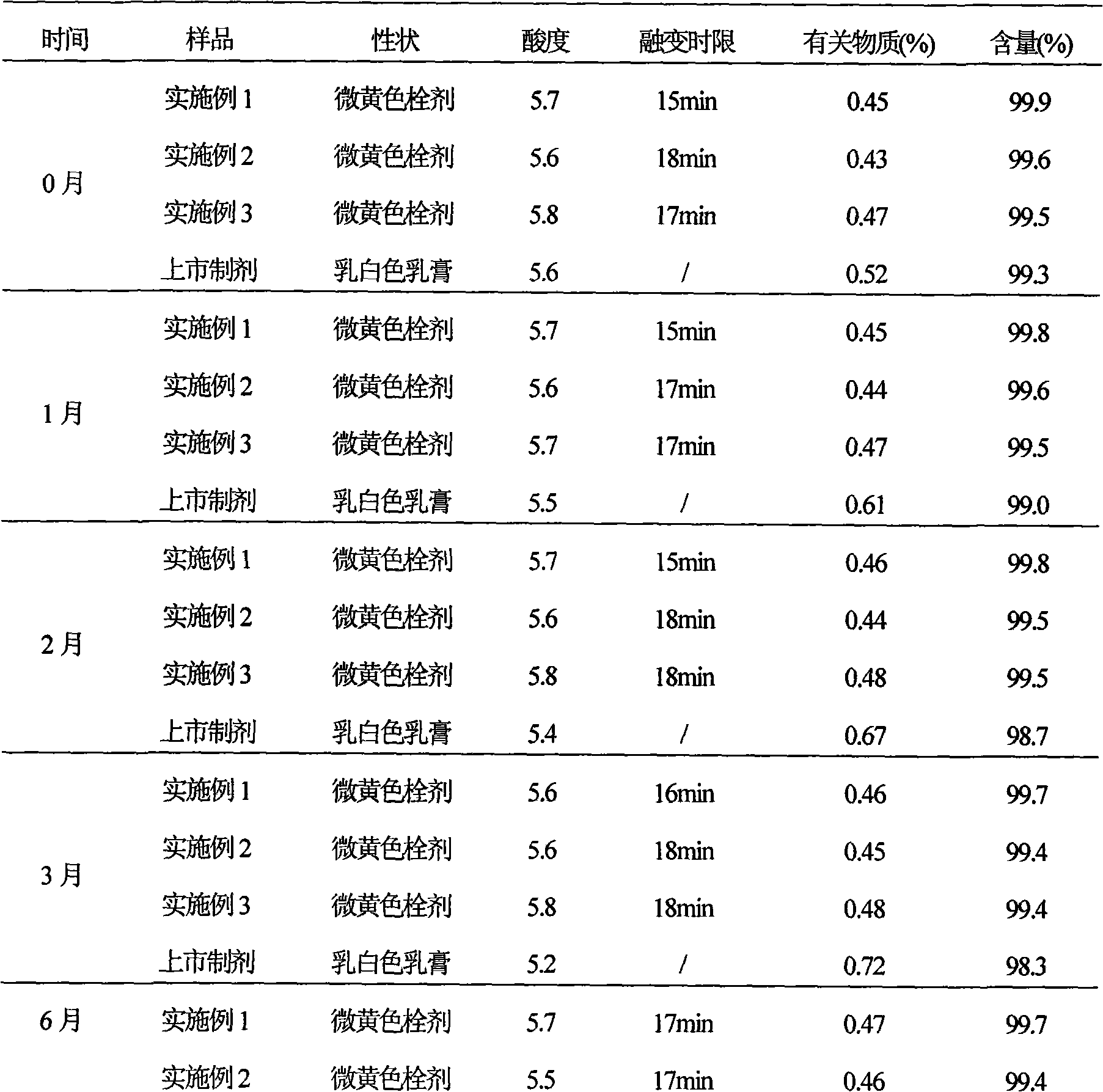
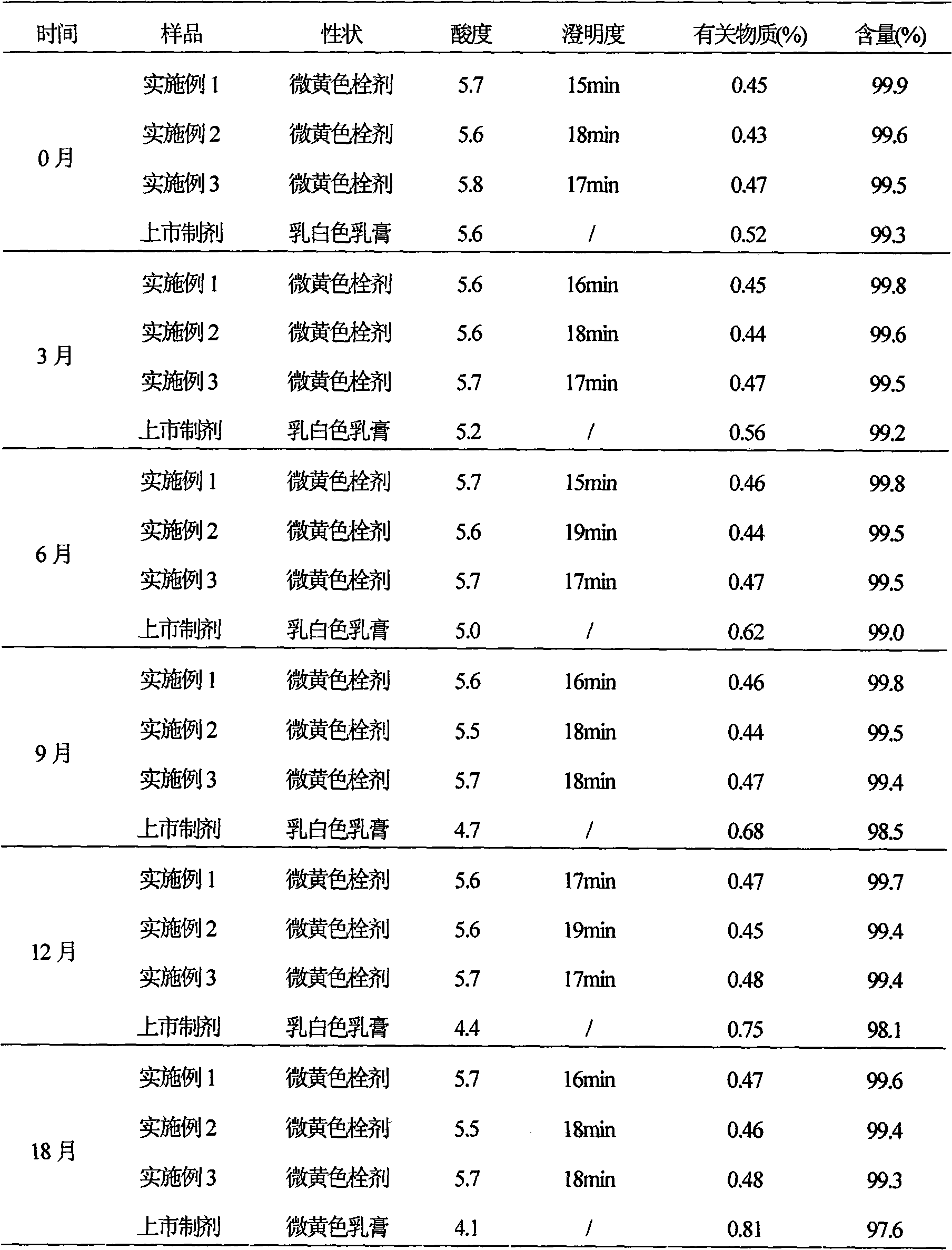
Secnidazole vaginal suppository and its preparation process
InactiveCN102266284ASimple recipeSimple production processOrganic active ingredientsAntimycoticsSide effectMonoglyceride
The secnidazole vaginal suppository of the present invention is composed of the following components: main ingredient secnidazole 500g, auxiliary material 36 type mixed fatty acid glyceride 2300g, made into 1000 suppositories, each weighing 2.8 grams, the 36 type mixed Fatty acid glycerides are a mixture of triglycerides, diglycerides and monoglycerides, and the melting point of type 36 mixed fatty acid glycerides is 35-37°C. The drug of the secnidazole suppository of the present invention is directly absorbed through the vagina, and will not cause damage to the heart, kidney and liver after testing. The curative effect is good, there is basically no side effect, the proportion is simple, and the use is convenient. The invention also discloses the preparation process of the secnidazole vaginal suppository at the same time.
Owner:HUBEI TUNGSHUN PHARMA
Pessary applicator providing low placement
A pessary applicator for positioning a pessary inside a vaginal cavity. The pessary applicator includes an outer member, an inner member which is slidable within the outer member, and a pessary which is housed within the pessary applicator. The inner member has an effective length of less than about 58 mm.
Owner:THE PROCTER & GAMBLE COMPANY
Use of 5-aminolevulinic acid and derivatives in a solid form for photodynamic treatment and diagnosis
The present invention relates to the use of a photosensitiser which is 5-ALA or a precursor or derivative thereof (e.g. an ALA ester), in the manufacture of a pharmaceutical product for use in the photodynamic treatment or diagnosis of cancer, an infection associated with cancer, or in the treatment or diagnosis of a non-cancerous condition, wherein said pharmaceutical product is in the form of a solid. The invention also relates to solid pharmaceutical products for use in such methods, e.g. suppositories, pessaries, tablets, pellets and capsules which comprise 5-ALA or a precursor or derivative thereof (e.g. an ALA ester) and at least one pharmaceutically acceptable carrier or excipient. Such products are particularly suitable for use in the photodynamic treatment or diagnosis of cancerous or non not cancerous conditions in the lower part of the gastrointestinal system or in the female reproductive system, e.g. in the treatment or diagnosis of colorectal cancer or cervical cancer.
Owner:PHOTOCURE
Ciclopirox olamine pessary and preparation method thereof
InactiveCN101579306AImprove stabilityFast drug effectOrganic active ingredientsAntimycoticsSide effectWhole body
The invention relates to a ciclopirox olamine pessary capable of reducing toxic and side effects of a medicament caused by systemic delivery, increasing local concentration of the medicament, fully playing the therapeutic effectiveness of the medicament and improving the adaptability of patients. The pessary can realize zero order release and is prepared by the following materials by weight portion: 1 portion of ciclopirox olamine, 65 to 90 portions of substrate, 0.2 to 3 portions of stearic acid, 0.1 to 5 portions of hydrogenated castor oil and 0.05 to 2 portions of sodium benzoate.
Owner:HAINAN YONGTIAN PHARMA INST
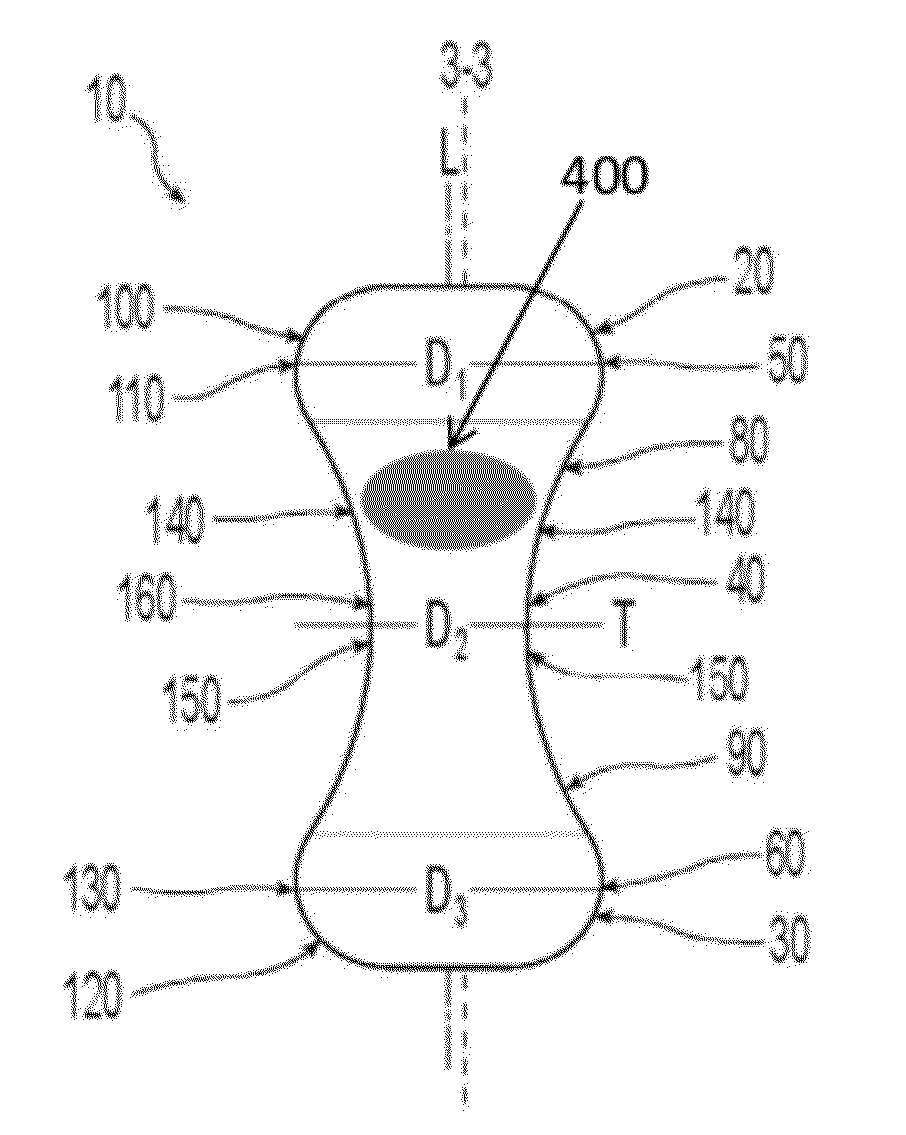
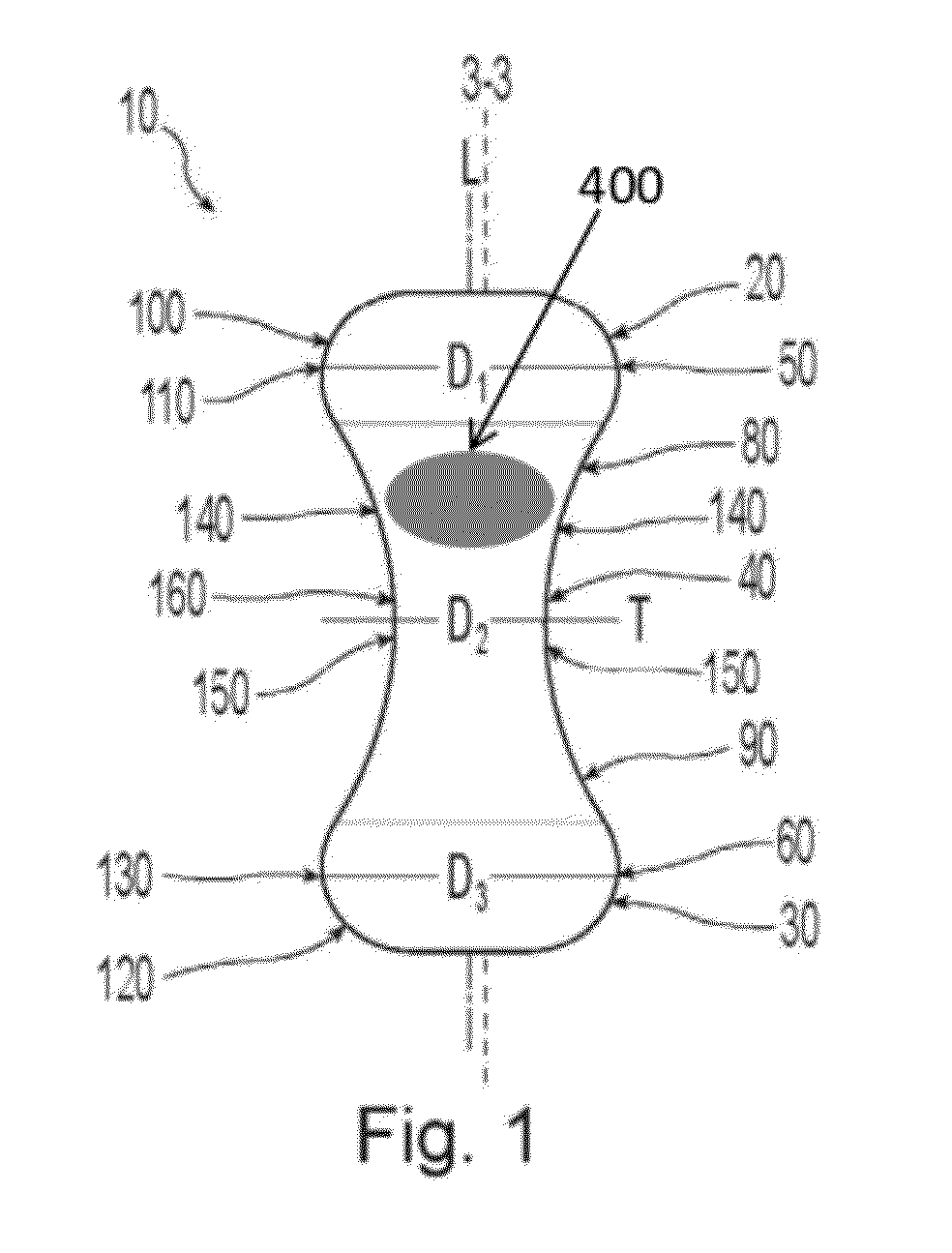
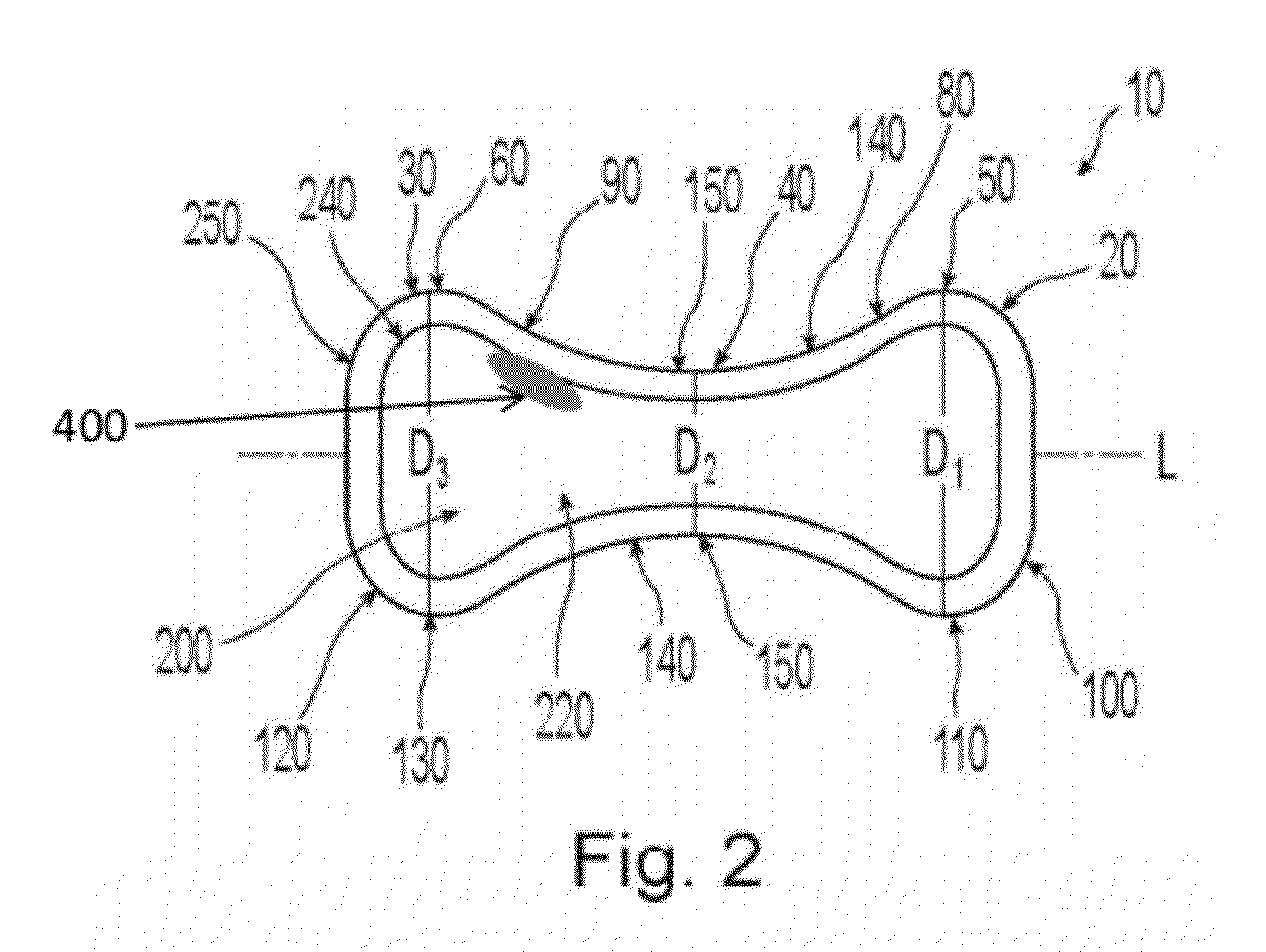
Pessary device
ActiveUS20120259159A1OptimizationAnti-incontinence devicesBed wetting preventionUrethraMaximum diameter
A non-expandable pessary device, the pessary device having a top, a base, a length, a longitudinal axis, a maximum diameter, and a minimum diameter that is less than the maximum diameter. The pessary device has a pressure region adapted to extend between an anterior vaginal wall and a posterior vaginal wall of a user to provide pressure on the user's urethra through the vaginal wall. The pressure region includes the maximum diameter, and the maximum diameter is less than 25 mm.
Owner:THE PROCTER & GAMBLE COMPANY
Pessary or intrauterine medicine release device containing antiestrogenic and anti-pregnant hormone composite preparation and its use
The present invention is a kind of pessary or intrauterine medicine releasing device containing one medicine part, and features the medicine part containing anti-estrogen in 40-70 weight portions and anti-progestogen in 30-60 weight portions. The present invention also provides the application of the pessary or the intrauterine medicine releasing device in preparing medicine for treating hysteromyma or endometriosis.
Owner:SHANGHAI INST OF PLANNED PARENTHOOD RES +4
Pessary with slow-release support
The invention relates to a pessary with a slow-release support. The slow-release support is installed inside the pessary, medicines are arranged on the support, medicines are separated from the outside through micro-hole films, and diameters of micro holes of micro-hole films are in a range from 0.1 nm to 10 mm in accordance with diameters of passing medicine. Due to own movement, organ oppression and micro hole diameter limitation, medicines slowly act on a vagina and a cervix and directly act on target organs in accordance with preset speed and the clinical effect is achieved. By the aid of the pessary, a physical mode and a chemical mode can be used for curing prolapsus and supplementing hormone.
Owner:江苏奥博金医药科技有限公司
Method for treating urinary incontinence
InactiveUS20120259163A1Reducing urinary incontinenceAnti-incontinence devicesBed wetting preventionUrethraMaximum diameter
A method for reducing urinary incontinence in a female. The method includes inserting into the female's vagina a first non-expandable intravaginal pessary device, the pessary device having a top, a base, a length, a longitudinal axis, a maximum diameter, and a minimum diameter that is less than the maximum diameter. The pessary device has a pressure region adapted to extend between an anterior vaginal wall and a posterior vaginal wall of a user to provide pressure on the user's urethra through the vaginal wall, and the pressure region comprising the maximum diameter, wherein the maximum diameter is less than 25 mm. The pessary device is retained within the vagina of the female for about 8 to about 12 hours, and removed and disposed. A second non-expandable intravaginal pessary device is then inserted into the female's vagina.
Owner:THE PROCTER & GAMBLE COMPANY
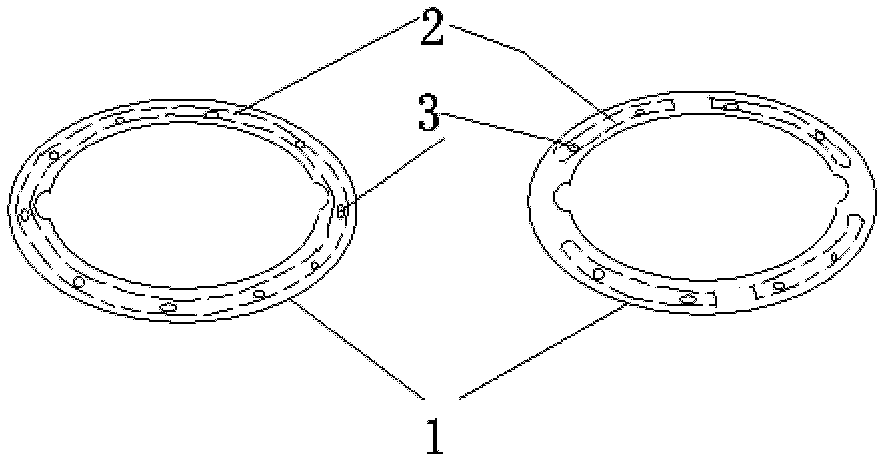
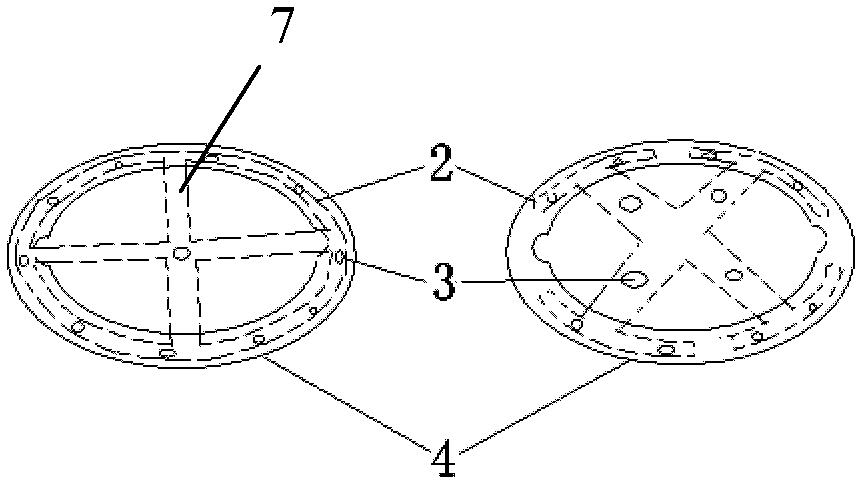
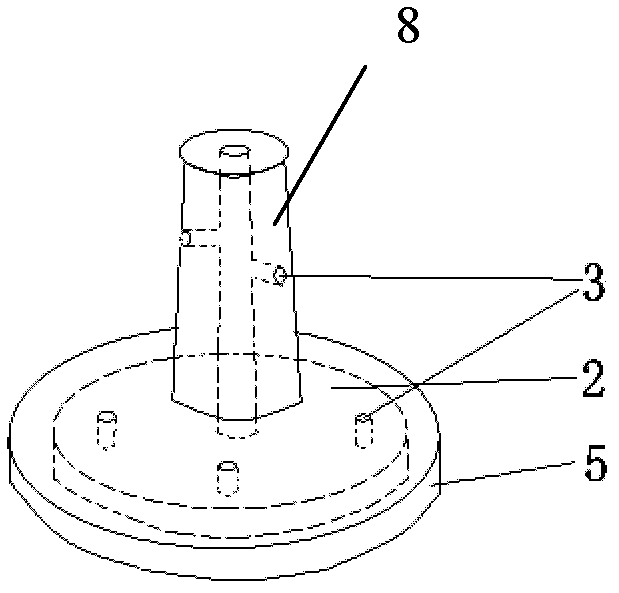
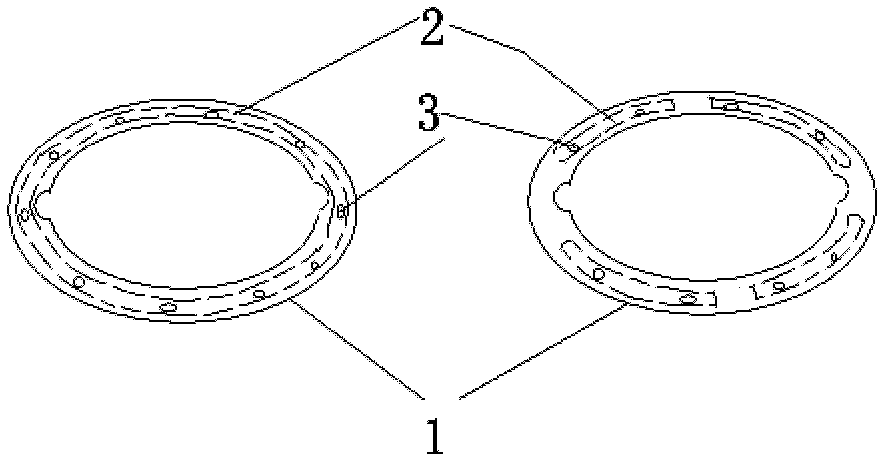
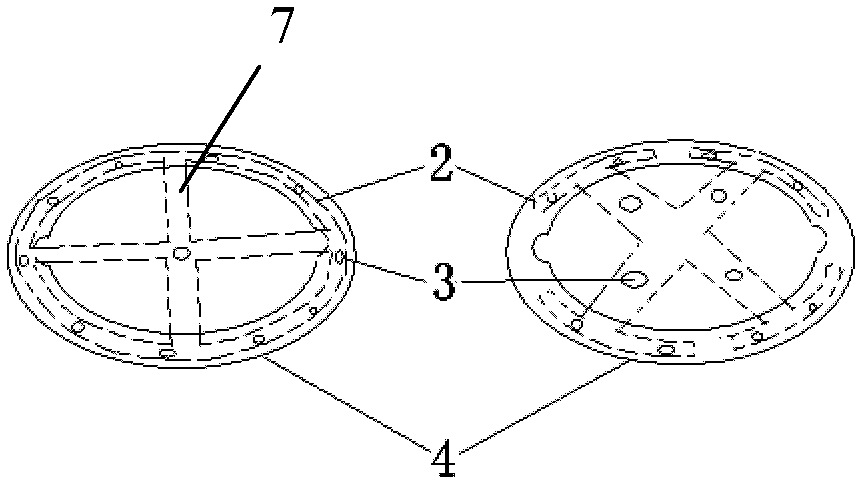
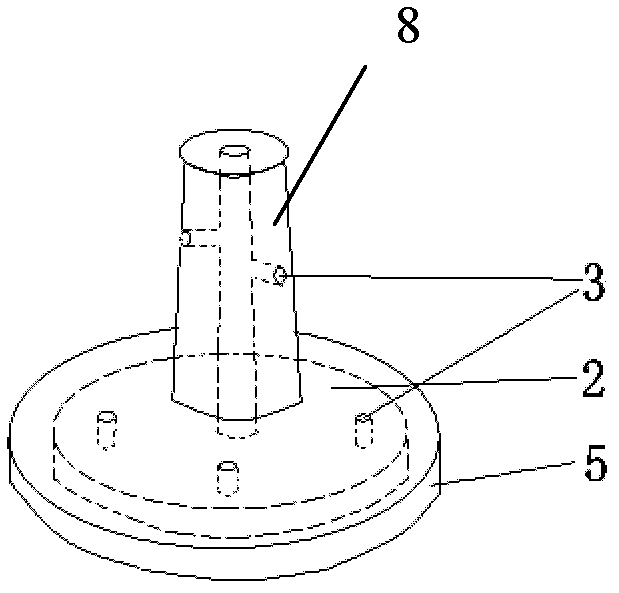
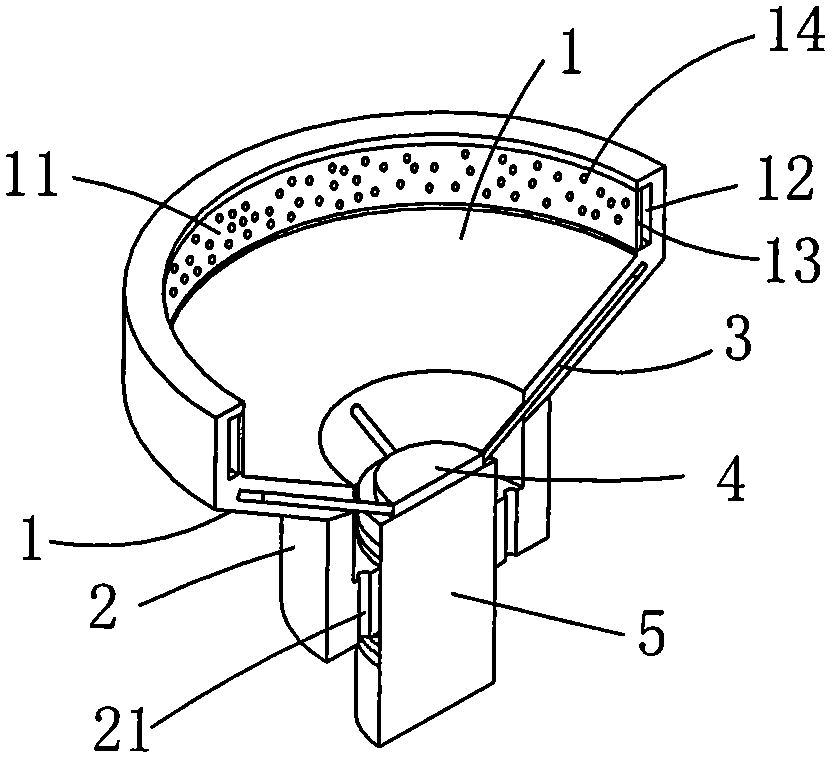
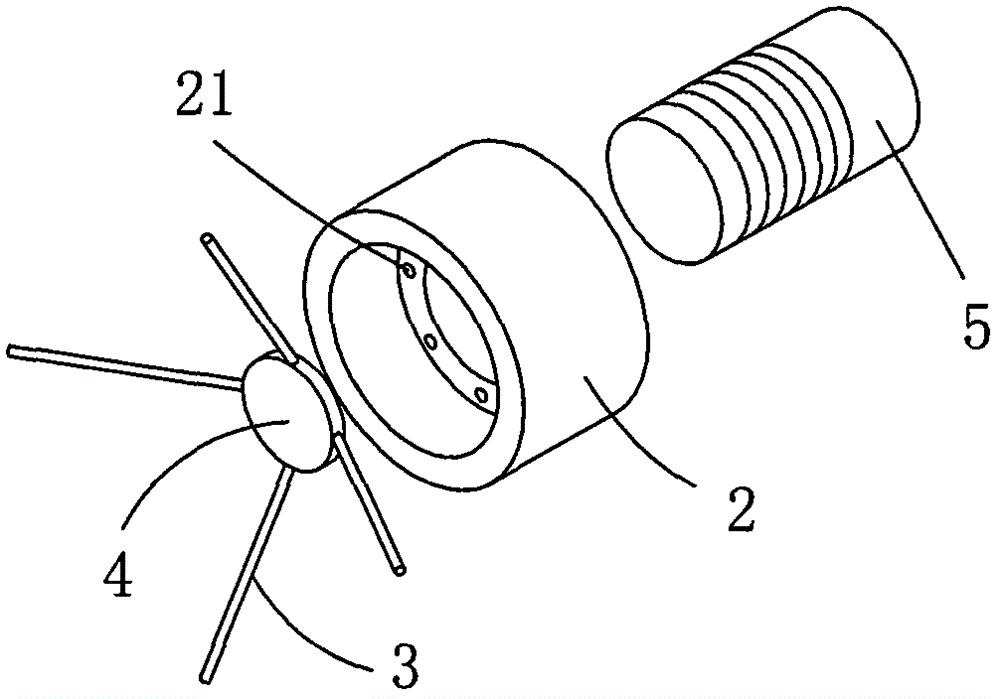
Adjustable pessary
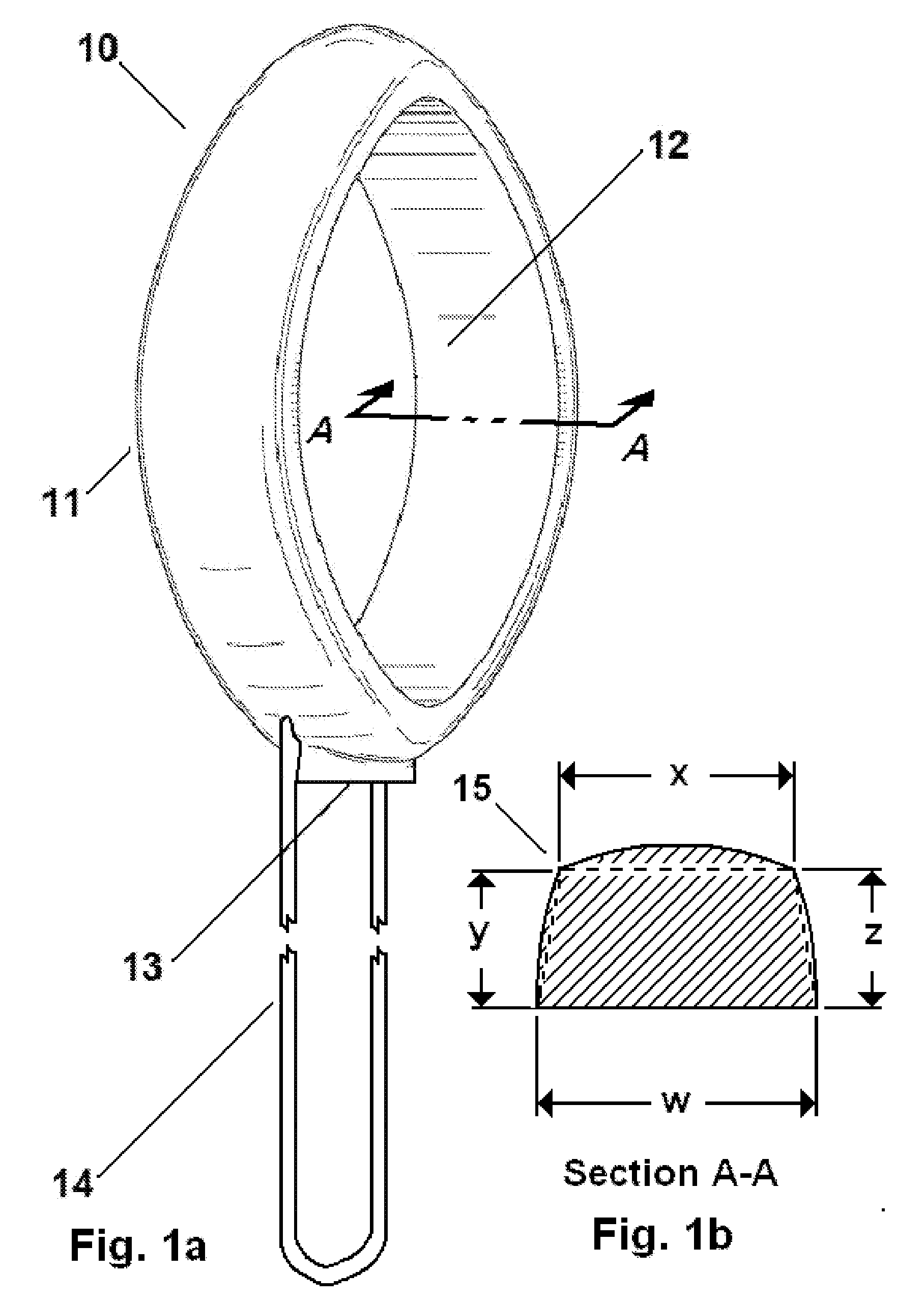
The invention relates to an adjustable pessary. A pessary body (1) of the pessary is in a horn mouth shape and is fixed to an annular body (2). A plurality of adjustment rods (3) are arranged in the pessary body (1). A rodless area (11) is formed between the upper ends of the adjustment rods (3) and the upper edge of the pessary body (1). The bottom ends of the adjustment rods (3) are exposed out of the pessary body (1) and are hinged to a movable piece (4). The movable piece (4) is located in the annular body (2) and can move up and down along the annular body (2). A movable piece push rod (5) is arranged in the annular body (2). The top of the movable piece push rod (5) is fixed to the movable piece (4). The movable piece push rod (5) pushes the movable piece (4) to move up and down so that the pessary body (1) can be transversely expanded or can transversely contract. The adjustable pessary has the advantages of being simple in structure, practical in function and obvious in effect, the size can be adjusted, the adjustable pessary is not prone to falling, and medicine in medicine containing space of the adjustable pessary is not prone to loss.
Owner:NINGBO COLLEGE OF HEALTH SCI
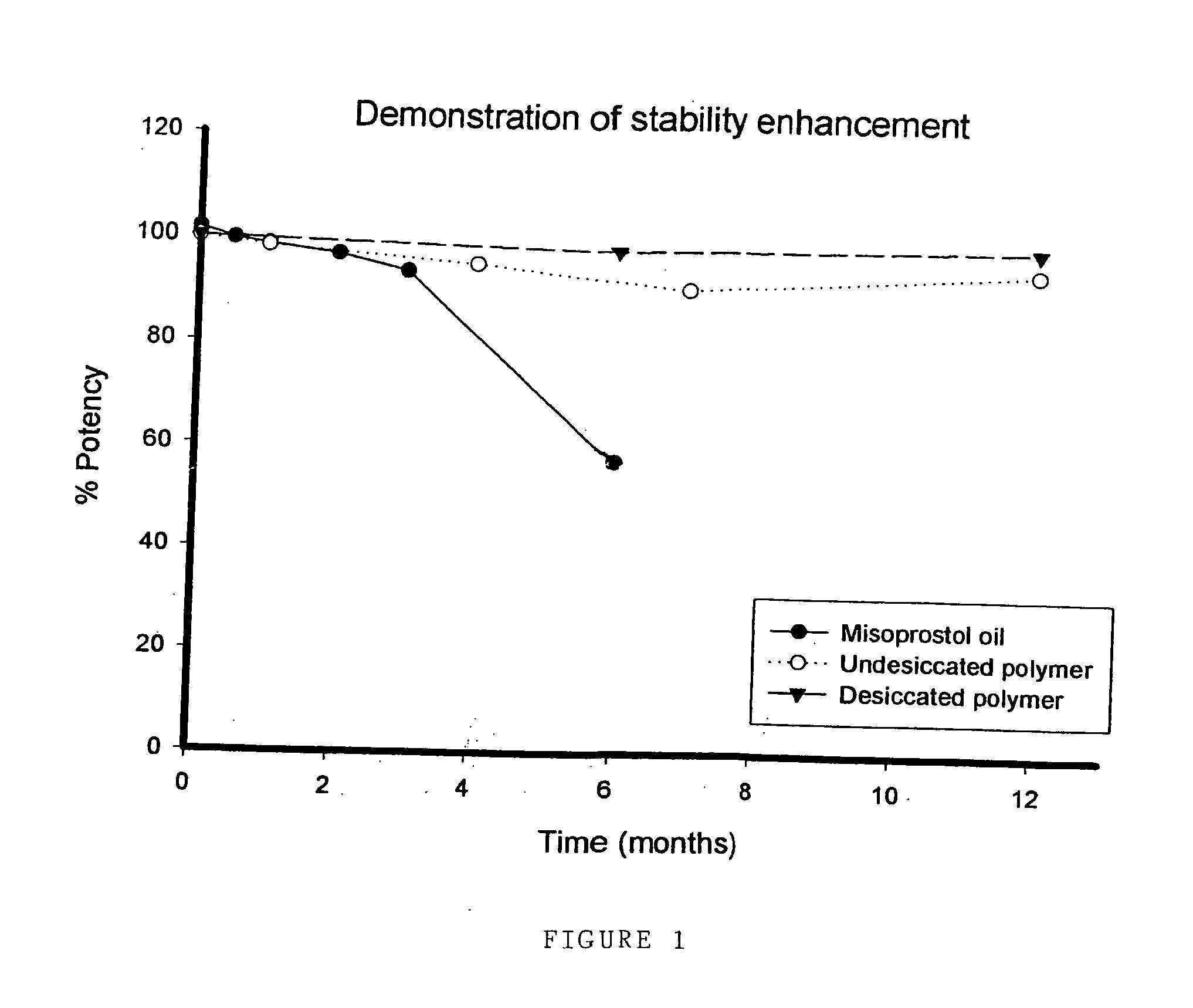
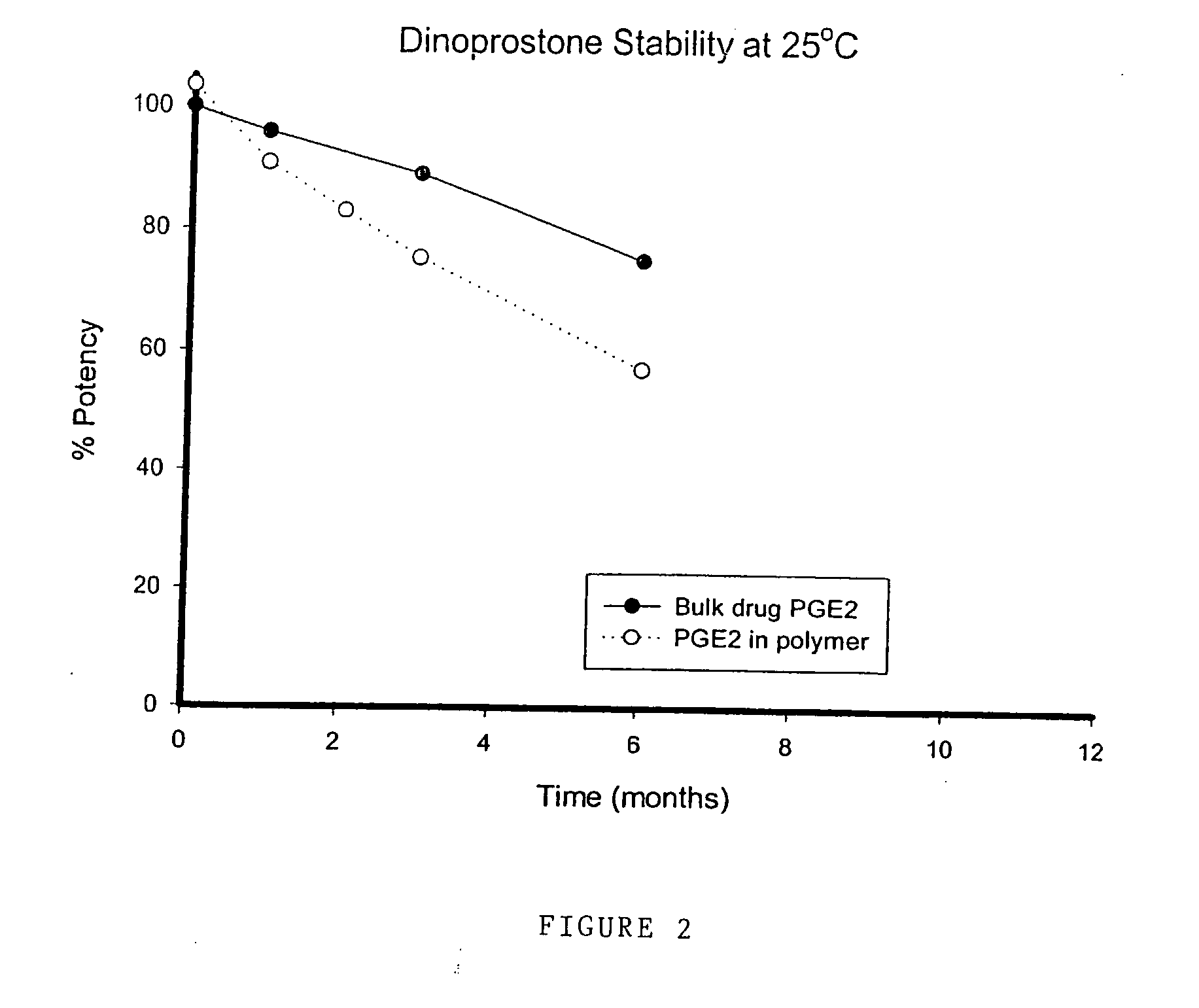
Stabilised prostaglandin composition
InactiveUS20070212391A1Improve propertiesGood storage stabilityBiocidePowder deliveryCross-linkSynthetic Prostaglandins
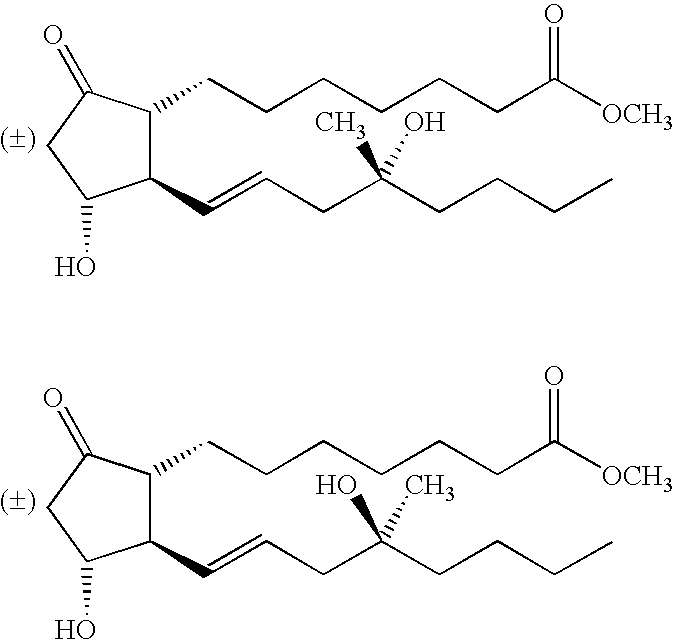
A pharmaceutical delivery device, such as a suppository or pessary, comprises a synthetic prostaglandin PGE1 analogue (e.g. misoprostol) in a solid polyurethane hydrogel. The polyurethane is either linear or cross-linked.
Owner:FERRING BV
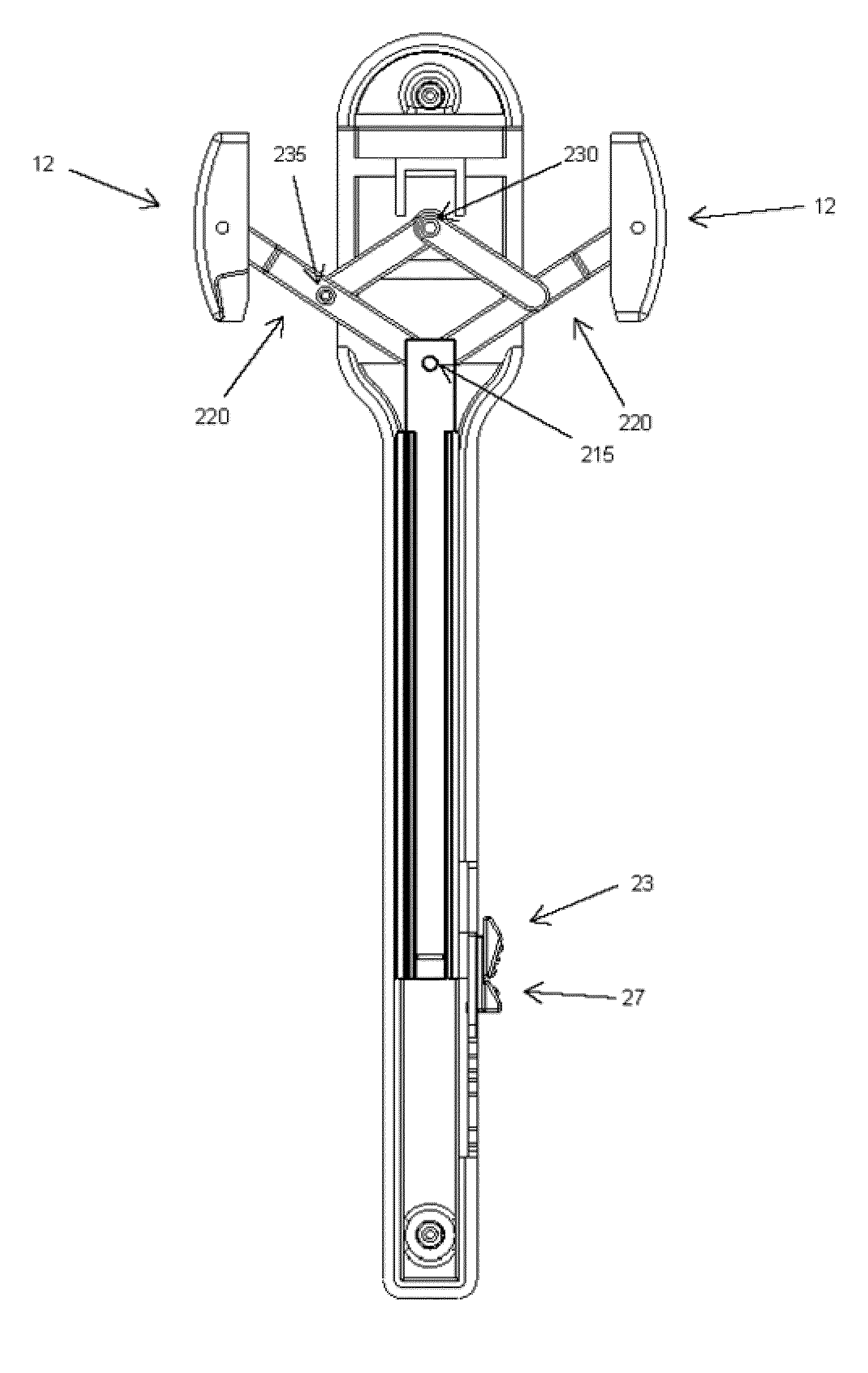
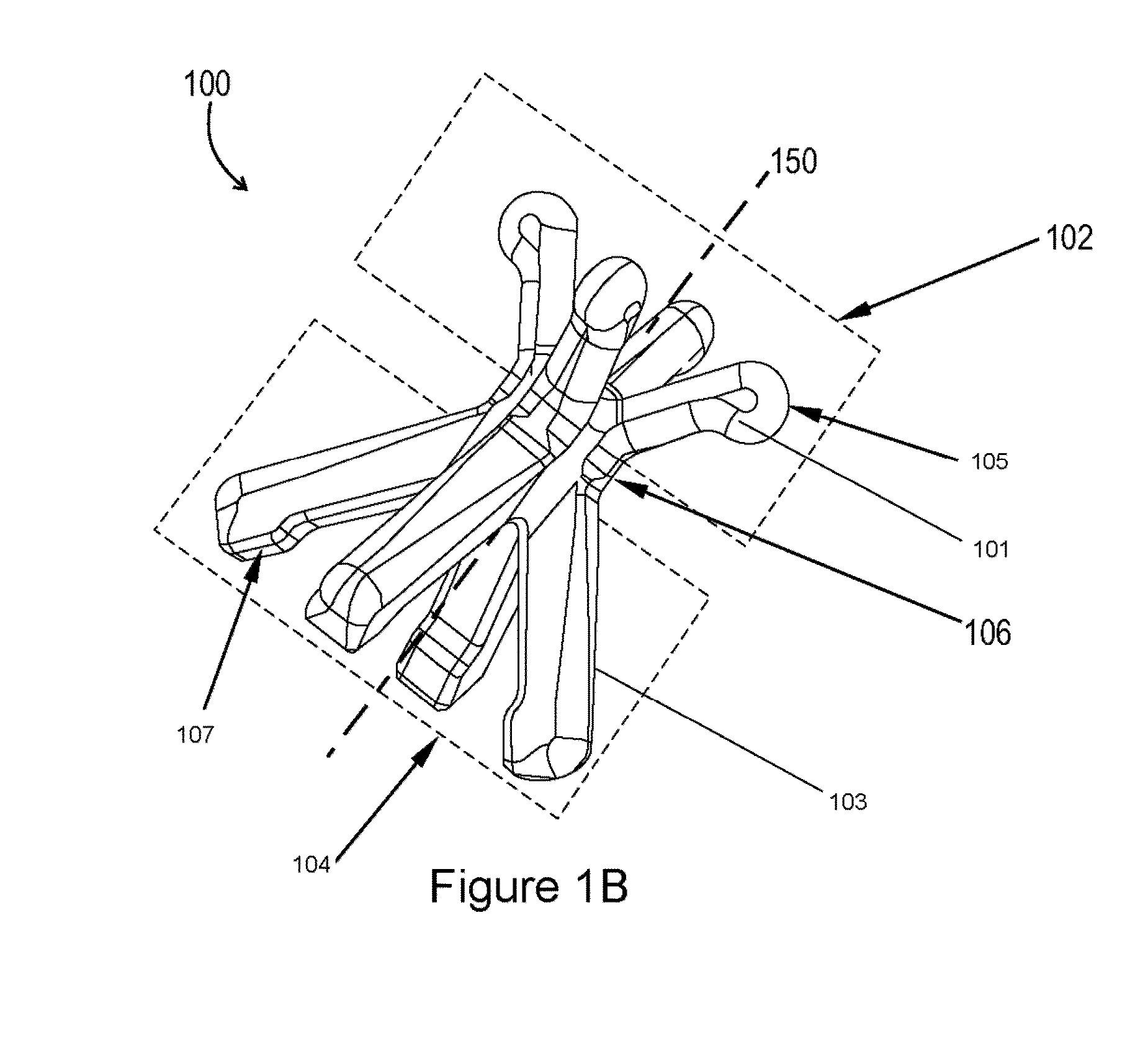
Pessary for pelvic organ prolapse
A collapsible pessary is provided, and can have a stem and at least one rotating petal member that can rotate between a collapsed state with a smaller diameter and a deployed state with a larger diameter. The collapsible pessary can have a plunger within the stem, and pushing the plunger upwards can cause the plunger to push upwards on a portion of the petal member, thereby causing the petal member to rotate into a deployed position. The pessary can be in a collapsed state wherein its overall diameter is at a minimum, or a deployed state, wherein its overall diameter is at a maximum.
Owner:DARTMOUTH HITCHCOCK CLINIC +1
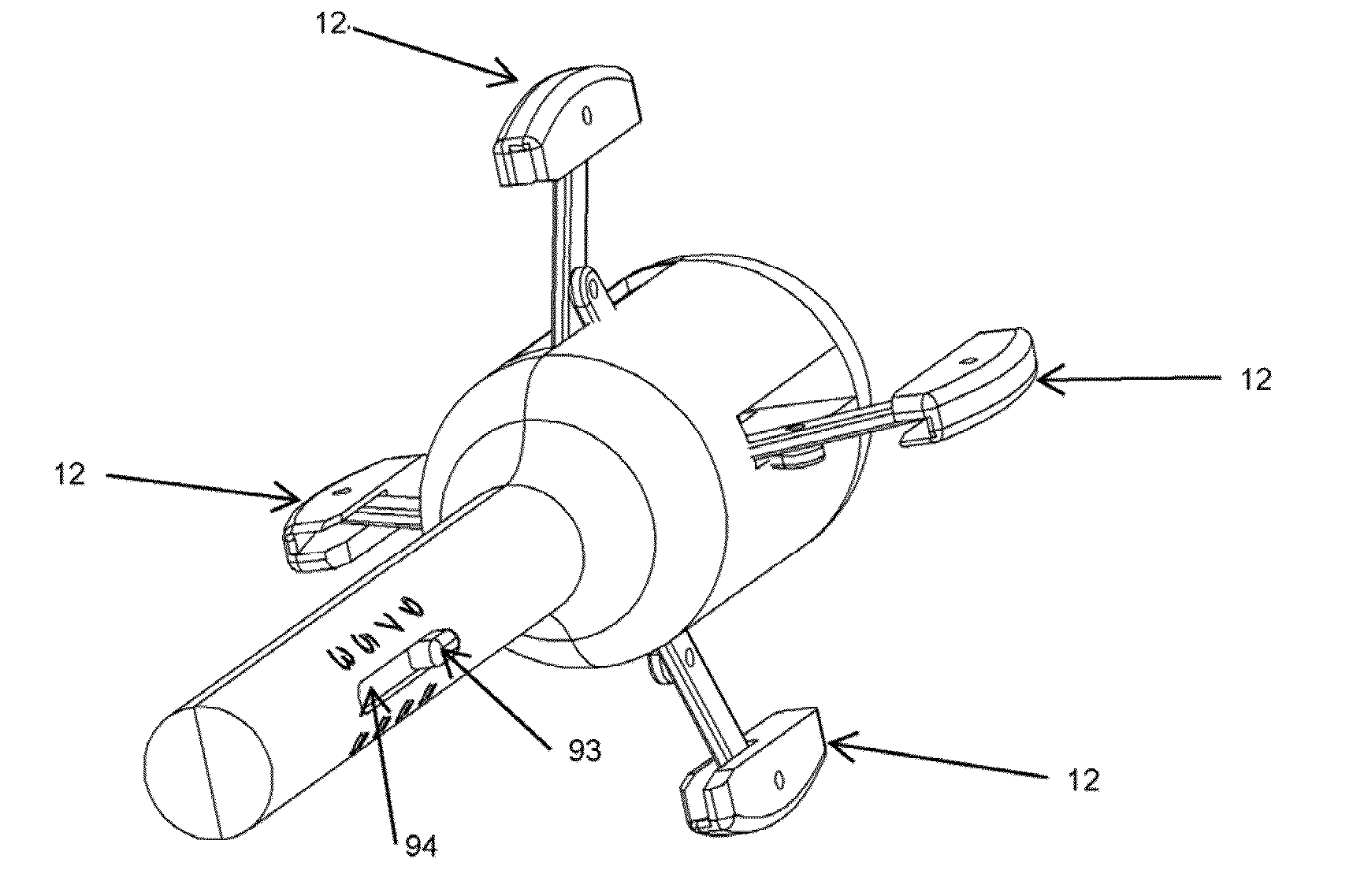
Pessary device
InactiveUS8887731B2Easy to inflateEasy to disassembleAnti-incontinence devicesSurgeryPessaryCheck valve
A pessary having a main stem and an inflatable bladder disposed about the proximal larger diameter section of the main stem. The main stem further provides a distal smaller diameter portion having a cap at its distal tip and a check valve disposed there under. The check valve communicates with a central fluid passage that may extend into the proximal larger diameter section of the main stem. Secondary fluid passages connect the central fluid passage to the inflatable bladder volume disposed about the outer circumference of the proximal larger diameter section of the main stem. The main stem provides support to the pelvic structures and the inflatable bladder acts to hold the device in position. At least one void through the proximal larger diameter portion of the main stem allows bodily material to pass from the vagina through the pessary device thereby decreasing the amount of vaginal discharge.
Owner:ZIPPER RALPH
Pessary applicator providing low placement
A pessary applicator for positioning a pessary inside a vaginal cavity. The pessary applicator includes an outer member, an inner member, a pessary, and a pessary positioning member. The outer member is disposed co-axially with the inner member for insertion of the pessary into the vaginal cavity. The inner member includes a first end, a second end, and an interior surface. The inner member is slidable within the outer member. The pessary is at least partially housed within the inner member. The pessary positioning member is positioned within the inner member. The pessary positioning member includes a pessary pushing surface positioned between the first end and the second end of the inner member.
Owner:THE PROCTER & GAMBLE COMPANY
Medical Exercise Device
InactiveUS20130324381A1Simple designFacilitate ease of insertion and flexibility and resistanceResilient force resistorsFemale contraceptivesRegimenDilator
Disclosed is an intra-vaginal device of a new and innovative design that in combination with kegel exercises or performing ones daily routine strengthens the muscles of the pelvic floor. The device employs a combination of unique shape, materials as well as carefully engineered deflection, frictional, testing and clean-ability characteristics with particular attention to a sexually neutral appearance, comfort during insertion and exercise as well as positive physical feedback to encourage prolonged and frequent use. Benefits of the device include improvement or prevention of urinary leakage and prolapse, increases in sexual intensity, to keep the uterus, bladder or rectum in place as a pessary as well as relief for women suffering from moderate prolapse. As a dilator to aid women who suffer from vaginismus. With its multiple features and benefits, the device is a vital tool in a woman's quest for a complete and balanced wellness regimen.
Owner:HORSLEY CARYN M
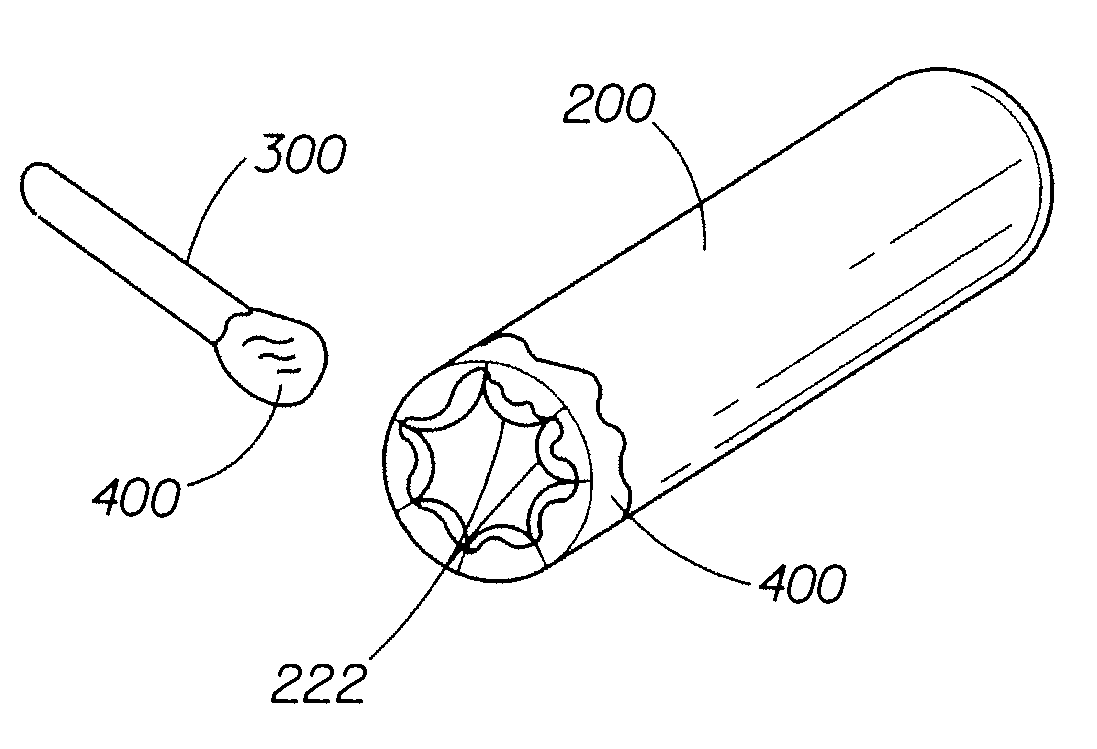
Method of conforming an overwrap to a pessary device
Owner:PROCTER & GAMBLE CO
Device and method for treatment of dysmenorrhea
InactiveCN1263454AIncrease drug concentrationReduce first pass metabolismSuppositories deliveryMedical devicesHigh concentrationMuscle contraction
Methods, devices, and compositions for treatment of dysmenorrhea comprise an intravaginal drug delivery system containing an appropriate pharmaceutical agent incorporated into a pharmaceutically acceptable carrier whereby the pharmaceutical agent is released into the vagina and absorbed through the vaginal mucosa to provide relief of dysmenorrhea. The drug delivery system can be a tampon device (42), vaginal ring, pessary, tablet, suppository, vaginal sponge, bioadhesive tablet, bioadhesive microparticle, cream lotion, foam, ointment, paste solution, or gel. The system delivers a higher concentration to the muscle of the uterus, the primary site for the dyskinetic muscle contraction, which is the pathophysiologic cause of dysmenorrhea.
Owner:UNIVERSITY OF MINNESOTA DULUTH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com