Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
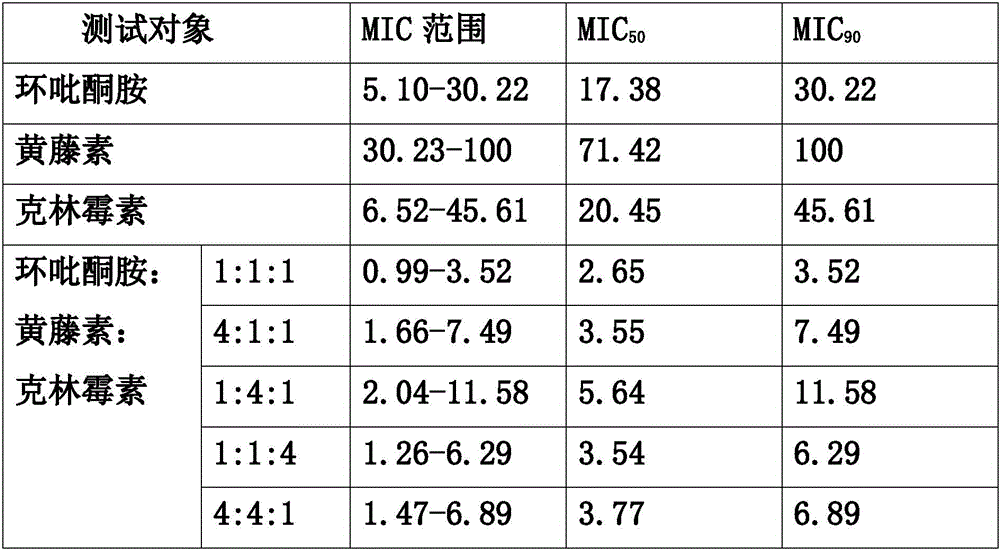
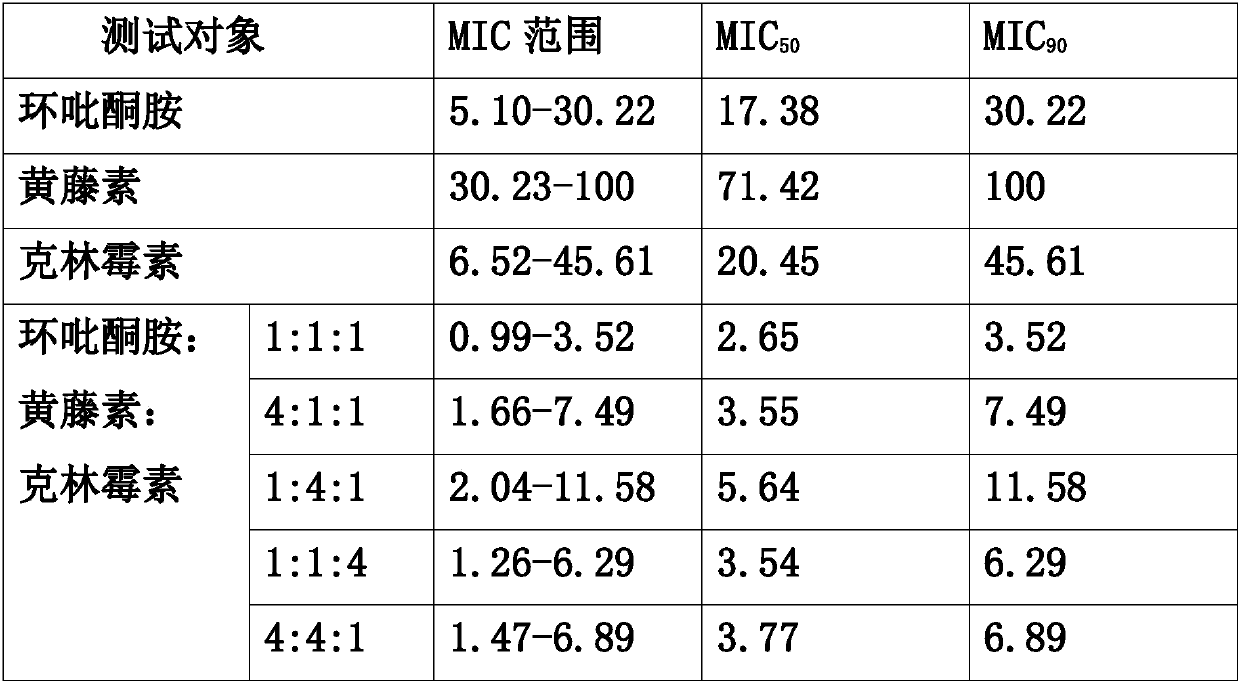
36 results about "Secnidazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
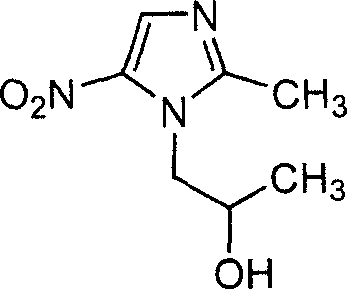
This medication is used to treat a certain type of vaginal infection (bacterial vaginosis).
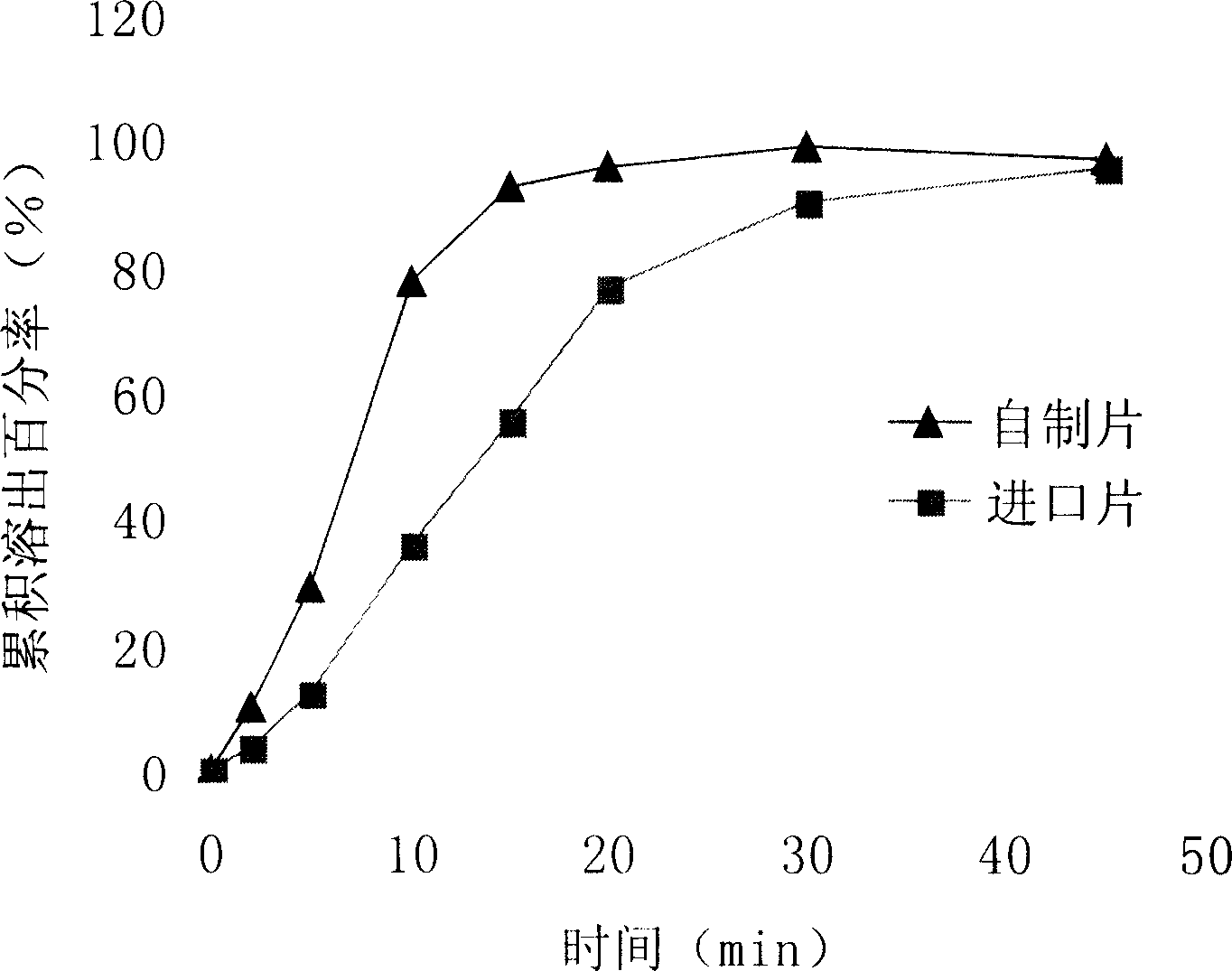
Secnidazole tablet and its prepn process
InactiveCN1973838AHigh dose of main drugPiece weight smallAntibacterial agentsOrganic active ingredientsCarboxymethyl celluloseMagnesium stearate
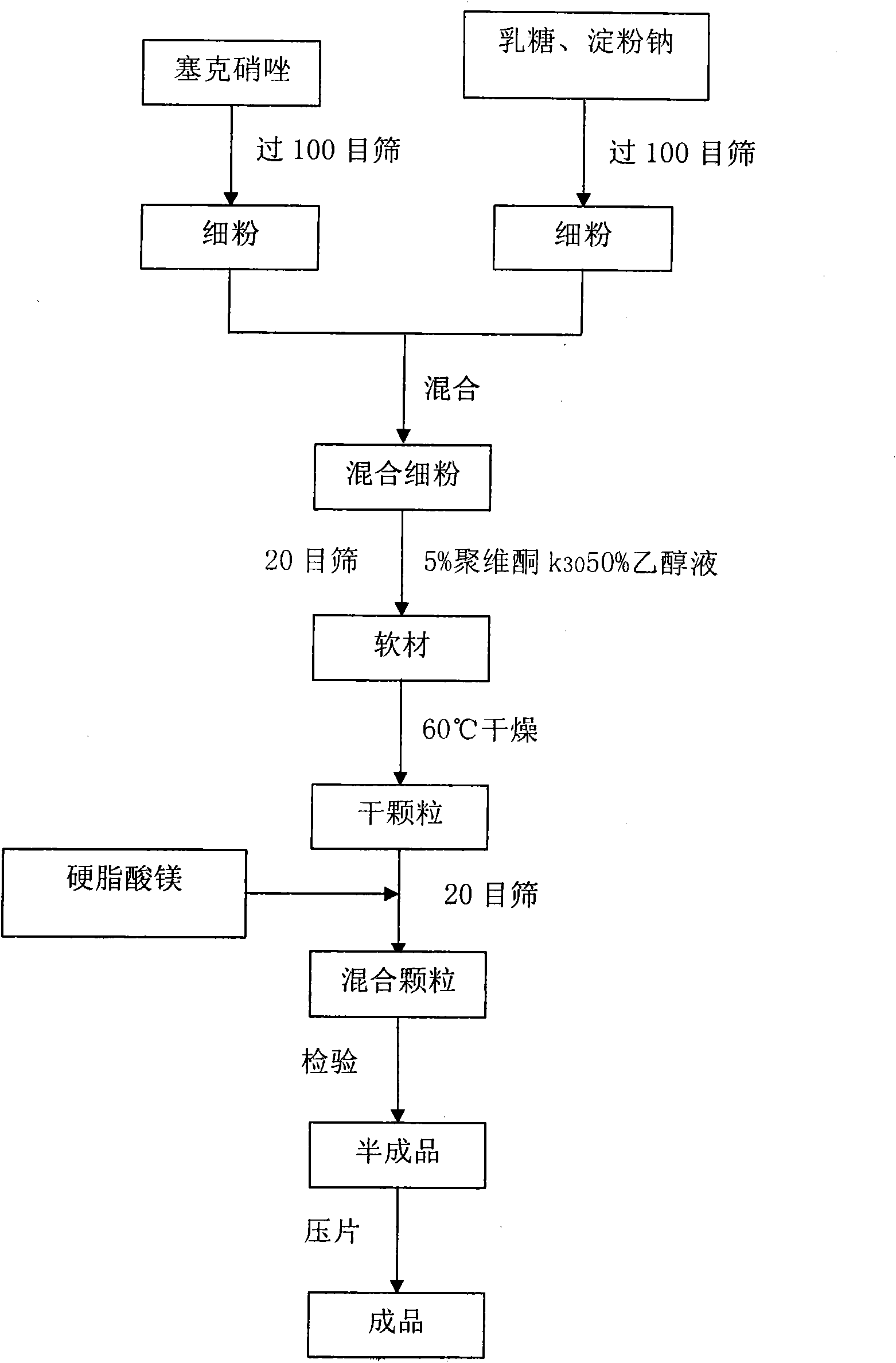
The secnidazole tablet contains secnidazole 50-90 wt%, microcrystalline cellulose, starch, pre-gelatinized starch and / or lactose 0.5-40 wt%, cross-linked sodium carboxymethyl cellulose and / or cross-linked polyvinyl pyrrolidone 0.5-9 wt%, polyvinyl pyrrolidone or hydroxypropylmethyl cellulose 0.1-2.5 wt%, magnesium stearate 0.5-1 wt%, and talcum powder and / or fine silica gel powder 0.5-1 wt%. The preparation process of the secnidazole tablet includes the steps of mixing the said materials, palletizing and tabletting. The secnidazole tablet has high effective component content, easy swallowing, no bitter taste and fast leaching.
Owner:湖北科益药业股份有限公司
Vaginal effervescence tablet of secnidazole and its preparation
InactiveCN1546020AStrong specificityGood curative effectOrganic active ingredientsPill deliveryEffervescent tabletCurative effect
The invention provides a Secnidazole vaginal effervescent tablet and its production method which comprises active constituent Secnidazole and effervescent tablet findings, wherein the Secnidazole amounts to 25-60% of the total weight.
Owner:苏州赫森医药科技有限公司
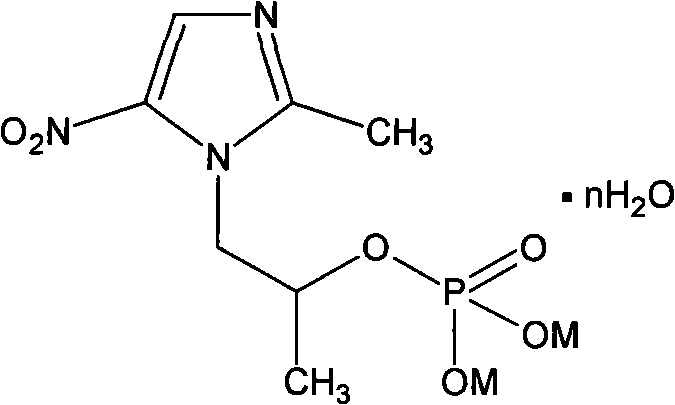
Secnidazole water-soluble salt and preparation method thereof
InactiveCN101255175AGood water solubilityEasy to reach dosageGroup 5/15 element organic compoundsAntiparasitic agentsWater solubleAmebic dysentery
The invention relates to a soluble derivate of secnidazole for curing amebic dysentery, that is secnidazole microcosmic salt or sufosuccinate monoester, wherein the compounds with above structure are chemical derivates of secnidazole which can act in the range of secnidazole. The aqueous solubility of the derivates is substantially better than secnidazole, so can be prepared to be a new type that the secnidazole can not, such as sterile powder for injecting. The invention further relates to preparation methods for secnidazole microcosmic salt or sufosuccinate monoester.
Owner:HEFEI UNIV OF TECH
Secnidazole vaginal suppository and its preparation process
InactiveCN102266284ASimple recipeSimple production processOrganic active ingredientsAntimycoticsSide effectMonoglyceride
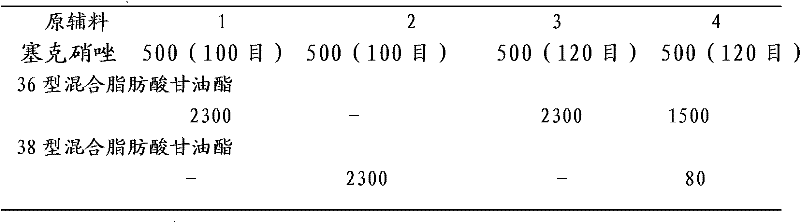
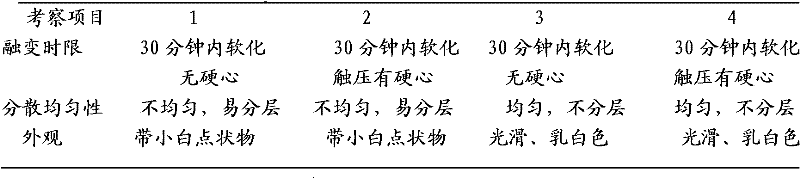
The secnidazole vaginal suppository of the present invention is composed of the following components: main ingredient secnidazole 500g, auxiliary material 36 type mixed fatty acid glyceride 2300g, made into 1000 suppositories, each weighing 2.8 grams, the 36 type mixed Fatty acid glycerides are a mixture of triglycerides, diglycerides and monoglycerides, and the melting point of type 36 mixed fatty acid glycerides is 35-37°C. The drug of the secnidazole suppository of the present invention is directly absorbed through the vagina, and will not cause damage to the heart, kidney and liver after testing. The curative effect is good, there is basically no side effect, the proportion is simple, and the use is convenient. The invention also discloses the preparation process of the secnidazole vaginal suppository at the same time.
Owner:HUBEI TUNGSHUN PHARMA
Association of fluconazole-tinidazole for the treatment of vaginal infections, its composition, preparation process and usage
The present invention refers to a treatment for mixed infectious diseases in the human reproductive system, wherein an association of compounds containing fluconazole and either tinidazole or secnidazole is used, the same being associated in doses lower to those commonly used therapeutically. The combination has proven to be highly efficacious and shown a good degree of tolerance.
Owner:ALPARIS S A DE
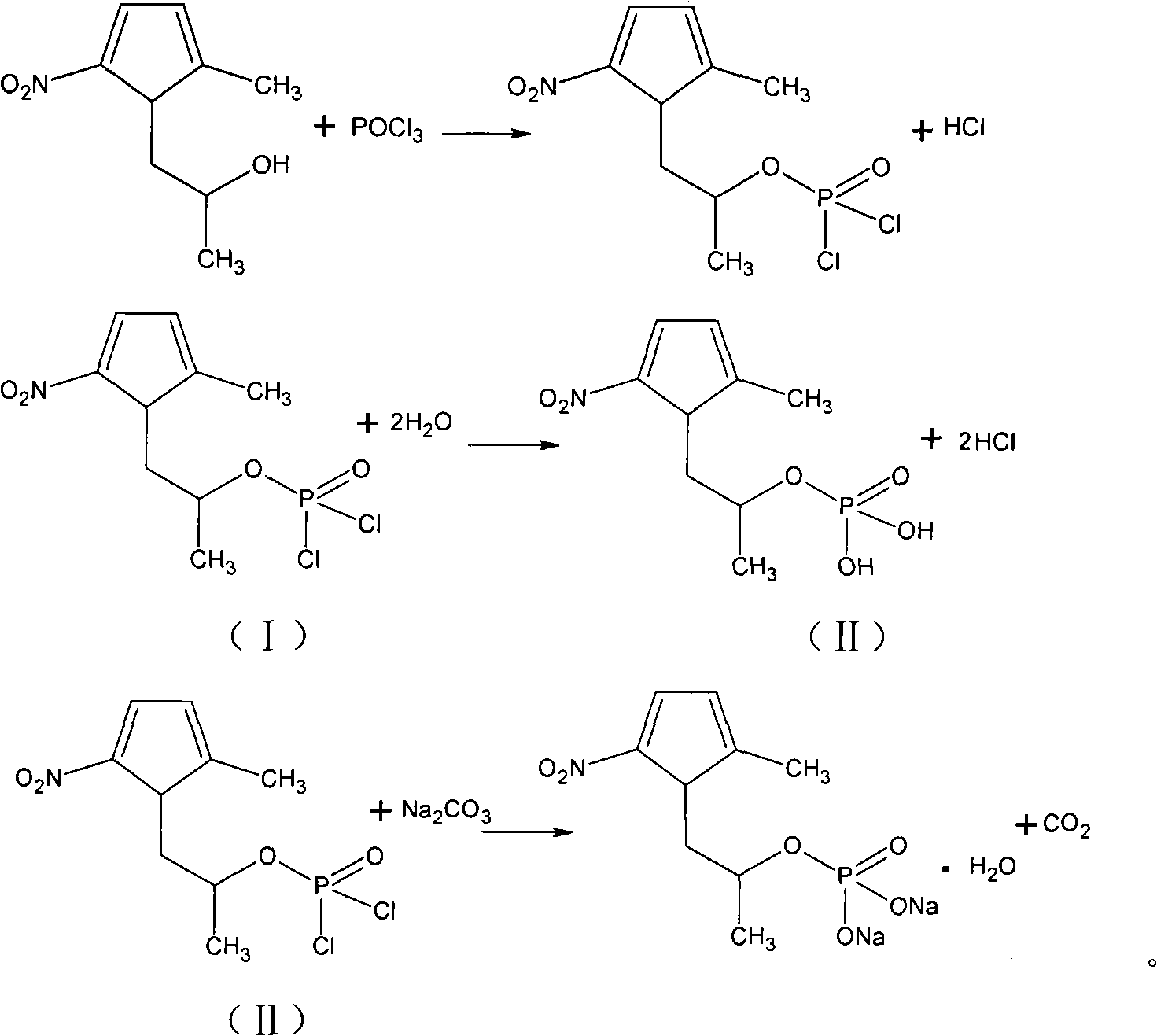
Method for preparing secnidazole
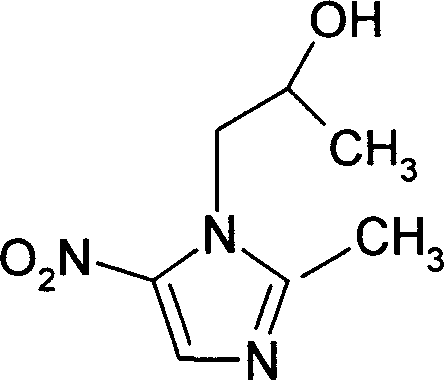
This invention relates to secnidazole [1-(2-hydroxy group propyl)-2-methyl-5- nitro imidazole] preparation method, it belongs to chemical pharmacy technique field. 2-methyl-5-nitro imidazole and 1-chlorine-2-propyl alcohol are materials, dry acid gas is let in to dissolve solid, then heated, distilled and recover 1-chlorine-2- propanol, pH is adjusted by sig water and cooled to 0 degree centigrade. Then it is filtrated, unreacted 2-methyl-5-nitro imidazole is recovered, filtrate pH value is adjusted to alkality, and then secnidazole crude is got after filtration. Reaction temperature is 85-95 degrees cenrigrade, reaction time is 6.0-7.5 hours, mol ratio of 2-methyl-5-nitro imidazole and 1-chlorine-2-propyl alcohol is 1:3.25-5.30. Compering to existing technique, the reaction preoceudre is only one step, generating period is shortened, and operation is simplified. Reaction whole yield is improved from less than 50 percent reported from document to 56-60 percent, and material mass using is reduced, so generating cost is reduced, generating safty is also improved.
Owner:ZHEJIANG SUPOR PHARM CO LTD
Interventional anti-infective water-based adhesive
InactiveCN102925064AGuaranteed bonding performanceAnti-inflammatoryNon-macromolecular adhesive additivesOrganic non-macromolecular adhesiveInflammationAnaerobic bacterium
The invention discloses an interventional anti-infective water-based adhesive which comprises the following components in parts by weight: 100 parts of n-octyl a-cyanoacrylate, 10-15 parts of polymethacrylate, 4-10 parts of tetracycline, 2-6 parts of secnidazole, 0.2-0.5 part of hydroquinone and 0.05-0.2 part of sulfur dioxide. The interventional anti-infective water-based adhesive disclosed by the invention enables the human body tissues to be easy for binding in an anaerobic and solvent environment, has counter force against anaerobes, can effectively relieve the infection of the human body tissues in or after operation, and has the function of diminishing inflammation, thereby ensuring the binding effect of the human body tissues.
Owner:无锡市天力胶带厂
Secnidazole vagina expansive suppository and preparation method and detection method thereof
ActiveCN103784390AGuaranteed effective concentrationPrevent outflowOrganic active ingredientsWeighing by removing componentTriethanolamine oleateTreatment effect
The invention relates to a secnidazole vagina expansive suppository and a preparation method and a detection method thereof. The secnidazole vagina expansive suppository comprises secnidazole, a matrix and an expansive carrier, wherein the medicine-containing matrix of the expansive suppository contains triethanolamine oleate so that the dispersion uniformity of the secnidazole in the matrix can be increased and the bioavailability of the suppository is increased; the thyme oil is capable of improving the lubricating property of the suppository and the dissolution rate of the secnidazole as an active ingredient, the absorption of a human body to the secnidazole is increased, and the bioavailability of the suppository is improved; the glucosan is capable of improving the treatment effect of the suppository on vulvovaginal candidiasis, promoting the recovery of the erosion and the anabrosis, and improving the immunity of the human body; the glucosan is capable of promoting the formation of the suppository, and improving the stability of the suppository; the secnidazole vagina expansive suppository is a hollow dual-layer expansive suppository, the medicine-containing matrix is wrapped in a matrix layer so that the suppository disclosed by the invention is more stable than a common suppository; meanwhile, the secnidazole vagina expansive suppository has the beneficial effects of preventing a medicine liquid from outflowing, being high in stability, remarkable in treatment effect, and the like by adopting six creative leading technologies.
Owner:哈尔滨田美药业股份有限公司
Preparation method of secnidazole
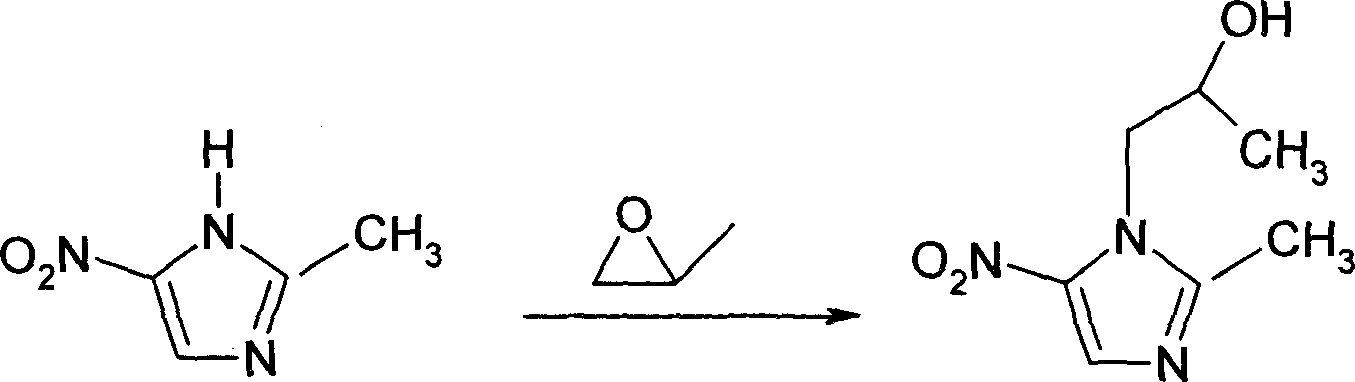
The invention discloses a preparation method of secnidazole, belonging to the field of medicine chemical technology. The preparation method comprises the following steps: (1) in the presence of lewis acid, reacting 2-methyl-5-nitroimidazole with epoxy propane in an organic solvent, separating an aqueous secnidazole solution from a reaction product; (2) adding hydrolysate into the aqueous secnidazole solution; after hydrolysis is completed, performing cooling and solid-liquid separation to remove unreacted 2-methyl-5-nitroimidazole; carrying out extraction, concentration, crystallization and solid-liquid separation to obtain crude secnidazole and crude mother liquor, wherein the mass ratio of hydrolate to 2-methyl-5-nitroimidazole is 5.5-8.0:1; (3) dissolving the crude secnidazole into dichloromethane, and carrying out solid-liquid separation, distillation, decoloring, crystallization and solid-liquid separation to obtain refined secnidazole and refined mother liquid. The preparation method for secnidazole has the advantages of avoiding the use of acid and alkali, lowering the cost, simplifying the process and improving the yield.
Owner:湖北金赛药业有限公司
Novel secnidazole soft gelatin capsule formulations and uses thereof
InactiveUS20190008784A1Antibacterial agentsOrganic active ingredientsIntravaginal administrationBacterial vaginosis
Embodiments described herein are directed to novel soft gelatin capsule formulations for intravaginal administration comprising secnidazole compounds and methods and uses of these pharmaceutical compositions in the treatment of bacterial vaginosis.
Owner:LUPIN INC
A kind of preparation method of secnidazole
The invention discloses a preparation method of secnidazole, belonging to the field of medicine chemical technology. The preparation method comprises the following steps: (1) in the presence of lewis acid, reacting 2-methyl-5-nitroimidazole with epoxy propane in an organic solvent, separating an aqueous secnidazole solution from a reaction product; (2) adding hydrolysate into the aqueous secnidazole solution; after hydrolysis is completed, performing cooling and solid-liquid separation to remove unreacted 2-methyl-5-nitroimidazole; carrying out extraction, concentration, crystallization and solid-liquid separation to obtain crude secnidazole and crude mother liquor, wherein the mass ratio of hydrolate to 2-methyl-5-nitroimidazole is 5.5-8.0:1; (3) dissolving the crude secnidazole into dichloromethane, and carrying out solid-liquid separation, distillation, decoloring, crystallization and solid-liquid separation to obtain refined secnidazole and refined mother liquid. The preparation method for secnidazole has the advantages of avoiding the use of acid and alkali, lowering the cost, simplifying the process and improving the yield.
Owner:湖北金赛药业有限公司
Venous injection of secnidazole
InactiveCN1679554AAchieve therapeutic effectOrganic active ingredientsAntimycoticsActive componentCurative effect
An intravenous injection of secindazole is prepared from the secnidazole as active component and the pharmacologically acceptable carrier. Its advantage is quickly taking its high curative effect.
Owner:湖南医药工业研究所有限公司
Preparation method of secnidazole and dexamethasone oral sustained-release medicine membrane
InactiveCN105477002AQuality improvementEasy to useOrganic active ingredientsDigestive systemDexamethasone acetateAcetic acid
The invention discloses a secnidazole and dexamethasone oral sustained-release medicine membrane. The secnidazole and dexamethasone oral sustained-release medicine membrane is prepared from the following raw materials in parts by weight: 0.5-1 part of secnidazole, 0.1-0.3 part of dexamethasone acetate, 2-4 parts of chitosan, 1-1.5 parts of gelatin, 4-6 parts of glycerol, 9-10 parts of ethanol, 10 parts of dilute acetic acid and about 80-100 parts of water for injection. The invention also discloses a preparation method of the secnidazole and dexamethasone oral sustained-release medicine membrane. The preparation method has the following advantages: the process is simple, the prepared secnidazole and dexamethasone oral sustained-release medicine membrane is stable in quality, convenient to use, definite in curative effect and low in manufacturing cost, has a good curative effect on treating oral inflammation and therefore is worthy of popularization.
Owner:张庆峰
Vaginal effervescence tablet of secnidazole and its preparation
InactiveCN1304002CStrong specificityGood curative effectOrganic active ingredientsPill deliveryEffervescent tabletCurative effect
Owner:苏州赫森医药科技有限公司
Composition of secnidazole and lactose and preparation method thereof
ActiveCN101874799ASignificant clinical effectGood disintegrationOrganic active ingredientsPharmaceutical non-active ingredientsCompressibilityMagnesium stearate
The invention relates to a composition of secnidazole and lactose. 1,000 tablets of the composition comprise 500 grams of secnidazole, 76 grams of lactose, 24 grams of sodium starch glycolate, a proper amount of polyvidone ethanol solution and 2.8 grams of magnesium stearate. A preparation method for the composition comprises a stepped production process. In the composition and the method, the lactose is quantitatively selected and used on the basis that the using amount of the secnidazole is determined, and both the secnidazole and the lactose have high fluidity and compressibility and can promote tablet disintegration; and meanwhile, the sodium starch glycolate is quantitatively selected and used as a disintegrating agent to endow the tablet with better disintegration and higher compressibility.
Owner:西安万隆制药股份有限公司
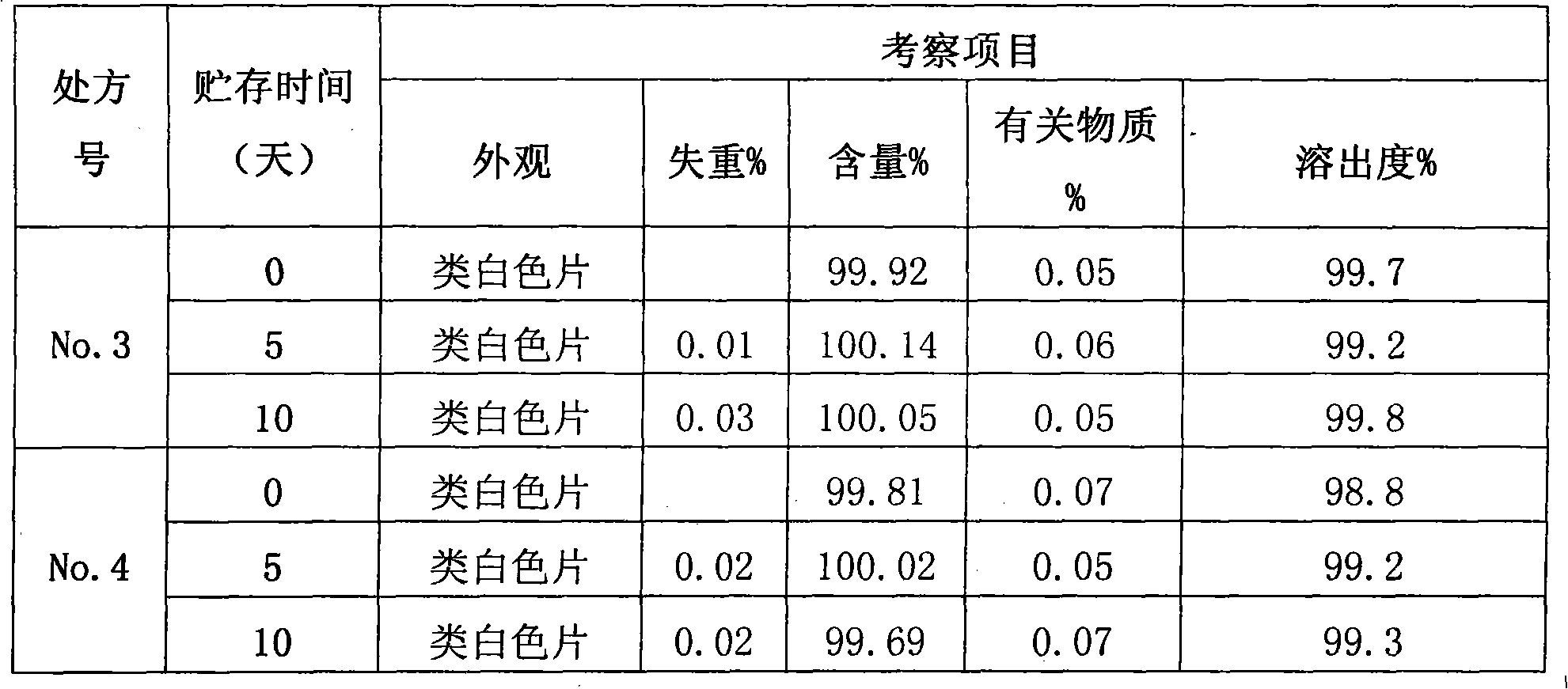
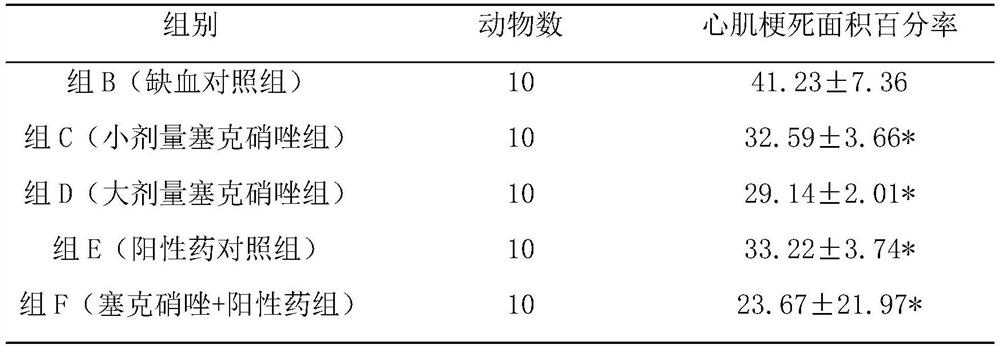
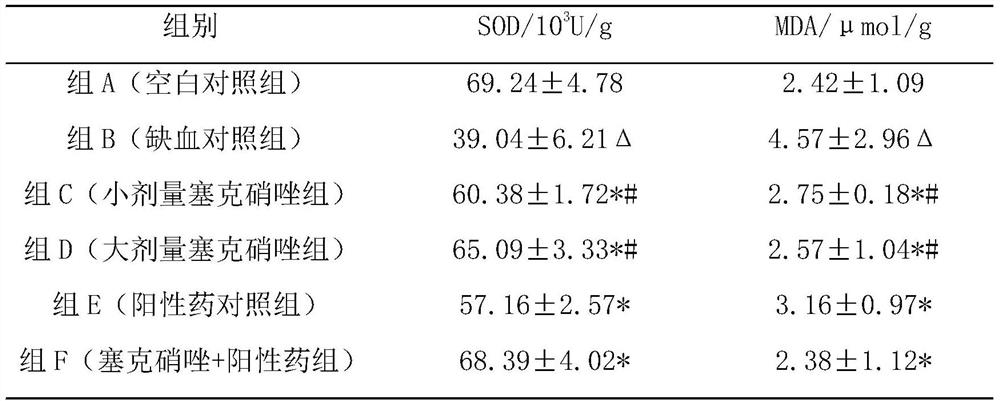
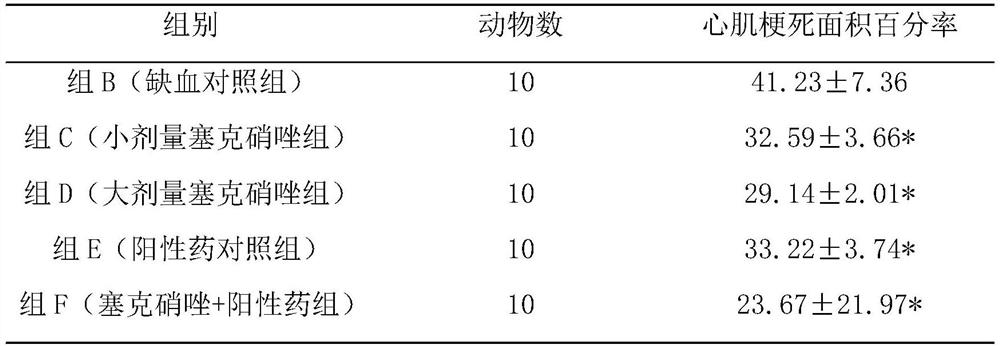
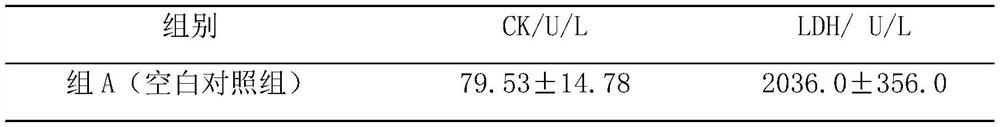
Pharmaceutical composition for preventing and treating myocardial ischemia and preparation method and application thereof
ActiveCN111759840AComprehensive treatmentOrganic active ingredientsPharmaceutical non-active ingredientsAcute myocardial ischaemiaPharmaceutical drug
The invention discloses an application of secnidazole to preparation of drugs for treating and / or preventing myocardial ischemia. The invention further discloses a pharmaceutical composition for treating and / or preventing the myocardial ischemia. The pharmaceutical composition comprises the secnidazole and a pharmaceutically acceptable excipient. A pharmacodynamic experiment result shows that thesecnidazole has a protective effect on an experimental myocardial ischemic injury, can significantly reduce the area of myocardial infarction, reduce the degree of myocardial damage, reduce the MDA content in serum and increase the content of active SOD, and can effectively resist acute myocardial ischemia and protect myocardium, so as to improve the symptoms of the myocardial ischemia.
Owner:WENZHOU PEOPLES HOSPITAL
Fructose injection of antibiotic medicine
InactiveCN108721625AEasy to useSuitable for useAntibacterial agentsPharmaceutical delivery mechanismFluconazoleNorfloxacin
The invention relates to fructose injection of an antibiotic medicine. The fructose injection of the antibiotic medicine consists of antibiotics, fructose and water and also comprises proper additives, wherein the antibiotics comprise gatifloxacin, levofloxacin, ciprofloxacin, pazufloxacin, fleroxacin, sparfloxacin, moxifloxacin, pefloxacin, rufloxacin, lomefloxacin, norfloxacin, caderofloxacin, azithromycin, telithromycin, ornidazole, secnidazole, tinidazole, metronidazole, clindamycin, lincomycin, fluconazole, etimicin, netilmicin, amikacin as well as medicinal acid addition salt, esterification compounds, derivatives and the like; the fructose injection is prepared from the antibiotics; the advantages that the injection is convenient to use and takes effect rapidly are achieved; and compared with the glucose injection, the fructose injection is easier to absorb and utilize, more suitable for antisepsis and anti-inflammation, energy supply and body liquid supplementation of patientssuffering from diabetes, heart diseases and liver diseases, and enlarges the use range.
Owner:WEIHAI HAOTONG MEDICAL SCI & TECH
Secnidazole injection and its prepn process
The present invention is the recipe and preparation process of Secnidazole injection as anaerobe resisting medicine. The Secnidazole injection is prepared with Secnidazole as main medicinal component and adding isotoic regulator and other supplementary material. The preparation process includes water dissolution, filtering and packing.
Owner:浙江南洋药业有限公司
Fructose injection of antibiotic drug
InactiveCN106668862AEasy to useSuitable for useAntibacterial agentsPharmaceutical delivery mechanismFluconazoleNorfloxacin
The invention provides a fructose injection of an antibiotic drug. The injection is composed of antibiotics, fructose and water. The injection also can contain proper additives. The antibiotic comprise gatifloxacin, levofloxacin, ofloxacin, ciprofloxacin, pazufloxacin, fleroxacin, sparfloxacin, moxifloxacin, pefloxacin, rufloxacin, lomefloxacin, norfloxacin, caderofloxacin, azithromycin, telithromycin, ornidazole, secnidazole, tinidazole, metronidazole, clindamycin, lincomycin, fluconazole, etimicin, netilmicin, amikacin and medicinal acid additive salts, esterified compounds, derivatives and the like. The antibiotics are prepared into the fructose injection. Besides the advantages of convenience in use and quickness to take effect of the injection, compared with a gluconic infection, the injection provided by the invention is more easily absorbed and utilized, is more suitable for preventing bacteria and diminishing inflammation, supplying energy and supplementing body fluids for patients with diabetes, heart disease and liver disease, so that the application range of the injection is expanded.
Owner:威海恒基伟业信息科技发展有限公司
Method for preparing secnidazole
ActiveCN100376559CShorten the production cycleEasy to operateOrganic chemistryNitroimidazoleFiltration
This invention relates to secnidazole [1-(2-hydroxy group propyl)-2-methyl-5- nitro imidazole] preparation method, it belongs to chemical pharmacy technique field. 2-methyl-5-nitro imidazole and 1-chlorine-2-propyl alcohol are materials, dry acid gas is let in to dissolve solid, then heated, distilled and recover 1-chlorine-2- propanol, pH is adjusted by sig water and cooled to 0 degree centigrade. Then it is filtrated, unreacted 2-methyl-5-nitro imidazole is recovered, filtrate pH value is adjusted to alkality, and then secnidazole crude is got after filtration. Reaction temperature is 85-95 degrees cenrigrade, reaction time is 6.0-7.5 hours, mol ratio of 2-methyl-5-nitro imidazole and 1-chlorine-2-propyl alcohol is 1:3.25-5.30. Compering to existing technique, the reaction preoceudre is only one step, generating period is shortened, and operation is simplified. Reaction whole yield is improved from less than 50 percent reported from document to 56-60 percent, and material mass using is reduced, so generating cost is reduced, generating safty is also improved.
Owner:ZHEJIANG SUPOR PHARM CO LTD
Compositions, devices and methods for treating obsessive-compulsive disorder
In alternative embodiments, provided are pharmaceutical compositions and methods for treating, ameliorating, reversing and / or preventing (acting as a prophylaxis) an Obsessive-Compulsive Disorder (OCD), with or without an accompanying autism or an autism spectrum disorder (ASD), e.g., a regressive autism. In alternative embodiments, these pharmaceutical compositions and methods are dosaged and administered to children in need thereof. In alternative embodiments, pharmaceutical compositions and methods are dosaged, formulated and dosaged as solid, liquid or aerosol preparations or formulations. In alternative embodiments, pharmaceutical compositions comprise rifaximin as the sole antibiotic, or rixafimin and other antimicrobial or antibiotic agent, for example, vancomycin, metronidazole, tinidazole, secnidazole or a combination thereof. As there are various molecular forms of rifaximins, all these are useful and used in methods and compositions as provided herein
Owner:CENT FOR DIGESTIVE DISEASES PTY LTD
Compositions, devices and methods for treating obsessive-compulsive disorder
In alternative embodiments, provided are pharmaceutical compositions and methods for treating, ameliorating, reversing and / or preventing (acting as a prophylaxis) an Obsessive-Compulsive Disorder (OCD), with or without an accompanying autism or an autism spectrum disorder (ASD), e.g., a regressive autism. In alternative embodiments, these pharmaceutical compositions and methods are dosaged and administered to children in need thereof. In alternative embodiments, pharmaceutical compositions and methods are dosaged, formulated and dosaged as solid, liquid or aerosol preparations or formulations. In alternative embodiments, pharmaceutical compositions comprise rifaximin as the sole antibiotic, or rixafimin and other antimicrobial or antibiotic agent, for example, vancomycin, metronidazole, tinidazole, secnidazole or a combination thereof. As there are various molecular forms of rifaximins, all these are useful and used in methods and compositions as provided herein
Owner:CENT FOR DIGESTIVE DISEASES PTY LTD
Improved method of preparing secnidazole
The present invention discloses an improved method for the preparation of butynidazole (i.e. 2-dimethyl-5-nitro-1H-imidazole-1 ethanol), which method comprises 2-dimethyl- 5-nitro-imidazole is reacted with propylene oxide, and the reactant is suspended in an organic ester solvent at a temperature range of 0-5, and the formed butynidazole is separated from the mixture by a conventional method. The other butinidazole prepared by the method of the invention has high purity and high yield, and is a high-efficiency anti-amoeba and antigen biomedicine.
Owner:UNICHEM LAB LTD
Chinese western medicine compound preparation for treating non-specific vaginitis and preparation method
InactiveCN106266541ASignificant effectShort course of treatmentAntibacterial agentsAntimycoticsAmpicillinAdditive ingredient
The invention discloses a Chinese western medicine compound preparation for treating non-specific vaginitis and a preparation method, and belongs to the technical field of the medicine. The Chinese western medicine compound preparation comprises the following effective ingredients of the raw materials: Secnidazole, vitamin B2, ampicillin, plain white rice roots, mango, white Paederia scandens, ageratum, water hyacinth grass, dodder, Spongilla, Ophiopogon, cynoglossum officinale, long bitter Mai, hibiscus root, Ficus pumila and arrowhead Bureau. The Chinese western medicine compound preparation is prepared by combining the traditional Chinese medicine and western medicine, has the efficacy of softening and resolving lumps, dispelling wind and arresting itching, activating blood circulation to dissipate blood stasis and clearing away heat and toxic material, is capable of effectively preventing the bacterial infection caused by the pathogenic bacteria invading to the vagina continuously, and has the remarkable curative effect to the non-specific vaginitis. The Chinese western medicine compound preparation also has the characteristics of shorter course of treatment, fast efficacy, convenient administration, safety and reliability, no relapse and lower cost.
Owner:刘岚
A kind of pharmaceutical composition for preventing and treating myocardial ischemia and its preparation method and application
ActiveCN111759840BComprehensive treatmentOrganic active ingredientsPharmaceutical non-active ingredientsActive enzymeAcute myocardial ischaemia
The invention discloses the application of secnidazole in the preparation of medicines for treating and / or preventing myocardial ischemia. The invention also discloses a pharmaceutical composition for treating and / or preventing myocardial ischemia, which comprises secnidazole and pharmaceutically acceptable excipients. The results of pharmacodynamic experiments show that secnidazole has a protective effect on experimental myocardial ischemic injury, can significantly reduce the size of myocardial infarction, reduce the degree of myocardial damage, reduce serum MDA content, increase SOD active enzyme content, and can effectively resist Acute myocardial ischemia, protect the myocardium, thereby improving the symptoms of myocardial ischemia.
Owner:WENZHOU PEOPLES HOSPITAL
Pharmaceutical composition for treating gynecologic inflammations as well as preparation method and application thereof
ActiveCN106580981ASolution rangeResolve Healing EffectsAntibacterial agentsOrganic active ingredientsAdditive ingredientCiclopirox Olamine
The invention relates to a pharmaceutical composition for treating gynecologic inflammations. The pharmaceutical composition comprises ciclopirox olamine, palmatine and clindamycin or secnidazole serving as active pharmaceutical ingredients, wherein a synergistic effect is achieved among the three active pharmaceutical ingredients. The preferable preparation form of the pharmaceutical composition is gel. The product is uniform, fine and smooth in texture and appropriate in thickness. The pharmaceutical composition can be used for effectively treating mixed infectious diseases, particularly mixed infective vaginitis.
Owner:MUDANJIANG MEDICAL UNIV
Secnidazole derivative as well as preparation method and application thereof
InactiveCN102180837ASimultaneous antimicrobial activitySimultaneously exert anti-inflammatory activityAntibacterial agentsOrganic active ingredientsNitroimidazoleAntibiosis
The invention discloses a secnidazole derivative which is synthesized and prepared from a salicylic acid derivative I and 2-methyl5-nitroimidazole. The structure of the derivative is as shown in one of general formula 1-general formula 4, wherein R is hydrogen, 4-methoxy, 4-chloride, 5-chloride, 3-methyl, 4-methyl, 5-methyl, 6-methyl or 4-trifluoromethyl. The secnidazole derivative has a secnidazole structure and a salicylic acid medicament structure, and can exert antibiosis activity and anti-inflammatory activity. The invention also discloses a preparation method of the derivative and application of the preparation method in preparing antibiosis and anti-inflammatory medicaments.
Owner:GUANGXI UNIV
Secnidazole vaginal expansion suppository and its preparation method and detection method
ActiveCN103784390BImprove stabilityImprove bioavailabilityWeighing by removing componentOrganic active ingredientsTreatment effectVulvovaginal Candidiasis
The present invention relates to a secnidazole vaginal expansion suppository and its preparation method and detection method; the expansion suppository comprises secnidazole, a matrix and an expansion carrier, and the drug-containing matrix of the expansion suppository contains triethanolamine oleate Can increase the dispersion uniformity of secnidazole in matrix, improve the bioavailability of suppository; Described thyme oil can improve the lubricity of suppository and the dissolution rate of active ingredient secnidazole, increase the human body's to secnidazole The absorption of azole, improve the bioavailability of suppository; Described dextran can improve the treatment of suppository vulvovaginal candidiasis, promote the healing of erosion, ulcer, improve the immunity of human body; Described dextran can also promote suppository forming, improving the stability of the suppository; the secnidazole vaginal expansion suppository is a hollow double-layer expansion suppository, and the drug-containing matrix is wrapped in the matrix layer, so that the suppository of the present invention is more stable than ordinary suppositories; and the The secnidazole vaginal expansion suppository adopts six original leading technologies, which have beneficial effects such as preventing the outflow of medicinal liquid, high stability, and remarkable curative effect.
Owner:哈尔滨田美药业股份有限公司
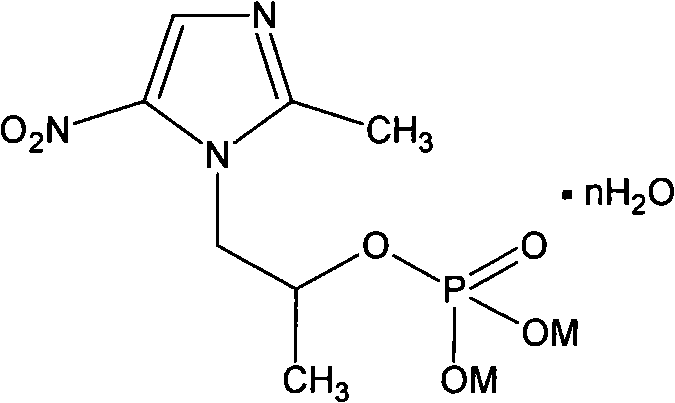
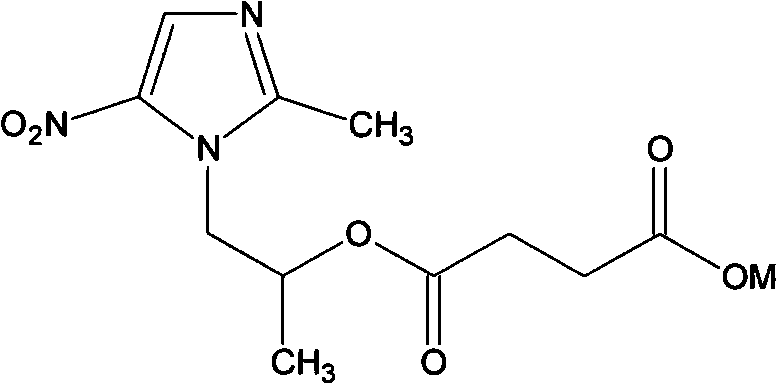
Water-soluble salt of secnidazole and preparation method thereof
The invention relates to a water-soluble derivative of a secnidazole medicament for treating amebic dysentery, namely secnidazole phosphate or succinic monoester salt. A compound with the structure is the chemical derivative of secnidazole, and can play the medicinal effect in a range of the secnidazole. The water solubility of the derivative is remarkably superior to that of the secnidazole, so the derivative can be prepared into formulations which cannot be prepared from the secnidazole such as injection powder. The invention also relates to a preparation method for the secnidazole phosphate and the secnidazole succinic monoester salt.
Owner:HEFEI UNIV OF TECH
A pharmaceutical composition for treating gynecological inflammation, its preparation method and application
ActiveCN106580981BImprove the effect of anti-infection treatmentHigh cure rateAntibacterial agentsOrganic active ingredientsAdditive ingredientCiclopirox Olamine
The invention relates to a pharmaceutical composition for treating gynecologic inflammations. The pharmaceutical composition comprises ciclopirox olamine, palmatine and clindamycin or secnidazole serving as active pharmaceutical ingredients, wherein a synergistic effect is achieved among the three active pharmaceutical ingredients. The preferable preparation form of the pharmaceutical composition is gel. The product is uniform, fine and smooth in texture and appropriate in thickness. The pharmaceutical composition can be used for effectively treating mixed infectious diseases, particularly mixed infective vaginitis.
Owner:MUDANJIANG MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com