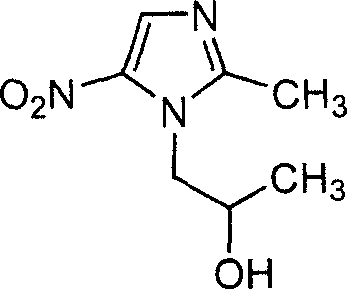
Method for preparing secnidazole
A technology of secnidazole and nitroimidazole, which is applied in the field of preparation of secnidazole [1--2-methyl-5-nitroimidazole], can solve the problems of difficult industrialization, easy volatility and high operation requirements problems, to avoid solvent residues, reduce a large amount of use, and improve safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 127.0g (1.0mol) of 2-methyl-5-nitroimidazole and 340.5ml (4.0mol) of 1-chloro-2-propanol into a 1000ml three-necked flask, stir at room temperature, and feed dry hydrogen chloride gas to until all solids are dissolved. Heat to 93°C and hold for 6.5 hours. 269.5 g of 1-chloro-2-propanol was distilled off under reduced pressure, which could be recycled after drying with anhydrous calcium chloride. Add 1 mol / L sodium hydroxide solution to the residue to adjust the pH to 3-4, and cool to 0°C. Filtrate, wash with water, and recover 2-methyl-5-nitroimidazole, which is 43.8g after drying (recoverable and applicable).
[0033] Continue to add 1 mol / L sodium hydroxide solution to the above filtrate to adjust the pH to 10, a solid is precipitated, and the crude secnidazole is obtained by filtration. The crude product was recrystallized with water to obtain 107.5 g of pure product, with a yield of 58.1% and a content of 99.3%.
Embodiment 2
[0035] Add 127.0g (1.0mol) of 2-methyl-5-nitroimidazole and 447.0ml (5.25mol) of 1-chloro-2-propanol into a 1000ml three-necked flask, stir at room temperature, and feed dry hydrogen chloride gas to until all solids are dissolved. Heat to 90°C and hold for 6 hours. 363.7 g of 1-chloro-2-propanol was evaporated under reduced pressure, and it could be recycled after drying with anhydrous calcium chloride. Add 1 mol / L sodium hydroxide solution to the residue to adjust the pH to 3-4, and cool to 0°C. Filtrate, wash with water, and recover 2-methyl-5-nitroimidazole, which is 44.6g after drying (recoverable and applicable).
[0036] Continue to add 1 mol / L sodium hydroxide solution to the above filtrate to adjust the pH to 10, a solid is precipitated, and the crude secnidazole is obtained by filtration. The crude product was recrystallized with water to obtain 109.3 g of pure product, with a yield of 59.1% and a content of 99.53%.
Embodiment 3
[0038] 127.0g (1.0mol) of 2-methyl-5-nitroimidazole and 293.7ml (3.45mol) of 1-chloro-2-propanol were added to a 1000ml three-necked flask, stirred at room temperature, and dry hydrogen chloride gas was introduced into the until all solids are dissolved. Heat to 88°C and hold for 7 hours. 224.6 g of 1-chloro-2-propanol was distilled off under reduced pressure, which could be recycled after drying with anhydrous calcium chloride. Add 1 mol / L sodium hydroxide solution to the residue to adjust the pH to 3-4, and cool to 0°C. Filtrate, wash with water, and recover 2-methyl-5-nitroimidazole, which is 45.9 g after drying (recoverable and applicable).
[0039] Continue to add 1 mol / L sodium hydroxide solution to the above filtrate to adjust the pH to 10, a solid is precipitated, and the crude secnidazole is obtained by filtration. The crude product was recrystallized with water to obtain 104.9 g of pure product with a yield of 56.7% and a content of 99.45%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com