Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
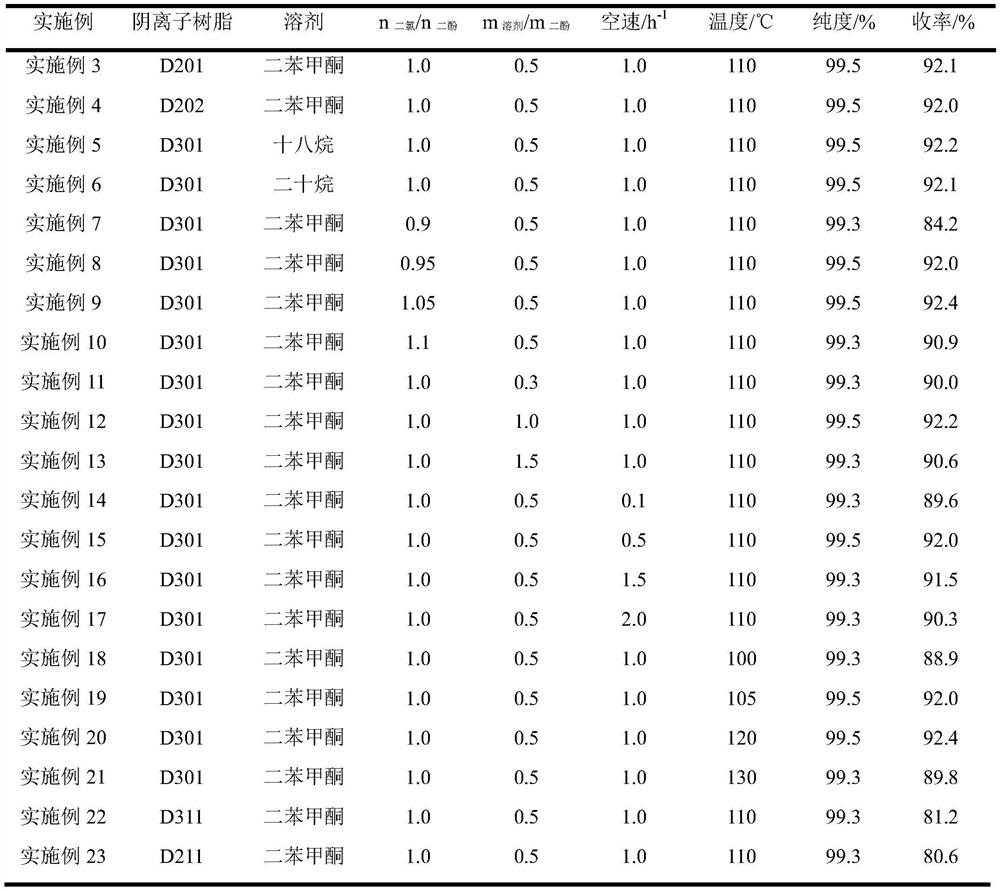
50 results about "Chloroacetone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chloroacetone is a chemical compound with the formula CH₃COCH₂Cl. At STP it is a colourless liquid with a pungent odour. On exposure to light, it turns to a dark yellow-amber colour. It was used as a tear gas in World War I.
Batch, semi-continuous or continuous hydrochlorination of glycerin with reduced volatile chlorinated hydrocarbon by-products and chloracetone levels
ActiveUS20080015369A1High selectivityQuick conversionOrganic compound preparationCarboxylic acid esters preparationSufficient timeChloroacetone
The present invention relates to a process for converting a multihydroxylated-aliphatic hydrocarbon or ester thereof to a chlorohydrin, by contacting the multihydroxylated-aliphatic hydrocarbon or ester thereof starting material with a source of hydrogen chloride at superatmospheric, atmospheric and subatmospheric pressure conditions for a sufficient time and at a sufficient temperature, preferably wherein such contracting step is carried out without substantial removal of water, to produce the desired chlorohydrin product; wherein the desired product or products can be made in high yield without substantial formation of undesired overchlorinated byproducts; said process carried out without a step undertaken to specifically remove volatile chlorinated hydrocarbon by-products or chloroacetone, wherein the combined concentration of volatile chlorinated hydrocarbon by-products and chloroacetone is less than 2000 ppm throughout any stage of the said process.
Owner:BLUE CUBE IP
Preparation method of indanylidene compound
A preparation method of an indanylidene compound (1) is disclosed. The indanylidene compound (1) is obtained through reacting a precursor compound (4) with phthalic anhydride (C). The preparation method is characterized in that the preparation method comprises the following steps: oxidizing a raw material delta 2,2'-bis-indoline-3,3'-dione (2) in the presence of an acidic aqueous solution with the pH value of less than 1 and an oxidant (A), filtering the reactant, dissolving the obtained intermediate compound (3) in an alkaline aqueous solution with the pH value of 8-10, reacting with chloroacetone (B) to obtain the precursor compound (4), and further reacting with the phthalic anhydride (C) to obtain the indanylidene compound (1). The synthetic route of the preparation method is shown in the specification.
Owner:CHENGDU KAOENSI SCI & TECH
New method for preparing thiabendazole
InactiveCN104557902ASolve problems that are not suitable for large-scale mass productionEasy to produceOrganic chemistryDisinfectantThiourea
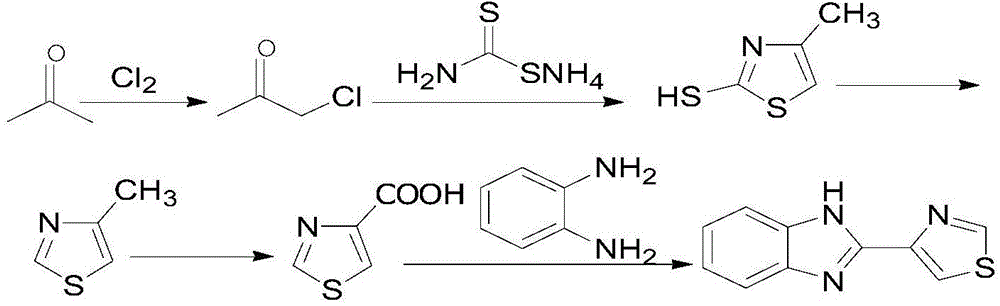
The invention relates to a new method for synthesizing thiabendazole. Acetone and chlorine are taken as starting materials to synthesize chloroacetone, chloroacetone not subjected to separation can directly react with thiocarbamide to obtain 2-Amino-4-methylthiazole which is subjected to diazotization to obtain 4-methylthiazole, and 4-methylthiazole is oxidized to produce 4-thiazolecarboxylic acid, and finally, 4-thiazolecarboxylic acid reacts with o-phenylenediamine to obtain the target object thiabendazole. The thiabendazole is a broad-spectrum anthelmintic, can repel roundworms, hookworms, whipworms, pinworms, strongyloises stercoralis and trichinization, is also a broad-spectrum efficient disinfectant and is widely used as a fruit fresh-keeping agent and a bactericidal mildew inhibitor in various fields over the past decade in China.
Owner:YANTAI BESTENPHARM TECH CO LTD
Batch, semi-continuous or continuous hydrochlorination of glycerin with reduced volatile chlorinated hydrocarbon by-products and chloracetone levels
ActiveUS7906690B2Minimize formationQuality improvementOrganic compound preparationCarboxylic acid esters preparationSufficient timeChloroacetone
The present invention relates to a process for converting a multihydroxylated-aliphatic hydrocarbon or ester thereof to a chlorohydrin, by contacting the multihydroxylated-aliphatic hydrocarbon or ester thereof starting material with a source of hydrogen chloride at superatmospheric, atmospheric and subatmospheric pressure conditions for a sufficient time and at a sufficient temperature, preferably wherein such contracting step is carried out without substantial removal of water, to produce the desired chlorohydrin product; wherein the desired product or products can be made in high yield without substantial formation of undesired overchlorinated byproducts; said process carried out without a step undertaken to specifically remove volatile chlorinated hydrocarbon by-products or chloroacetone, wherein the combined concentration of volatile chlorinated hydrocarbon by-products and chloroacetone is less than 2000 ppm throughout any stage of the said process.
Owner:BLUE CUBE IP
Method for synthesizing (+/-)-Murracarpin and anti-inflammatory and analgesic effect thereof
InactiveCN101824015AMild reaction conditionsEasy to operateOrganic active ingredientsOrganic chemistryEpoxyAcetic anhydride
The invention relates to the field of natural medicinal chemistry, in particular to a method for synthesizing (+ / -)-Murracarpin and anti-inflammatory and analgesic effect thereof. The method for preparing the (+ / -)-Murracarpin comprises the following steps of: reacting 7-hydroxy coumarin, acetic anhydride, trifluoroacetic acid and hexamethylenetetramine to obtain 7-hydroxy-8-formoxyl coumarin; reacting the 7-hydroxy-8-formoxyl coumarin with (Me)2SO4 to obtain 7-methoxy-8-formoxyl coumarin; reacting the obtained 7-methoxy-8-formoxyl coumarin with 1-chloroacetone to obtain 8-(2'-acetyl epoxy ethyl)-7-methoxy coumarin, and reacting the 8-(2'-acetyl epoxy ethyl)-7-methoxy coumarin, methyltriphenyl phosphorous bromide and LDA to obtain 8-(2'-propenyl epoxy ethyl)-7-methoxy coumarin; and performing acid hydrolysis on the obtained 8-(2'-propenyl epoxy ethyl)-7-methoxy coumarin in methanol to obtain the (+ / -)-Murracarpin. Research shows that the obtained compound has anti-inflammatory and analgesic effect.
Owner:许瑞安
Preparation method of CDK (Cyclin-dependent Kinase) inhibitor
ActiveCN107118207AThe synthesis method is simpleMild reaction conditionsOrganic active ingredientsGroup 3/13 element organic compoundsChloroacetoneCoupling reaction
The invention discloses a preparation method of a CDK (Cyclin-dependent Kinase) inhibitor. The preparation method comprises the following steps: enabling monomethylthiourea and 1-chloroacetone to react to obtain N,4-dimethylthiazole-2-amine; carrying out bromination reaction to obtain 5-bromo-N,4-dimethylthiazole-2-amine; enabling the 5-bromo-N,4-dimethylthiazole-2-amine and borate to react under the action of a catalyst, so as to obtain an aromatic borate intermediate; enabling the aromatic borate intermediate and 2,4-dichloro-5fluorouracil to be subjected to suzuki coupling reaction under the catalysis of a palladium series catalyst, so as to obtain a coupled product; enabling the coupled product and aromatic amine to be subjected to Buchwald-Hartwig reaction to finally obtain a target product.
Owner:苏州东南药业股份有限公司
Method of synthesizing 1, 3-dichloroacetone with catalysis of silicon dioxide-carried phosphotungstic heteropoly acid
InactiveCN107954847AReduce pollutionHigh reusabilityOrganic compound preparationCatalyst activation/preparationHeteropoly acidPhosphoric acid
The invention discloses a method for catalytically synthesizing 1,3-dichloroacetone supported by silica-supported phosphotungstic heteropolyacid, which comprises the following steps: (1) dissolving sodium tungstate dihydrate in water, and injecting into the system under stirring Phosphoric acid was added dropwise in the water, and then refluxed after the dropwise addition; (2) Natural cooling and crystallization, redissolving the crystals in water at 80°C, then adding ether for extraction, recrystallizing in water, and obtaining phosphotungstic heteropoly acid; (3) Tetraethyl orthosilicate, deionized water, absolute ethanol, and phosphotungstic heteropolyacid are mixed and stirred to form a gel, then left to stand for aging and dried to obtain silica-loaded phosphotungstic heteropoly Acid catalyst; (4) Mix 1,3-dichloropropanol with hydrogen peroxide solution, add silica-supported phosphotungstic heteropolyacid catalyst, react at constant temperature for 10 hours, filter, and the filtrate is 1,3-dichloroacetone . The method provided by the invention has the advantages of simple operation, less environmental pollution, high yield and good purity of 1,3-dichloroacetone.
Owner:SHAANXI HUANKE BIOLOGICAL TECH CO LTD
Method for preparation of medetomidine with chloroacetone
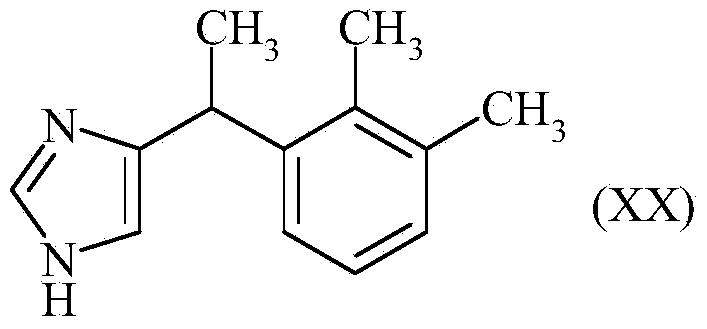
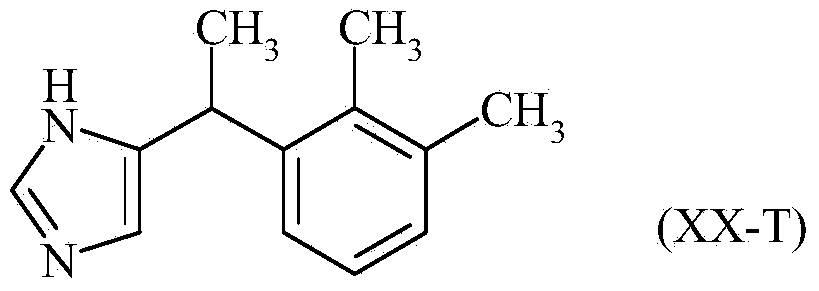
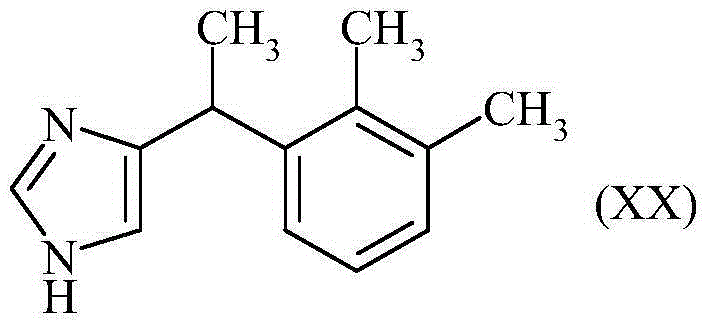
InactiveCN104245678AReduce the numberReduce in quantityOrganic active ingredientsOrganic chemistryChloroacetoneBromine
The invention discloses a method for the preparation of medetomidine starting from 1-bromo 2,3-dimethylbenzene and chloroacetone.
Owner:LONZA LTD
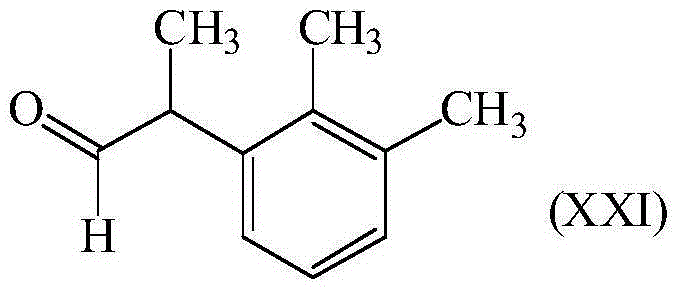
Method for preparation of 2-(2,3-dimethylphenyl)-1-propanal with chloroacetone
InactiveCN104220408AReduce usageIncrease the batch sizeOrganic compound preparationPreparation from heterocyclic compoundsChloroacetoneBromine
The invention discloses a method for the preparation of 2-(2,3-dimethylphenyl)-1-propanal starting from 1-bromo 2,3-dimethylbenzene and chloroacetone, its use in perfumes and its use for the preparation of medetomidine.
Owner:LONZA LTD
Synthesizing method of N-methoxyphenyl-N-(acetyl)methylamine
InactiveCN102491912AOvercome the problems of high toxicity, cumbersome process and unsuitable for industrial productionSimple processOrganic chemistryOrganic compound preparationEthyl acetateLigroin
The invention relates to a synthesizing method of N-methoxyphenyl-N-(acetyl)methylamine. According to the invention, para-anisidine and chloroacetone are adopted as raw materials. The method comprises steps that: 1, para-anisidine, and ethyl acetate and triethylamine used as reaction solvents are added into a reaction vessel, wherein a dosage amount of the reaction solvents is 2-4 times that of para-anisidine; the mixture is heated while stirring; chloroacetone is added to the mixture when the temperature is 65-85 DEG C; the mixture is then subject to a reaction with maintained temperature for 30-60min; a proper amount of water is added to the reaction vessel to stop the reaction; 2, the obtained mixture is settled and layered; a lower-layer water phase is removed; ligroin is added to an organic phase, and the mixture is stirred for 1-2h, such that crystals can grow; 3, the mixture is subject to pump filtration, a filter cake is leached by using ligroin and then leached by using water; the filter cake is dried by pump filtration, such that light yellow crystals, which are N-methoxyphenyl-N-(acetyl)methylamine products, are obtained. According to the method provided by the invention, the solvents are advantaged in low toxicity and easy recycling. The technical processes of the method are simple, and the method is suitable for industrialized productions. With the method, a molar yield is higher than 75%. As a result of HPLC detection, the purity of the product is higher than 93%.
Owner:SHANGHAI NEW ASIA PHARMA +1
Preparation method of 4-methylthiazol
InactiveCN103232404AEasy to recycle and reuseAvoid pollutionOrganic chemistryChemical industryChloroacetone
The invention relates to a preparation method of 4-methylthiazol. The invention aims at solving the problem that in the existing method for preparing the methylthiazol, the reaction route is long, the reaction is complicated and not easy to control, the production cost of the used catalyst is high and the yield is high. The preparation method comprises the following steps of A, mixing formamide, anhydrous glycol dimethyl ether and phosphorus pentasulfide, heating the mixture to be reacted at the temperature of 45 to 50 DEG C, and filtering the mixture to obtain thioformamide; B, adding chloroacetone into the thioformamide to be reacted at the temperature of 50 to 60 DEG C, adjusting the pH value to 6 to 7, and cooling, filtering, washing and dehydrating the mixture to obtain the 4-methylthiazol. Raw materials are cheap and easy to obtain, the reaction condition is moderate, the synthesis step is simple, no catalyst is used, no pollution exists, the yield is high, operation is simple and convenient, and the solvent can be recycled. The preparation method is applied to the technical field of chemical industry.
Owner:HEILONGJIANG UNIV
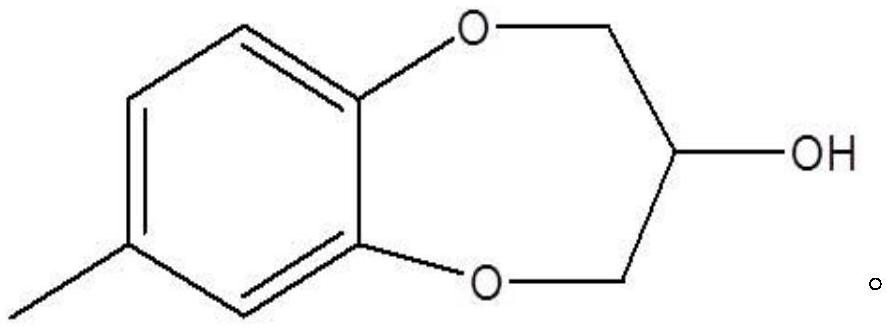
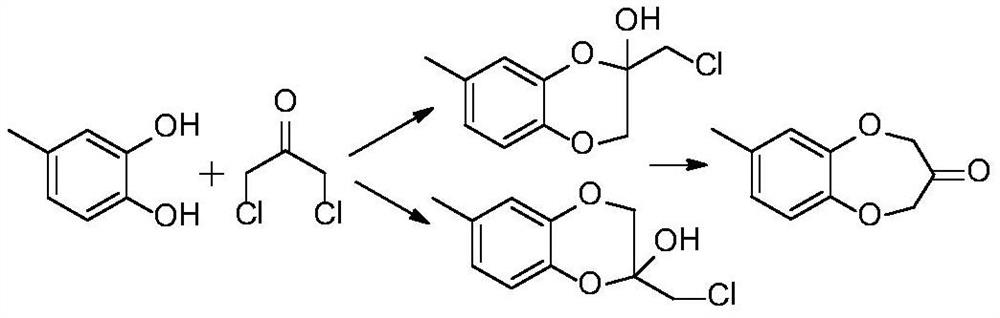
Synthesis process of watermelon ketone precursor
The invention provides a synthesis process of a watermelon ketone precursor. According to the invention, cheap 1,3-dihalopropanol and 4-methyl catechol are subjected to a condensation reaction under an alkaline condition to synthesize a watermelon ketone precursor 3,4-dihydro-7-methyl-2H-1,5-benzoxazole-3-ol. According to the method, the use of highly toxic 1,3-dichloroacetone is avoided, the yield of the watermelon ketone precursor is greatly increased, the production cost of the watermelon ketone spice is reduced, and the quality of watermelon ketone is improved; and the synthesis process issimple to operate and environment-friendly.
Owner:江西开源香料有限公司
Method for preparing pymetrozine
ActiveCN108707137AShort reaction pathAtom economy is highOrganic chemistryOrganic synthesisChloroacetone
The invention discloses a method for preparing pymetrozine with an aim to provide a synthesis method short in reaction route of the pymetrozine, small in environmental pollution and simple in technology operation, and belongs to the technical field of organic synthesis. The method includes the steps of 1), allowing dimethyl carbonate serving as a raw material to be subjected to hydrazinolysis withhydrazine hydrate to obtain carbazide; 2), subjecting carbazide and smoke aldehyde to condensation reaction to obtain (E)-N'-(pyridine-3-kiya methyl) hydrazine carbon hydrazide; 3), subjecting (E)-N'-( pyridine-3-kiya methyl) hydrazine carbon hydrazide and chloroacetone to condensation reaction to obtain (E)-N'-(Z)-1-chloropropyl-2-subunit)-2-(pyridine-3-kiya methyl) diazanyl-1-carbohydrazide; 4), subjecting (E)-N'-(Z)-1-chloropropyl-2-subunit)-2-(pyridine-3-kiya methyl) diazanyl-1-carbohydrazide to ring formation under the alkaline condition to obtain the pymetrozine.
Owner:广东立威化工有限公司
Production method of mexiletine hydrochloride
InactiveCN107641074AIncrease spawn rateMaximize production efficiencyOrganic compound preparationCarbonyl compound preparationKetoneSolvent
The invention provides a production method of mexiletine hydrochloride. The method comprises: a etherification step, namely dissolving 2,6-dimethylphenol in a solid-liquid heterogeneous reaction system to obtain a mixed solution, mixing chloroacetone with the mixed solution, and performing reflux reaction to obtain ether ketone, wherein the solid-liquid heterogeneous reaction system comprises a solvent, a solid-liquid phase transfer promoter, inorganic base and an alkali metal halide; an amination reduction step, namely under suitable reaction conditions, contacting the ether ketone with ammonia methanol to carry out amination reduction reaction to obtain ether amine; and a salting step, namely reacting the ether amine with hydrogen chloride in the solvent to obtain the mexiletine hydrochloride. The method disclosed by the invention improves the reaction efficiency and the production efficiency, and has the advantages of low production cost, small resource consumption and high production efficiency.
Owner:CHANGZHOU YABANG PHARMA
A method for synthesizing intermediate impurities of antigout drug febuxostat and applications of the synthesized impurities
InactiveCN108033929AHigh purityThe synthesis method is simpleOrganic chemistryComponent separationSynthesis methodsHexamethylenetetramine
The invention relates to the field of medicines, and particularly relates to a method for synthesizing intermediate impurities of antigout drug febuxostat and applications of the synthesized impurities. The method includes (1) reacting 4-hydroxy thiobenzamide adopted as an initial material with chloroacetone, and subjecting an obtained reaction product, trifluoroacetic acid and hexamethylenetetramine to a Duff reaction to generate the impurity A; (2) reacting 4-hydroxy thiobenzamide adopted as an initial material with ethyl 2-chloro-3-oxopropanoate, and subjecting an obtained reaction product,trifluoroacetic acid and hexamethylenetetramine to a Duff reaction to generate the impurity B; and (3) determining structures of the impurity A and the impurity B through nuclear magnetic resonance.The method is simple, raw materials are easily available, and purity of the obtained impurity A and purity of the impurity B are high and are 99.55% and 98.13% respectively.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Preparation method of hexachloroacetone
ActiveCN109942392AHigh yieldLow impurity contentOrganic compound preparationCarbonyl compound preparationImpurityAcetone
The invention relates to a preparation method of hexachloroacetone, belonging to the technical field of the preparation of organic chemical gas. The preparation method comprises the following steps: mixing a compound A with a chlorine molecule B, and reacting at 30-150 DEG C under the effect of a catalyst, wherein a reaction product is a mixture containing hexachloroacetone and the catalyst; and separating the catalyst from the mixture, so as to obtain a crude product; purifying the crude product, so as to obtain hexachloroacetone, wherein the compound A is at least one of acetone and chloroacetone with the chlorine atomic number of 1-5, the chlorine molecule B is chlorine or a mixture of chlorine and diluent gas, the diluent gas is inert gas or nitrogen, and the catalyst is pyrimidine, 2-chloropyrimidine or symtriazine. The method has the beneficial effects that the catalyst is easily recycled, and hexachloroacetone with extremely low impurity content can be obtained in a high-yield manner.
Owner:中国船舶集团有限公司第七一八研究所
Production method of 1,1,3-trichloroacetone
ActiveCN113548949ATo diluteDistributeOrganic compound preparationCarbonyl compound separation/purificationPhoto catalyticSorbent
The invention discloses a method for producing 1,1,3-trichloroacetone, belonging to the technical field of chemical product preparation. The method comprises the following steps: with acetone as a raw material, synthesizing 1,1,3-trichloroacetone in an inert solvent through photocatalytic chlorination, recovering a solvent, acetone, monochloroacetone, 1,1-dichloroacetone and other light components from a chlorination solution in a rectification manner, separating the intermediate component 1,3-dichloroacetone from 1,1,3-trichloroacetone in a simulated moving bed filled with an adsorbent to obtain 1,1,3-trichloroacetone, conducting eluting with a desorption agent to obtain a desorption solution containing 1,3-dichloroacetone and 1,1,3-trichloroacetone, distilling the desorption solution to remove the solvent, and then returning the desorption solution to a chlorination kettle for indiscriminate application. The method has the characteristics of mild reaction conditions, high reaction rate, high selectivity of 1,1,3-trichloroacetone, low wastewater discharge amount, environment-friendly process and the like.
Owner:常州新东化工发展有限公司
4-furfurylthiopentanone-2 preparation method
The invention belongs to the technical field of fine chemistry, and provides a 4-furfurylthiopentanone-2 preparation method, which specifically comprises: adding a raw material chloroacetone to a toluene solution of triphenylphosphine or to triethyl phosphite, and carrying out a heating reflux reaction to obtain a phosphorus salt; dissolving the obtained phosphorus salt in an organic solvent, adding a strong alkali, stirring for 2-3 h, and obtaining phosphorus ylide after completing a reaction; adding the phosphorus ylide to an acetaldehyde aqueous solution or paraldehyde, and stirring until the reaction is completed to obtain 3-pentene-2-ketone; adding piperidine to the 3-pentene-2-ketone, adding a dichloromethane solution of furfuryl mercaptan in a dropwise manner, and controlling the temperature of the system during the adding at 35-40 DEG C; and after the adding, stirring until the reaction is completed so as to obtain the product 4-furfurylthiopentanone-2. According to the present invention, with the method, the problems of high risk, high cost and being unsuitable for industrial production of the 4-furfurylthiopentanone-2 synthesis route in the prior art are solved.
Owner:石家庄和中科技有限公司
Preparation method and application of pteridine folic acid
ActiveCN107353299AHigh purityImprove quality controlOrganic chemistryPreparing sample for investigationChemical synthesisPteridine synthesis
The invention relates to a preparation method and application of pteridine folic acid. The preparation method has the advantages that by using p-aminobenzoyl glutamic acid, triamino pyrimidine sulfate and trichloroacetone as raw materials, and adopting the chemical synthesizing method, the content of crude product of pteridine folic acid is obviously increased; by using preparation chromatography to separate, the pteridine folic acid with purity more than 95% is obtained; the production mechanism and control method of the pteridine folic acid are favorably further studied, and the quality control level of the folic acid is effectively increased.
Owner:ZHEJIANG SHENGDA BIO PHARM
Bis(3,2-hydroxy-pyridone) derivative, and preparation method and application thereof
InactiveCN106748995AUnique tooth structureGood water solubilityOrganic active ingredientsOrganic chemistryChloroacetoneCarboxylic acid
The invention discloses bis(3,2-hydroxy-pyridone) derivative, and a preparation method and application thereof. The preparation method is characterized by comprising the following steps: performing Michael addition and amidation reaction for chloroacetone, oxalacetic acid sodium salt derivatives and ammonia gas under the catalysis of Lewis acid, building 2,3-hydroxy-pyridone fragments, protecting the fragments by virtue of benzyl, hydrolyzing ethyl ester to obtain four carboxylic acids by virtue of saponification under a strong alkaline condition, then performing amidation reaction for lysine and the activated 3,2-hydroxy-pyridone derivative to obtain a target product precursor protected by benzyl, and finally removing the benzyl by virtue of Pd / C under the condition of H2 to obtain the target bis(3,2-hydroxy-pyridone) derivative. A compound of the invention has the structural characteristics that bi-hydroxyl pyridone has a unique toothed structure; by introducing the lysine group, the bis(3,2-hydroxyl-pyridone) derivative can form a stable complex with metal ions so as to be used as a potential chelating agent in the field such as metal chelating in living bodies and the like.
Owner:INST OF NUCLEAR PHYSICS & CHEM CHINA ACADEMY OF
Method for synthesizing watermelon ketone
ActiveCN114456146AGood reaction selectivityLess side effectsOrganic chemistryChemical recyclingOrganic solventPtru catalyst
The invention discloses a method for synthesizing watermelon ketone. 4-methylcatechol and 1, 3-dichloroacetone are used as raw materials to react in an organic solvent to obtain the watermelon ketone, the reaction is carried out in the presence of a catalyst, and the catalyst is anion exchange resin. The boiling point of the organic solvent is 300-350 DEG C. The method further comprises recrystallization post-treatment combining dynamic recrystallization and traditional static recrystallization under stirring. The method is a one-step reaction, the used catalyst is high in reaction selectivity, the product yield and purity are high, and the fragrance of the product is not influenced.
Owner:SHANDONG NHU PHARMA +1
Method for preparing 2-(2,3-dimethylphenyl)-1-propanal with chloroacetone
InactiveCN104220408BReduce usageIncrease the batch sizeOrganic compound preparationPreparation from heterocyclic compoundsChloroacetoneMedetomidine
The invention discloses a method for preparing 2-(2,3-dimethylphenyl)-1-propanal by using 1-bromo-2,3-dimethylbenzene and chloroacetone as starting materials. Use in fragrances and its use in the preparation of medetomidine.
Owner:LONZA LTD
Folic acid synthesis method
PendingCN113816961AReduce generationReduce pollutionOrganic chemistryPalladium on carbonNitrobenzene
The invention discloses a folic acid synthesis method which comprises the following steps: mixing p-nitrobenzoyl chloride and L-glutamic acid in water, carrying out condensation reaction under the condition that the pH value is 10-11, and separating a water phase in a product obtained by the condensation reaction; carrying out reduction reaction on the water phase, ammonium formate and palladium on carbon, and then removing palladium on carbon to obtain a reduction product; and carrying out cyclization reaction on the reduction product, 2, 4, 5-triamino-6-hydroxypyrimidine sulfate, 1, 1, 3-trichloroacetone and pyrosulfurous acid under the condition that the pH value is 2 to 4.
Owner:北京斯利安药业有限公司
Preparation process of 2-methyl-3-hydroxyquinoline-4-carboxylic acid
The invention discloses a preparation process of 2-methyl-3-hydroxyquinoline-4-carboxylic acid, belonging to the field of organic synthesis. The process comprises the steps of adding water, calcium hydroxide, sodium hydroxide and isatin into a container, stirring for over half an hour, dropwise adding chloroacetone after 1-2 hours, stirring for over 2 hours after the dropwise adding, directly adjusting the pH to 1.0+ / -0.2 with hydrochloric acid, filtering, and washing, so as to obtain the product. The preparation process has the beneficial effects that the heating and cooling processes are omitted in the whole process, the operation and the equipment are simplified, the energy cost is lowered, the optimal molar proportion of calcium hydroxide to sodium hydroxide is controlled at 3 to 1;onone hand, the reaction fluidity is facilitated, and on the other hand, the quality stability of the product is improved.
Owner:JIANGSU HUAER CHEM
A kind of preparation method and application of trans folic acid
ActiveCN107365311BHigh purityImprove quality controlOrganic chemistry methodsChemical synthesisChloroacetone
The present invention relates to a preparation method of trans folic acid and its application. Using p-aminobenzoyl glutamic acid, triaminopyrimidine sulfate and trichloroacetone as raw materials, the chemical synthesis method can significantly improve the trans folic acid crude product. content, and then separated by preparative chromatography to obtain trans folic acid with a purity greater than 95%. The research of the invention is conducive to further research on the production mechanism and control method of trans folic acid, and can effectively improve the quality control level of folic acid.
Owner:ZHEJIANG SHENGDA BIO PHARM
A kind of synthetic method of 2-amino-6-chloropurine
Owner:SHANGHAI LINKCHEM TECH CO LTD
The preparation method of 4-furfurylthiopentanone-2
ActiveCN110357840BReduce manufacturing costImprove securityOrganic chemistryPhosphorous acidEngineering
The invention belongs to the technical field of fine chemistry, and provides a preparation method of 4-furfurylthiopentanone-2, which specifically comprises the following steps: adding raw material chloroacetone into a toluene solution of triphenylphosphine or adding triethyl phosphite , the temperature is raised and refluxed to obtain a phosphorus salt; the obtained phosphorus salt is dissolved in an organic solvent, a strong base is added, and stirred for 2 to 3 hours, the reaction is completed, and phosphorus ylide is obtained; Stir until the reaction is completed to obtain 3-pentene-2-ketone; piperidine is added to 3-pentene-2-ketone, the dichloromethane solution of furfuryl mercaptan is added dropwise, and the temperature of the system is controlled to be 35~ 40° C., after the dropwise addition, the mixture was stirred until the reaction was completed to obtain the product 4-furfurylthiopentanone-2. The invention solves the problems in the prior art that the synthesis route of 4-furfuryl pentanone-2 is high in danger, high in cost and unsuitable for industrial production.
Owner:石家庄和中科技有限公司
Preparation method of high-temperature lubricant
PendingCN112500351AIncrease stickinessHigh decomposition temperatureOrganic chemistryAdditivesIonic liquidMethyl palmoxirate
The invention discloses a preparation method of a high-temperature lubricant. The preparation method comprises the following steps: 1. synthesis of 1-chloroacetone: sequentially adding calcium carbonate and acetone into a three-necked flask, sufficiently oscillating at the heating temperature of 60 DEG C, uniformly mixing, installing a condenser pipe above the three-necked flask, and connecting adevice for absorbing chlorine to the other side of the condenser pipe; absorbing chlorine with sodium hydroxide, collecting a finished product, filtering with diethyl ether to fully change a solid into powder, and filtering to obtain organic powder; and 2, synthesis of 1-methyl-3-ethylene methylene imidazole ammonium chloride ionic liquid: adding N-methyl imidazole and the 1-chloroacetone obtainedin the step 1 into a round-bottom flask, adding toluene into the round-bottom flask, putting the in-situ flask into a constant-temperature oil bath device with a magnetic stirrer, and purifying to obtain the lubricant. According to the prepared lubricant, the lubricant is high in viscosity during high-temperature movement, high in decomposition temperature which can reach 300 DEG C, free of deflagration at the critical decomposition temperature and good in conductivity.
Owner:江苏聚千新材料科技有限公司
A kind of production method of folic acid
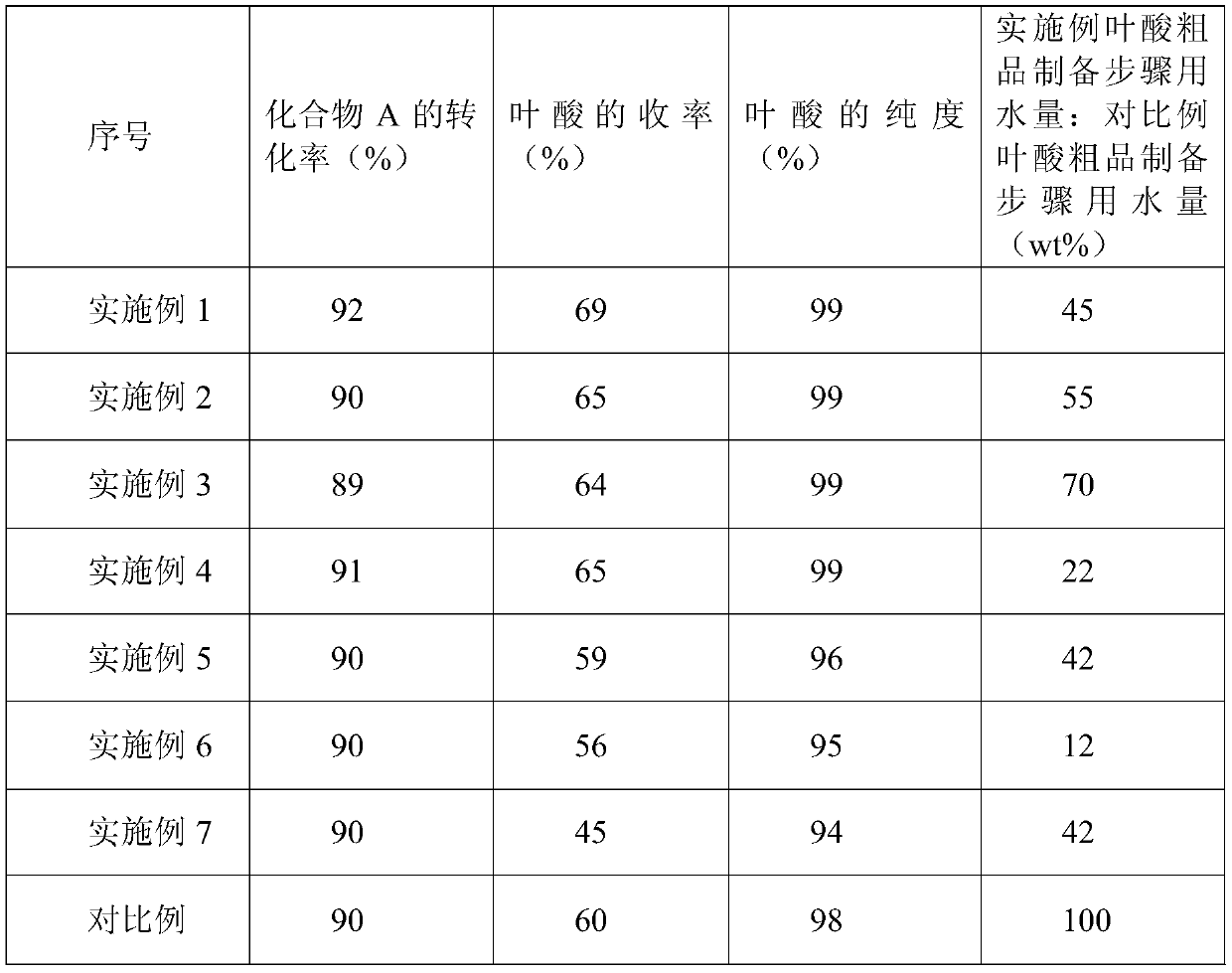
The invention relates to a production method folic acid. The production method sequentially includes following steps: synthesizing a crude product: using N-(p-aminobenzoyl)-L-glutamic acid shown as a compound A in a formula I, 2, 4, 5-triamino-6-hydroxy pyrimidine sulfate shown as a compound B in the formula I and trichloroacetone shown as a compound C in the formula I as raw material, sodium metabisulfite as an antioxidant and water as a solvent, and adopting a mode of charging in batches for graded cyclization reaction to obtain reaction liquid; subjecting the reaction liquid to cooling and press filtering to obtain a crude folic acid product; subjecting the crude folic acid product to acid dissolution, alkali dissolution and refining to obtain the folic acid. The mode of charging in batches is adopted to maintain concentration of materials in the reaction liquid within a reasonable range, so that water amount needed at the stage of synthesizing the crude folic acid product is reduced to 1 / 4 of that needed by existing synthesis processes.
Owner:JIHENG PHARMA HENGSHUI CITY
Infant food enhancer and preparation method thereof
InactiveCN104544119AWidely producedAcidic food ingredientsFood ingredient functionsBiotechnologySodium acetate
The invention discloses an infant food enhancer and a preparation method thereof. The infant food enhancer is prepared by stirring and refining methyl naphthoquinone, p-amino-benzoyl glutamic acid, acetic anhydride, trichloroacetone, zinc gluconate, sodium acetate, menadiol, a neutralizer, 2,4-diamino-6-hydroxypyrimidine, sodium metabisulfite, acetyl, folic acid, 3,4,5-triamino-6-hydroxypyrimidine sulfate and auxiliary materials. The content of a product is 98-104 percent, the content of water is 4-8 percent, the content of ignition residues is 0.01-0.1 percent, the content of heavy metal is 0.0001-0.001 percent, the pH value is 4-4.8, and the content of arsenic is 0.0001-0.0003 percent.
Owner:NANTONG SHUANGHE FOOD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com