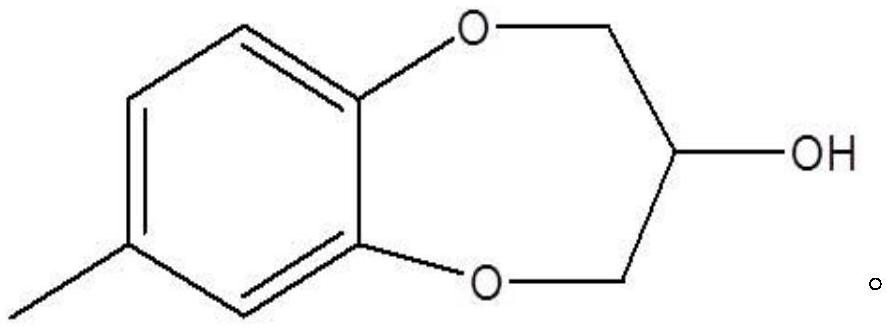
Synthesis process of watermelon ketone precursor
A synthesis process and technology of watermelon ketone are applied in the field of synthesis technology of watermelon ketone precursor to achieve the effects of reducing production cost, improving quality and high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The synthesis process of watermelon ketone precursor 3,4-dihydro-7-methyl-2H-1,5-benzoxazol-3-ol:
[0029] The reaction vessel is a 2000ml four-necked flask with a thermometer, a mechanical stirrer, a reflux condenser and a constant pressure dropping funnel. The reaction flask was evacuated, replaced with nitrogen three times, then under the protection of nitrogen, the dimethyl sulfoxide of 500ml was added in the reaction flask, the mechanical stirring was started, and 124.13g (1.0mol) 4-methylcatechol was added in the reaction flask, And 212g sodium carbonate (2.0mol), add solid to be slow, make reaction solution can not form viscous solid. After adding sodium carbonate, slowly increase the temperature of the reaction solution to make the temperature of the reaction solution reach 100°C. Then, the mixed solution of 154.77g (1.2mol) and 200ml dimethyl sulfoxide was added dropwise from the constant pressure dropping funnel into the reaction flask, and the dropping rate ...
Embodiment 2
[0031] The synthesis process of watermelon ketone precursor 3,4-dihydro-7-methyl-2H-1,5-benzoxazol-3-ol:
[0032] The reaction vessel is a 2000ml four-necked flask with a thermometer, a mechanical stirrer, a reflux condenser and a constant pressure dropping funnel. Reaction flask is evacuated, nitrogen replacement three times, then the dimethyl sulfoxide of 500ml is added in reaction flask, starts mechanical stirring, adds 156.4 (1.26mol) 4-methylcatechols in reaction flask, and 212.1 sodium carbonate ( 2.0mol), the solid should be added slowly so that the reaction solution cannot form a viscous solid. After the addition of sodium carbonate, the temperature of the reaction solution was slowly increased until the temperature of the reaction solution reached 90°C. Then the mixed solution of 172.6g (1.34mol) 1,3-dichloropropanol and 200ml dimethyl sulfoxide is put into the reaction flask at a low price from the constant pressure dropping funnel, and the rate of addition is contr...
Embodiment 3
[0034] The synthesis process of watermelon ketone precursor 3,4-dihydro-7-methyl-2H-1,5-benzoxazol-3-ol:
[0035]The reaction vessel is a 2000ml four-necked flask with a thermometer, a mechanical stirrer, a reflux condenser and a constant pressure dropping funnel. The reaction flask was evacuated, replaced with nitrogen three times, then 500ml of dimethyl sulfoxide was added to the reaction flask, mechanical stirring was started, and 146.2g (1.18mol) of 4-methylcatechol and 248g of sodium carbonate were added to the reaction flask (2.34mol), the solid should be added slowly so that the reaction solution cannot form a viscous solid. After the sodium carbonate was added, the temperature of the reaction solution was slowly increased to make the temperature of the reaction solution reach 95°C. Then, the mixed solution of 167.8 (1.3mol) 1,3-dichloropropanol and 200ml dimethyl sulfoxide is put into the reaction flask at a low price from the constant pressure dropping funnel, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com