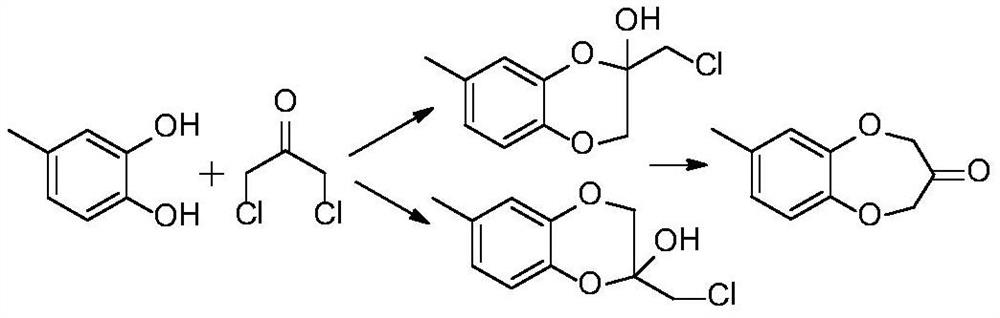
Method for synthesizing watermelon ketone
A technology of watermelon ketone and dichloroacetone, which is applied in the direction of organic chemistry, can solve the problems of complicated purification steps, undisclosed watermelon ketone purity, and chemical reactions involved, and achieve the effect of improving purity, good selectivity, and less side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Add 20g of anion resin D301 to a fixed-bed reactor with an inner diameter of 12mm and a length of 40cm. The particle size of the anion resin is 10-20 mesh, it is filled from the bottom of the reactor, the filling height is 25cm, and the filling density is 0.7g / cm 3 . Under the protection of nitrogen, 4-methylcatechol and 1,3-dichloroacetone dissolved in benzophenone (the moles of 1,3-dichloroacetone and 4-methylcatechol than n 二氯 / n 二酚 1:1, the mass ratio m of benzophenone and 4-methylcatechol 溶剂 / m 二酚 0.5:1) into the fixed bed reactor, under normal pressure, at a space velocity of 1.0h -1 , The reaction was carried out under the condition that the reaction temperature was 110°C. The reaction solution was collected for a period of time (124 g of raw material 4-methylcatechol was introduced in total), and the watermelon ketone fraction was collected by rectification under reduced pressure, which was 173.3 g. The watermelon ketone cut that 173.3g collects is transf...
Embodiment 2
[0067] Add 20g of anion resin D301 to a fixed-bed reactor with an inner diameter of 12mm and a length of 40cm. The particle size of the anion resin is 10 to 20 mesh. Filling starts from the bottom of the reaction tube, the filling height is 25cm, and the filling density is 0.7g / cm 3 . Under the protection of nitrogen, 4-methylcatechol and 1,3-dichloroacetone dissolved in benzophenone (the moles of 1,3-dichloroacetone and 4-methylcatechol than n 二氯 / n 二酚 1:1, the mass ratio m of benzophenone and 4-methylcatechol 溶剂 / m 二酚 0.5:1) into the fixed bed reactor, under normal pressure, at a space velocity of 1.0h -1 , The reaction was carried out under the condition that the reaction temperature was 110°C. Collect the reaction solution for a period of time (a total of 124 g of raw material 4-methylcatechol was introduced), and rectify under reduced pressure to collect 173.3 g of the watermelon ketone fraction; transfer the watermelon ketone fraction to a two-necked bottle, add 200...
Embodiment 3-23
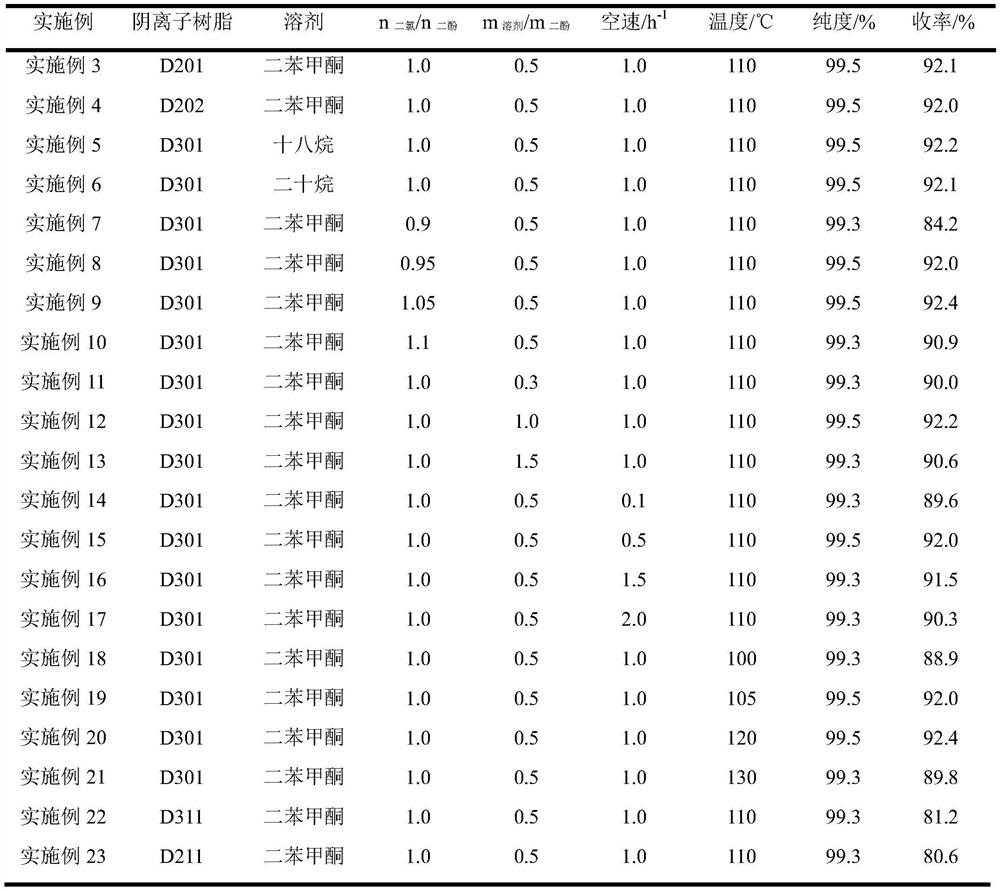
[0069] On the basis of Example 2, the reaction conditions were changed without changing the post-treatment separation method, and the purity and yield results are shown in Table 1. Each catalyst is operated for a long period, and after 1000 hours, the purity of the product watermelon ketone fluctuates by ±0.1%, and the yield fluctuates by ±0.2%, that is, the catalyst has good stability.
[0070] The reaction conditions and reaction result of table 1 watermelon ketone synthesis
[0071]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com