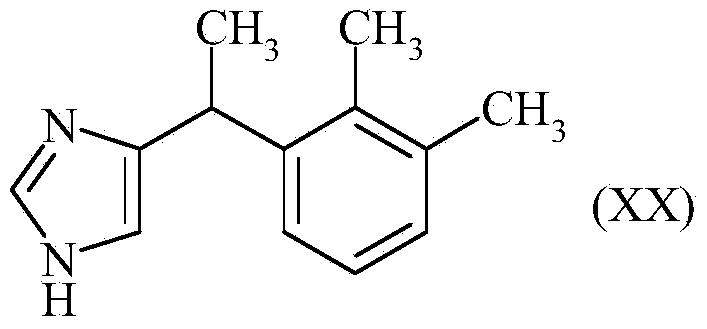
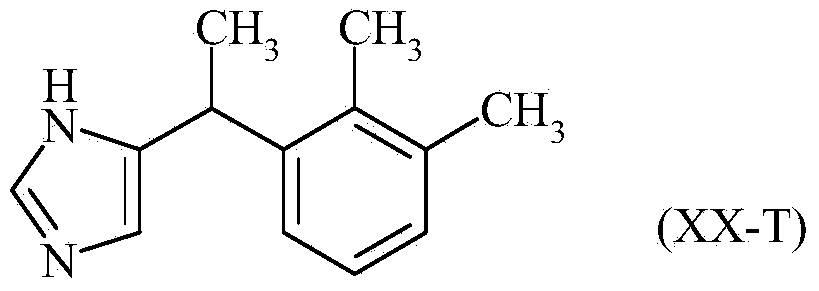
Method for preparation of medetomidine with chloroacetone
A technology of reaction and compound, applied in the field of preparation of medetomidine, can solve the problems of relatively expensive use, high material consumption, long time consumption and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0241] Example 1: 2-(2,3-Dimethylphenyl)methyloxirane, compound of formula (XXII), metal substitution with butyllithium in THF
[0242] To a solution of 1-bromo-2,3-dimethylbenzene (0.27ml, 2.0mmol) in THF (4.0ml) was added n-butyllithium (2.0ml of a 1.6M solution in hexane at -78°C , 3.2 mmol). The mixture was stirred at -78°C for 30 minutes, then a solution of chloroacetone (0.24ml, 3.0mmol) in toluene (0.42ml) was added dropwise over 20 minutes. The mixture was stirred at -78°C for 1 hour, then allowed to warm to room temperature. Analysis of a sample after 3 hours at room temperature showed the title epoxide to be the major reaction product. After stirring at room temperature for 3 days, the mixture was poured into water (20ml) and the product was extracted with ethyl acetate (1 x 10ml, 2 x 5ml). The combined extracts were washed with MgSO 4 Drying and concentration under reduced pressure afforded the title epoxide as an oil in quantitative yield.
[0243] 1 H NMR: 1...
Embodiment 2
[0245] Example 2: 2-(2,3-Dimethylphenyl)methyloxirane, compound of formula (XXII), metallized with magnesium in THF
[0246] To a suspension of magnesium (89 mg, 3.66 mmol) in THF (4.0 ml) was added NaH (81 mg, 60% in oil, 2.0 mmol), and after stirring at room temperature for 10 minutes, 1-bromo-2,3 - Dimethylbenzene (0.40ml, 2.96mmol). An exothermic reaction ensued and the resulting mixture was stirred at room temperature for 1 hour. The mixture was then cooled to -20°C and a solution of chloroacetone (0.26ml, 3.3mmol) in toluene (0.63ml) was added dropwise over 10 minutes. The mixture was then stirred at room temperature for 2 hours. The samples were worked up by mixing with water, extracting with ethyl acetate, evaporating the ethyl acetate with a stream of nitrogen. use 1 H NMR analysis of the residue showed it to be a mixture of xylene and the title oxirane compound.
Embodiment 3
[0247] Example 3: 2-(2,3-Dimethylphenyl)propanal, compound of formula (XXI)
[0248] The 2-(2,3-dimethylphenyl)methyloxirane prepared according to Example 1, the compound of formula (XXII) (158mg, 0.97mmol) was dissolved in toluene (1.57mL), and at room temperature Join BF under 3 OEt 2 (0.006ml, 0.05mmol). After 2 hours at room temperature the sample was mixed with solid NaHCO 3 Mix, filter, concentrate under reduced pressure, use 1 H NMR analysis of the residue. The crude product consisted essentially of pure 2-(2,3-dimethylphenyl)propanal.
[0249] 1 H NMR: 1.40(d, J=7.1Hz, 3H), 2.25(s, 3H), 2.32(s, 3H), 3.89(qd, J=7.1, 1.0Hz, 1H), 6.89 to 6.92(m, 1H ), 7.12 (m, 2H), 9.67 (d, J=1.0Hz, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com