Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
43 results about "Pazufloxacin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
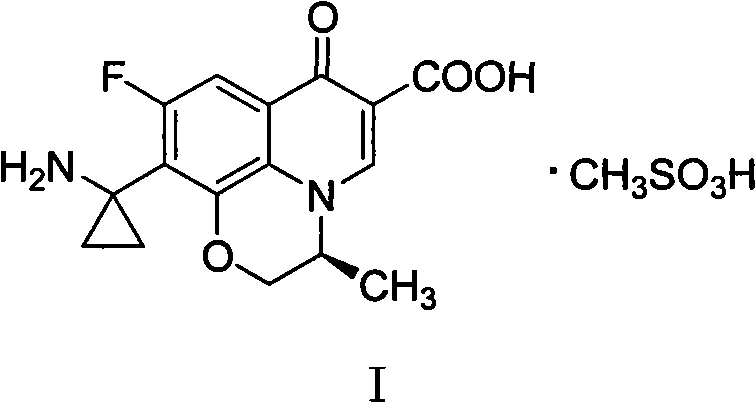
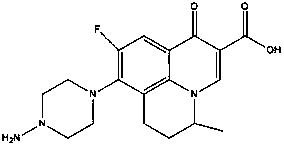
Pazufloxacin (INN) is a fluoroquinolone antibiotic. It is sold in Japan under the brand names Pasil and Pazucross.
Pazufloxacin mesylate tablet and preparation method and detection method thereof
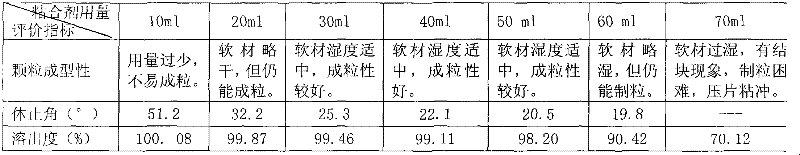
ActiveCN102125533ANice appearanceHigh dissolution rateAntibacterial agentsOrganic active ingredientsSide effectAdhesive
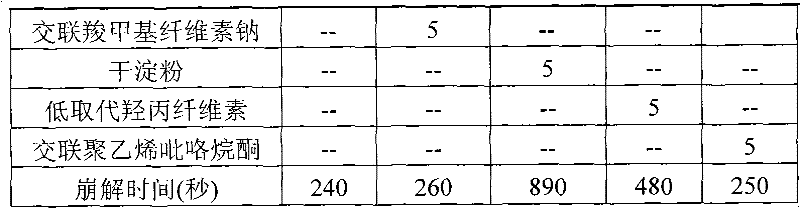
The invention relates to a pazufloxacin mesylate tablet and a preparation method and a detection method thereof, which belong to the technical field of medicines. A medicinal oral tablet with high dissolvability and high stability is prepared by the following steps of: screening the formula components of the pazufloxacin mesylate tablet; and selecting suitable adhesives and adopting specific coating materials. Clinical trials show that the pazufloxacin mesylate tablet has an exact curative effect, a few side effects and high safety.
Owner:BEIJING SIHUAN KEBAO PHARM CO LTD
Preparation method of pazufloxacin intermediate
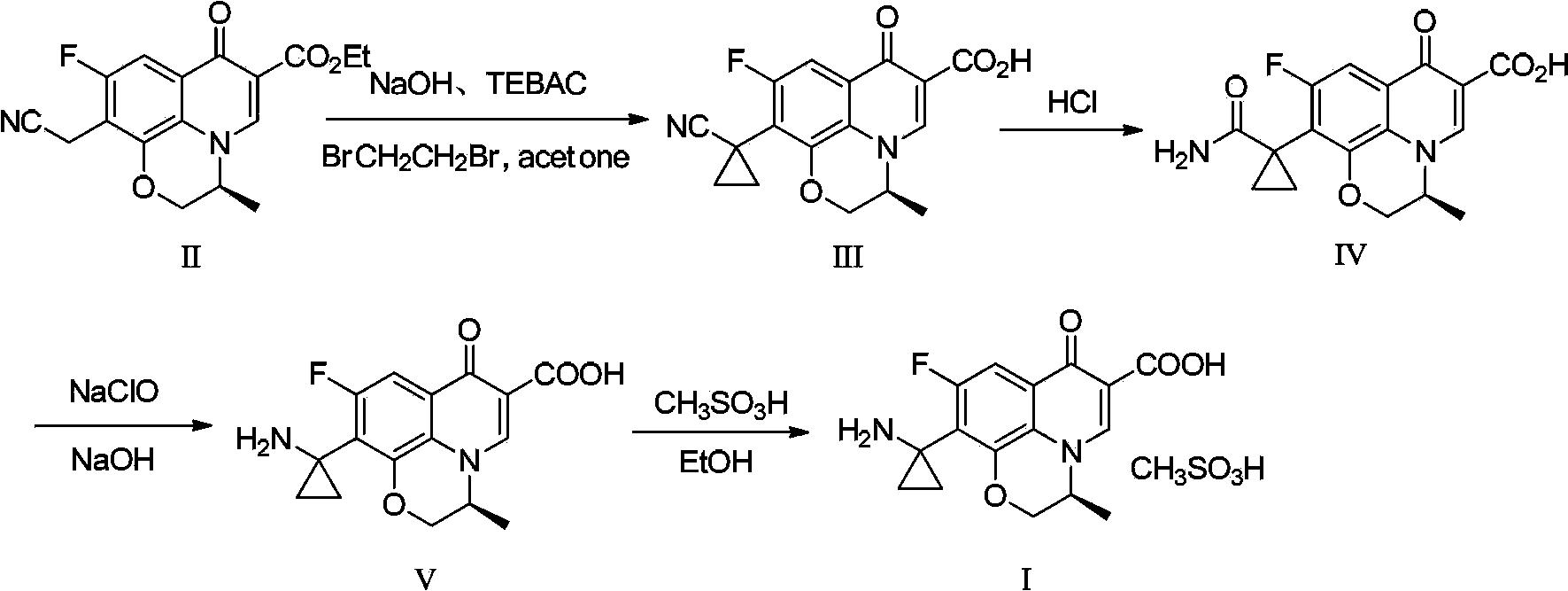
The invention relates to a preparation method of a pazufloxacin intermediate, the preparation method is as folows: in a suitable solvent and at a suitable temperature, under the effects of a phase transfer catalyst and an inorganic alkali, a compound II reacts with 1, 2 -dichloroethane for cyclopropanation, the reaction system is direct heated and refluxed for hydrolysis reaction, a pazufloxacin intermediate compound IV is obtained by postprocessing; the weight ratio of inorganic alkali to compound II is 1.5-2.5:1; the ratio of solvent to compound II is 14-16mL:1g. Compared with the prior art, according to the preparation method, a specified concentration and the specific amount of the alkali is used, a two-step method is performed by a 'one pot' method, the unit operation is simplified; and in the preparation process, the three wastes are less, the environmental protection pressure is low, the yield is high, and the method is suitable for industrialized production.
Owner:ZHEJIANG HAISEN PHARMACY CO LTD
Container-holding mesylate pazufloxacin aqueous solution and preparation method thereof
InactiveCN103027889ASolve unqualifiedGood compatibilityAntibacterial agentsOrganic active ingredientsForeign matterPolyethylene glycol
The present invention relates to a container-holding mesylate pazufloxacin aqueous solution. The mesylate pazufloxacin aqueous solution is stored in a container with a seal, and is characterized in that the pH of the aqueous solution is in the range of 1-4, the concentration of mesylate pazufloxacin in the aqueous solution is 0.001-0.01 g / ml, the container is sealed by complex membrane or a coating film rubber stopper, and the film used by the complex membrane or the coating film rubber stopper is fluoro-polymer film, poly-p-xylene-containing film or composite polyethylene glycol terephthalate film. The present invention solves the problem that visible foreign matter appears in the mesylate pazufloxacin injection products during placement.
Owner:SHANDONG XINHUA PHARMA CO LTD
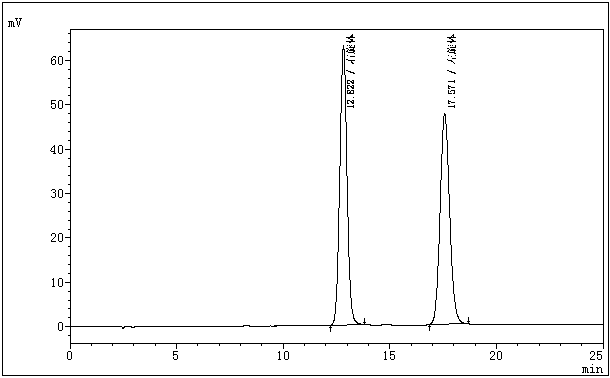
Chiral stationary-phase detection method for dextroisomer of pazufloxacin mesilate injection
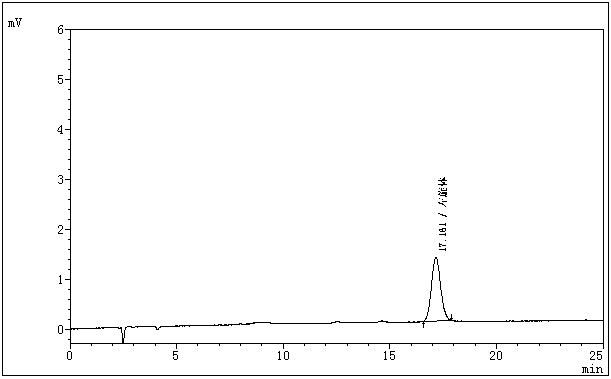
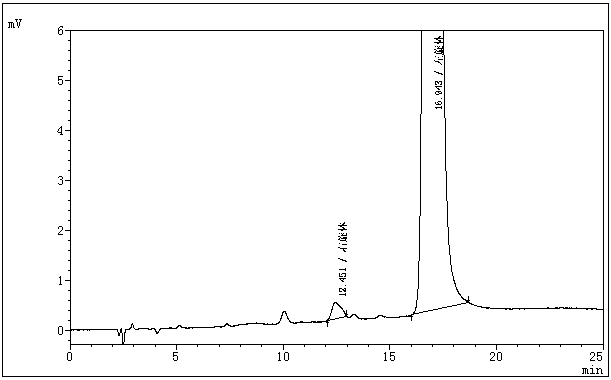
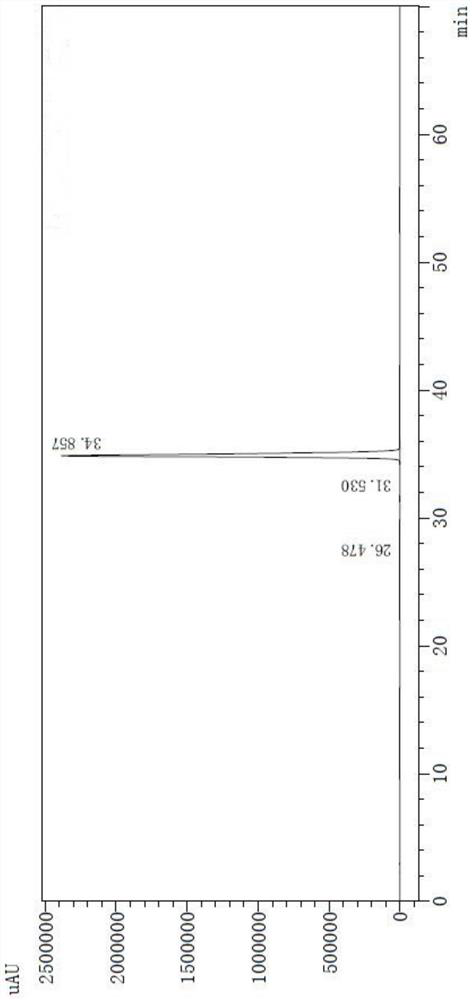
The invention belongs to the field of medicine analysis, and particularly relates to a chiral stationary-phase detection method for the dextroisomer of pazufloxacin mesilate injection. The invention aims at providing a method which is simple and convenient to operate, and capable of rapidly and accurately detecting a dextroisomer, wherein HPLC (high performance liquid chromatography) detection conditions are as follows: 5mu m silica gel coated with amylose-tri(3,5-dimethylphenyl carbamate) on the surface or 5mu m silica gel coated with amylose-tri[(S)-alpha-methylphenyl carbamate] on the surface is used as a filler in a stationary phase; a mobile phase A is a phosphate buffered solution; a mobile phase B is acetonitrile or ethanol, wherein the phosphate buffered solution contains 10-100 mM of monopotassium phosphate; the pH value is adjusted to 1.5-4.0 by phosphoric acid; a mobile phase is prepared from the mobile phase A and the mobile phase B in a volume ratio of (50 to 90): (50 to 10); a flow speed is 1.0 ml / min; a column temperature is 10-25 DEG C; a detection wavelength is 240 nm; and the number of theoretical plates is not less than 2500 counted by the peaks of the laevoisomer of pazufloxacin, and a separation degree between the laevoisomer and the dextroisomer of pazufloxacin needs to meet requirements.
Owner:CHENGDU BAIYU PHARMA CO LTD
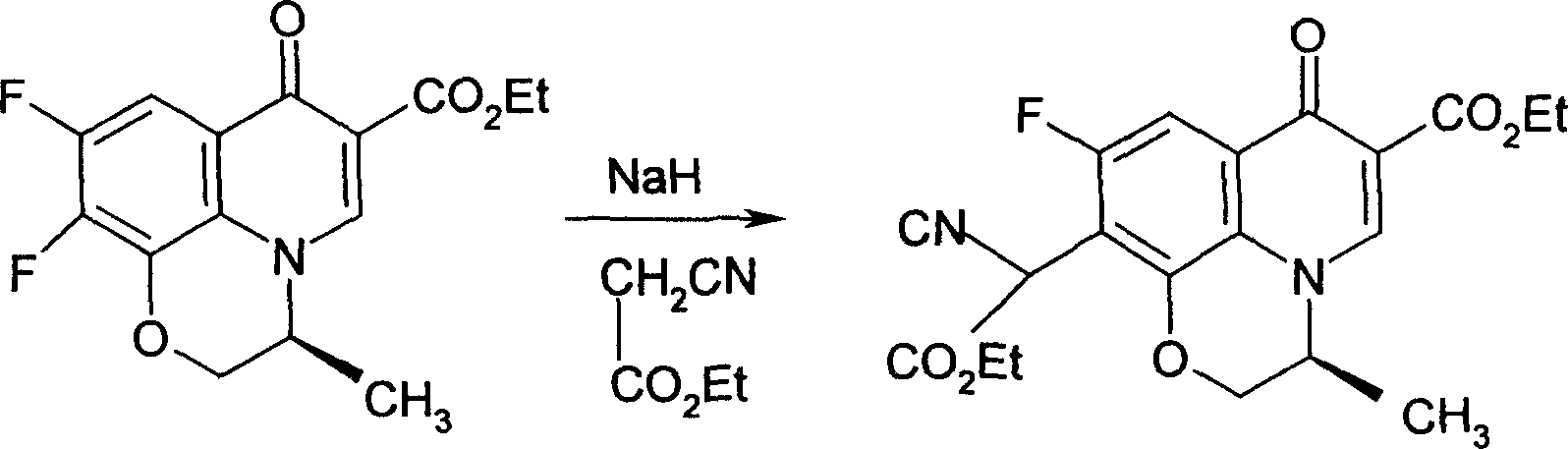
Method of synthesizing methanesulfonic acid parzhushaxing intermedinte
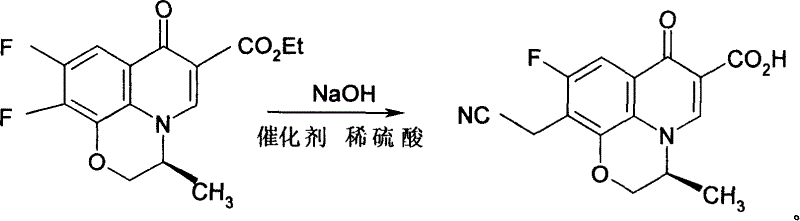
The invention provides a process for synthesizing pazufloxacin mesylas which comprises, preparing (S)-9,10-difluoro-3-methyl-7-oxy-2,3-dihydro-7H-pyrido[1,2,3-d,e][1,4]benzene oxazine-6-carboxylic acid ethyl ester (difluoro), charging NaOH, cooling down to below 0 deg. C with ice and salt mixture, stirring and charging phase transition catalyst PEG200, ethyl cyanoacetate, stirring and adjusting pH with dilute sulphuric acid, slowly elevating the temperature to 25-60 deg. C, reacting 4-10 hours, cooling down to 2-10 deg. C, stirring 2-5 hours at below 0 deg. C, elevating the temperature to 20-25 deg. C, thermal insulating and reacting 3-8 hours, freezing, evoluting crystal, filtering by suction, finally drying to obtain the product.
Owner:武汉人福药业有限责任公司
Pazufloxacin mesilate injection composition and preparation method thereof
ActiveCN105287371AImprove stabilityLow content of related substancesAntibacterial agentsOrganic active ingredientsSuccinic acidPazufloxacin
The invention provides a pazufloxacin mesilate injection composition and a preparation method thereof. The pazufloxacin mesilate injection composition comprises pazufloxacin mesila, methanesulfonic acid, succinic acid and common salt. The pazufloxacin mesilate injection composition has the characteristics of high stability, low content of relevant substances and suitability for industrialized production.
Owner:HEBEI ZHITONG BIOLOGICAL PHARMA
Anti-quinolone antibiotic class specific monoclonal antibody hybridoma cell strain YH6 and application thereof
ActiveCN106520704AHigh sensitivityBiological material analysisMicroorganism based processesOrbifloxacinEnzyme linked immunoassay
An anti-quinolone antibiotic class specific monoclonal antibody hybridoma cell strain YH6 and an application thereof belong to the technical field of immunochemistry. The monoclonal cell strain YH6 is preserved in China General Microbiological Culture Collection Center with the preservation number of CGMCC No.12024. A monoclonal antibody secreted by the YH6 is detected by indirect competitive enzyme-linked immunosorbent assay, and has cross reaction with the following 21 pyrethroids: norfloxacin, ofloxacin, enrofloxacin, ciprofloxacin, flumequine, nafloxacin, enoxacin, lomefloxacin, levofloxacin, pefloxacin, nalidixic acid, danofloxacin, pyridine acid, cinoxacin, oxolinic acid, marbofloxacin, pazufloxacin, sparfloxacin, gatifloxacin, orbifloxacin and fleroxacin, and the IC50 value of the monoclonal antibody is 0.1-50 ng / mL. The class specific monoclonal antibody can be used for developing colloidal gold immunochromatographic test strips and immunosensors, provides a raw material for the immunodetection of quinolone antibiotic residues in foods, and has practical application values.
Owner:JIANGNAN UNIV
Pazufloxacin mesilate and quality control method of injection preparation
ActiveCN103675184AReliable evaluationAccurate evaluationComponent separationFluid phaseQuantitative determination
The invention discloses pazufloxacin mesilate and a quality control method of an injection preparation. According to the method, high performance liquid chromatography is employed for performing quantitative determination on pazufloxacin mesilate and an impurity pazufloxacin mesilate dextroisomer in the preparation. The technical scheme provided by the invention is capable of relatively reliably and relatively accurately evaluating and controlling the quality of pazufloxacin mesilate and the injection preparation.
Owner:SHANDONG QIDU PHARMA
Eye drop of pazufloxacin mesilate, its preparing method and application
InactiveCN1634070AInhibition formationAvoid developmentOrganic active ingredientsSenses disorderFiltrationPreservative
The disclosed eye drop is prepared from Pazufloxacin mesilate, preservative agent, stabilizer, viscosity increaser and solvent through the steps of, (1) preparing boric acid and cushion liquid, (2) charging preservative into buffer agent, heating, stirring, dissolving and charging into stabilizing agent, charging Pazufloxacin mesilate, heating and stirring till dissolving, obtaining solution 1, (3) charging viscosity increaser into buffer agent or boric acid water, stirring till dissolving to obtain solution 2, (4) filtering the solution to obtained filtration liquid, (5) filtering the solution 2 to obtain filtration liquid, (6) merging the filtration liquid obtained in step (4) and (5), stirring, dissolving and charging into boric acid water or cushion agent, adjusting pH with hydrochloric acid or NaOH.
Owner:NANJING MEDICAL UNIV +1
New application of quinolone compounds in prevention and treatment of plant bacterial diseases such as citrus canker
PendingCN111771895AStrong antibacterial activityHigh antibacterial activityBiocideDisinfectantsPipemidic acidFleroxacin
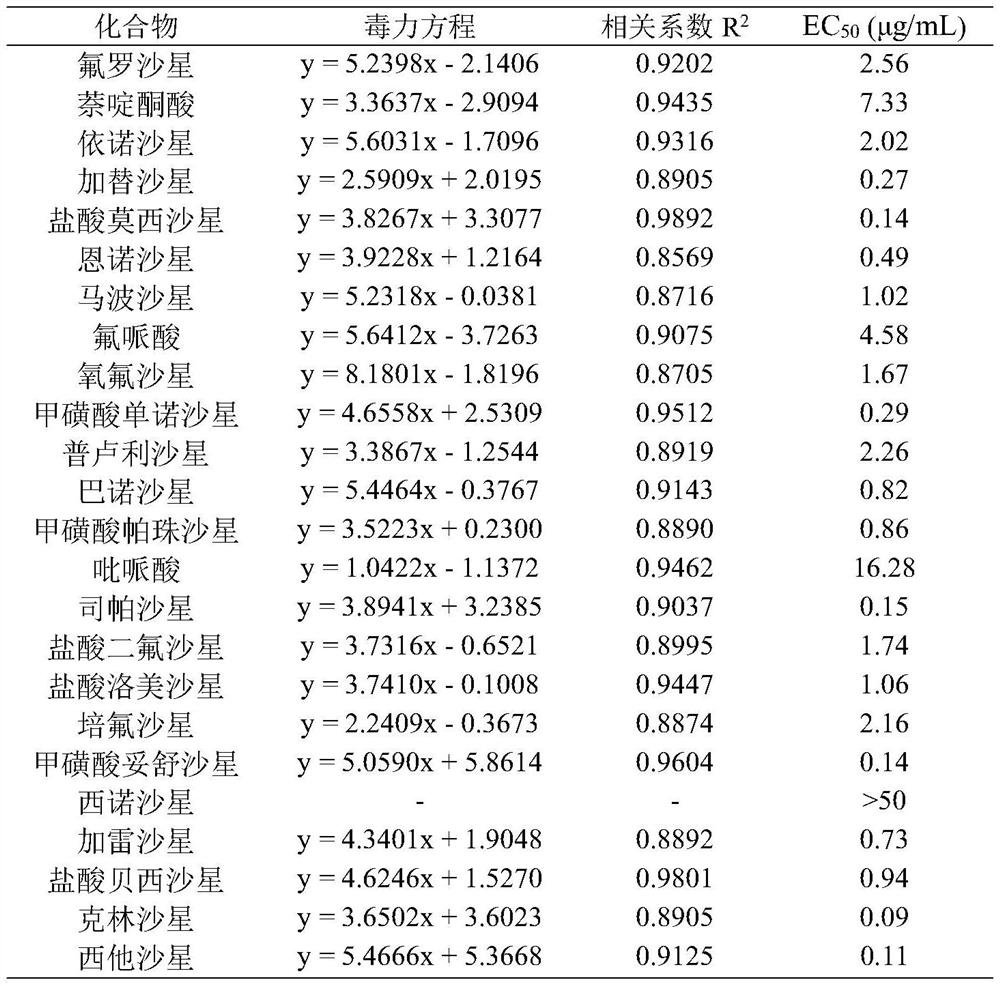
The invention discloses a new application of quinolone compounds as bactericides in prevention and treatment of bacterial diseases and citrus canker of crops. The quinolone compounds comprise floroxacin, enofloxacin, gatifloxacin, moxifloxacin hydrochloride, enrofloxacin, marbofloxacin, floxacin, mononorfloxacin mesylate, prulifloxacin, Balofloxacin, pazufloxacin mesylate, pipemidic acid, sparfloxacin, difloxacin hydrochloride, lomefloxacin hydrochloride, pefloxacin, tosufloxacin mesylate, Cinoxacin, galafloxacin, besifloxacin hydrochloride, ofloxacin, nalidixic acid, Clinafloxacin and Sitafloxacin. The quinolone compounds can be used for preventing and treating bacterial diseases caused by citrus canker pathogens, especially gatifloxacin, moxifloxacin hydrochloride, mononorfloxacin mesylate, sparfloxacin, tosufloxacin mesylate, clinafloxacin and sitafloxacin, has excellent bacteriostatic activity on citrus canker pathogens, and can be used for preventing and treating bacterial diseases of crops.
Owner:LANZHOU UNIVERSITY
Application of fluoroquinolone medicine used as polymyxin-type antibiotic sensitizer
The invention discloses novel application of a fluoroquinolone medicine and application of the fluoroquinolone medicine in preparation of a sensitizer of a pseudomonas aeruginosa (P.aeruginosa) inhibitor. The fluoroquinolone medicine is prepared from gemifloxacin, sparfloxacin, enrofloxacin, ciprofloxacin, sarafloxacin, moxifloxacin, pefloxacin, tosufloxacin, orbifloxacin, prulifloxacin, marbofloxacin, levofloxacin, flumequine or / and pazufloxacin; the pseudomonas aeruginosa is pseudomonas aeruginosa DK2 or PAO1; the pseudomonas aeruginosa inhibitor is polymyxin B or colistin.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Pazufloxacin mesilate for injection and preparation method
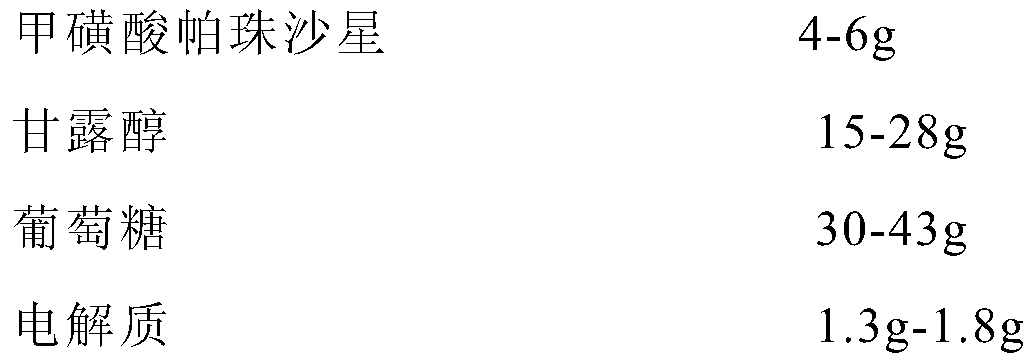
InactiveCN110801435AAvoid active ingredientsAvoid turbidityAntibacterial agentsOrganic active ingredientsHypoglycemiaChloride potassium
The invention relates to pazufloxacin mesilate for injection. The pazufloxacin mesilate comprises the following components as shown in the description, further eletrolyte comprises the following components in parts by weight of 0.6-0.8g of sodium chloride, 0.5-0.7g of potassium chloride and 0.2-0.3 of magnesium chloride. An injection agent can avoid separation of effective components, can preventturbidity in the long-term storage process, and can reduce the appearance of situations of electrolyte disturbance and hypoglycemia after medication. The invention further provides a preparation method of the pazufloxacin mesilate for injection.
Owner:NANJING CHENGONG PHARM CO LTD
Western medicine composition for treating leucoderma
InactiveCN106511973ANo discomfortReduce economic pressurePeptide/protein ingredientsHydroxy compound active ingredientsTreatment effectSide effect
The invention discloses a western medicine composition for treating leucoderma. The composition is prepared from, by weight, 0.1-1 part of vitamin C, 0.1-2 parts of vitamin p, 0.1-1 part of vitamin E, 2-5 parts of placentin, 2-8 parts of hair, 10-25 parts of thymosin injection, 0.2-5 parts of retinoic acid, 1-3 parts of netilmicin, 2-5 parts of acetylspiramycin, 2-10 parts of pazufloxacin mesilate, 1-5 parts of asparaginic acid, 0.5-1 part of glycine, 2-5 parts of compound kaliziran tincture and 1-5 parts of vindesine. The medicine for treating leucoderma has the advantages that the medicine is good in treatment effect, takes effect quickly and is short in treatment period and small in toxic and side effects, leucoderma is not likely to relapse after drug withdrawal, and the medicine is convenient to take. Formation of melanin around a leukoplakia portion is benefited, and the effective rate is as high as 80%. A preparation method is simple, cost is low, and economic burden of patients is effectively reduced.
Owner:郑州莉迪亚医药科技有限公司
Fructose injection of antibiotic medicine
InactiveCN108721625AEasy to useSuitable for useAntibacterial agentsPharmaceutical delivery mechanismFluconazoleNorfloxacin
The invention relates to fructose injection of an antibiotic medicine. The fructose injection of the antibiotic medicine consists of antibiotics, fructose and water and also comprises proper additives, wherein the antibiotics comprise gatifloxacin, levofloxacin, ciprofloxacin, pazufloxacin, fleroxacin, sparfloxacin, moxifloxacin, pefloxacin, rufloxacin, lomefloxacin, norfloxacin, caderofloxacin, azithromycin, telithromycin, ornidazole, secnidazole, tinidazole, metronidazole, clindamycin, lincomycin, fluconazole, etimicin, netilmicin, amikacin as well as medicinal acid addition salt, esterification compounds, derivatives and the like; the fructose injection is prepared from the antibiotics; the advantages that the injection is convenient to use and takes effect rapidly are achieved; and compared with the glucose injection, the fructose injection is easier to absorb and utilize, more suitable for antisepsis and anti-inflammation, energy supply and body liquid supplementation of patientssuffering from diabetes, heart diseases and liver diseases, and enlarges the use range.
Owner:WEIHAI HAOTONG MEDICAL SCI & TECH
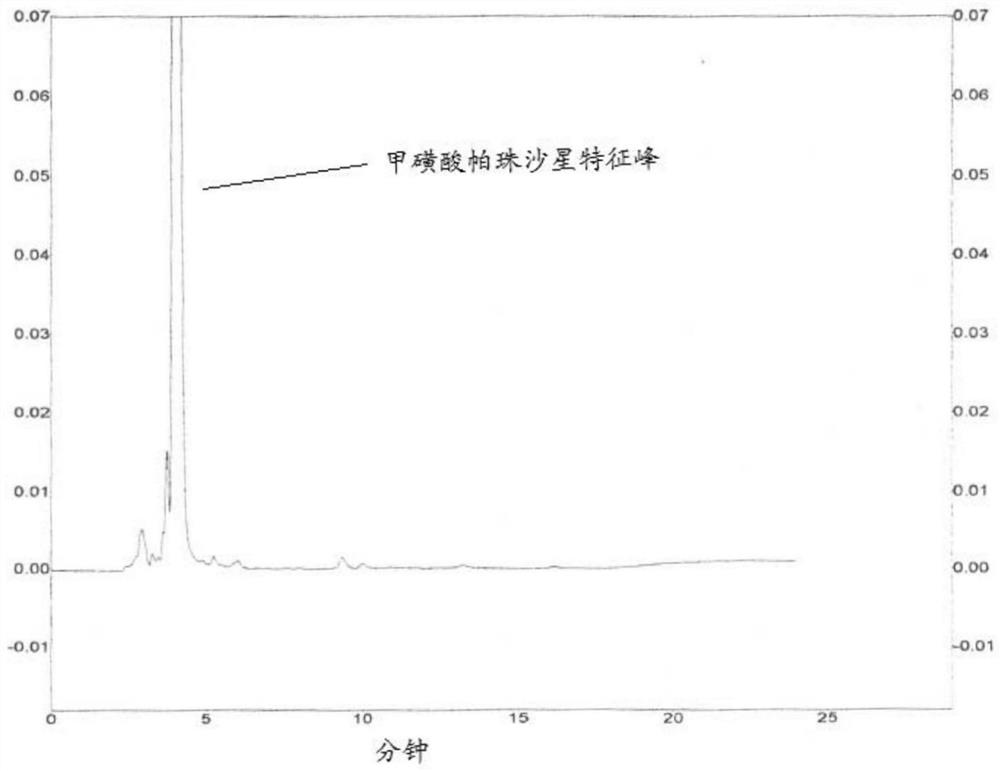
Method for detecting related substances in pazufloxacin mesylate bulk drug by adopting HPLC
InactiveCN113624859AModerate retention timeEfficient separationComponent separationDipotassium phosphateCombinatorial chemistry
The invention discloses a method for detecting related substances in pazufloxacin mesylate by adopting HPLC, which comprises the following steps: preparing a test solution of a pazufloxacin mesylate bulk drug, and diluting the test solution to obtain a contrast solution; and according to the method, detecting a pazufloxacin mesylate bulk drug by taking acetonitrile-10% triethylamine mesylate solution and 1.0 mol / L dipotassium phosphate-water in a volume ratio of 30: 10: 7:170 as a first mobile phase and taking acetonitrile-10% triethylamine mesylate solution and 1.0 mol / L dipotassium phosphate-water in a volume ratio of 45:10:7:138 as a second mobile phase. Therefore, the first mobile phase and the second mobile phase are combined for use, so that the related substances of the pazufloxacin mesilate bulk drug can be detected more accurately and scientifically.
Owner:HAINAN HAISHEN TONGZHOU PHARM CO LTD
Preparation method for pazufloxacin mesilate for injection
ActiveCN103271883AImprove product qualityGood repeatabilityAntibacterial agentsOrganic active ingredientsMANNITOL/SORBITOLPazufloxacin
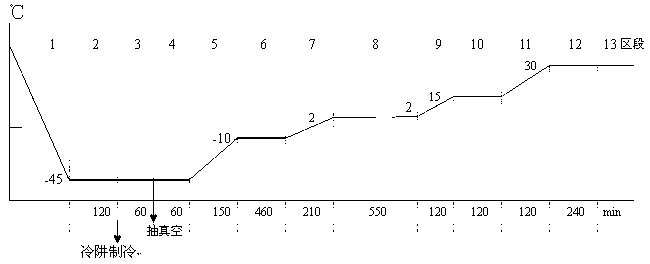
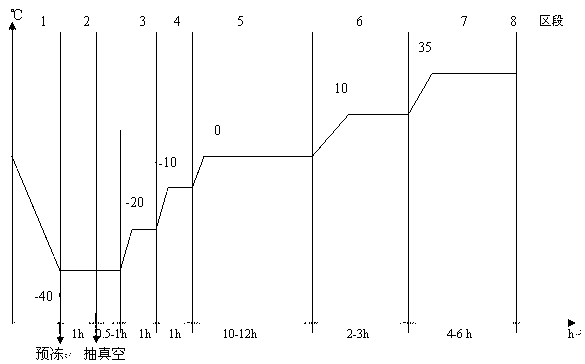
The invention provides a preparation method for pazufloxacin mesilate for injection. The preparation method comprises the following steps of: preparing the pazufloxacin mesilate and mannitol according to a prescription, and regulating pH values to be 3.0-4.0; adding a proper amount of needle active carbon, heating the mixture to be 70-80 DEG C, and carrying out decoloring after stirring for 15-20 minutes; putting an encapsulated semi-finished product into a freezing vacuum drier, controlling the temperature to be below -30 DEG C and carrying out prefreezing for 1-1.5 hours; when the temperature of a cold trap of the drier is lower than -40 DEG C, vacuumizing until the pressure is 20 Pa, and carrying out constant-temperature drying for 0.5-1 hour; raising the temperature of a separation board of the drier to be -10 DEG C, carrying out the constant-temperature drying for 0.5-1 hour; and raising the temperature of the separation board to be 0 DEG C, carrying out the constant-temperature drying until the waterline of the product reaches the bottom, raising the temperature to be 10 DEG C in a temperature manner, after carrying out the constant-temperature drying until the temperature of the product approximates to 0 DEG C, raising the temperature of the separation board to be 35 DEG C, and judging that the product is qualified after carrying out the constant-temperature drying until reaching the end point. The preparation method has the advantages that the production cost is lowered and the product quality is improved and is more suitable for industrial production.
Owner:SICHUAN BAILI PHARM CO LTD
Pazufloxacin mesylate and preparation method of powder for injection
InactiveCN101381372AReduce the temperatureAvoid instabilityAntibacterial agentsPowder deliveryFreeze-dryingSolvent
The invention discloses a method for preparing pazufloxacin mesilate and a powder injection thereof. The method is as follows: a reaction system which is formed by pazufloxacin and methane-sulforic acid as well as a solvent of acetone is subjected to heating and reflux, cooling and crystallization, and separation to obtain the pazufloxacin mesilate, and the pazufloxacin mesilate is then subjected to aseptic filling and freeze drying to obtain the powder injection of pazufloxacin mesilate. The preparation method of the invention can produce pazufloxacin mesilate for injection which has stable quality.
Owner:HAINAN YONGTIAN PHARMA INST
Method for purifying pazufloxacin mesylate
InactiveCN101928290AEasy to operateHigh economic and practical valueOrganic chemistryPurification methodsMedicine
The invention relates to a method for preparing pure pazufloxacin mesylate. The method comprises the step of treating the pazufloxacin mesylate or pazufloxacin by using N,N-dimethyl acetamide.
Owner:ZHEJIANG STARRY PHARMA
Fructose injection of antibiotic drug
InactiveCN106668862AEasy to useSuitable for useAntibacterial agentsPharmaceutical delivery mechanismFluconazoleNorfloxacin
The invention provides a fructose injection of an antibiotic drug. The injection is composed of antibiotics, fructose and water. The injection also can contain proper additives. The antibiotic comprise gatifloxacin, levofloxacin, ofloxacin, ciprofloxacin, pazufloxacin, fleroxacin, sparfloxacin, moxifloxacin, pefloxacin, rufloxacin, lomefloxacin, norfloxacin, caderofloxacin, azithromycin, telithromycin, ornidazole, secnidazole, tinidazole, metronidazole, clindamycin, lincomycin, fluconazole, etimicin, netilmicin, amikacin and medicinal acid additive salts, esterified compounds, derivatives and the like. The antibiotics are prepared into the fructose injection. Besides the advantages of convenience in use and quickness to take effect of the injection, compared with a gluconic infection, the injection provided by the invention is more easily absorbed and utilized, is more suitable for preventing bacteria and diminishing inflammation, supplying energy and supplementing body fluids for patients with diabetes, heart disease and liver disease, so that the application range of the injection is expanded.
Owner:威海恒基伟业信息科技发展有限公司
Pazufloxacin mesylate tablet and preparation method and detection method thereof
The invention relates to a pazufloxacin mesylate tablet and a preparation method and a detection method thereof, which belong to the technical field of medicines. A medicinal oral tablet with high dissolvability and high stability is prepared by the following steps of: screening the formula components of the pazufloxacin mesylate tablet; and selecting suitable adhesives and adopting specific coating materials. Clinical trials show that the pazufloxacin mesylate tablet has an exact curative effect, a few side effects and high safety.
Owner:BEIJING SIHUAN KEBAO PHARM CO LTD
Quality control method of pazufloxacin mesylate injection
PendingCN114354794AThe result is accurateSensitive methodComponent separationIsocratic elutionGradient elution
The invention relates to the technical field of pharmaceutical analysis and provides a quality control method of a pazufloxacin mesilate injection, which is used for solving the problem that the existing pazufloxacin mesilate quality method cannot separate photodegradable impurities, and comprises the following steps: (1) preparing a test solution; preparing a contrast solution; preparing a reference solution; (2) recording a chromatogram of a reference substance of the reference substance solution; (3) recording chromatograms of the test solution and the contrast solution; and (4) calculating the content of pazufloxacin mesylate by peak area according to an external standard method. According to the method, the proportion of the two mobile phases in the gradient is adjusted according to the polarity of each impurity, and isocratic elution is changed into gradient elution, so that the impurities with different polarities are well separated.
Owner:四川美大康佳乐药业有限公司
Pazufloxacin methane-sulfonate eye ointment and its producing process-anti-infection
InactiveCN1491650AStable and controllable qualityQuality, safety and effectivenessOrganic active ingredientsSenses disorderMethane sulfonateActive component
The present invention relates to pazufloxacin methane-sulfonate eye ointment as a kind of quinolone antibiotic and its preparation process. It is eyeí»s ointment preparation prepared with pazufloxacin methane-sulfonate as active component and proper eye ointment matrix.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Acne removing lotion and production technology and application thereof
InactiveCN108143803AEasy to useQuick resultsOrganic active ingredientsPharmaceutical delivery mechanismFrostChlorogenic acid
The invention discloses acne removing lotion and a production technology and application thereof. The acne removing lotion is prepared from, by weight, 3-8 parts of chlorogenic acid, 4-7 parts of croton seeds, 5-10 parts of persimmon frost, 5-11 parts of pazufloxacin mesilate, 8-13 parts of fructus trichosanthes, 6-15 parts of evodia lepta roots, 6-10 parts of garden balsam seeds, 6-12 parts of gynura formosana and 95-480 parts of water. The acne removing lotion has the advantages of being convenient to use, rapid in effect, remarkable in treatment effect, small in skin irritation, not likelyto be allergic, capable of treating both symptoms and root causes and not prone to relapse; the lotion is worthy of being popularized, the preparation process is simple, and industrial production is facilitated.
Owner:广州市尚昇生物科技有限公司
Pazufloxacin mesilate medicinal preparation and preparation method thereof
InactiveCN102805748ASimple prescriptionSimple processAntibacterial agentsOrganic active ingredientsMedicineAdhesive
The invention provides a pazufloxacin mesilate oral medicinal preparation which comprises the components by weight as follows: 8-15 parts of pazufloxacin mesilate, 0.7-2.0 parts of diluent 0-2.0 parts of a disintegrating agent, 0.03-0.1 parts of lubricant, 0.06-0.2 parts of glidant, 0-0.2 parts of an antisticking agent and 0.18-0.6 parts of adhesive. The preparation is simple in prescription and process, low in production cost, and high in stability and bio-availability, and completely meets the requirements of pharmacopeia. The invention provides a novel pazufloxacin oral medicinal preparation which is more safe and stable and more convenient for being taken.
Owner:CHENGDU JUNXIANG MEDICAL SCI & TECH
Preparation method for pazufloxacin mesilate for injection
ActiveCN103271883BGood repeatabilityImprove product qualityAntibacterial agentsOrganic active ingredientsMANNITOL/SORBITOLWork in process
The invention provides a preparation method for pazufloxacin mesilate for injection. The preparation method comprises the following steps of: preparing the pazufloxacin mesilate and mannitol according to a prescription, and regulating pH values to be 3.0-4.0; adding a proper amount of needle active carbon, heating the mixture to be 70-80 DEG C, and carrying out decoloring after stirring for 15-20 minutes; putting an encapsulated semi-finished product into a freezing vacuum drier, controlling the temperature to be below -30 DEG C and carrying out prefreezing for 1-1.5 hours; when the temperature of a cold trap of the drier is lower than -40 DEG C, vacuumizing until the pressure is 20 Pa, and carrying out constant-temperature drying for 0.5-1 hour; raising the temperature of a separation board of the drier to be -10 DEG C, carrying out the constant-temperature drying for 0.5-1 hour; and raising the temperature of the separation board to be 0 DEG C, carrying out the constant-temperature drying until the waterline of the product reaches the bottom, raising the temperature to be 10 DEG C in a temperature manner, after carrying out the constant-temperature drying until the temperature of the product approximates to 0 DEG C, raising the temperature of the separation board to be 35 DEG C, and judging that the product is qualified after carrying out the constant-temperature drying until reaching the end point. The preparation method has the advantages that the production cost is lowered and the product quality is improved and is more suitable for industrial production.
Owner:SICHUAN BAILI PHARM CO LTD
Methanesulfonic acid pazufloxacin gel droplet for ear and its preparation
The invention discloses a ethanesulfonic acid Pazufloxacin ad aurem gel auristilla, which take ethanesulfonic acid Pazufloxacin for the major components, and is produced complemented by Carbomer, glycerin, sodium hydroxide, propanediol and sterile water for injection or purified water or distilled water. The invention also discloses the preparation method of the said ethanesulfonic acid Pazufloxacin ad aurem gel auristilla. The invention of this medicine is a new generation quinolone synthesis antibacterial drugs, with high bioavailability, can reduce the dosage in clinical application, dosage interval extension, reduction the adverse reactions of systemic absorption, helping improving patients medication compliance. Meanwhile, the invention of the drug overcomes the resistance which the old varieties drugs bring to patients, solves the need of new medicines for the clinical treatment of ear infections diseases.
Owner:广州博济新药临床研究中心有限公司
Method for detecting small polar impurities in pazufloxacin mesylate bulk drug
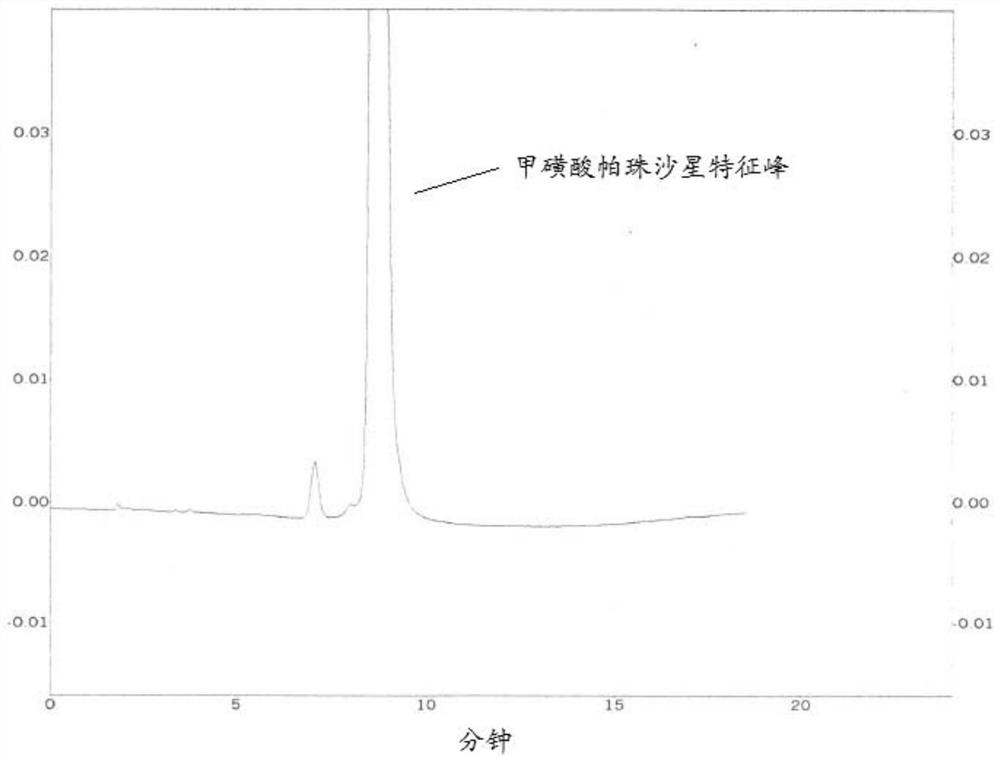
InactiveCN113702517AAchieve quality controlEfficient detectionComponent separationAgainst vector-borne diseasesDipotassium hydrogen phosphatePazufloxacin
The invention discloses a method for detecting small polar impurities in pazufloxacin mesylate bulk drug, which adopts high performance liquid chromatography for detection, and the mobile phase is acetonitrile-10% triethylamine mesylate solution-1. 0mol / L dipotassium phosphate-water with the volume ratio of 45: 10: 7: 138; the method comprises the following steps: respectively taking a test solution and a contrast solution, injecting the test solution and the contrast solution into a high performance liquid chromatograph, and recording the retention time of a chromatogram to 7 times of a pazufloxacin mesylate characteristic peak. According to the present invention, the pazufloxacin mesylate bulk drug is detected by using the HPLC, and the small polar impurity with the retention time greater than the characteristic peak of the pazufloxacin mesylate can be effectively detected within the 7-time retention time of the pazufloxacin mesylate by using the specific ratio of the mobile phase; therefore, the quality control of the pazufloxacin mesilate bulk drug can be realized more accurately and scientifically.
Owner:HAINAN HAISHEN TONGZHOU PHARM CO LTD
A group-selective monoclonal antibody hybridoma cell line yh6 against quinolone antibiotics and its application
ActiveCN106520704BHigh sensitivityBiological material analysisMicroorganism based processesOrbifloxacinNorfloxacin
An anti-quinolone antibiotic class specific monoclonal antibody hybridoma cell strain YH6 and an application thereof belong to the technical field of immunochemistry. The monoclonal cell strain YH6 is preserved in China General Microbiological Culture Collection Center with the preservation number of CGMCC No.12024. A monoclonal antibody secreted by the YH6 is detected by indirect competitive enzyme-linked immunosorbent assay, and has cross reaction with the following 21 pyrethroids: norfloxacin, ofloxacin, enrofloxacin, ciprofloxacin, flumequine, nafloxacin, enoxacin, lomefloxacin, levofloxacin, pefloxacin, nalidixic acid, danofloxacin, pyridine acid, cinoxacin, oxolinic acid, marbofloxacin, pazufloxacin, sparfloxacin, gatifloxacin, orbifloxacin and fleroxacin, and the IC50 value of the monoclonal antibody is 0.1-50 ng / mL. The class specific monoclonal antibody can be used for developing colloidal gold immunochromatographic test strips and immunosensors, provides a raw material for the immunodetection of quinolone antibiotic residues in foods, and has practical application values.
Owner:JIANGNAN UNIV
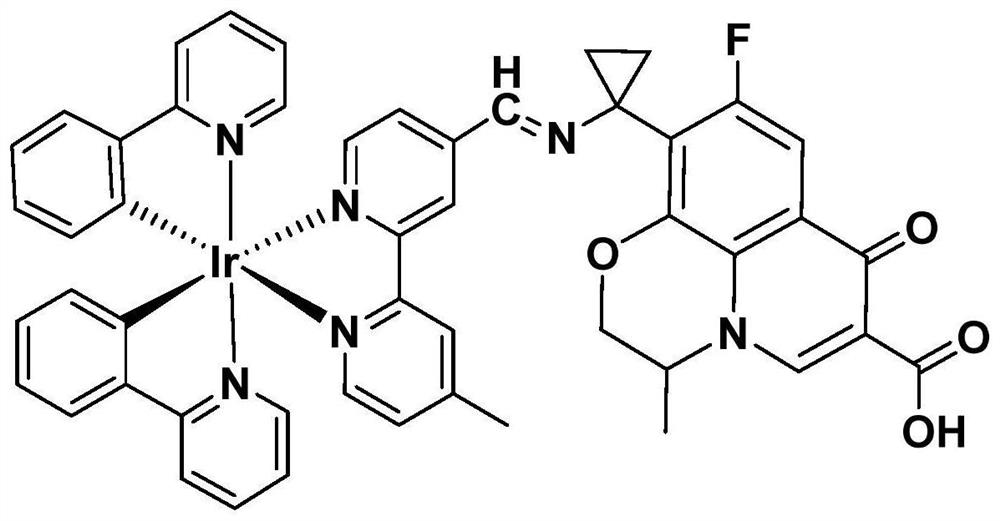
Preparation method and application of pazufloxacin iridium complex
PendingCN114315911AAvoid infectionSelectiveAntibacterial agentsOrganic active ingredientsNormal cellCombinatorial chemistry
The invention discloses a preparation method and application of a pazufloxacin iridium complex. The pazufloxacin iridium complex at least contains one of the following chemical formulas: [Ir (ppy) 2 (mbpyPAZ)] PF6, [Ir (bzq) 2 (mbpyPAZ)] PF6; [Ir (dfppy) 2 (mbpyPAZ)] PF6; [Ir (thpy) 2 (mbpyPAZ)] PF6; [Ir (pq) 2 (mbpyPAZ)] PF6. The pazufloxacin iridium complex has antibacterial and anti-tumor dual functions, has certain selectivity, and has extremely low cytotoxicity to normal cells L-02.
Owner:GUANGDONG ZHANJIANG PROVINCIAL LAB OF SOUTHERN MARINE SCI & ENG
Pazufloxacin mesilate xylitol injection and preparation method thereof
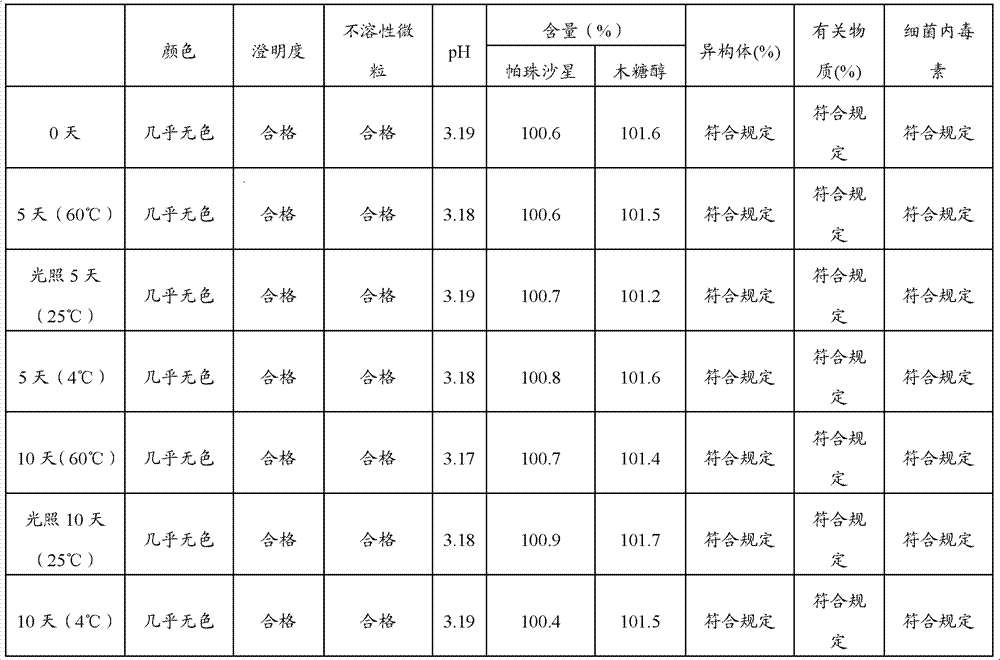
The invention discloses a pazufloxacin mesilate xylitol injection which is characterized by containing the components as follows: 1g to 10g of pazufloxacin mesilate and 43g to 100g of xylitol, wherein methanesulfonic acid is used for regulating the PH value to 2.0 to 6.0, and water for injection is added to reach 1000ml. Furthermore, the invention further provides a preparation method of the pazufloxacin mesilate xylitol injection. Compared with the prior art, the pazufloxacin mesilate xylitol injection and the preparation method thereof have the advantage that: the isomer and relevant substances of the pazufloxacin mesilate xylitol injection under illumination and at high temperature are within the qualified scope by means of study and test on thermal stability, light stability and low temperature.
Owner:四川美大康佳乐药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com