Quality control method of pazufloxacin mesylate injection
A technology of pazufloxacin mesylate and quality control method, which is applied in the field of quality control of pazufloxacin mesylate injection, can solve problems such as the generation of photodegradable impurities and the separation of non-degradable impurities, and achieve accurate results and methods Sensitive and repeatable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
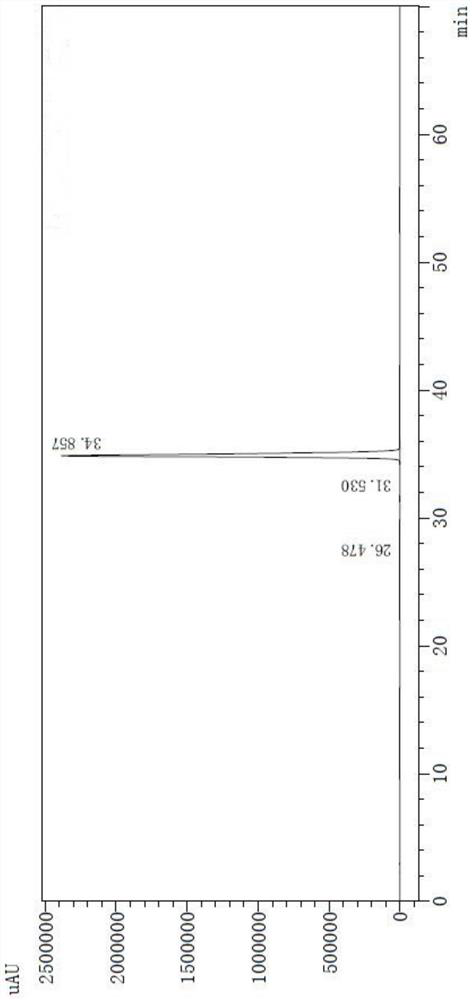
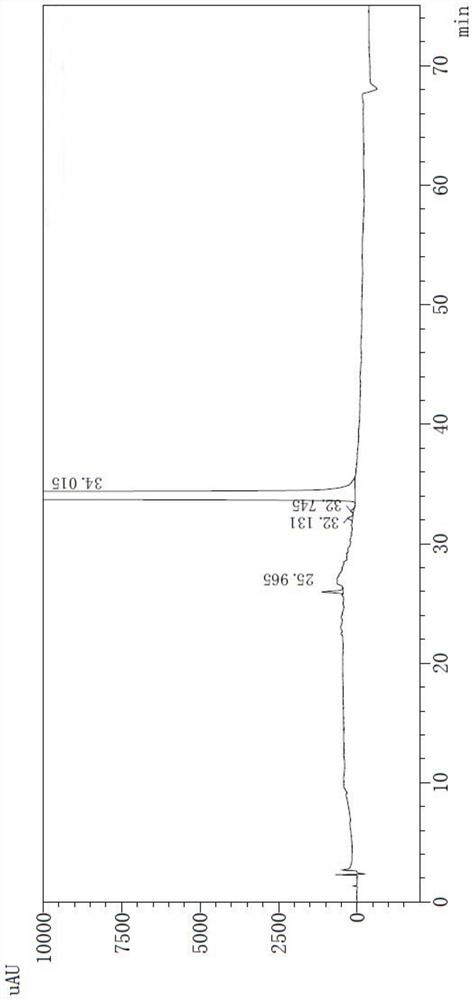
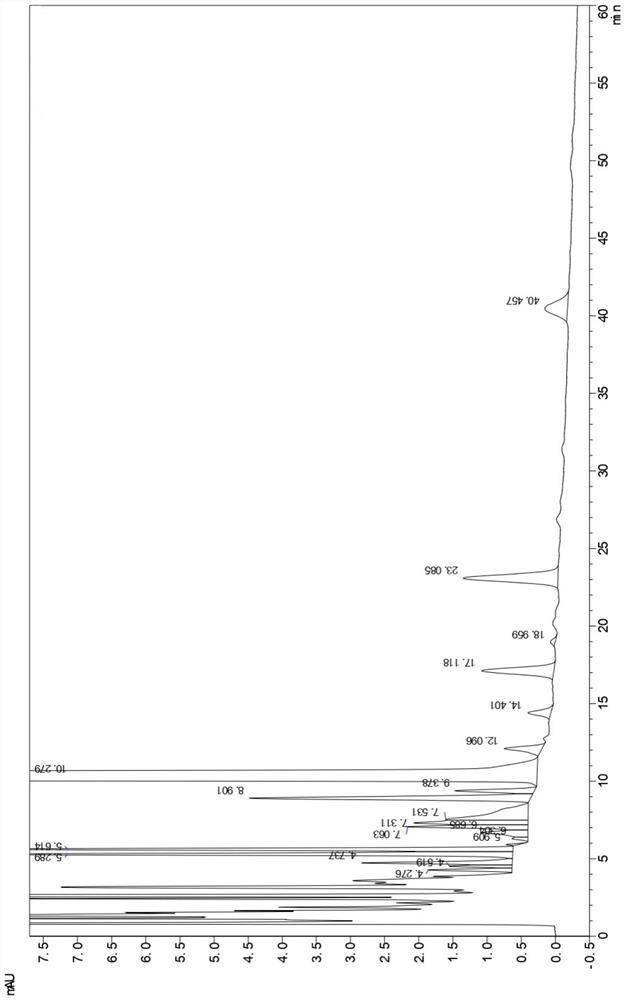
[0055] Related substances were determined according to high performance liquid chromatography (Chinese Pharmacopoeia 2020 Edition Sibu General Rules 0512).
[0056] 1. Mixed solvents Take 85ml of mobile phase A and 15ml of mobile phase B, mix well, and get ready.
[0057] 2. The test solution Take an appropriate amount of this product (pazufloxacin mesylate injection) and dilute it with a mixed solvent to make a solution containing about 0.24mg of pazufloxacin per 1ml.
[0058] 3. Control solution Accurately measure an appropriate amount of the test solution, and quantitatively dilute it with a mixed solvent to prepare a solution containing about 0.48 μg of pazufloxacin mesylate per 1 ml.
[0059] 4. Sensitivity solution Accurately measure an appropriate amount of Pazufloxacin mesylate reference substance solution under the content determination item, and quantitatively dilute it with a mixed solvent to make a solution containing about 0.06μg per 1ml.
[0060] 5. Chromatograp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mobile phase a | aaaaa | aaaaa |
| Mobile phase a | aaaaa | aaaaa |
| Mobile phase a | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com