Method of synthesizing methanesulfonic acid parzhushaxing intermedinte
A technology for pazufloxacin mesylate and intermediates, which is applied in the field of synthesizing pazufloxacin mesylate intermediates, can solve the problems of high price of organic chemical reagents, difficult operation by transport workers, large losses in the extraction process, and the like. The effect of low cost, low risk factor and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
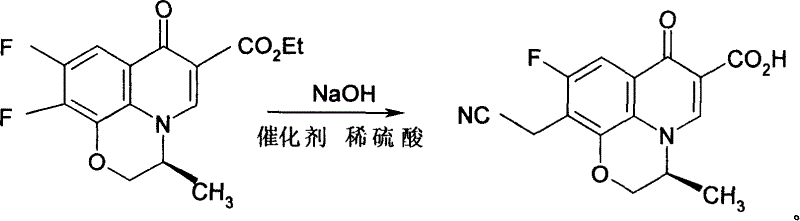
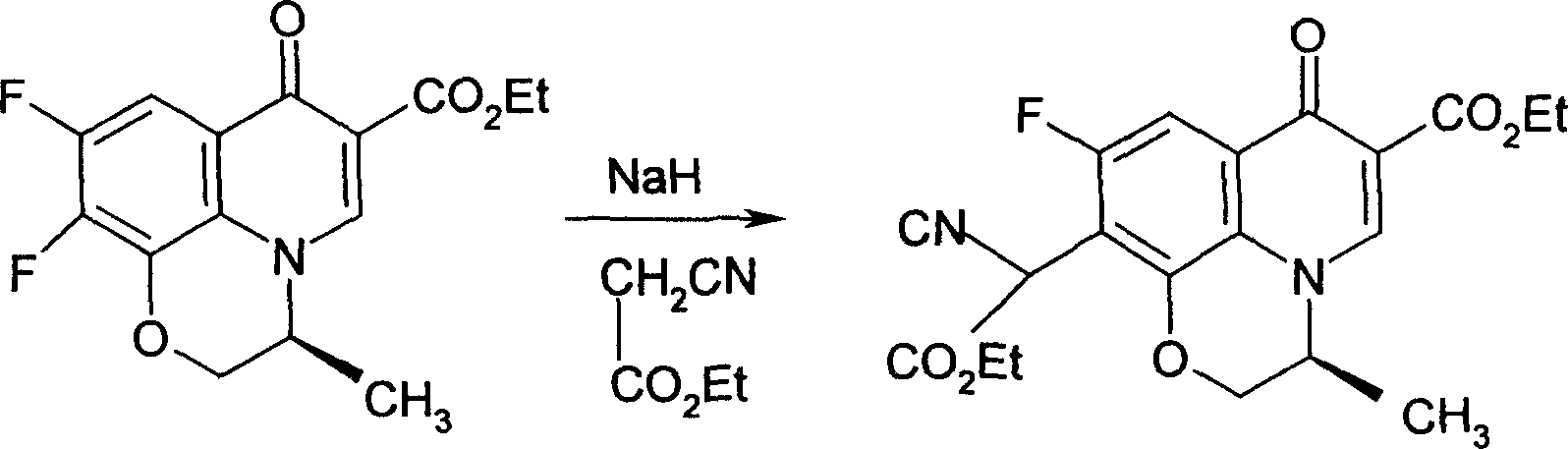
[0026] Example 1, take 140g (S)-9,10-difluoro-3-methyl-7-oxo-2,3-dihydro-7H-pyrido[1,2,3-d,e][1, 4] Ethyl benzoxazine-6-carboxylate (difluoride), 50% NaOH 500ml, use a mixture of ice and salt, the mixing ratio is 5:1, cool to below 0°C, add 20g of phase transfer catalyst PEG200 under stirring , 100g ethyl cyanoacetate, stir, carefully adjust the pH value of the solution to 6.0 with 6% dilute sulfuric acid, and slowly heat to 50°C, react for 4 hours, cool to 9°C after all the bubbles overflow, and keep the temperature at 0 Below ℃, stir slowly for 2 hours, then gradually increase the temperature to 20 ℃, keep the temperature for 4 hours, and use TLC (thin layer chromatography) to control the completion of the reaction. Freeze to -10°C, crystals precipitate out, filter with suction, and dry at 55°C to obtain 115.4g of the product, with a yield of 93.06%.
[0027] The reaction formula is as follows:
[0028]
[0029] Example 2, method and reaction formula are the same as Exa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com