Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
59 results about "Sitafloxacin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
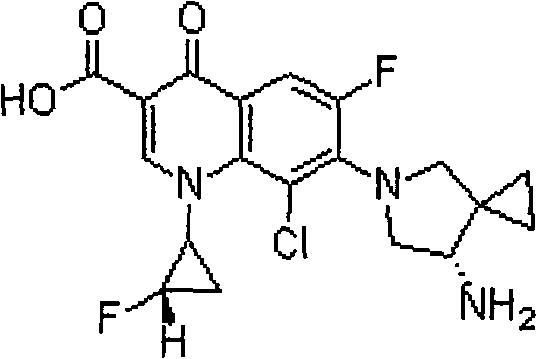
Sitafloxacin (INN; also called DU-6859a) is a fluoroquinolone antibiotic that shows promise in the treatment of Buruli ulcer. The molecule was identified by Daiichi Sankyo Co., which brought ofloxacin and levofloxacin to the market. Sitafloxacin is currently marketed in Japan by Daiichi Sankyo under the tradename Gracevit.
Sitafloxacin preparation method
ActiveCN103524487AReduce pollutionReduce stepsOrganic compound preparationAmino-carboxyl compound preparationQuinolineCarboxylic acid
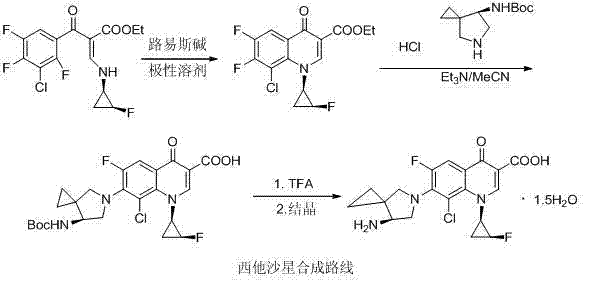
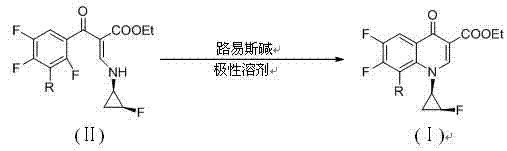
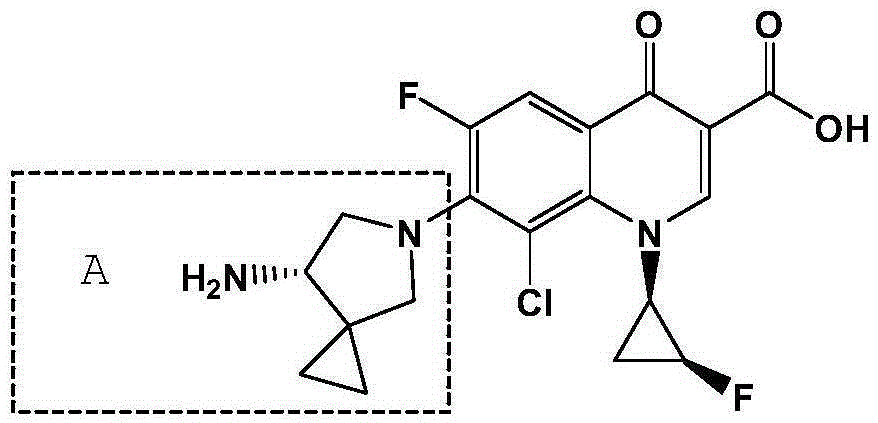
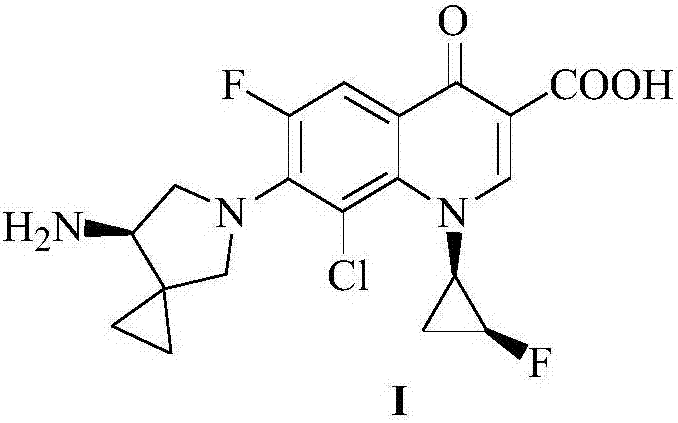
The invention discloses a sitafloxactin preparation method which comprises taking 2,4,5-trifluoro-3-chlorobenzoic acid as a starting material and conducting a series of steps like acylation, esterification, substitution, cyclization, substitution, deprotection, so that 7-[(7S)-7-amino-5-azaspiro[2.4]heptan-6-yl]-8-chloro-6-fluoro-1-[(1R,2S)-2-fluorocyclopropyl]-1,4-oxo-3-quinoline carboxylic acid, namely the sitafloxacin, is prepared. The method is relatively low in cost, simple in operation, mild in reaction, high in reaction yield and product purity, and meets the requirements of large-scale industrial production.
Owner:NANJING YOUKE BIOLOGICAL MEDICAL RES
Stable sitafloxacin medicinal composition and preparation method thereof
InactiveCN101732277AImprove stabilityReduce generationAntibacterial agentsOrganic active ingredientsMedicinePhotodegradation
The invention relates to a stable medicinal composition of sitafloxacin or salt thereof or a hydrate thereof and a preparation method. The composition contains the sitafloxacin or the salt thereof or the hydrate thereof and a medicinal pharmaceutical adjunct, wherein the medicinal pharmaceutical adjunct contains a diluting agent, a disintegrating agent, a lubricant and a powder coating material containing an opacifier. The preparation method of the composition is characterized by comprising the following steps: firstly, adopting a powder coating technology to carry out powder coating on the raw materials and part of the auxiliary materials and then carrying out the next preparation process. The opportunities that the raw materials contact with light are reduced after coating processing, thereby reducing the generation of a photodegradation product and enhancing the stability of the preparation greatly.
Owner:CHIATAI QINGCHUNBAO PHARMA
Sitafloxacin hydrate injection and preparation method thereof
ActiveCN101637447ALess impuritiesImprove stabilityAntibacterial agentsPowder deliveryCurative effectSurface-active agents
The invention discloses a sitafloxacin hydrate injection and a preparation method thereof. Each product contains 1-50mg of sitafloxacin hydrate per ml, 0.5-30mg of antioxygens, 0.1-0.75 mg of metal complexing agent, 2-20mg of complex solubilizer, 6-100mg of osmotic pressure modifier, and 0.1-1mg of surface active agent. The sitafloxacin hydrate injection is prepared by the preparation method of the invention can obviously reduce generated impurities, increases the stability, is not only beneficial to increase the quality of the product and but also can increase the curative effect.
Owner:WUHAN WUYAO SCI & TECH
Stabilized liquid preparation
InactiveUS7304075B2Reduce contentReduce transmissionAntibacterial agentsBiocideAqueous solutionNuclear chemistry
A liquid preparation having improved light stability is provided, which comprises an aqueous solution containing sitafloxacin and sodium chloride.
Owner:DAIICHI PHARMA CO LTD
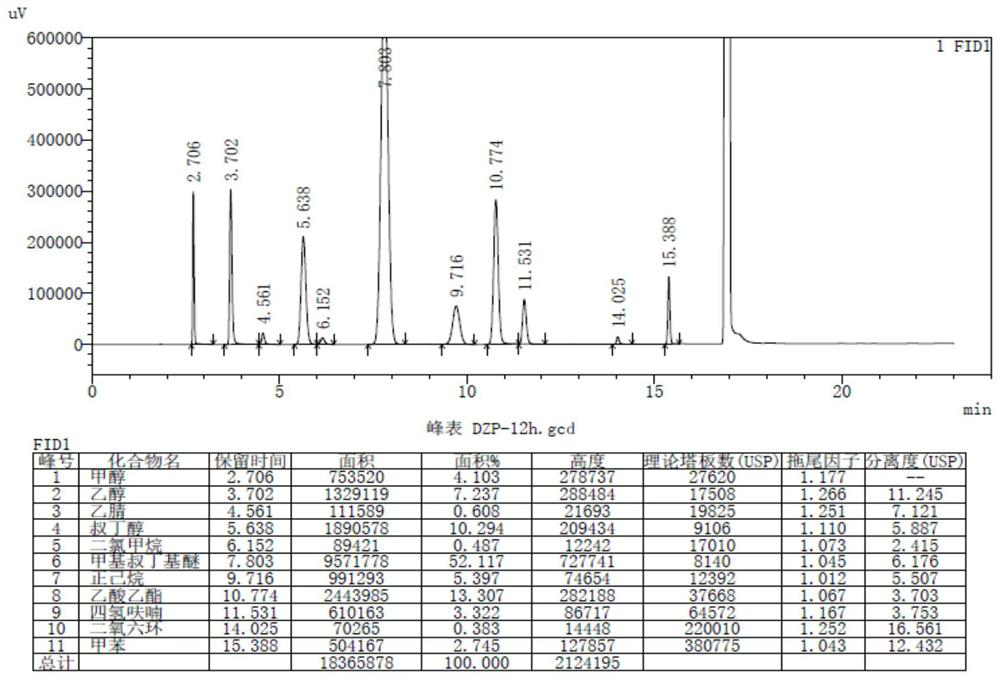
Method for simultaneously detecting multiple residual solvents in sitafloxacin
PendingCN111751459AAccurate and effective detectionRich varietyComponent separationGas liquid chromatographicEthyl acetate
The invention belongs to the technical field of analytical chemistry, and relates to a method for simultaneously detecting multiple residual solvents in sitafloxacin. Specifically, the method comprises the following steps: 1) determining gas chromatography conditions; 2) preparing a reference solution and a test solution; 3) performing methodology verification; and 4) determining the residual solvent in the sitafloxacin sample. The method can effectively and accurately detect 11 residual solvents such as methanol, ethanol, acetonitrile, tert-butyl alcohol, dichloromethane, methyl tert-butyl ether, n-hexane, ethyl acetate, tetrahydrofuran, dioxane and toluene at the same time, and is wide in application range and high in detection efficiency. A weak-polarity capillary chromatographic columnis selected, so that a relatively good separation effect can be achieved. Meanwhile, a headspace sample injection method is adopted, so that the interference of direct sample injection of a solutionsample to detection and the pollution to a chromatographic column are reduced.
Owner:JUMPCAN PHARMA GRP
Novel preparation method for sitafloxacin intermediate
InactiveCN101759629AHigh yieldHigh optical purityChemical recyclingOptically-active compound separationMedicinal chemistryHeptane
The invention provides a novel preparation method for sitafloxacin intermediate (7S)-amino-5-[1(R)-5-phenethyl] 4-oxo-5-azaspiro [2.4]-heptane. The method comprises the following steps: adopting raceme (7R, 7S)-amino-5-[1(R)-5-phenethyl] 4-oxo-5-azaspiro [2.4]-heptane as the raw material, and obtaining (7S)-amino-5-[1(R)-5-phenethyl] 4-oxo-5-azaspiro [2.4]-heptane after optical resolution. The method has the advantages that the cost is low, the operation is simple, the reaction is mild, toxic and harmful substances are not used and generated, the yield is high, and the optical purity of the product is high, thereby being suitable for large-scale industrialized production.
Owner:PEKING UNIV FOUNDER GRP CO LTD +2
Sitafloxacin sustained-release pellet and preparation method thereof
ActiveCN101496789AWidely distributedEvenly distributedAntibacterial agentsOrganic active ingredientsSustained release pelletsPharmacology
The invention discloses a slow-release micropill of sitafloxacin, which comprises a slow-release drug-containing micropill, wherein the slow-release drug-containing micropill consists of a pill core of sitafloxacin and a slow-release coating layer; the pill core of sitafluoacin is coated with the slow-release coating layer; and the weight of the slow-release coating layer is 2 to 100 percent thatof the pill core of sitafloxacin. According to different coating materials and coating thicknesses, the slow-release micropill of sitafloxacin can be prepared into the micropills with different release curves, and according to clinical need, the preparation unit finally formed is added with one or more of the micropills with different release curves so as to achieve the best clinical effect.
Owner:CHONGQING LUMMY PHARMA
Preparation method of sitafloxacin intermediate
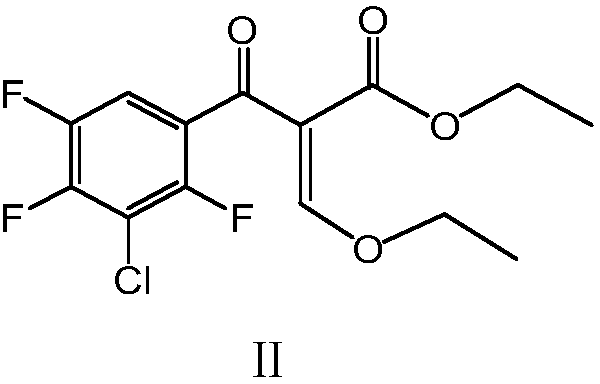
ActiveCN109293513AHigh recovery rateThe synthetic route is simpleOrganic compound preparationCarboxylic acid esters preparationEthyl esterChloride
The invention discloses a preparation method of a sitafloxacin intermediate, wherein the preparation method includes the steps: in the presence of low-valence metals or transition metals, 3-chloro-2,4,5-trifluorobenzoyl chloride undergoes a reaction with ethyl 3-bromo-2-ethoxyacrylate to obtain the sitafloxacin intermediate. Compared with the prior art, the preparation method of the sitafloxacin intermediate II has the advantages of simple synthetic route, mild reaction conditions, simple post-treatment, low energy consumption, high solvent recovery rate, low cost and easy availability of adopted raw materials and reagents. The preparation method provided by the invention has the yield of 96% or more, has the purity of the obtained product 99% or more after simple conventional post-treatment, is suitable for industrialized mass production and has good market prospects.
Owner:JIANGXI FUSHINE PHARMA CO LTD +1
Preparation method for sitafloxacin hydrate
InactiveCN105061395AHigh yieldReduce dosageOrganic chemistryHydrogenTert-Butyloxycarbonyl protecting group
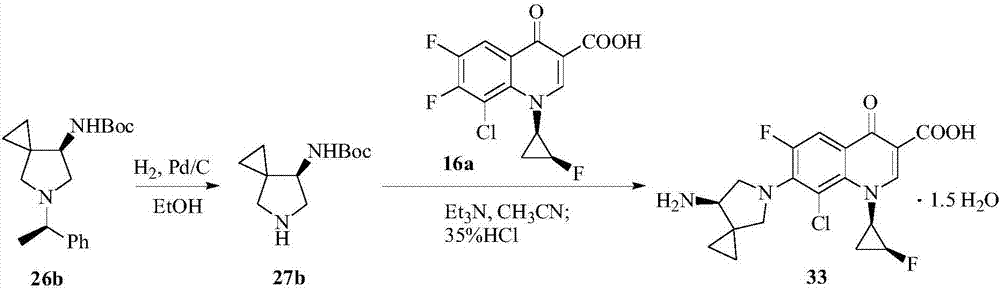
The present invention provides a preparation method for sitafloxacin hydrate. The preparation method comprises: taking a compound II as a raw material, and orderly performing debenzylation, substitution, chlorination and tert-butoxycarbonyl removal to finally obtain the sitafloxacin hydrate. According to the preparation method provided by the present invention, hydrogen gas and an autoclave are avoided, thereby greatly simplifying process operation, and eliminating hidden danger at the same time. All process steps of the preparation method are quick in response, and have no side reactions, and separation and purification are convenient and quick, so that the preparation method disclosed by the present invention is not only high in yield, more importantly but also has advantages of environmentally friendliness, simple process and low cost, and can meet the demand of industrial mass production.
Owner:SUZHOU SIXTH PHARMA PLANT OF JIANGSU WUZHONG PHARMA GROUP
Sitafloxacin intermediate, preparation method of sitafloxacin and sitafloxacin pharmaceutical composition
ActiveCN103360310AEasy to handleSimple processAntibacterial agentsOrganic active ingredientsPharmaceutical drugEngineering
The invention discloses a sitafloxacin intermediate, a preparation method of sitafloxacin and a sitafloxacin pharmaceutical composition. The preparation method can be used for solving the problems of low yield, troublesome aftertreatment, poor safety and higher cost in the existing sitafloxacin preparation. The preparation method disclosed by the invention is simple in process, easily available in raw materials, lower in cost, the solvent after reaction is easy to treat, high yield and quite suitable for large-scale industrial production. The sitafloxacin pharmaceutical composition obtained by virtue of the preparation method provided by the invention can be used for further improving the product dissolution effect, improving the in-vivo bioavailability of the sitafloxacin and enhancing the exertion of medical effect.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
Sitafloxacin hydrate tablet composition and preparation method thereof
InactiveCN103156821ALow costNo side effectsAntibacterial agentsOrganic active ingredientsCelluloseMagnesium stearate
The invention relates to the field of preparation of medicaments and specifically relates to a sitafloxacin hydrate tablet composition and a preparation method thereof. The medicament comprises the following active ingredients in percentage by weight: 33-34% of sitafloxacin, 37-39% of mannitol, 20-21% of starch, 6-7% of hydroxypropyl cellulose, 1.3-1.4% of hydroxypropyl methyl cellulose and 0.9-1% of magnesium stearate; and the method for making the sitafloxacin hydrate tablets comprises the steps of crushing, screening, pelletizing, drying, stabilizing size, totally mixing, pressing tablets and the like. The sitafloxacin hydrate tablet composition disclosed by the embodiment of the invention selects a small quantity of excipients with low cost to prepare the tablets having the same efficacy with the existing sitafloxacin hydrate tablets, the medicament cost is low, and the side effects can be avoided basically.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
Method for preparing sitafloxacin
The invention discloses a method for preparing sitafloxacin. The method comprises the following steps: (1) dissolving a compound II and a compound III in acetonitrile, adding triethylamine, heating to reflux, slowly cooling to the temperature of 0 DEG C after reaction is completed, filtering, drying, thereby obtaining a light yellow compound IV; (2) dissolving a compound IV in a non-protonic solvent, slowly adding the compound into the pre-cooled hydrochloric acid, standing and layering the reaction solution after reaction is completed, regulating the pH value of an aqueous phase by using a strong alkaline solution, regulating the pH value by using ammonium hydroxide, performing vacuum concentration on the system at room temperature so as to remove ammonia gas, separating out lots of white solids, filtering and drying, thereby obtaining sitafloxacin. According to the method for preparing sitafloxacin, disclosed by the invention, impurities produced in the reaction process can be effectively reduced, and the method is simple and convenient in after-treatment, good in refining effect, high in yield and suitable for industrial production.
Owner:SHANDONG QIDU PHARMA
Preparation method of sitafloxacin hydrate five-membered ring side chain intermediate
The invention relates to a preparation method of a sitafloxacin hydrate five-membered ring side chain intermediate. The preparation method comprises following steps: keto carbonyl groups of a raw material 1 are reacted with sodium cyanoborohydride or sodium triacetoxyborohydride in the presence of ammonium acetate or ammonium chloride; reduction of amide carbonyl groups of an obtained production is realized with lithium aluminum hydride; free amino groups of a reduction product are reacted with di-tert-butyl dicarbonate ester in the presence of an alkali; phenethyl groups of an obtained compound are subjected to reductive destruction with formic acid or a formate in the presence of palladium-carbon so as to obtain the sitafloxacin hydrate intermediate (product 5). Reaction conditions of the preparation method are mild; equipment requirements are low; preparation process is safe; stereoselectivity is excellent; the raw material reagents are cheap and easily available; and production cost is low.
Owner:广州朗启生物科技有限公司
Preparation method of sitafloxacin hydrate granule composition
InactiveCN102988298ASimple preparation processLow costAntibacterial agentsOrganic active ingredientsWestern medicineCarrageenan
The invention relates to a preparation method of a sitafloxacin hydrate granule composition, belonging to the technical field of Western medicine prepration. The sitafloxacin hydrate granule composition is formed by a base tablet and a coating, and is prepared into a sitafloxacin hydrate inclusion compound by adopting carrageenan. The sitafloxacin hydrate granule composition does not have bitter taste.
Owner:QINGDAO CENT HOSPITAL
Preparation method of sitafloxacin hydrate
ActiveCN109232530AAvoid too muchFew reaction stepsOrganic chemistryBulk chemical productionCarboxylic acidEthyl acetate
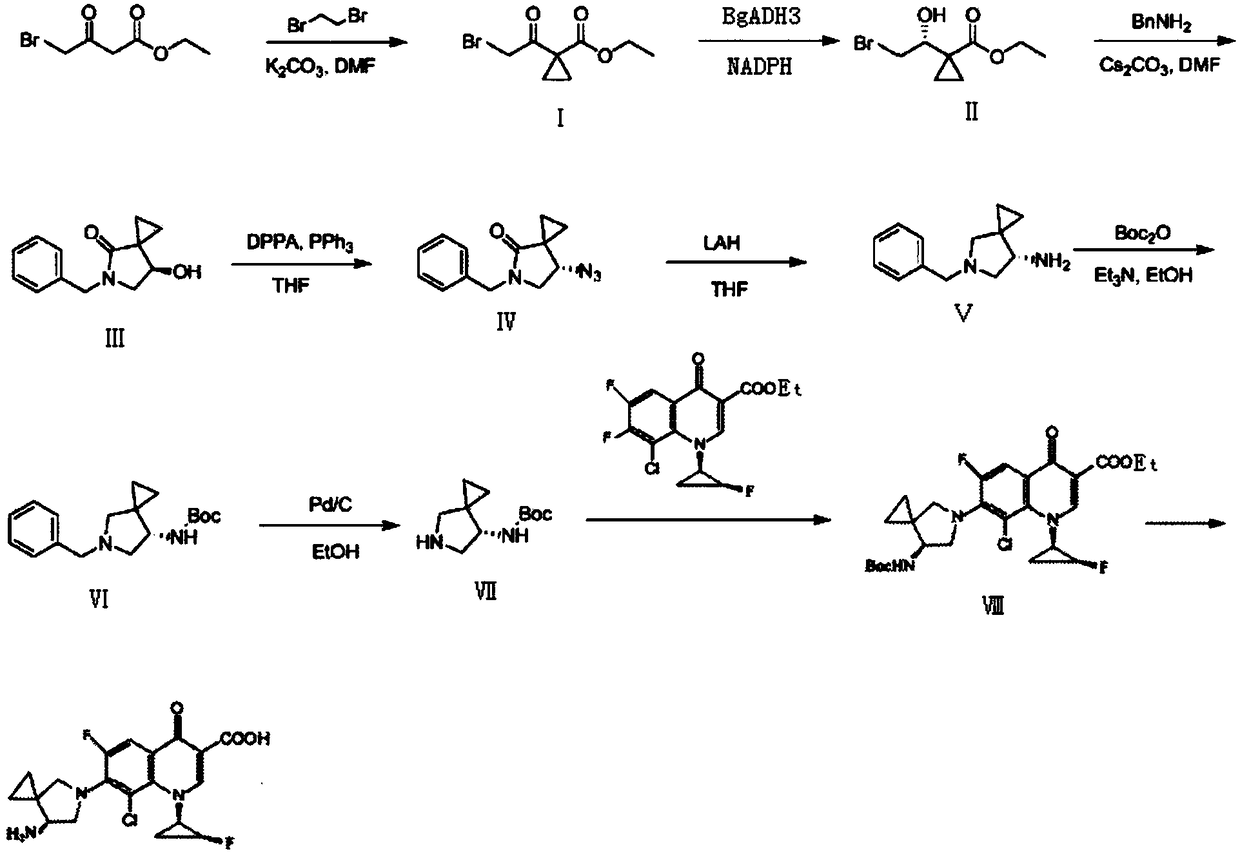
The invention discloses a preparation method of sitafloxacin hydrate. The method comprises the following steps: taking ethyl 4-bromoacetoacetate used as a raw material, enabling ethyl 4-bromoacetoacetate to be fully reacted with 1,2-dibromoethane and preparing the obtained product into a compound II in the presence of carbonyl reduction enzyme; taking the compound II, enabling the compound II to carry out cyclization reaction with benzylamine in a solvent in the presence of cesium carbonate, enabling the obtained product to be reacted with DPPA and preparing a compound IV; reducing nitrine group of the compound IV to prepare a compound V; connecting primary amine group of the compound V with a BOC protection group to obtain a compound VI; reducing the compound VI through Pd / C, enabling theobtained product to be reacted with 8-chlorine-6,7-difluoro-1-[(1R,2s)-2-fluorocyclopropyl]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid ethyl ester to prepare a compound VIII; and carrying out deprotection of the compound VIII to obtain sitafloxacin hydrate. The sitafloxacin hydrate is few in preparation steps, simple in post-treatment and relatively high in yield.
Owner:SUZHOU DAWNRAYS PHARM CO LTD
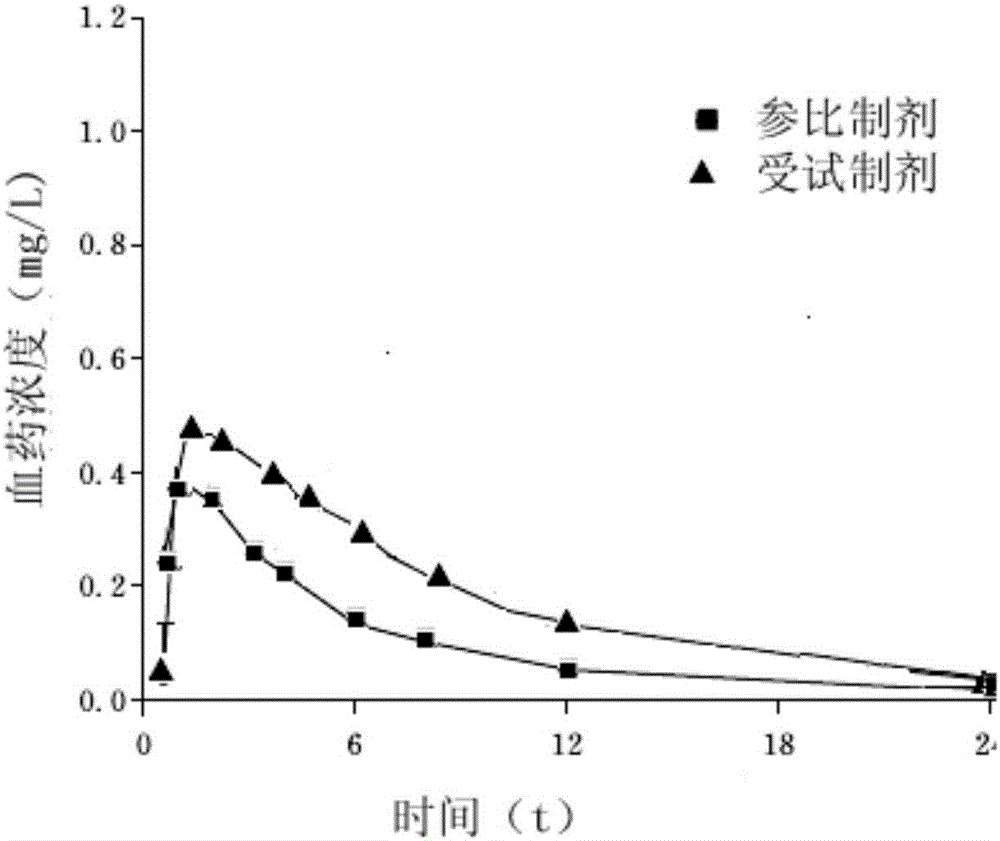
Sitafloxacin dihydrate crystal, and preparation method and composition tablet thereof
ActiveCN106749174AImprove stabilitySimple processAntibacterial agentsOrganic active ingredientsCelluloseMagnesium stearate
The invention belongs to the technical field of medicines, and particularly relates to a sitafloxacin dihydrate crystal, and a preparation method and a composition tablet thereof. The preparation method of the sitafloxacin dihydrate crystal provided by the invention comprises the dissolving and crystallizing steps. The invention further provides a composition containing the sitafloxacin dihydrate crystal; the sitafloxacin dihydrate crystal as an active component comprises the following components in percent by weight: 32-33% of sitafloxacin dihydrate, 38-39% of mannitol, 19-20% of starch, 6-7% of hydroxy propyl cellulose, 1.3-1.4% of hydroxypropyl methyl cellulose and 0.9-1% of magnesium stearate; and a method for preparing the sitafloxacin tablet comprises the following steps of crushing, sieving, granulating, drying, granulating, totally mixing, tableting and the like. Compared with the prior art, the sitafloxacin tablet prepared through the method provided by the invention has the advantages of improved dissolvability and bioavailability.
Owner:SHANDONG YUXIN PHARMA CO LTD
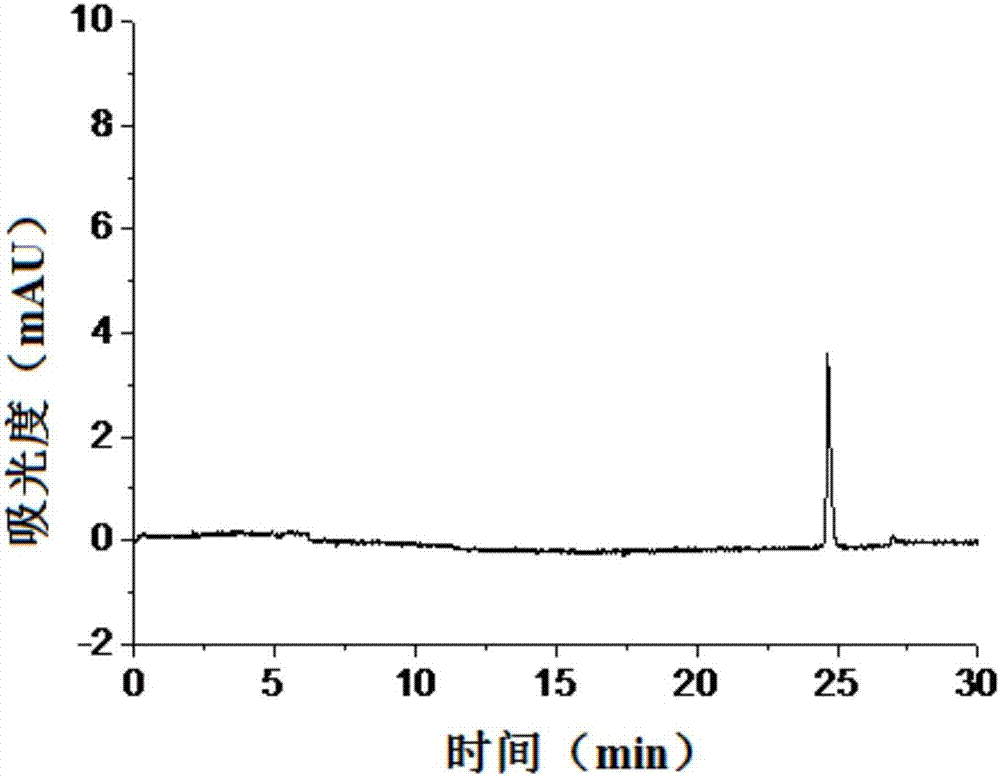
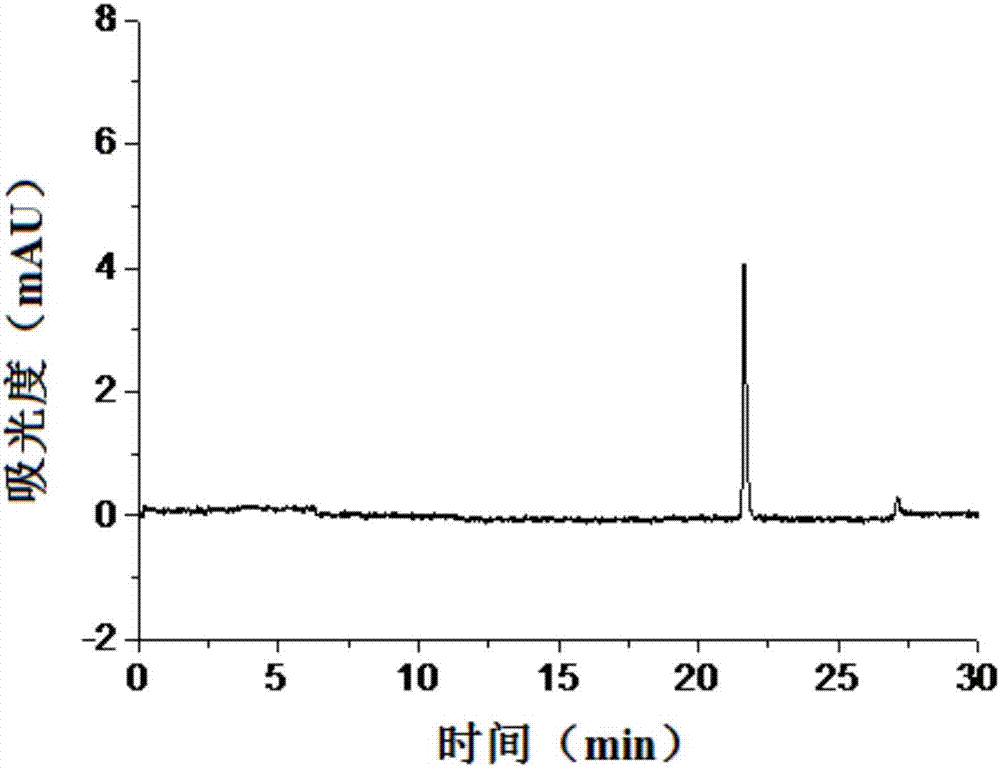
Method for detecting content of isomer impurities in sitafloxacin
InactiveCN107966489ALow costEasy to operateMaterial analysis by electric/magnetic meansLinearityPeak area
The invention discloses a method for detecting the content of isomer impurities in sitafloxacin. The method comprises respectively preparing control solutions of isomers and sitafloxacin and a test sample solution, respectively detecting the control solutions and the test sample solution through a capillary electrophoresis method, and calculating contents of isomer impurities through an external standard method according to a peak area or a standard curve of the peak area and the concentration. The method has the advantages of low cost, simple processes, high resolution degree, good flexibility, good linearity, specificity, precision, stability, sensitivity, repeatability, high sample recovery rate and accurate and reliable detection result. The method realizes simultaneous separation of sitafloxacin and its isomers such as IMA, IMB, IMC, IMD and IME, provides an effective method for sitafloxacin product quality monitoring and has a practical value.
Owner:SHANGHAI INST OF ORGANIC CHEMISTRY - CHINESE ACAD OF SCI
Cracking catalyst and method for preparing sitafloxacin intermediate with same
ActiveCN110026212AIncrease profitImprove conversion ratePhysical/chemical process catalystsOrganic chemistryNitrogen gasTube reactor
The invention discloses a cracking catalyst, which is prepared by mixing an active component with a carrier at a weight ratio of 1 to 1-3, wherein the active component is any two or more of sulfate, nitrate, halide and oxide of metal lithium, magnesium, chromium, iron, nickel, zinc, copper, aluminum, silver or palladium; the carrier is one or more of activated carbon, graphite powder and gypsum powder; the invention further discloses a method for preparing a sitafloxacin intermediate 2-chloro-2-fluoro-1-benzyloxymethylcyclopropane with the cracking catalyst; and the method comprises the following steps: step (1), filling the fully mixed catalyst into a tubular reactor equipped with a copper wire mesh, and performing nitrogen replacement treatment at 300-500 DEG C; and step (2), contactingdichlorodifluoromethane with allyl benzyl ether gas in a gas cabinet, feeding into the tubular reactor in the step (1), and continuously reacting at 500-700 DEG C to obtain the sitafloxacin intermediate 2-chloro-2-fluoro-1-benzyloxymethylcyclopropane, wherein the reaction can be carried out continuously.
Owner:LINHAI LIMIN CHEM
Highly absorptive solid preparation
A solid preparation improved in oral absorption and reduced in fluctuations of oral absorption can be obtained by incorporating an organic acid, particularly tartaric acid, in a solid preparation having, as an active ingredient, a high content of a quinolone compound, particularly sitafloxacin, having poor water solubility at pH around neutrality.
Owner:DAIICHI PHARMA CO LTD
Preparation method of sitafloxacin hydrate
InactiveCN107513053AHigh yieldEasy to operateOrganic chemistryHydrogenTert-Butyloxycarbonyl protecting group
The invention provides a preparation method of a sitafloxacin hydrate. The preparation method takes a compound II as a raw material, debenzylation, substitution, chlorination and removal of t-butyloxycarboryl are sequentially carried out, and finally the sitafloxacin hydrate is obtained. The preparation method provided by the invention has the advantages that use of hydrogen and an autoclave is avoided, technological operation is greatly simplified, and hidden danger is also eliminated; and reaction in each technological step is rapid, no side reaction is generated, and separation and purification are simple and rapid, so that the preparation method provided by the invention not only has high yield, more importantly, the preparation method also has the advantages of environment friendliness, simple technology and low cost, and industrial large-scale mass production requirement can be met.
Owner:SUZHOU SIXTH PHARMA PLANT OF JIANGSU WUZHONG PHARMA GROUP
New application of quinolone compounds in prevention and treatment of plant bacterial diseases such as citrus canker
PendingCN111771895AStrong antibacterial activityHigh antibacterial activityBiocideDisinfectantsPipemidic acidFleroxacin
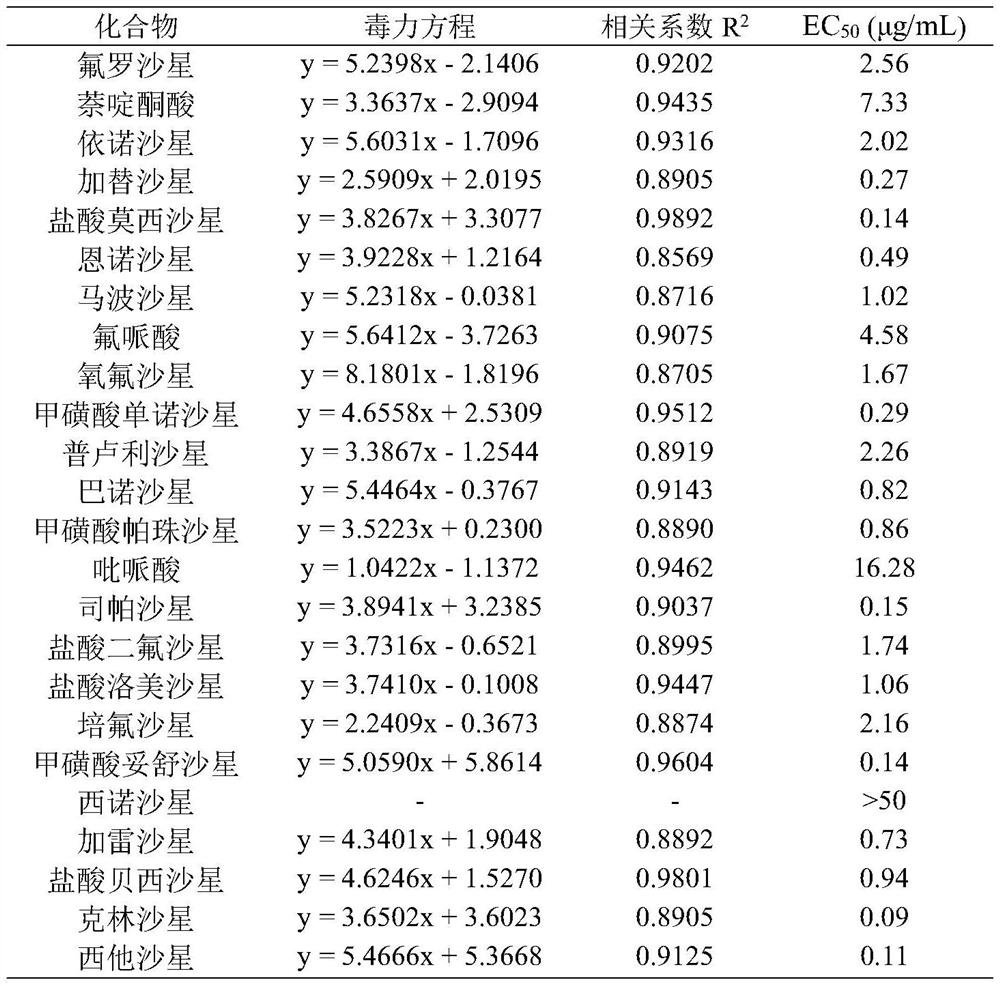
The invention discloses a new application of quinolone compounds as bactericides in prevention and treatment of bacterial diseases and citrus canker of crops. The quinolone compounds comprise floroxacin, enofloxacin, gatifloxacin, moxifloxacin hydrochloride, enrofloxacin, marbofloxacin, floxacin, mononorfloxacin mesylate, prulifloxacin, Balofloxacin, pazufloxacin mesylate, pipemidic acid, sparfloxacin, difloxacin hydrochloride, lomefloxacin hydrochloride, pefloxacin, tosufloxacin mesylate, Cinoxacin, galafloxacin, besifloxacin hydrochloride, ofloxacin, nalidixic acid, Clinafloxacin and Sitafloxacin. The quinolone compounds can be used for preventing and treating bacterial diseases caused by citrus canker pathogens, especially gatifloxacin, moxifloxacin hydrochloride, mononorfloxacin mesylate, sparfloxacin, tosufloxacin mesylate, clinafloxacin and sitafloxacin, has excellent bacteriostatic activity on citrus canker pathogens, and can be used for preventing and treating bacterial diseases of crops.
Owner:LANZHOU UNIVERSITY
Sitafloxacin medicament composition for injection and preparation method thereof
InactiveCN102475685AReduces hydrogen bond formationReduce wasteAntibacterial agentsPowder deliveryPharmaceutical medicineMannitol
The invention relates to a stable sitafloxacin medicament composition. The medicament composition comprises a therapeutically effective amount of a novel oral quinolone antibiotic medicine selected from sitafloxacin, and pharmaceutically acceptable auxiliary materials of mannitol and the like. The medicament composition of the invention can be used to treat single or mixed bacterial infection of the respiratory tract, the urogenital tract, the abdominal cavity, soft skin tissues and the like.
Owner:JIANGSU CAREFREE PHARM CO LTD
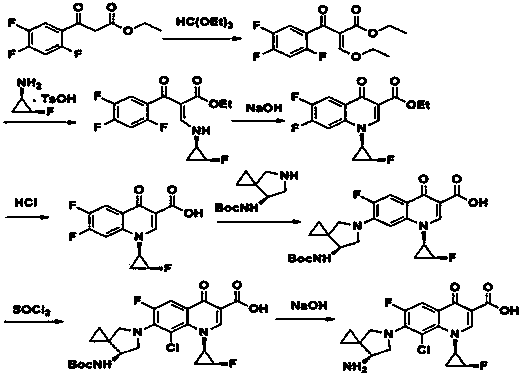
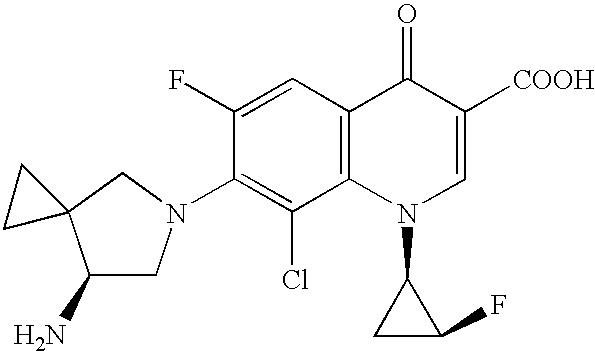
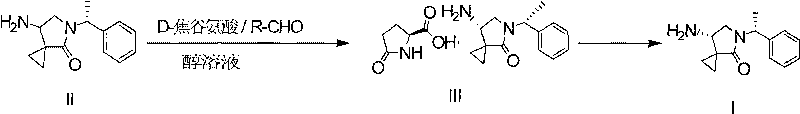
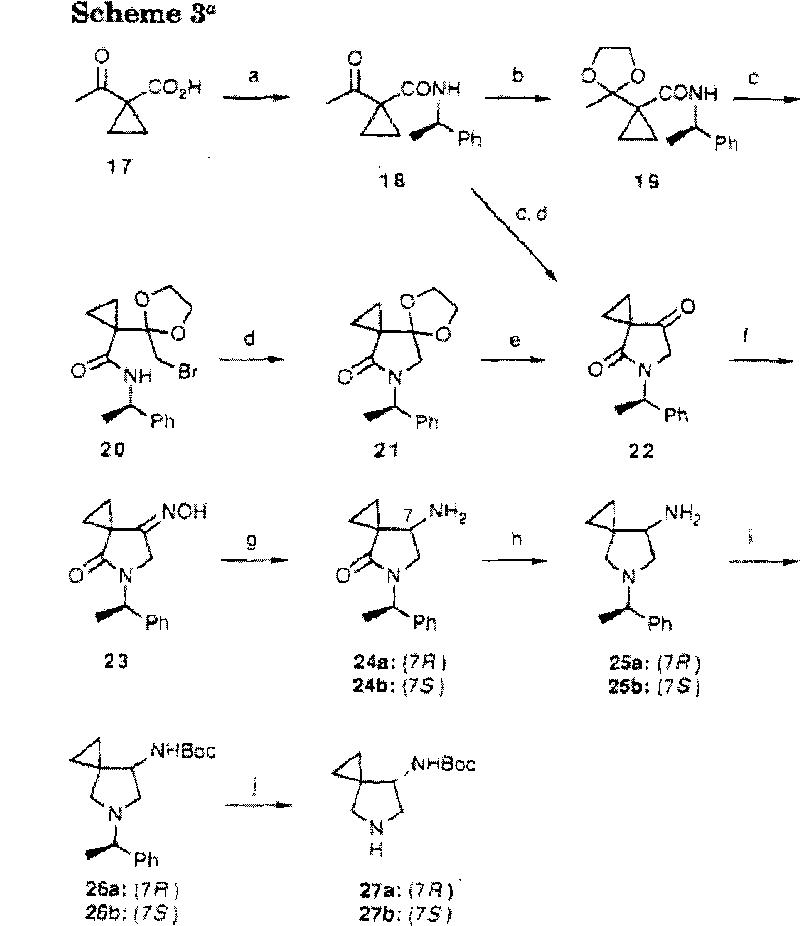
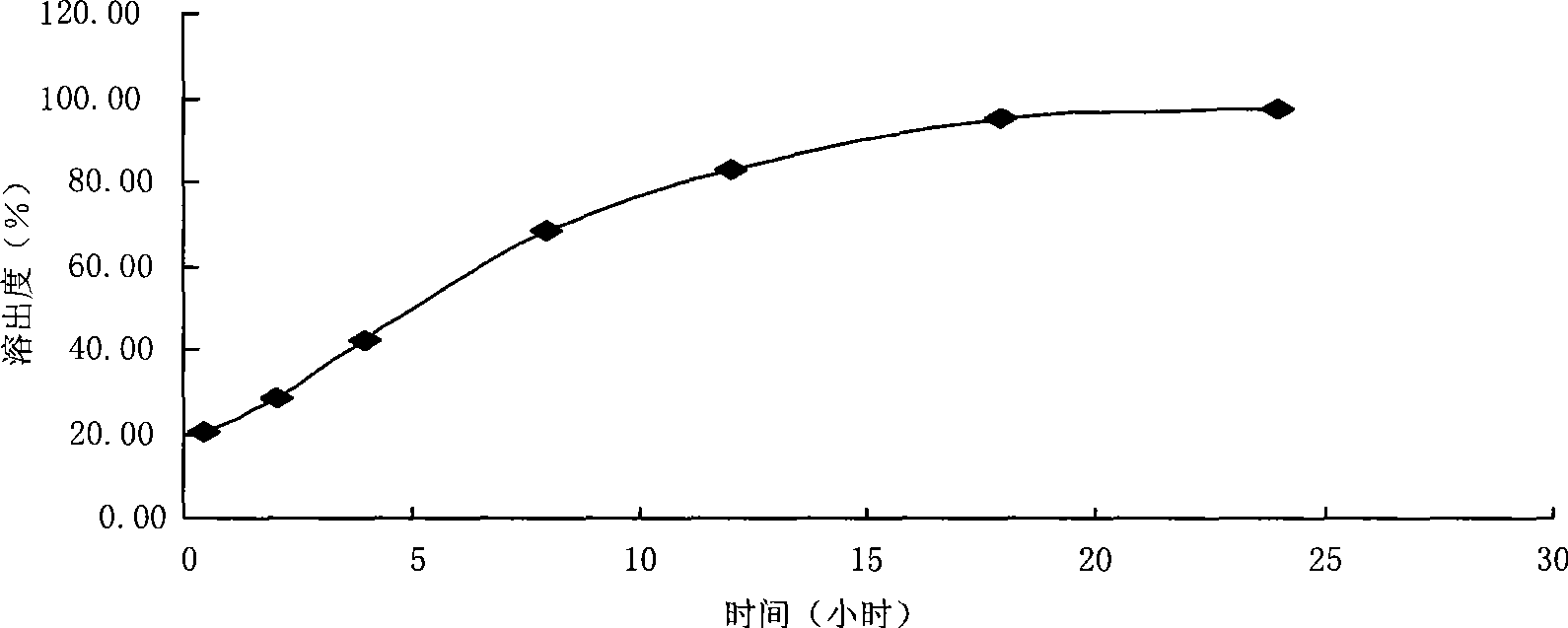
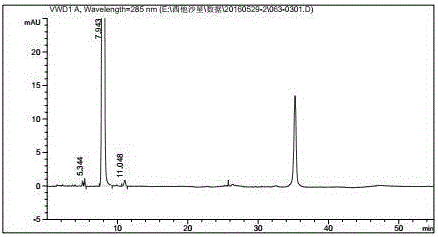
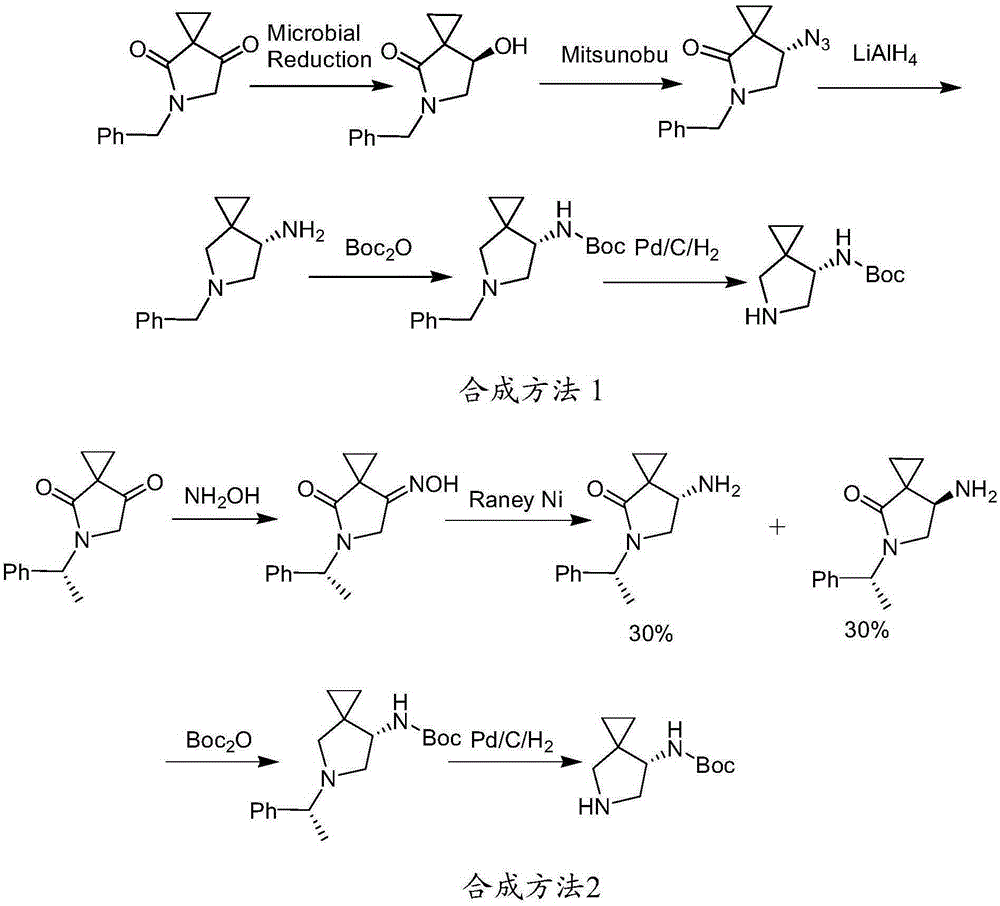
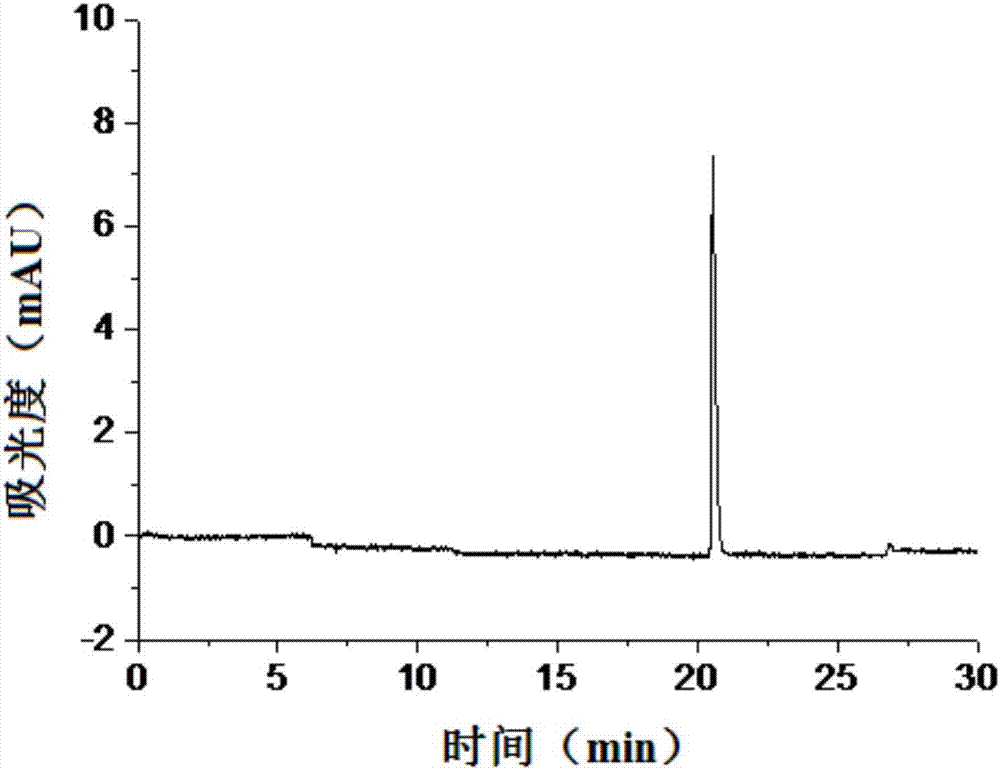
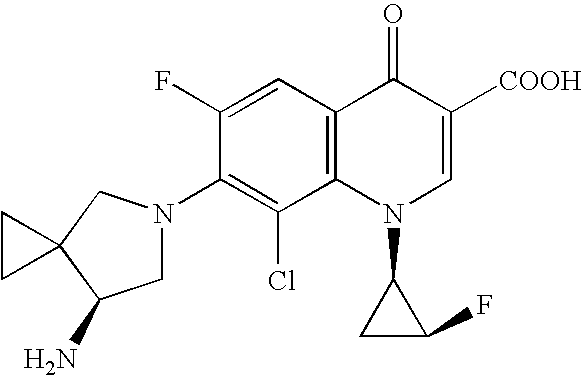
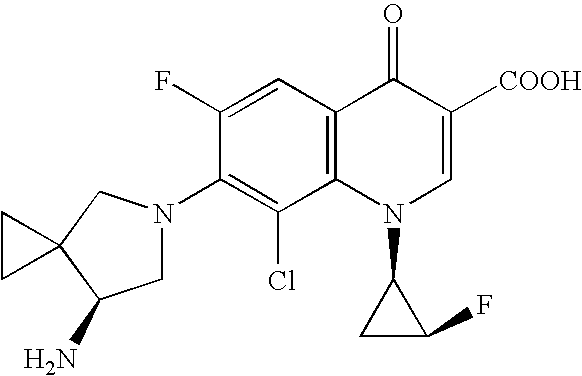
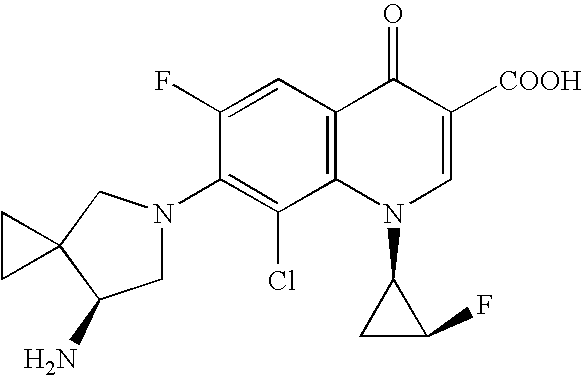
Method for preparing efficiently synthetic sitafloxacin intermediate (7S)-5-azaspiro[2.4]heptane-7-yl tert-butyl carbamate
ActiveUS20180370914A1High ee valueAvoid material wasteOrganic chemistryBulk chemical productionCarbamateTert butyl
The present invention discloses a preparation method for efficient synthesis of sitafloxacin intermediate (7S)-5-azaspiro[2.4]heptane-7-yl-tert-butyl carbamate, comprising the following steps: reactingto obtainreactingto obtainreactingto obtainand reactingto obtainIn the present invention, a single compound with a relatively high ee value can be obtained, the unnecessary waste of materials is avoided, the yield is significantly improved, the operation is simple, the industrial scale-up is easy, and the production cost is reduced.
Owner:CHEN STONE GUANGZHOU CO LTD
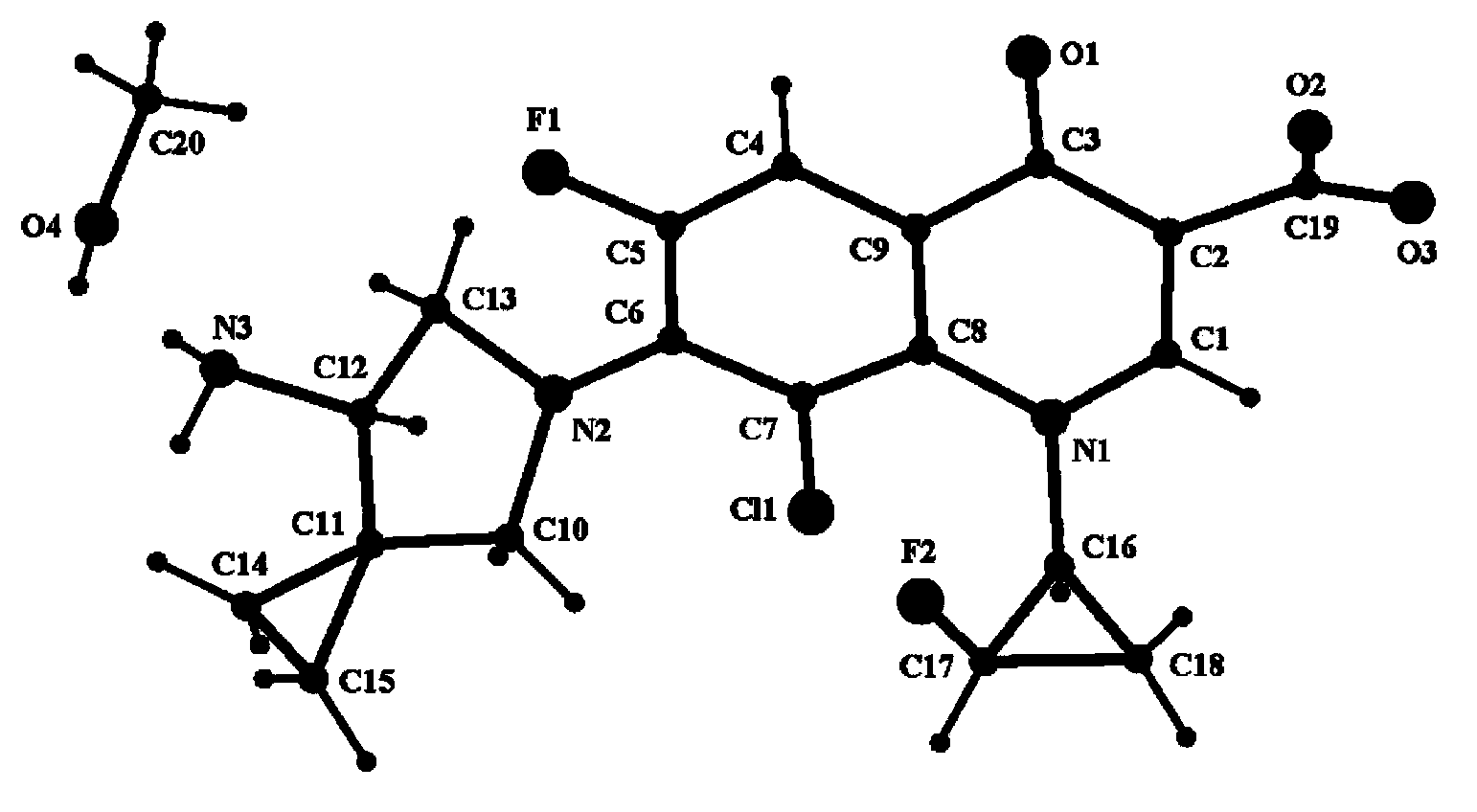
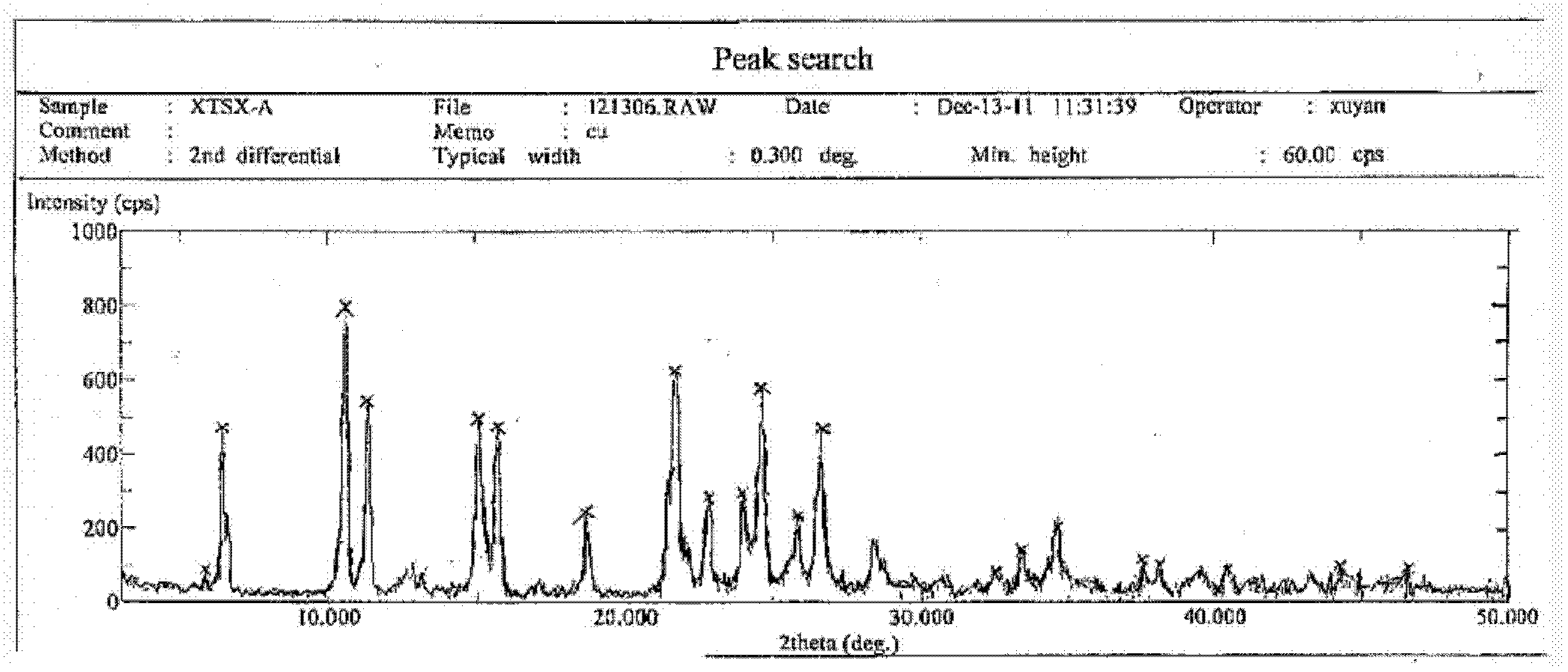
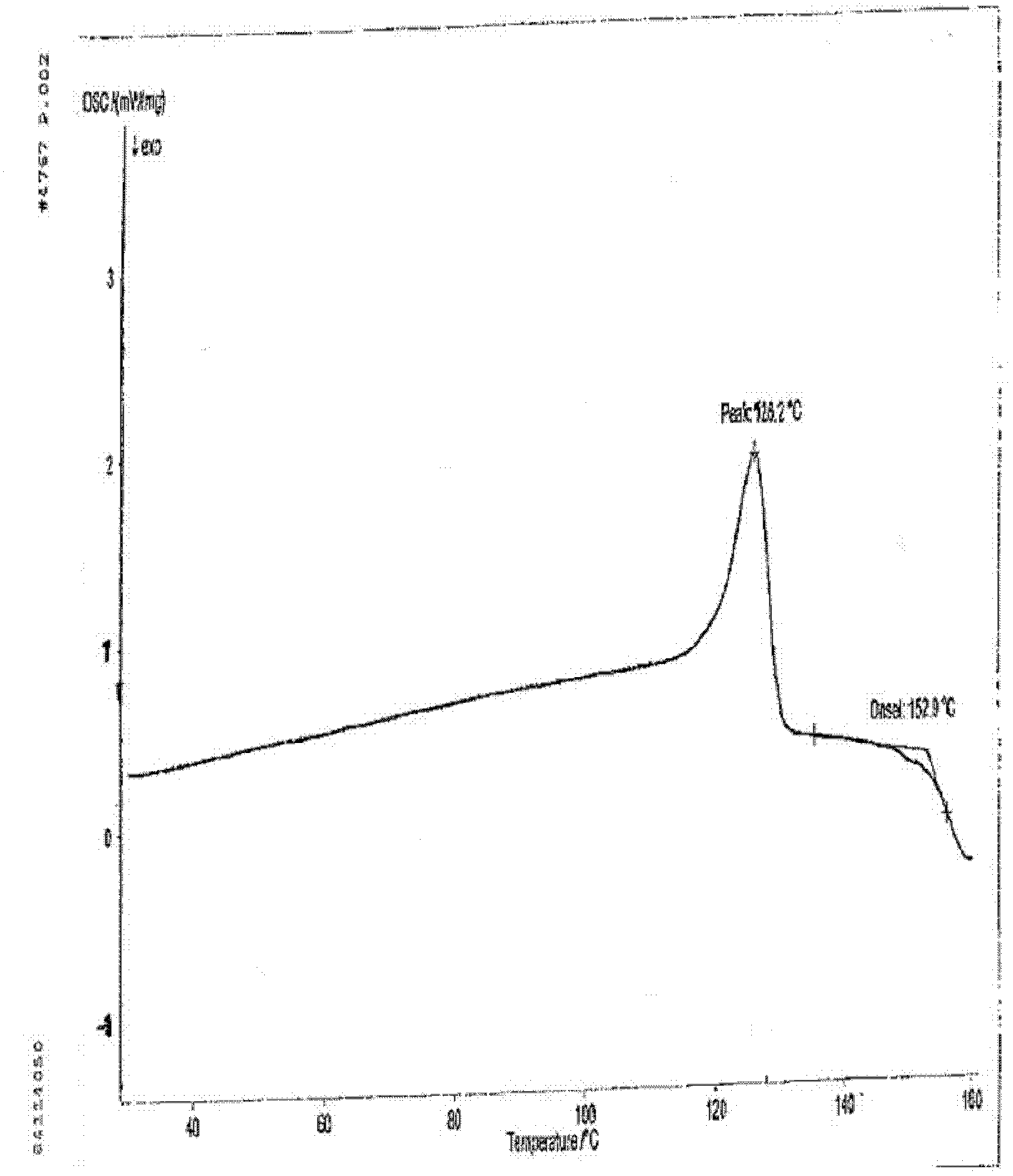
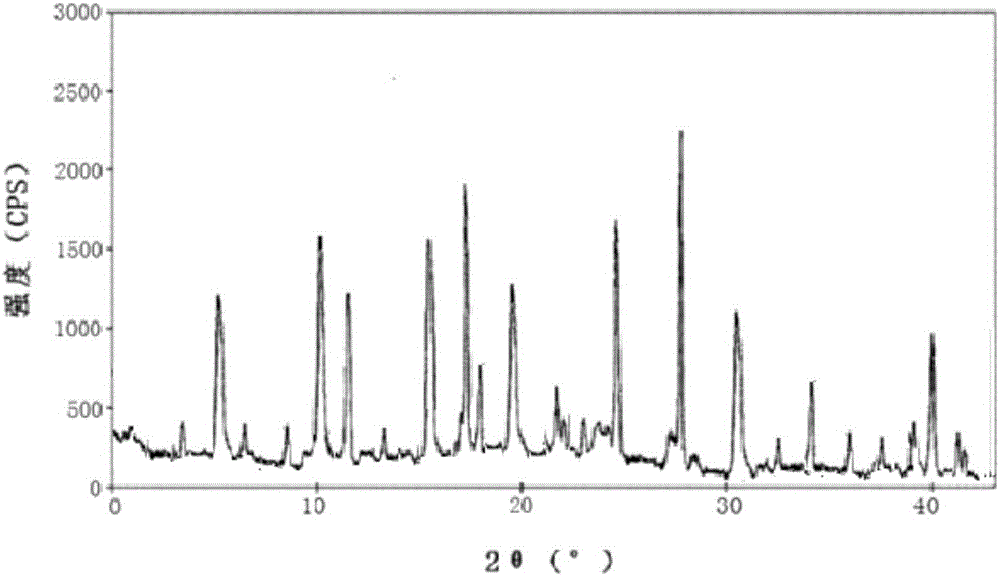
Novel sitafloxacin crystal form and preparation method thereof
InactiveCN103539776AEnsure safetyGuarantee the quality of medicinesOrganic chemistryState of artAbsolute configuration
The invention discloses a novel sitafloxacin crystal form and a preparation method thereof. Peaks appear in an X-ray powder diffraction spectrum of the novel crystal form when the angle 2theta is at 10. 6 degrees, 11.4 degrees, 21.7 degrees and 24.6 degrees; and in a differential thermal analysis spectrum of the novel crystal form, the endothermic peak appears at 126.2 DEG C and the exothermic peak of the novel crystal form is at 152.9 DEG C. The novel crystal form disclosed by the invention is used for solving the problem that the absolute configuration of sitafloxacin medicinal hydrate is difficult to determine in the prior art. The preparation method of the novel sitafloxacin crystal form is simple to operate, and the crystal form prepared by the method is stable, and residue solvent risk is avoided; therefore, the preparation method is very suitable for large-scale industrial production.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
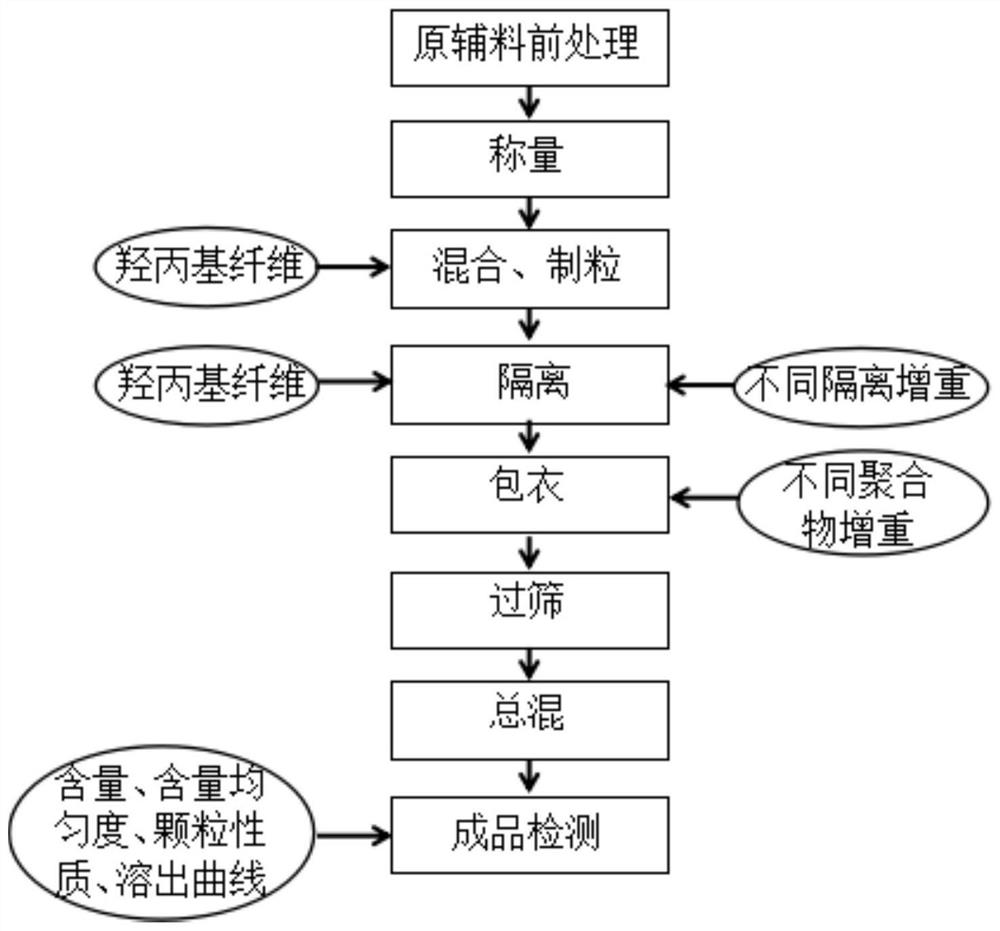
Sitafloxacin fine granule and preparation process thereof
PendingCN112915068AGood dissolution effectNarrow particle size distributionAntibacterial agentsOrganic active ingredientsBiochemical engineeringProcess engineering
The invention discloses a sitafloxacin fine granule and a preparation process thereof. By adjusting the dosage and component proportion of an isolation layer and a coating in the granulation process, the problems of too fast disintegration, caking and poor dispersibility easily caused by a large variety of auxiliary materials and large prescription dosage in the preparation process of the sitafloxacin fine granule can be effectively solved, and the obtained finished product is narrow in particle size distribution, uniform in mixing and good in dissolution effect. The auxiliary materials are cheap and easy to obtain, the preparation is easy, no special equipment is needed, the production cycle is short, the cost is low, and the fine granule is very suitable for industrial production.
Owner:JUMPCAN PHARMA GRP
Sitafloxacin fumarate crystal form a and its pharmaceutical use
ActiveCN102718746BImprove stabilityHigh dissolution rateAntibacterial agentsOrganic active ingredientsCrystal planeCondensed matter physics
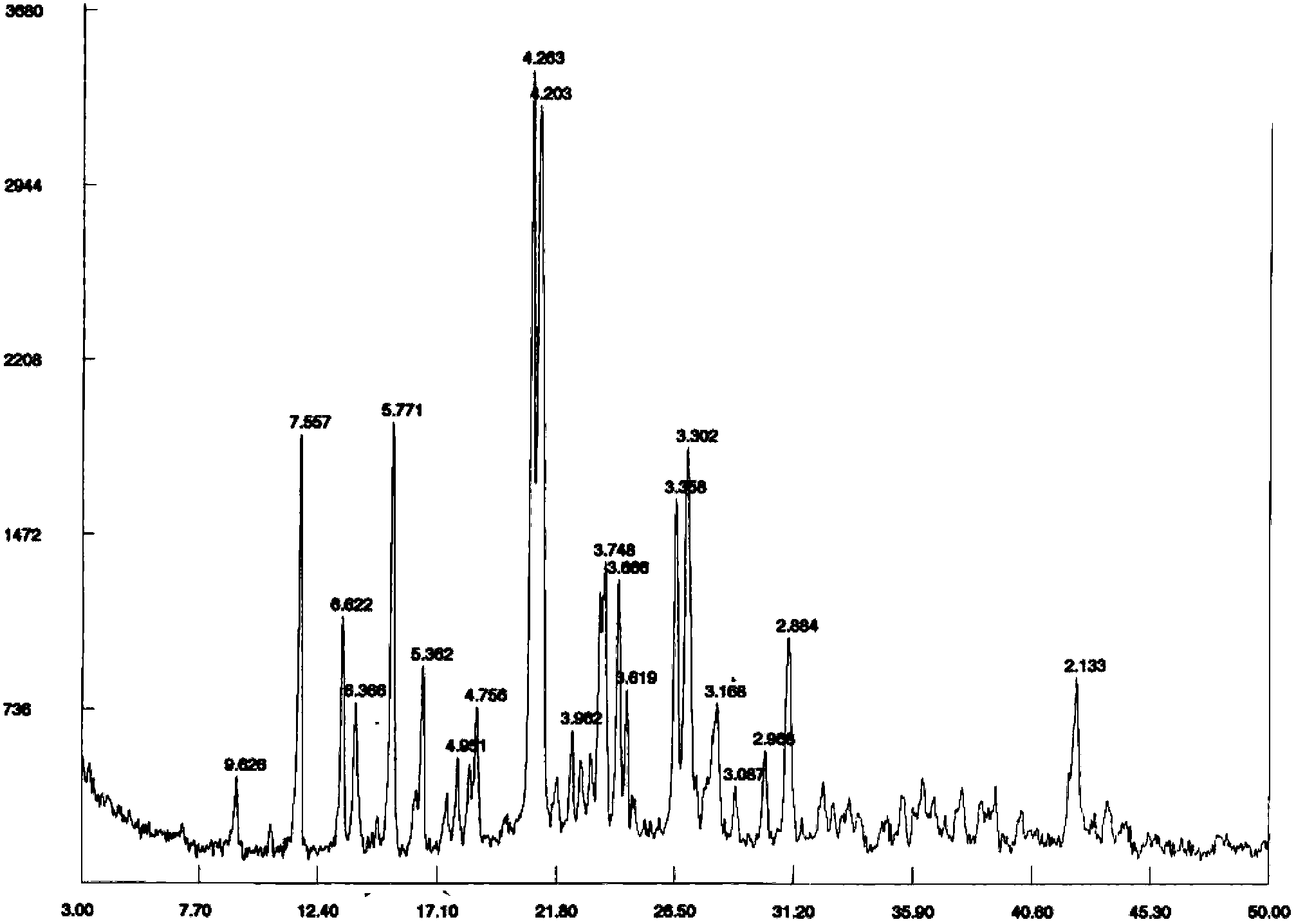
The invention mainly relates to trioxymethylene fumarate crystal form A. Cu-Ka radiation is adopted, and in an X-ray powder diffraction graph of the trioxymethylene fumarate crystal form A, diffraction peaks exist when the crystal plane is away from a value d by about 7.557 angstroms, 6.622 angstroms, 5.771 angstroms, 4.263 angstroms, 4.203 angstroms, 3.748 angstroms, 3.666 angstroms, 3.358 angstroms, 3.302 angstroms and 2.884 angstroms. The invention further relates to a preparation method and the pharmaceutical usage of the trioxymethylene fumarate crystal form A. The trioxymethylene fumarate crystal form A has the advantages of high stability, high dissolution rate after being made into preparation and simplicity in preparation process.
Owner:NANJING YOUKE BIOLOGICAL MEDICAL RES
Sitafloxacin tablet and preparation method thereof
ActiveCN113181125ASimple processEasy to operateAntibacterial agentsOrganic active ingredientsMannitolPharmacology
The invention relates to a sitafloxacin tablet and a preparation method thereof. Common opacifying agent ferric oxide is added in the crystallization and purification process of a crude product of the sitafloxacin tablet, so the purification time of the sitafloxacin tablet can be effectively shortened, and the effect of the ferric oxide serving as the opacifying agent is not influenced; moreover, the refined sitafloxacin powder is added step by step and is ground together with one to two times of mannitol, so the finally formed tablet has good dissolution rate under the condition of using a small amount of mannitol, and meanwhile, the taste of the tablet can be adjusted through the mannitol, so that the compliance of a patient is improved.
Owner:CHINA MEHECO SANYANG PHARMA CO LTD
Racemic recovery method of by-product in resolution mother liquor of intermediate of sitafloxacin
ActiveCN108911972ARealize rational utilizationHigh yieldOrganic compound preparationPreparation by hydrogenolysisBenzeneRecovery method
The invention discloses a racemic recovery method of the by-product in a resolution mother liquor of an intermediate of sitafloxacin. The method specifically comprises following steps: (1) carrying out resolution on a raceme 2-fluorocyclopropanecarboxylic acid to obtain by-products (1R,2R)-2-fluorocyclopropanecarboxylic acid and (1S,2R)-2-fluorocyclopropanecarboxylic acid; (2) carrying out a reduction reaction on the recovered by-products to obtain trans-2-fluorocyclopropane formaldehyde; (3) converting the trans-2-fluorocyclopropane formaldehyde into trans(3-(2-fluorocyclopropyl)allyl)benzene; and (4) carrying out racemization on the trans(3-(2-fluorocyclopropyl)allyl)benzene, and carrying out further oxidation and resolution to obtain the sitafloxacin intermediate which is (1S,2S)-2-fluorocyclopropanecarboxylic acid. Therefore, the invention provides the racemic recovery method of the trans-intermediate of the by-product of the sitafloxacin, and the racemic recovery method is mild incondition and simple and convenient in operation, and is capable of industrialized application and production.
Owner:WUHAN UNIV
Sitafloxacin hydrate degradation impurity preparation method
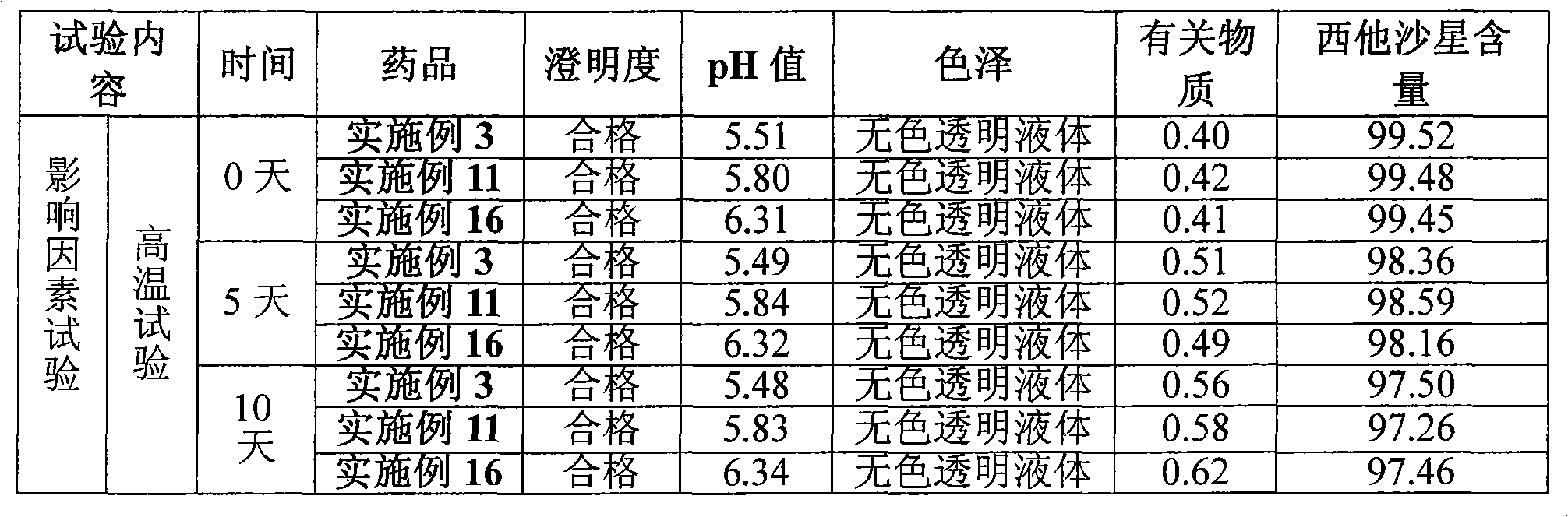
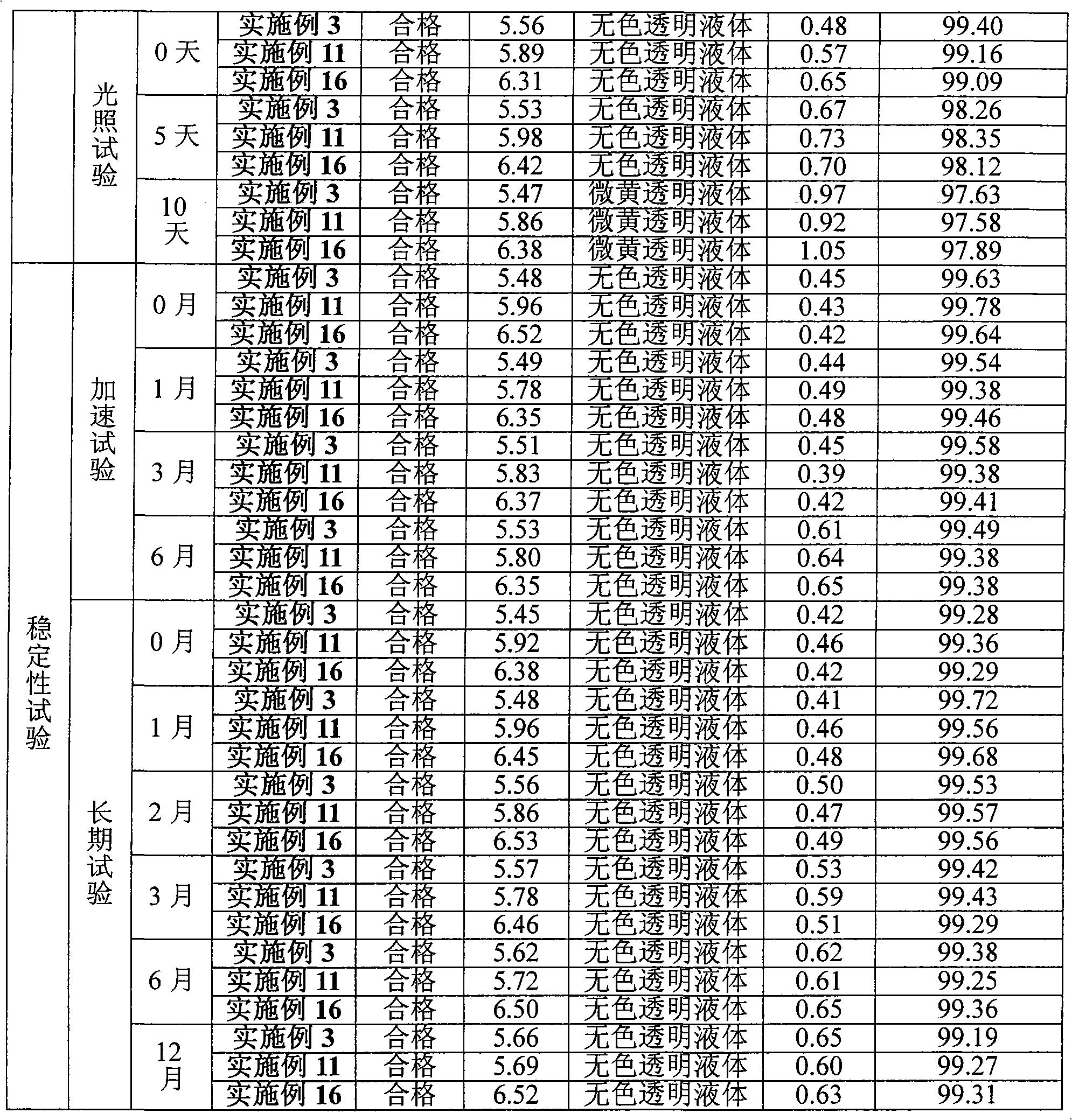
The invention belongs to the field of medicinal chemistry and particularly relates to a sitafloxacin hydrate degradation impurity preparation method. The sitafloxacin hydrate degradation impurity preparation method includes: dissolving sitafloxacin hydrate in a solvent; illuminating for 10-20 hours; performing column chromatography chromatography separation to obtain degradation impurity P1 and impurity P2. The sitafloxacin hydrate degradation impurity preparation method is simple and highly operable, and products conforming to the requirements of the quality standards can be obtained via simple purification.
Owner:SHANDONG QIDU PHARMA
Sitafloxacin hydrate granules and preparation method of sitafloxacin hydrate granules
ActiveCN105663054AMask bitternessUniform granulesAntibacterial agentsOrganic active ingredientsMANNITOL/SORBITOLDissolution
The invention relates to sitafloxacin hydrate granules. The formula comprises 10 parts of sitafloxacin hydrate, 50 to 100 parts of mannitol, 0.5 to 1 part of silicon dioxide, other pharmaceutically acceptable auxiliary materials and 15 to 30 parts of coating liquid. Compared with the prior art, the sitafloxacin hydrate granules produced by the invention effectively cover the bitter taste of the sitafloxacin hydrate, and have uniform granules and high dissolution rate. A mixing method of the materials in a preparation process of the sitafloxacin hydrate granules, provided by the invention, effectively prevents dust dispersion and static bonding, and the problem that screening is difficult is overcome; the flowability of the materials is improved and the mixing uniformity is improved; the preparation process is simple and is applicable to industrial production.
Owner:JUMPCAN PHARMA GRP
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com













![Method for preparing efficiently synthetic sitafloxacin intermediate (7S)-5-azaspiro[2.4]heptane-7-yl tert-butyl carbamate Method for preparing efficiently synthetic sitafloxacin intermediate (7S)-5-azaspiro[2.4]heptane-7-yl tert-butyl carbamate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/1b6ba8c9-47b1-4f9d-bc90-14643f4bebc8/US20180370914A1-D00001.png)
![Method for preparing efficiently synthetic sitafloxacin intermediate (7S)-5-azaspiro[2.4]heptane-7-yl tert-butyl carbamate Method for preparing efficiently synthetic sitafloxacin intermediate (7S)-5-azaspiro[2.4]heptane-7-yl tert-butyl carbamate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/1b6ba8c9-47b1-4f9d-bc90-14643f4bebc8/US20180370914A1-D00002.png)
![Method for preparing efficiently synthetic sitafloxacin intermediate (7S)-5-azaspiro[2.4]heptane-7-yl tert-butyl carbamate Method for preparing efficiently synthetic sitafloxacin intermediate (7S)-5-azaspiro[2.4]heptane-7-yl tert-butyl carbamate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/1b6ba8c9-47b1-4f9d-bc90-14643f4bebc8/US20180370914A1-D00003.png)