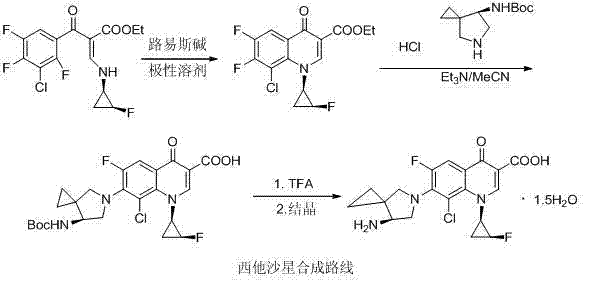
Sitafloxacin intermediate, preparation method of sitafloxacin and sitafloxacin pharmaceutical composition
A technology for sitafloxacin and intermediates, applied in the field of pharmaceutical compositions containing sitafloxacin, can solve the problems of complex post-processing, poor safety, and low yield, and achieve simple post-processing, low cost, and high yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
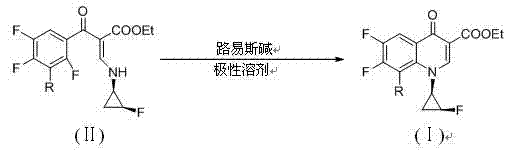
[0047] Example 1 8-chloro-6,7-difluoro-1-[(1R,2S)-2-fluorocyclopropyl]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid Preparation of ethyl ester
[0048] Take 3.65g of ethyl 2-(3-chloro-2,4,5-trifluorobenzoyl)-3-(1R,2S)-2-fluorocyclopropaneamino]acrylate in 40ml N,N-di In methylformamide (DMF), add 3.18g Na 2 CO 3 , stirred and heated to 40°C, TLC monitored the reaction until the raw material point disappeared to stop the reaction, spin-dried DMF under reduced pressure, added 50ml of water and 100ml of dichloromethane to separate phases, spin-dried the organic layer, and dried to obtain 3.2g of the product, with a yield of 92.7% . Melting point: 179.5-181℃, m / e 346.7[M+H] + , 1 H-NMR (500MHz, DMSO) δ (ppm): 8.64(d,J=2.5, 1H) ,8.13(m,1H), 5.11(m,1H), 4.30(m,2H),4.23(m,1H ), 1.72(m,2H), 1.33(t,J=7.5,3H).
Embodiment 2
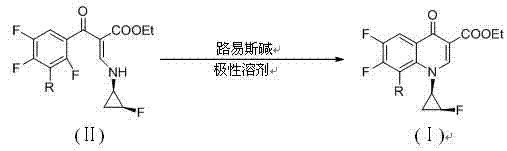
[0049] Example 2 8-fluoro-6,7-difluoro-1-[(1R,2S)-2-fluorocyclopropanyl]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid Preparation of ethyl ester
[0050] Dissolve 3.49 g of 2-(3-fluoro-2,4,5-trifluorobenzoyl)-3-(1R,2S)-2-fluorocyclopropaneamino]ethyl acrylate in 40ml of tetrahydrofuran, and add 2.8 ml triethylamine, stirred and heated to reflux, TLC monitored the reaction until the raw material point disappeared to stop the reaction, spin-dried tetrahydrofuran, added 50ml water and 100ml dichloromethane for phase separation, spin-dried the organic layer, and dried to obtain 2.87g of product, yield 87% , m / e 330.3[M+H] + , 1 H-NMR (500MHz, DMSO) δ (ppm): 8.54(d,J=2.5, 1H) , 8.23(m,1H), 5.21(m,1H), 4.30(m,2H),4.33(m,1H ), 1.82(m,2H), 1.34(t, J=7.5,3H).
Embodiment 3
[0051] Example 3 Preparation of 6,7-difluoro-1-[(1R,2S)-2-fluorocyclopropanyl]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid ethyl ester
[0052] Dissolve 3.31g of 2-(2,4,5-trifluorobenzoyl)-3-(1R,2S)-2-fluorocyclopropaneamino]ethyl acrylate in 50ml of N,N-dimethylacetamide (DMA), add 9.7g potassium oxalate, stir and heat to 50°C, monitor the reaction by TLC until the raw material point disappears to stop the reaction, spin the DMA to dryness under reduced pressure, add 50ml water and 100ml dichloromethane to separate phases, spin the organic layer to dry, After drying, 2.7g of the product was obtained, with a yield of 86.8%, melting point: 249-251°C, m / e 312.2[M+H] + , 1 H-NMR (500MHz, DMSO)δ(ppm): 8.66(d, J=2.5, 1H), 8.25(m,1H), 8.15(m,1H), 5.13(m,1H), 4.32(m,2H ), 4.25(m,1H), 1.75(m,2H), 1.35(t,J=7.5,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com