Racemic recovery method of by-product in resolution mother liquor of intermediate of sitafloxacin
A technology of sitafloxacin and recovery method, applied in the direction of organic racemization, organic chemical method, chemical instrument and method, etc., to achieve the effect of reducing production cost, obvious economic and social benefits, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
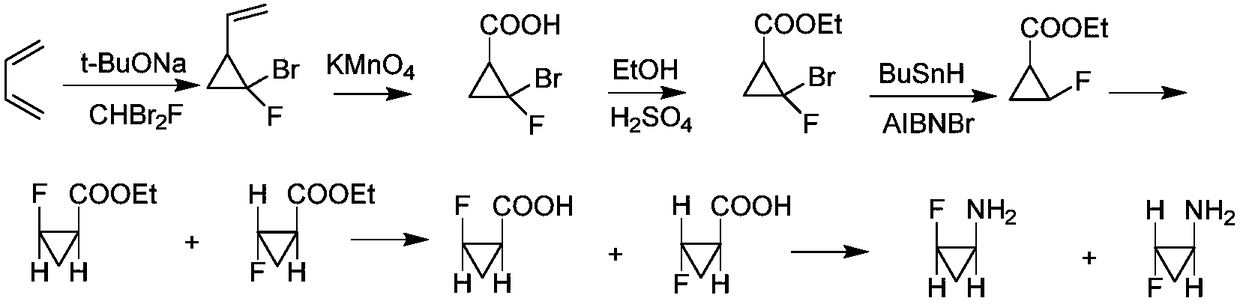
[0035] [Example 1] Preparation of 2-fluorocyclopropane-1-carbaldehyde (VII)
[0036]Under anhydrous and oxygen-free conditions, 7.62 g of oxalyl chloride was slowly added to a mixture of 50 mL of DCM and 3.07 g of DMF, stirred at 0°C for 1 h, and spin-dried at room temperature for use. Under anhydrous and oxygen-free conditions, add 45mL acetonitrile, 80mL THF, and cool to -30°C. Mix 4.16g trans-2-fluoro-cyclopropanecarboxylic acid, 3.21g pyridine, and 80mL THF evenly, add the mixture into the reaction flask within 30min, stir at -30°C for 1h, and stir at -20°C for 30min. Cool to -90°C, add 34mL of 0.046mol tri-tert-butoxylithium aluminum hydride into the reaction flask within 30 minutes, and stir at -90°C for 30 minutes. After the reaction, add 50mL of 2M dilute hydrochloric acid to the reaction flask, remove from the ice bath, extract three times with 50mL of ether, add 50mL of saturated sodium bicarbonate solution (twice) and 50mL of H 2 After washing with O, anhydrous so...
Embodiment 2
[0037] [Example 2] Preparation and racemization treatment of 3-(2-fluorocyclopropyl) allyl)benzene (VIII)
[0038] Under anhydrous and oxygen-free conditions, add 2-bromoethylbenzene into the reaction flask of phosphorus oxychloride, use toluene as solvent, reflux for 12 hours, and spin dry to obtain phosphorus ylide solid with a yield of 72%. 1 H NMR (400MHz, CDCl 3 ( m,2H). Then, under anhydrous and oxygen-free conditions, 0.367g of the phosphorus ylide intermediate obtained above, 2mL THF and 0.56 potassium tert-butoxide were added, refluxed for 1h, cooled to room temperature, 0.1g trans-2-fluorocyclopropanecarbaldehyde, refluxed for 2h. After the reaction was completed, it was washed with saturated sodium bicarbonate and extracted with ethyl acetate. After drying the organic phase the product is purified by chromatography.
[0039] Racemization preparation process: In a three-necked flask equipped with a dropping device, add 1.5g of 3-(2-fluorocyclopropyl)allyl)benzene...
Embodiment 3
[0040] [Example 3] Preparation of 2-fluorocyclopropane-1-carboxylic acid (II)
[0041] 1.0 g of potassium permanganate was added in batches to the ethanol solution of 3-(2-fluorocyclopropyl)allyl)benzene (VIII), the mixture was heated to 70° C. for 3 h, then heated to 90° C. for 4 h. After the reaction was completed, the reactant was filtered and washed with hot water. Rotary evaporation under reduced pressure until about 10 mL of the reaction solution remained, slowly added 1M HCl until pH = 4, and dried. After purification by column chromatography, a white solid product was obtained with a yield of 49%. The relative contents of the four isomers are: (1S, 2S) 4.5, (1R, 2R) 4.5, (1R, 2S) 45.46, (1S, 2SR) 45.54. 1 H NMR (400MHz, DMSO-d 6 )δ 11.06(s,1H), 5.05–4.67(m,1H), 2.13–1.90(m,1H), 1.56–1.31(m,1H), 1.26–1.08(m,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com