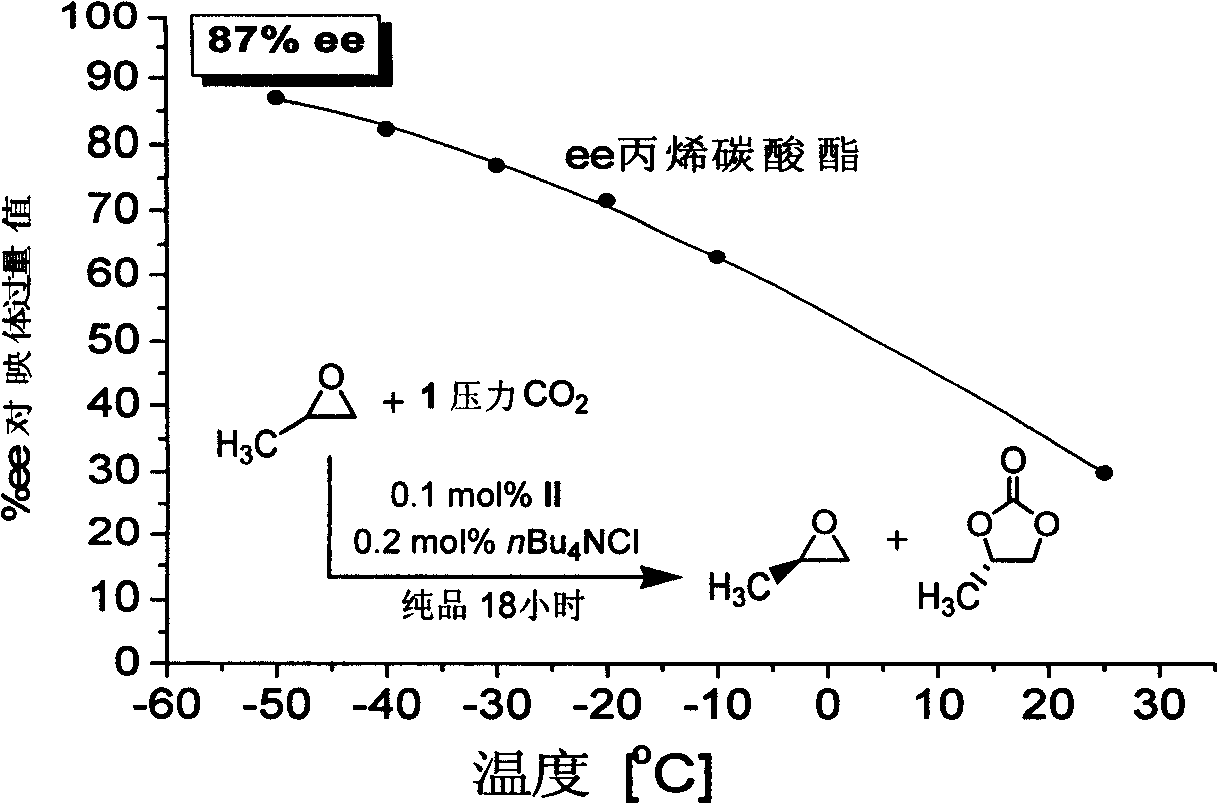
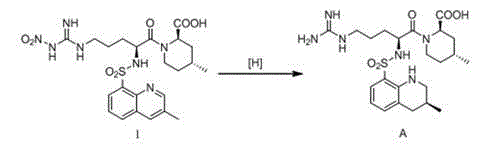
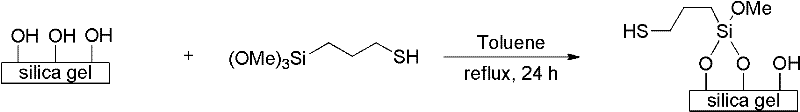
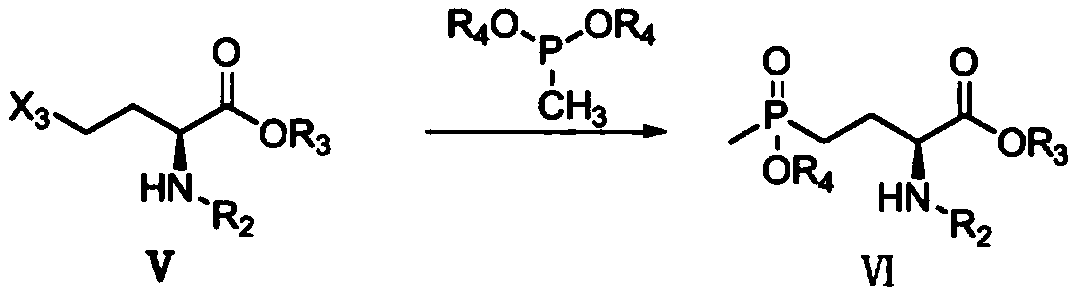
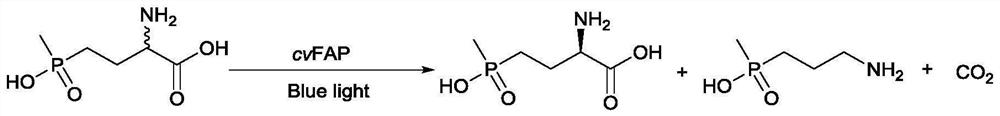
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
209results about How to "High ee %value" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
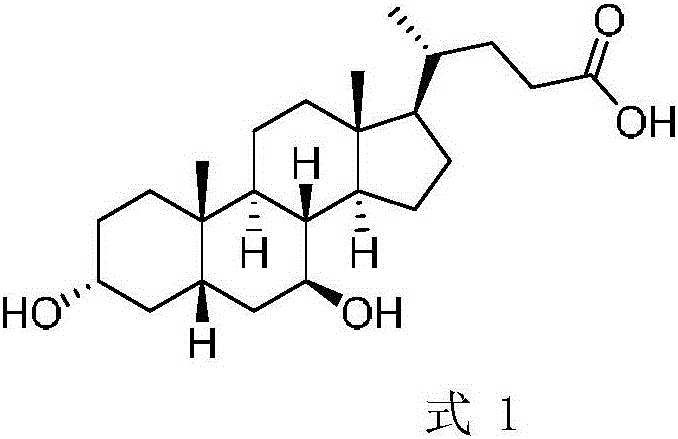
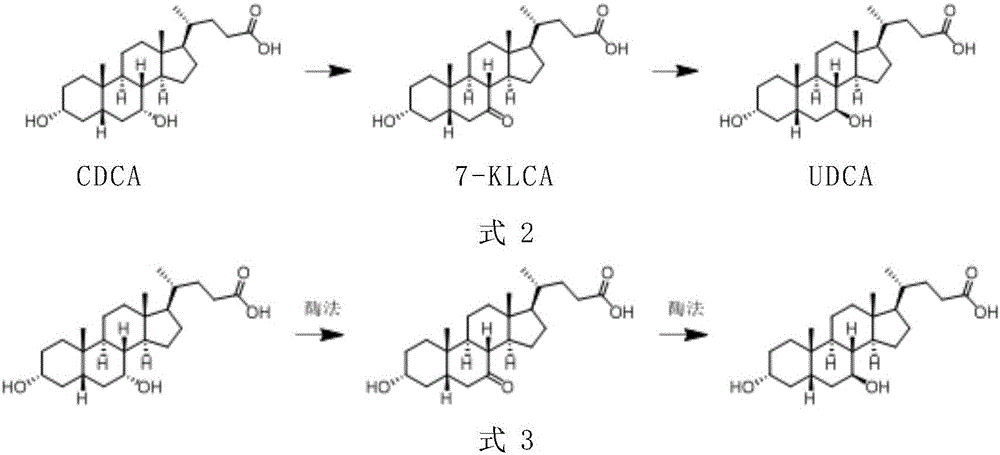
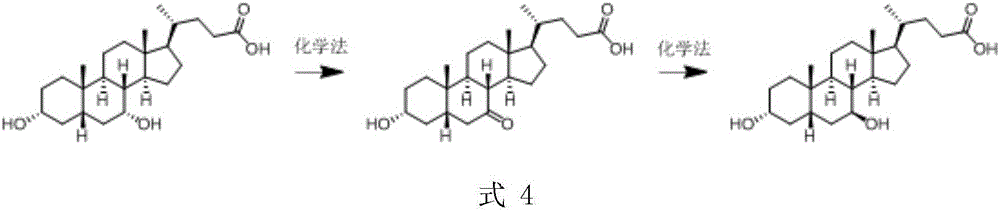
A chemical-enzyme method of preparing ursodeoxycholic acid
ActiveCN106086149AHigh ee valueEliminate enzyme inactivationSteroidsFermentationChenodeoxycholic acidOxidizing agent
A chemical-enzyme method of preparing ursodeoxycholic acid is disclosed. The method adopts chenodeoxycholic acid as an initial substrate, and prepares the ursodeoxycholic acid through a chemical process and a bio-enzyme process in order, wherein 7-KLCA reductase is adopted as a biological catalyst. A situation that an oxidant residual in a process of preparing 7-ketolithocholic acid through a chemical manner causes later enzyme inactivation in the prior art does not occur. A product prepared by the method is high in ee value and low in comprehensive cost.
Owner:ENZYMEWORKS
Transaminase mutant and application thereof
The invention discloses a transaminase mutant and application thereof. An amino acid sequence of the transaminase mutant is an amino acid sequence obtained by mutation of an amino acid sequence shownin SEQ ID NO:1, and mutation at least comprises one of the following mutation site combinations: T7C+S47C, Q78C+A330C, V137C+G313C, A217C+Y252C and L295C+C328C; or the amino acid sequence of the transaminase mutant is an amino acid sequence which has a mutation site in a mutant amino acid sequence and has 80% homology or above with the mutant amino acid sequence. The transaminase mutant realizes change of protein structure and functions, reduces enzyme dose, increased the ee value of the product and reduces after treatment difficulty, so that the transaminase mutant can be suitable for industrial production.
Owner:ASYMCHEM LAB TIANJIN
Preparation of multi-chiral catalyst, preparation and application of cyclic carbonates with optical activity
InactiveCN101270113AHigh optical purityGood effectOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric synthesesEpoxyAlkane
The present invention discloses a preparation method of carbonate with high optical activity, a main catalyst that is used in the method, and a preparation method of the main catalyst. In the method, racemic epoxy alkane and carbon dioxide have catalytic cycloaddition reaction under chiral double-component catalysts to prepare the optically active cyclic carbonate. The main catalyst is the metal complex of double-chiral Schiff base with coordinated four teeth; the auxiliary catalyst is four substituted tribromide or quaternary ammonium salt.
Owner:LANZHOU UNIVERSITY
Method for preparing L-glufosinate-ammonium
ActiveCN111662325AHigh ee valueSimple stepsGroup 5/15 element organic compoundsHerbicides and algicidesChemical synthesisBiochemical engineering
The invention relates to a method for preparing L-glufosinate-ammonium. Compared with an existing method, the method of the invention is a new chemical synthesis route, is simple in steps, easily available in raw materials and controllable in cost, can obtain the L-glufosinate-ammonium product with the high ee value without chiral catalysis, and has potential industrial application value.
Owner:LIER CHEM CO LTD +1
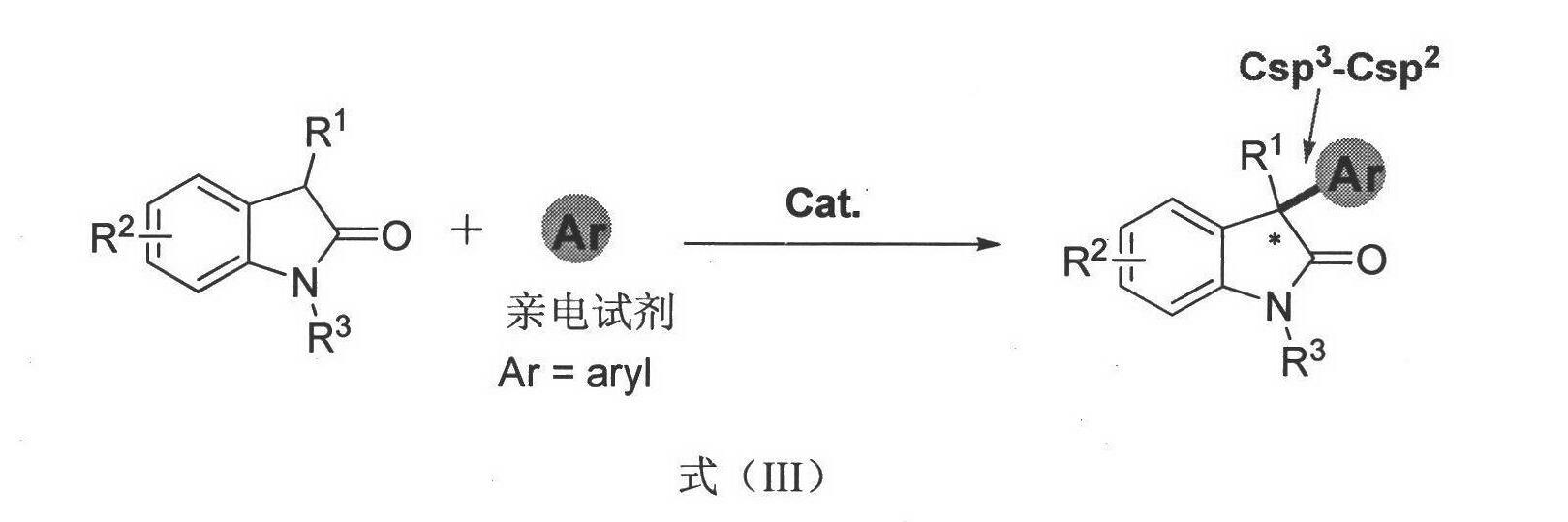
Method for asymmetric synthesis of 3,3-disubstituted-2-oxindole compound
InactiveCN102659494AHigh ee valueSimple post-reaction handlingAsymmetric synthesesNatural productEnantioselective synthesis
The invention discloses a method for asymmetric synthesis of a 3,3-disubstituted-2-oxindole compound. The method is characterized in that a 3-monosubstituted-2-oxindole compound and a 1,4-naphthoquinone compound as reaction raw materials undergo a reaction in the presence of chiral organic catalysts in air to produce the 3,3-disubstituted-2-oxindole compound. The method has mild reaction conditions and adopts easily available raw materials. The 3,3-disubstituted-2-oxindole compound obtained by the method has a very high ee value, provides a key skeleton structure for the synthesis of many natural products and drugs, and can be widely used for large-scale industrial production.
Owner:EAST CHINA NORMAL UNIV +1
Synthesis method for chiral intermediate of atorvastatin calcium
ActiveCN105153110AEasy to operateGood repeatabilityOrganic chemistry methodsChemical synthesisCyanide
The invention discloses a synthesis method for a chiral intermediate of atorvastatin calcium, and belongs to the technical field of medical intermediate synthesis. The synthesis method is characterized in that according to the process route, not only are dangerous, highly toxic and expensive chemicals such as butyl lithium, editpotassium cyanide and periodic acid in chemical synthesis prevented from being used, but also an ee value of the chiral intermediate is effectively improved due to usage of a mixed chiral catalysts of titanium iso-propylate and S-xenol. According to the synthesis method, the raw materials are low in cost and easy to obtain, the route operation is easy, the repeatability is good, the yield is very high, and the synthesis method is suitable for industrial production.
Owner:北京华素制药股份有限公司
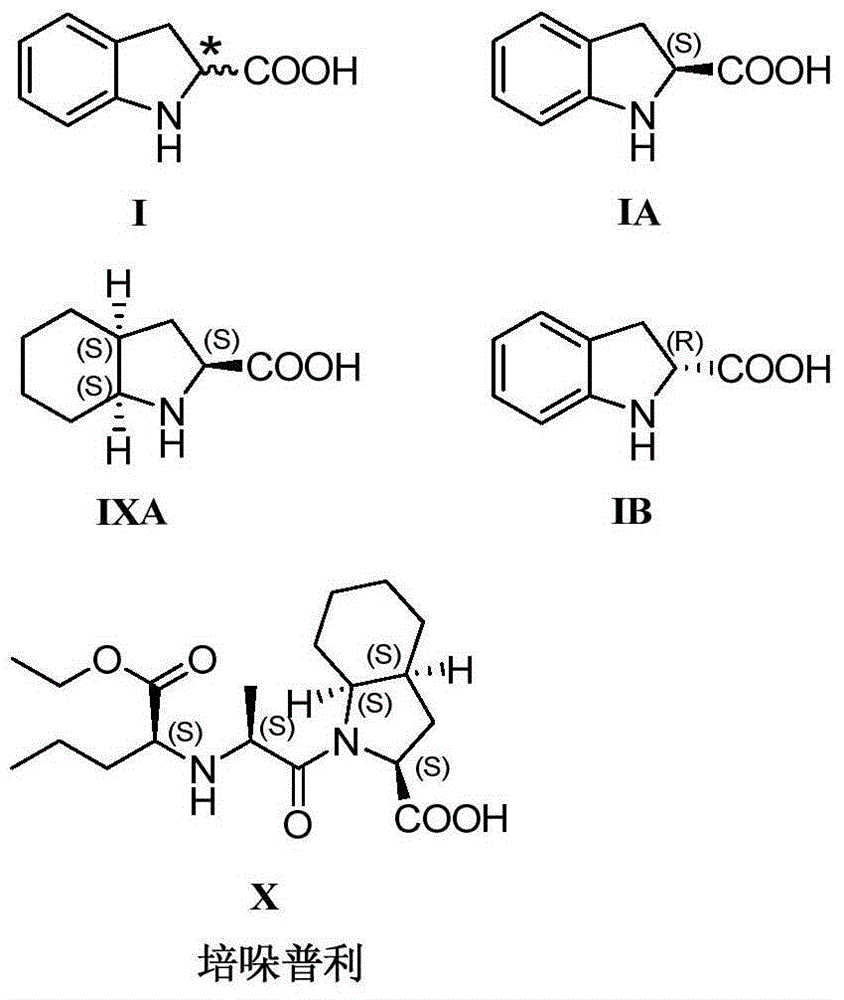
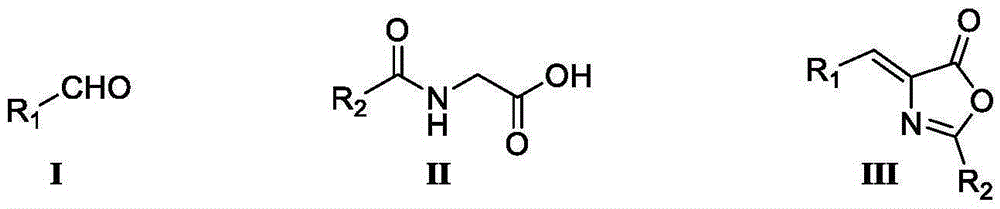
Synthesis method of enantiomer-enriched indoline-2-formic acid
ActiveCN104672124ASimple reaction conditionsImprove conversion rateOrganic chemistryEnantiomerSynthesis methods
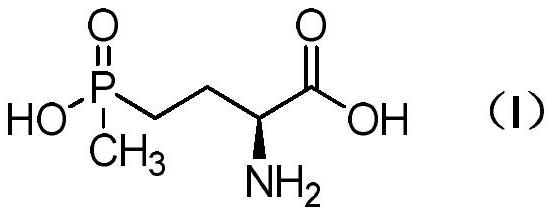
The invention discloses a synthesis method of enantiomer-enriched indoline-2-formic acid shown in a formula (I). The synthesis method of the enantiomer-enriched indoline-2-formic acid comprises the following steps: by adopting low-cost and available ortho-position halogen substituted benzaldehyde and N-benzoyl substituted glycine as starting materials, carrying out Erlenmeyer-Plochl cyclization, alkaline hydrolysis and asymmetric catalytic hydrogen for constructing a chiral center, and then carrying out acid catalysis, deprotection and cyclization sequentially or cyclization, acid catalysis and deprotection sequentially, so that the enantiomer-enriched indoline-2-formic acid is obtained. The synthesis method of the enantiomer-enriched indoline-2-formic acid has the advantages that raw materials used in the whole process route are low-cost and easily available, harmful substances or multiple danger special processes are not used, reaction conditions are mild, technological operation is simple, production is safe and stable, the product yield is high, the purity is high, less three wastes are produced, and the energy consumption is low, so that the synthesis method of the enantiomer-enriched indoline-2-formic acid is a process route especially applicable to industrial production. The formula (1) is described in the specification.
Owner:ZHEJIANG CHANGMING PHARMA
Preparation method of chiral alpha-amino acid
InactiveCN105330557ASimple processSafe, stable and reliable productionOrganic compound preparationAmino-carboxyl compound preparationGlycineAcid hydrolysis
The invention discloses a preparation method of chiral alpha-amino acid. Initial raw materials comprising aldehyde and N-acryl substituted glycine undergo Erlenmeyer-Plochl cyclization, hydrolysis or alcoholysis, asymmetric catalytic hydrogenation and acid hydrolysis to obtain the chiral alpha-amino acid compound. The method adopting the above synthesis route has the advantages of mild reaction conditions, simple technological operation, safe and stable production, realization of high yield, good chemical purity and good optical purity of the above obtained product, wide application range, and suitableness for industrial production.
Owner:天台宜生生化科技有限公司
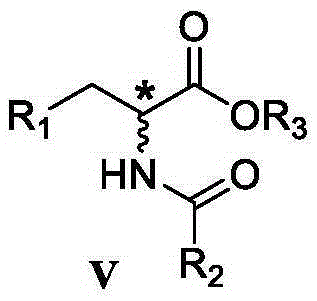
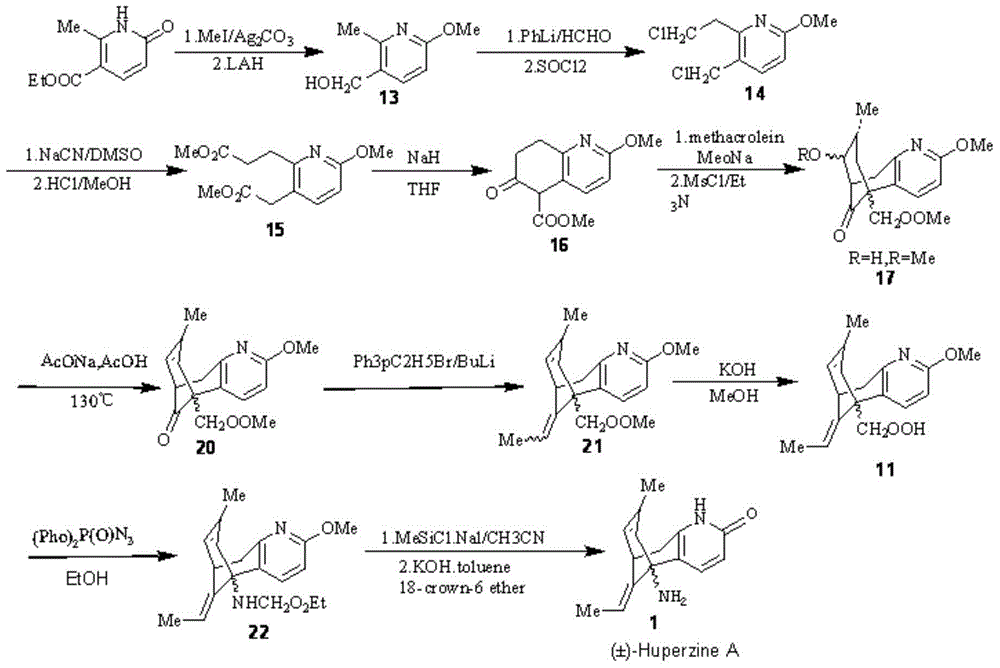
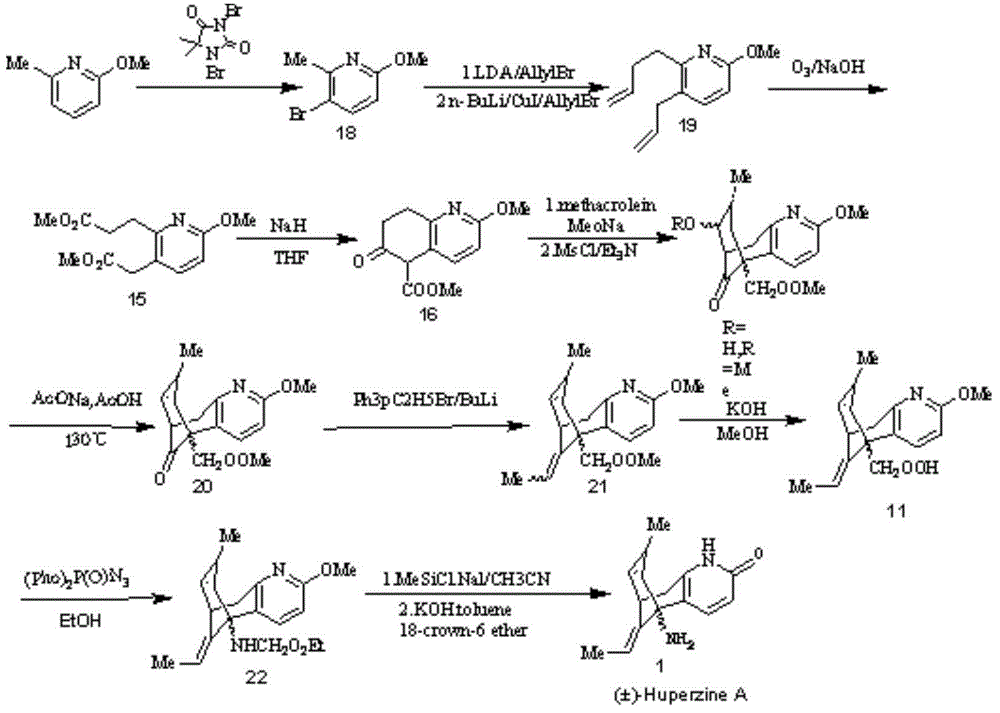
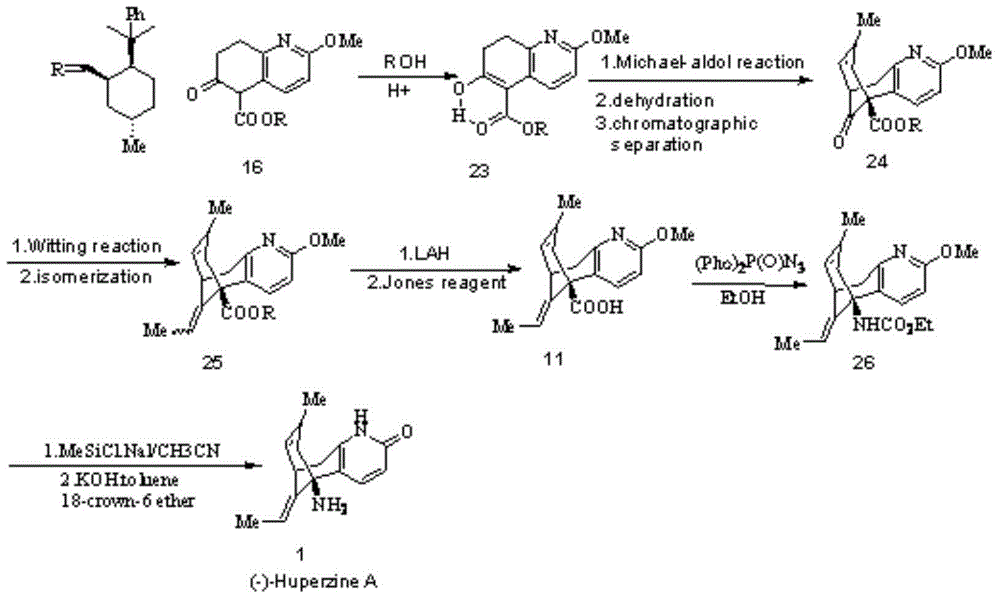
Reversible acetylcholinesterase inhibitor huperzine-A synthesis method
InactiveCN105399672AHigh ee valueLow costOrganic chemistryCholinesterase inhibitionSynthesis methods
The present invention discloses a reversible acetylcholinesterase inhibitor huperzine-A synthesis method, wherein the route is defined in the specification. The method of the present invention has advantages of easily-available raw materials, simple operation, high yield, low cost, high purity of the final product, easy quality control and the like, and is suitable for industrial production.
Owner:SHANGHAI HONGJING BIOTECH CO LTD
Ketoreductase and application thereof in preparation of (S)-2-chloro-1-(3,4-difluorophenyl) ethanol
ActiveCN109423484AIncrease concentrationHigh ee valueOxidoreductasesFermentationHigh concentrationAlcohol
The invention relates to preparation of chiral alcohol by an enzymic method, belongs to the field of preparation of a medical intermediate by a gene engineering technology, and provides ketoreductaseand application thereof in preparation of (S)-2-chloro-1-(3,4-difluorophenyl) ethanol. Ketoreductase of which the source is different from that of ketoreductase in the prior art is provided. The concentration of a substrate 2-chloro-1-(3,4-difluorophenyl) ethanone which can be converted by the ketoreductase reaches 350 g / L or above, moreover, ee value of a product (S)-2-chloro-1-(3,4- difluorophenyl) ethanol can reach 99% or above, and meanwhile, the conversion rate of the substrate is 99% or above. According to the technical scheme, by the ketoreductase which is from the new source, the problem that the substrate concentration of (S)-2-chloro-1-(3,4-difluorophenyl) ethanol prepared by enzymatic hydrolysis in industrial application is not high is solved, and the conversion rate of the high-concentration (S)-2-chloro-1-(3,4-difluorophenyl) ethanol and the ee value of the product are increased.
Owner:SYNCOZYMES SHANGHAI
Method for synthesizing chiral bisaryl alcohol compound
ActiveCN109837317AReduce usageHigh yieldOxidoreductasesGenetic engineeringAlcoholSporobolomyces species
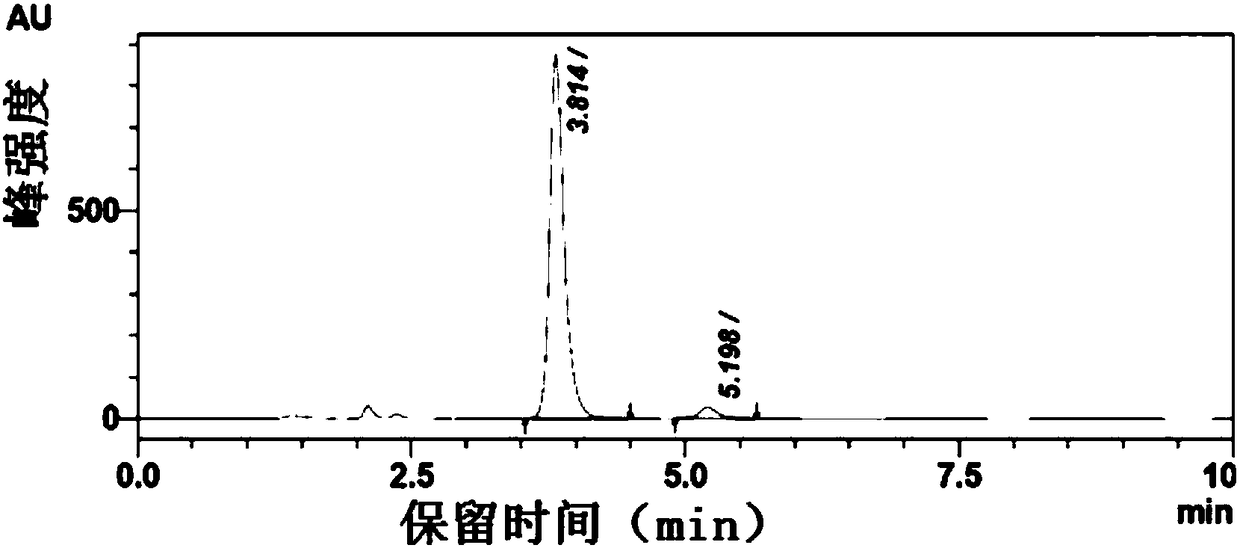
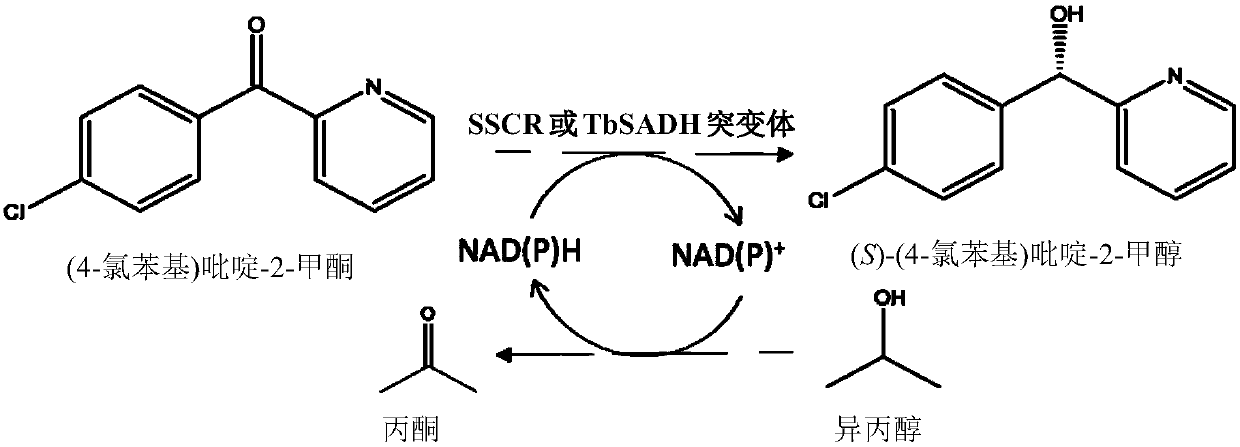
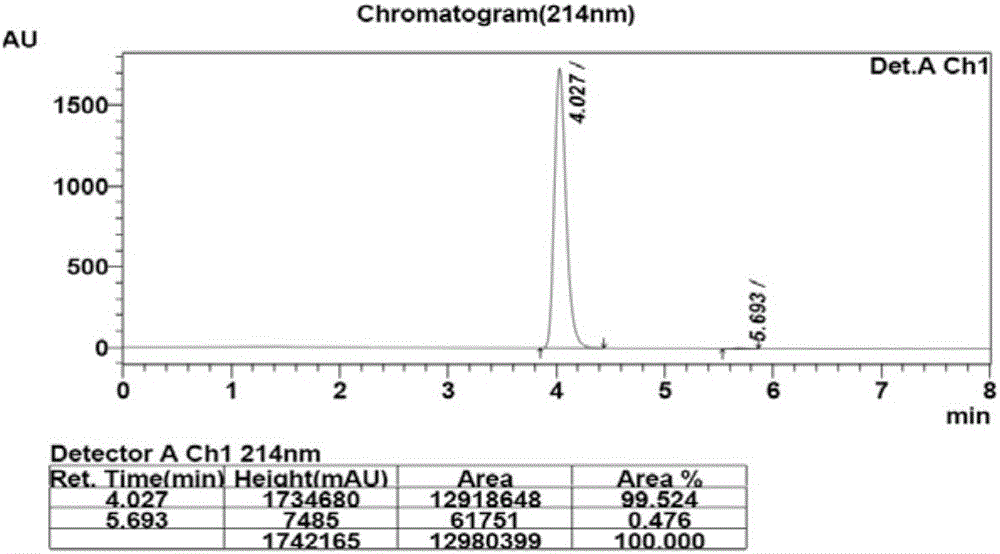
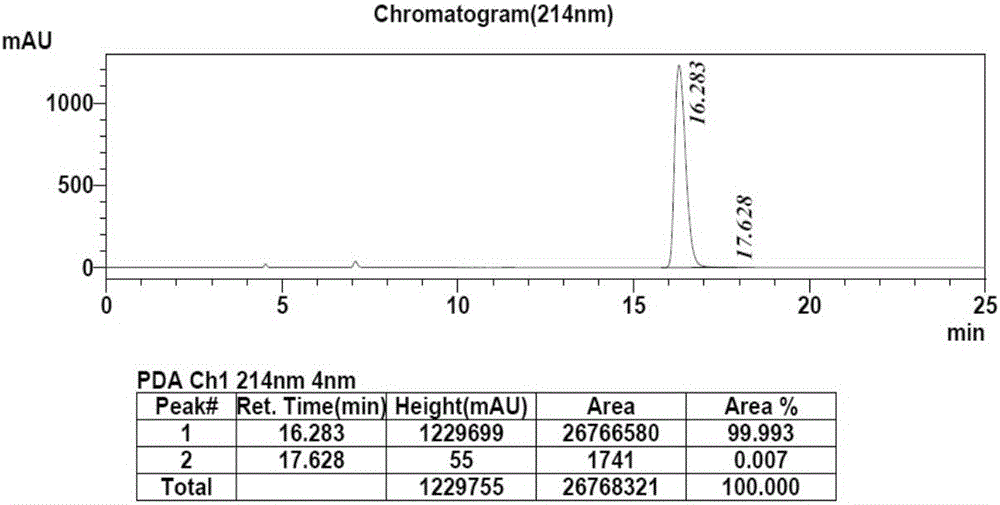
The invention discloses a method for synthesizing a chiral bisaryl alcohol compound. The method provided by the present invention specifically uses a mutant of a carbonyl reductase SSCR derived from Sporobolomyces Salmonicolor and a mutant of alcohol dehydrogenase TbSADH derived from Thermoanaerobacter brockii to catalyze asymmetric reduction of (4-chlorophenyl)pyridine-2-ketone to form (S)-(4-chlorophenyl) pyridine-2-methanol. The experiment proves that the method provided by the invention has high yield and high ee value, can reduce the addition amount of coenzyme, and does not need to add enzyme such as glucose alcohol dehydrogenase for cofactor circulation, the reaction condition is mild, and the operation is simple.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Biological preparation method of (S)-1-(2,6-dichloro-3-fluorophenyl)ethanol
The invention discloses a biological preparation method of (S)-1-(2,6-dichloro-3-fluorophenyl)ethanol. The method comprises the following steps: proportionally mixing co-expressed whole cells, 2,6-dichloro-3-fluoroacetophenone, glucose and a buffer solution, reacting at 30-40 DEG C under the pH value of 5-8, and carrying out after-treatment to obtain the target product, wherein the co-expressed whole cells are gene engineering bacteria containing carbonyl reductase and glucose dehydrogenase. Compared with the prior art, the method disclosed by the invention does not need any exogenous coenzyme, and the catalyst can be easily separated from the reaction solution, so that the technique is greatly simplified and the production cost is lowered. The product has higher yield and ee value, and thus, has favorable industrial application value.
Owner:SYNCOZYMES SHANGHAI
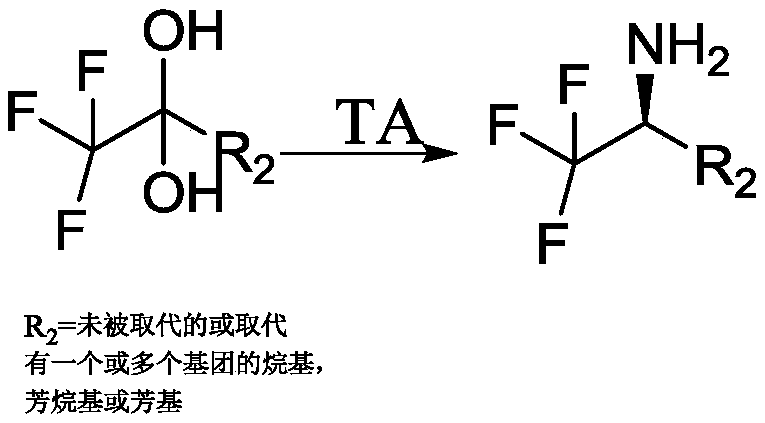
Method for synthesizing (R)-1-tert-butoxycarbonyl-3-aminopiperidine by adopting transaminase catalyst and enzymatic way
ActiveCN110724675AStrong specificityHigh catalytic activityTransferasesGenetic engineeringPtru catalystTert-Butyloxycarbonyl protecting group
The invention relates to the technical field of enzyme catalysis, and specifically relates to a transaminase catalyst; and the invention further relates to a method for synthesizing (R)-1-tert-butoxycarbonyl-3-aminopiperidine by adopting an enzymatic way, as well as a production method of the (R)-1-tert-butoxycarbonyl-3-aminopiperidine. The method for synthesizing the (R)-1-tert-butoxycarbonyl-3-aminopiperidine by adopting the enzymatic way comprises a step of, in the presence of pyridoxal phosphate and the transaminase catalyst, allowing reaction of N-tert-butoxycarbonyl-3-piperidinone as a reaction substrate with an amino donor so as to produce the (R)-1-tert-butoxycarbonyl-3-aminopiperidine. The method for synthesizing the (R)-1-tert-butoxycarbonyl-3-aminopiperidine by adopting the enzymatic way utilizes relatively few reagents, and is mild in reaction conditions, so that tedious steps needed for chemical process synthesis are greatly simplified; and moreover, a target product withan ee value up to 99.77% or above can be obtained without performance of separation. Therefore, the transaminase catalyst and the process method utilizing the transaminase catalyst to synthesize the (R)-1-tert-butoxycarbonyl-3-aminopiperidine provided by the invention have broad application prospects as well as great market values.
Owner:ENZYMASTER NINGBO BIO ENG CO LTD
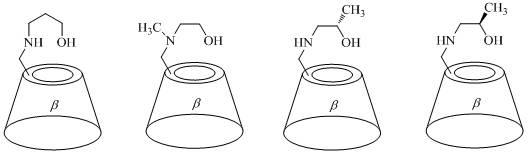
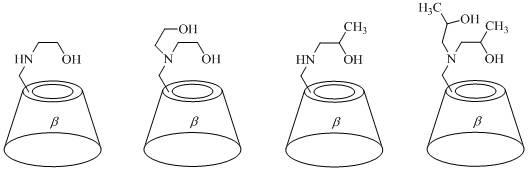
Beta-cyclodextrin derivative and preparation method and application thereof
InactiveCN102627704AHigh
<i>ee</i>
%valueHigh purityAsymmetric synthesesCatalytic oxidationEthyl group
The invention discloses a beta-cyclodextrin derivative and a preparation method and application thereof. The beta-cyclodextrin derivative is a single [6-(2-hydroxy ethyl) amino-6-desoxy]-beta-cyclodextrin, single [6-di(2-hydroxy ethyl) amino-6-desoxy]-beta-cyclodextrin, single [6-(2-hydroxy propyl) amino-6-desoxy]-beta-cyclodextrin, single [ 6 di(2-hydroxy propyl ) amino-6- desoxy]-beta-cyclodextrin, single [6-(3-hydroxy propyl) amino-6-desoxy)-beta-cyclodextrin, single [6-methyl ( 2-hydroxy ethyl ) amino-6-desoxy]-beta-cyclodextrin, single {6- [(2S)-2-hydroxy propyl] amino-6-desoxy}-beta-cyclodextrin and single {6[(2R)-2-hydroxy propyl] amino-6- desoxy}-beta- yclodextrin. The beta-cyclodextrin derivative can be prepared by a nucleophilic substitution reaction of single (6-O-p-toluene sulfonyl)-beta-cyclodextrin and corresponding aminoalcohol. The synthesis method has the advantages of simple operation, mild reaction conditions, simple purification, high yield and good purity of object products. The beta-cyclodextrin derivative can be used as a water-soluble ligand of metal mimic enzyme in aqueous metallic catalytic oxidation and aqueous metallic catalytic reduction reaction.
Owner:SUN YAT SEN UNIV
Preparation method of optically-pure argatroban
The invention belongs to the technical field of medicine, and provides a preparation method of optically-pure argatroban represented by the formula A. In the preparation method, a compound represented by a formula I is subjected to a transfer hydrogenation reaction in the presence of a chiral catalyst so as to obtain the argatroban represented by the formula A. The preparation method provided by the invention has the advantages of mild conditions, simple operation, high yield, low production cost, and suitability for massive production.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Method for preparing (3S)-3-(4-aminophenyl)-piperidyl-1-tert-butyl formate
The invention discloses a method for preparing (3S)-3-(4-aminophenyl)-piperidyl-1-tert-butyl formate. The method is characterized by comprising the following steps: 1) performing a contact reaction on a 3-(4-aminophenyl)-piperidyl-1-tert-butyl formate racemate and (R)-(-)-binaphthyl-2,2-diyl hydrogen phosphate, cooling, crystallizing, and filtering to obtain a solid compound A; (2) hydrolyzing the solid compound A obtained in the step (1) under an acidic condition, adjusting the pH to be 8 to 10 after the hydrolysis reaction is completed, extracting with ethyl acetate, and concentrating to obtain the (3S)-3-(4-aminophenyl)-piperidyl-1-tert-butyl formate. The method provided by the invention is high in yield and high in product optical purity, and provides a new way for preparing the (3S)-3-(4-aminophenyl)-piperidyl-1-tert-butyl formate.
Owner:QINGDAO YUNTIAN BIOTECH
Preparation method of load type chiral ketone catalyst and application thereof
InactiveCN102580773AWide variety of sourcesLow priceOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric synthesesPtru catalystCombinatorial chemistry
The invention discloses a preparation method of load type chiral ketone catalyst, which comprises the step of carrying out surface modification on a carrier to obtain the carrier by means of surface modification, and also comprises the following steps of: 1) preparing chiral ketone: in solution, preparing the chiral ketone by midbody ketone and allyl chlorocarbonate under the catalysis of catalyst and the condition of acid-binding agent; and 2) carrying out solid load on the chiral ketone catalyst: carrying out reflux reaction on the chiral ketone, the carrier obtained by means of surface modification and the radical initiator in inert solvent, cooling a product into room temperature, filtering, washing the obtained solid by mixed solvent of dichloromethane and diethyl ether in a soxhlet extractor, and carrying out vacuum drying under room temperature to obtain the load type chiral ketone catalyst. The load type chiral ketone catalyst prepared by the invention is applicable to the asymmetric epoxidation reaction of olefin.
Owner:ZHEJIANG UNIV
Preparing method of (2R,4R)-4-methylpiperidine-2-ethyl formate compound
InactiveCN108047125AEasy to routeSimple and fast operationOrganic chemistry methods4-methylpiperidineEthyl nipecotate
The invention relates to a preparing method of a (2R,4R)-4-methylpiperidine-2-ethyl formate compound. The method comprises the following steps of 1, regarding 4-methyl-2-cyanopiperidine as an initialraw material to be subjected to a hydrolysis reaction through hydrochloric acid to obtain 4-methyl-2-piperidinecarboxylicacid hydrochloride; 2, using ethyl alcohol to esterify 4-methyl-2-piperidinecarboxylicacid hydrochloride to obtain 4-methyl-2-ethyl nipecotate hydrochloride; 3, adding a mixed solvent of methyl tertiary butyl ether and ethyl alcohol, reacting the mixed liquid for pulping, filtering to remove cis-form 4-methyl-2-ethyl nipecotate hydrochloride solid, and collecting mother liquor to obtain anti-form 4-methyl-2-ethyl nipecotate hydrochloride; 4, using L-tartaric acid to split anti-form 4-methyl-2-ethyl nipecotate to obtain the target product (2R,4R)-4-methylpiperidine-2-ethyl formate.
Owner:BEIJING VOBAN PHARMA TECH CO LTD
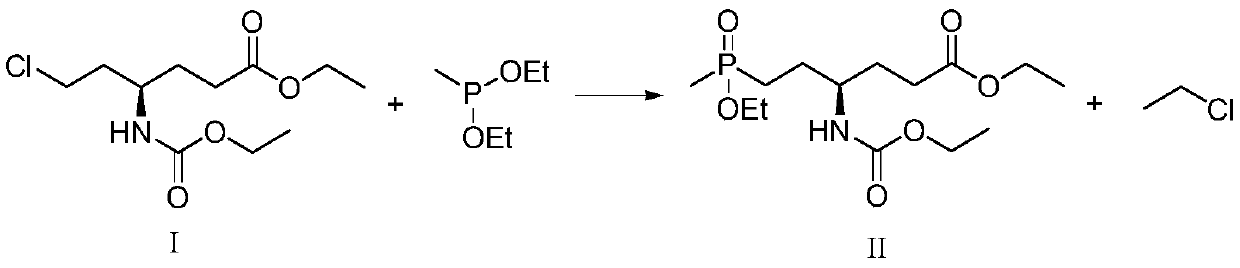
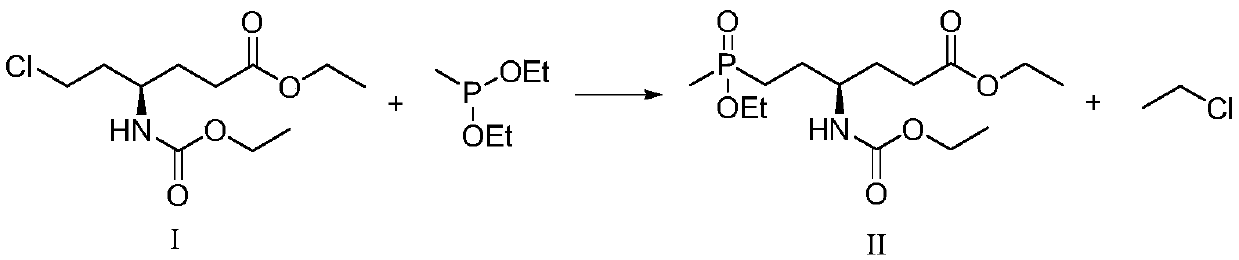
Synthetic method of L-glufosinate intermediate
PendingCN109912649AReduce dosageImprove securityGroup 5/15 element organic compoundsOrganic synthesisReaction rate
The invention provides a synthetic method of L-glufosinate intermediate shown as a formula II and belongs to the technical field of organic synthesis. The synthetic method comprises the step: under aprotective gas atmosphere, reacting a compound in a formula I with diethyl methyl phosphonate in the presence of a catalyst to obtain an intermediate shown as the formula II; the catalyst is MgBr2+PEG-400, LiBr+PEG-400, KCl+PEG-400, MgBr2+TBAB, MgCl2,MgBr2, TMSBr+TBAB, LaCl3+TBAB or CaCl2. The synthetic method provided by the invention has the benefits that as the catalyst is added, the dosage ofthe diethyl methyl phosphonate is reduced, and the process safety and the reaction rate are improved; meanwhile, the problem of reaction racemization can be solved, so that the ee value of a product is improved, the post-processing operation is simplified, and the process cost is reduced, therefore, the industrial application is more easy to perform.
Owner:LIER CHEM CO LTD +1
Novel chiral open-chain pyridoxamine catalyst and synthesis method and application thereof
InactiveCN106083703AImprove biological activitySynthesis shortcutOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydrogenHalogen
The invention relates to a novel chiral open-chain pyridoxamine catalyst and a synthesis method and application thereof. The structural general formula of the pyridoxamine catalyst is shown in the specification, wherein R1, R2, R3 and R4 are one of hydrogen, C1-24 alkyl, C1-24 alkyl containing substituent groups, substances shown in the specification and halogen, the substituent groups on C1-24 alkyl are a substance shown in the specification or a substance shown in the specification or a substance shown in the specification or O-Rw or S-Rw' or halogen, and Rx, Rx', Ry, Ry', Ry'', Rz, Rz', Rw and Rw' are one of hydrogen, methyl, ethyl, n-propyl, isopropyl, n-butyl, tertiary butyl, cyclopentyl, cyclohexyl, cycloheptyl, phenyl, benzyl, (1-phenyl)ethyl, 1-naphthyl, 2-naphthyl and halogen. Compared with the prior art, the pyridoxamine catalyst can achieve rapid and efficient synthesis of chiral amino acid, the preparation raw materials are easy to obtain, reaction conditions are mild, cost is low, and when the novel chiral open-chain pyridoxamine catalyst is used for a transamination reaction, the conditions are mild, and the reaction is stable.
Owner:SHANGHAI NORMAL UNIVERSITY
Fatty acid photodecarboxylase mutant and application thereof to synthesis of L-phosphinothricin
ActiveCN112063608AIncrease enzyme activityHigh ee valueBacteriaMicroorganism based processesGlycineArginine
The invention discloses a fatty acid photodecarboxylase mutant and an application thereof to synthesis of L-phosphinothricin. The fatty acid photodecarboxylase mutant is obtained by mutating glycine at the 402nd site of amino acid shown in SEQ ID No.2 into phenylalanine; or, mutating threonine at the 370 site into arginine when mutating on the 402nd site; or mutating serine at the 513rd sitewhen mutating on the 402nd site and the 370 site into glycine and the like. A fatty acid photodecarboxylase gene is mutated by utilizing a site-specific saturation mutation technology, the 370th site, the 371st site, the 402nd site, the 513rd site and the 514 site are found to be key sites influencing enzyme activity and stereoselectivity, a mutant of which the enzyme activity and ee valueare far higher than those of female parent fatty acid photodecarboxylase is obtained, the highest conversion rate is 50%, and the product ee value is 96%.
Owner:ZHEJIANG UNIV OF TECH
Method for preparing L-glufosinate-ammonium
ActiveCN111662326AHigh ee valueSimple stepsGroup 5/15 element organic compoundsChemical synthesisBiochemical engineering
The invention relates to a method for preparing L-glufosinate-ammonium. Compared with an existing method, the method of the invention is a new chemical synthesis route, is simple in steps, easily available in raw materials and controllable in cost, can obtain the L-glufosinate-ammonium product with the high ee value without chiral catalysis, and has potential industrial application value.
Owner:LIER CHEM CO LTD +1
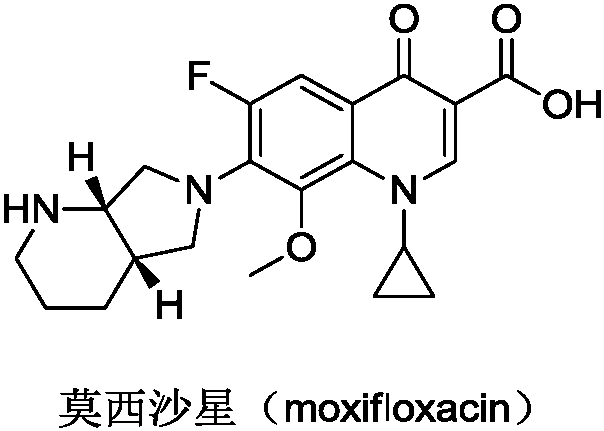
Preparation method of moxifloxacin intermediate compound
The invention discloses a preparation method of a moxifloxacin intermediate compound. The preparation method comprises the following step of under the actions of omega-transaminase and / or immobilizingtype thereof and ammonia donor, performing the following ammonia conversion reaction on a compound shown in a formula (III) in a solvent, so as to prepare a compound shown in a formula (II), whereinan amino acid sequence of the omega-transaminase is shown in SEQ ID NO.1, SEQ ID NO.2 or SEQ ID NO.3 in a sequence table; X is chloride, bromine, iodine, methanesulfonate or tosylate; R is C1-4 carbalkoxy, carbobenzoxy or benzyl. The preparation method has the advantage that the cost is low, the fewer steps are required, the operation is simple, the ee value of a product reaches 99% or above, andthe preparation method is more suitable for industrialization production. (The formulas are shown in the attached figures.).
Owner:SHANGHAI PUYI CHEM CO LTD
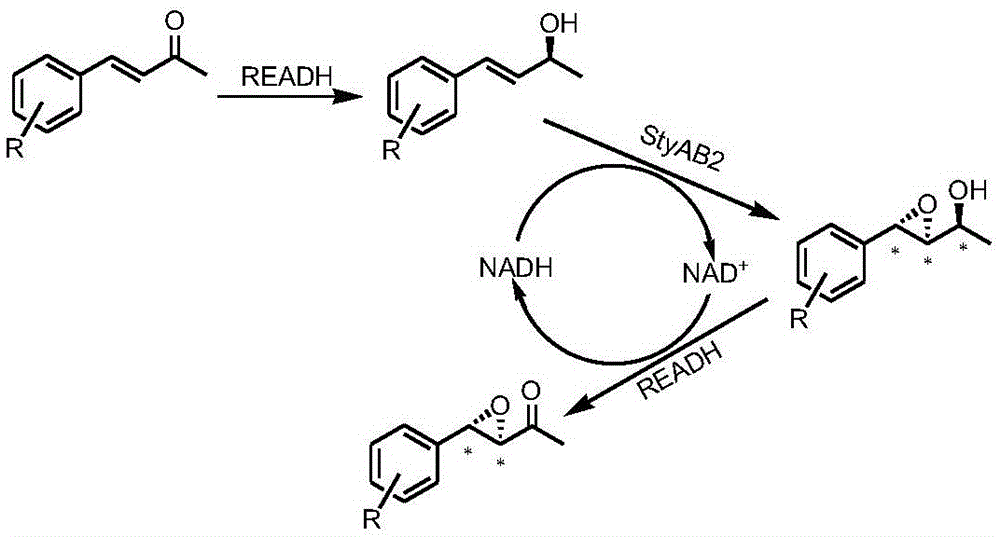
New method for using controllable multi-enzyme cascade reaction to synthesize optical pure allylic epoxy ketone or alcohol
ActiveCN105087704AHigh ee valueHigh de valueBacteriaMicroorganism based processesStyrene monooxygenaseEpoxy
The invention discloses a new method for using controllable multi-enzyme cascade reaction to synthesize two kinds of optical pure epoxy compounds. The method is characterized in that coexpression carbonyl reductase in escherichia coli and the recombinant bacteria of styrene monooxygenase are used as biocatalysts, alpha, beta-unsaturated ketone compounds are used as substrate, isopropanol is used as a switch for controlling, and chirality pure allylic epoxy ketone / alcohol is synthesized.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
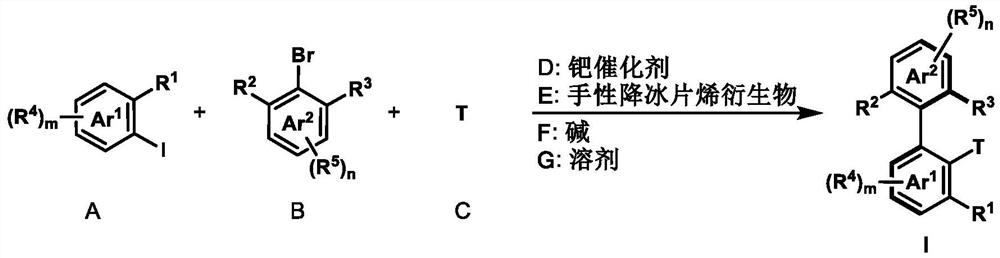
Preparation method of axially chiral biaryl compound and chiral fluorenol compound
ActiveCN111662158ANo special handling requiredLow priceSilicon organic compoundsCarboxylic acid nitrile preparationPtru catalystIodide
The invention discloses a preparation method of an axially chiral biaryl compound and a chiral fluorenol compound. The preparation method comprises the following steps: stirring and reacting aryl iodide, aryl bromide and olefin which are taken as starting raw materials in an organic solvent at 50-150 DEG C under the action of a palladium catalyst, a phosphine ligand, a chiral norbornene derivativeand an alkali to obtain the biaryl axial chiral compound and the chiral fluorenol compound. The method has the advantages of cheap and easily available raw materials, mild reaction conditions, good substrate universality, high yield and simple preparation process. The prepared biaryl axial chiral compound can be applied to synthesis of novel chiral ligands and chiral catalysts.
Owner:WUHAN UNIV
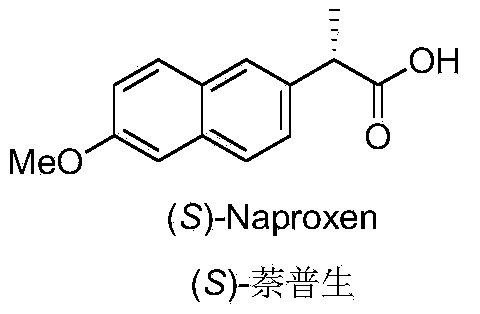
Novel asymmetric catalytic synthesis method of (S)-naproxen
InactiveCN103755553AHigh ee valueOrganic compound preparationCarboxylic acid esters preparationPropionatePropanoic acid
The invention discloses a novel asymmetric catalytic synthesis method of (S)-naproxen. According to the method, racemic 2-halogenated propionate is used as an initial raw material to perform an asymmetric Kumada cross-coupling reaction with 6-methoxyl-naphthyl grignard reagent in the catalysis of bisoxazoline / cobalt to prepare (S)-naproxen ester, and the ee value is increased by using a re-crystallization method, and the (S)-naproxen is prepared by catalytic hydrogenation. The synthetic route is simple and short, is a two-step reaction, the total yield of the whole synergetic route is 43 percent, and the optical purity of the product is more than 99 percent.
Owner:CHINA AGRI UNIV
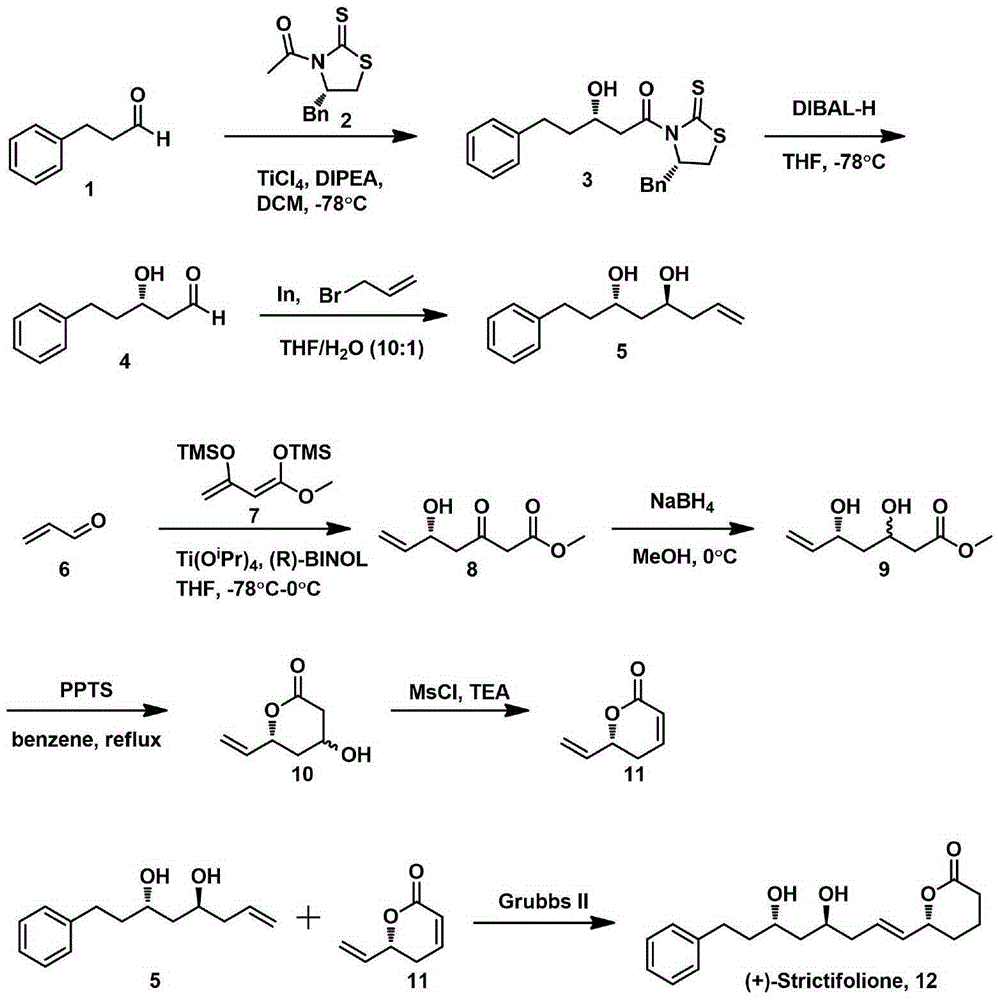
Natural product (+)-Strictifolione synthetic method
InactiveCN105254604ASimple and fast operationHigh yieldOrganic chemistryChemical recyclingIndiumStrictifolione
The invention discloses a natural product (+)-Strictifolione synthetic method. The method comprises the steps that 3-benzenepropanal and an Evans chiral auxiliary reagent are subjected to an aldol reaction for diisobutyl aluminum hydride reduction and then subjected to an addition reaction with metal indium activated 3-propylene bromine to obtain a compound shown in the formula 5; silyl enol ether reacts with acrolein under the action of titanium tetraisopropoxide and (R)-1,1-binaphthol, then carbonyl is reduced through sodium borohydride, p-toluenesulfonic acidpyridinium salt is used for cyclization, and acidification treatment is carried out after a methylsulfonyl group becomes a leaving group to obtain a compound shown in the formula 11; a Grubbs second-generation catalyst is used for carrying out a metal coupling reaction on the compounds shown in the formulas 5 and 11 to obtain (+)-Strictifolione. According to the bisection synthetic method, the design is simple and reasonable, operation is easy and convenient, the reaction condition is moderate, linear steps are simple, the product yield is high, and the production cost is greatly reduced.
Owner:JIANGXI SCI & TECH NORMAL UNIV
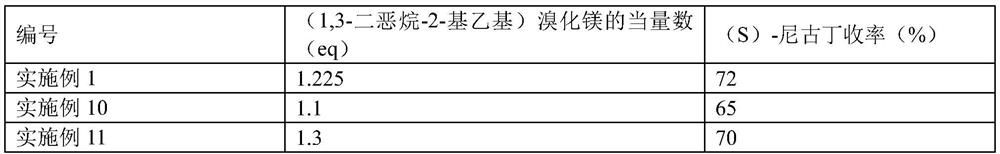
Preparation method for synthesizing chiral nicotine from chiral tert-butyl sulfinamide
PendingCN113444070AHigh yieldHigh ee valueOrganic chemistry methodsPtru catalystCombinatorial chemistry
The invention discloses a preparation method for synthesizing chiral nicotine from chiral tert-butyl sulfinamide. The preparation method comprises the following steps: carrying out a condensation reaction on 3-pyridylaldehyde and chiral tert-butyl sulfinamide under the action of titanate; then carrying out a reaction on a condensation product and (1,3-dioxan-2-ylethyl)magnesium bromide; performing cyclization under an acidic condition; and finally, carrying out reduction and aminomethylation to obtain the chiral nicotine. The novel synthesis method of chiral nicotine provided by the invention has the advantages that a reaction route is short, raw materials are easy to obtain and low in price since chiral tert-butyl sulfinamide is taken as a starting raw material, expensive or complex chiral catalysts do not need to be prepared, each step of reaction operation is simple, and the chiral nicotine generated by the reaction is high in yield, high in ee value and reduced in production cost.
Owner:SHENZHEN ZINWI BIO-TECH CO LTD
Avalopam intermediate as well as preparation method and application thereof
PendingCN114163380AInnovative designHigh yieldOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsCombinatorial chemistryOrganic chemistry
The invention provides an avalcopam intermediate (a compound shown in a formula I). The compound can be used as an avalcopam raw material or intermediate for synthesizing avalcopam. The invention further provides a preparation method of the atavapam intermediate compound shown in the formula I. The target product atavapam intermediate compound shown in the formula I is obtained through three-step reaction, the whole route design is novel, the chiral resolution process adopted in the prior art is avoided, practicability is high, the yield is high, the reaction speed is high, few by-products are generated, and the preparation method is very suitable for industrial application.
Owner:CHONGQING MEDICAL UNIVERSITY
Preparation method of (3S, 4R)-3-amido-4-methyl piperidine-1-tertiary butyl carboxylate
ActiveCN103664743ASimple reaction conditionsHigh stereoselectivityOrganic chemistryCarboxylateCarbonyl group
The invention discloses a high-stereoselectivity preparation method of (3S, 4R)-3-amido-4-methyl piperidine-1-tertiary butyl carboxylate, which is mainly used for solving the problems that compounds reported by conventional documents have long synthesis routes and poor stereoselectivity. The preparation method comprises the following steps: carrying out ylide reaction under an alkaline condition by taking a compound R)-1-(1-tert-butyloxycarbonyl)-4-oxo-3-piperidyl hydrazine-1,2-dibenzyl dicarboxylate as a starting material to obtain (S)-1-(1-tert-butyloxycarbonyl)-4-methylene -3-piperidyl hydrazine-1,2-dibenzyl dicarboxylate; then, obtaining (3S, 4R)-3- diazanyl-4-methyl piperidine-1-tertiary butyl carboxylate by catalytic hydrogenation under action of a metal hydrogenation catalyst and hydrogen gas; and finally, reducing under action of the metal hydrogenation catalyst and hydrogen gas to obtain the (3S, 4R)-3-amido-4-methyl piperidine-1-tertiary butyl carboxylate. And the total yield is 34.5%.
Owner:上海药明康德新药开发有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com